Abstract
Background/Aim:
Anastomotic leak after esophagectomy is one of the most challenging complications resulting in a high morbidity and mortality and prolonged hospitalization. The study intended to assess the outcome of endoluminal self-expanding stent in the treatment of this problem.
Settings and Design:
Department of Thoracic and Cardiovascular Surgery, Arhus University Hospital, Skejby, Arhus, Denmark. A retrospective study.
Patients and Methods:
From January 2007 to December 2010, 209 patients underwent esophagectomy for malignant disease of the esophagus or the cardia. Twenty patients developed anastomotic leak. Treatment consisted of conservative measures, surgery, and stent placement. Details of treatment, clinical outcome, complications, and mortality were evaluated.
Statistical analysis:
None.
Results:
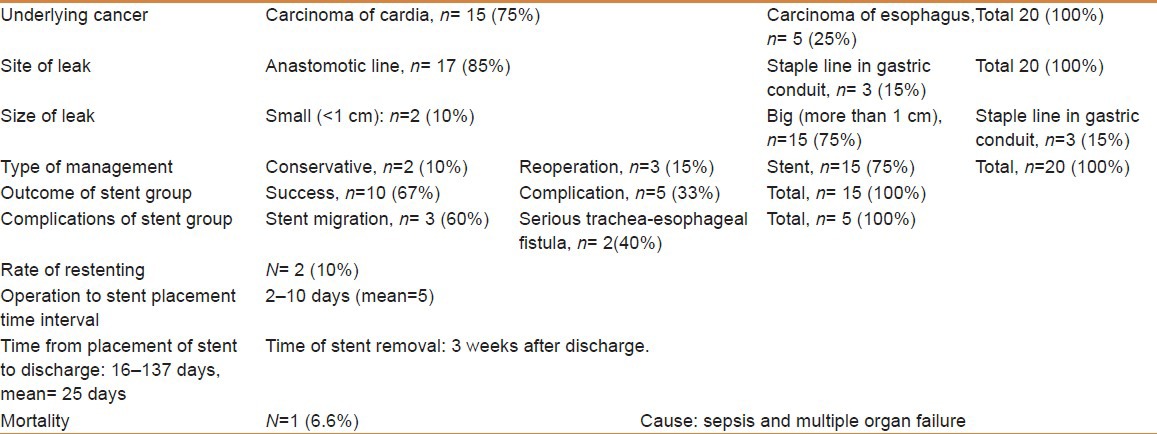
One hundred and forty-seven patients (70.3%) had carcinoma of the cardia, whereas 62 patients (29.7%) had esophageal carcinoma. Twenty patients (9.5%) developed anastomotic leak; small (<1 cm) in two patients (10%); managed conservatively and bigger than 1 cm in 15 patients (75%); treated with an esophageal stent (Hanaro stent, DIAGMED Healthcare, Thirsk, YO7 3TD, United Kingdom). In three patients (15%), perforation of the staple line of the intrathoracic gastric conduit was found and managed by reoperation. Functional sealing of anastomoses after stent placement could be achieved in 10 patients (67%). Stent-related morbidity developed in five patients (33%): Migration of the stent, n=3 and tracheoesophageal fistula, n=2. Stents were smoothly removed 3 weeks after discharge. The mean hospital stay was 25 days. There was only one stent-related death (6.6%).
Conclusion:
Endoluminal stent implantation is an effective and safe option in the management of postesophagectomy leaks.
Keywords: Anastomosis, esophagus, endoscopy
Postoperative leakage at surgical anastomoses in the gastrointestinal tract represents a serious clinical problem. Clinical leakage from anastomoses after esophagectomy remains one of the most challenging complications resulting in a high morbidity and mortality leading to prolonged hospitalization.
Traditional conservative management has most often consisted of effective drainage of the pleural space, antibiotic therapy, and oral intake restrictions for small leaks. Operative repair, or if unsuccessful, esophageal diversion are considered for large leaks.[1] Such treatments postpone oral intake and prolong hospitalization with significant rate of morbidity.[2]
Recently, temporary endoscopic self-expanding covered stent placement is well accepted as an effective treatment for postoperative anastomotic leak following esophageal surgery as well as for malignant tracheaesophageal fistula with low morbidity and mortality.[1,3]
Early experience with endoluminal esophageal stent for spontaneous and iatrogenic esophageal perforations has drawn attention of their value in the treatment of intrathoracic leak after esophagectomy.[4]
The aim of intraluminal stent implantation is to seal leaks, maintain protection of the esophageal mucosa from the irritating gastrointestinal sections while healing takes place, as well as preventing soiling of the mediastinum and pleural cavity. However, the use of endoluminal stent placement is sometimes associated with complications such as tissue overgrowth, erosion into the adjacent organs, and stent migration necessitating repositioning.[4,5]
In this study, we aim to assess the safety and clinical efficacy of treating anastomotic leaks after esophagectomy with temporary intraluminal self-expanding covered stent.
PATIENTS AND METHODS
Between January 2007 and December 2010, 209 patients underwent transthoracic esophagectomy, with gastric pull up for malignant diseases (either cancer of the esophagus or cardia). Patients with benign diseases were excluded from the study. Twenty patients developed postoperative anastomotic leak. They were the subject of this study.
Data on patients, demographics, type of cancer, time interval between the primary operation and manifestation of leak, details and type of treatment with stent, clinical success, complications, and mortality were retrospectively reviewed.
The diagnosis of an anastomotic leak was usually made initially on clinical grounds when fever developed with a marked increase in infection parameters, alteration in the amount or type of chest drain fluid, atrial fibrillation, respiratory or circulatory insufficiency. Clinical suspicion of anastomotic leak would lead to prompt computed tomography (CT) scanning of the chest and upper abdomen with peroral contrast study. Cases with radiological evidence of contrast leak into the mediastinum or pleura were better evaluated by gastroscopy to select the appropriate treatment. For cases with a very high clinical suspicion of anastomotic leak, gastroscopy was directly chosen (without CT scan) for confirmation of diagnosis and selection of the treatment option.
Flexible esophagoscopy under general anesthesia was routinely performed to find the site of a leak (whether from a gastric conduit or from a gastroesophageal anastomosis). The former would be treated with prompt surgical re-exploration and repair, whereas the latter (if bigger than 1 cm) usually necessitated intraluminal self-expanding esophageal stent (Hanaro stent) [Figure 1] and nasojejunal feeding tube (Fresenius kabi) placement with the aid of fluoroscopy for subsequent enteral nutrition. Stents were placed endoscopically utilizing general anesthesia and fluoroscopy by a team of upper gastrointestinal surgeon and a thoracic surgeon. Leak occlusion was confirmed by esophagogram. Small anastomotic leaks (<8 mm) were managed conservatively.
Figure 1.
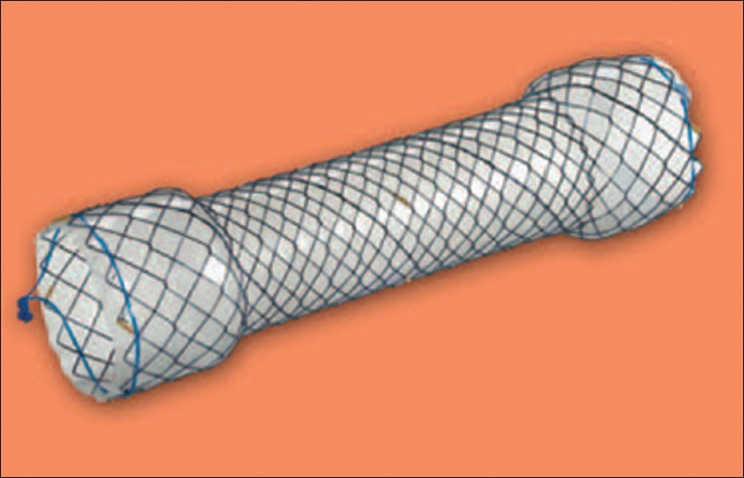
Hanaro stent
All patients received intravenous antibiotics, selective gastrointestinal decontamination for 10 days, as well as drainage of the affected pleural cavity by chest drain or ultrasound-guided percutaneous catheter drainage. Fluid diet was initiated when there was a good clinical response and leak ceased. Stent removal was usually done 2–3 weeks after discharge with the aid of flexible esophagoscopy under general anesthesia.
RESULTS
One hundred and forty-seven patients (70.3%) had adenocarcinoma of the cardia, whereas 62 patients (29.7%) had esophageal carcinoma. Twenty patients (9.5%) developed a postoperative anastomotic leak. They were 15 males and 5 females, between 61 to 84 years of age with a mean age of 68 years. Sixteen patients had conventional surgery (thoracotomy and laparotomy), whereas four had had minimal invasive procedures (thoracoscopy and laparoscopy). Fifteen patients (75%) underwent esophagectomy for cancer of the cardia, whereas the remaining five patients (25%) had surgery for esophageal cancer. The leak was small (<1 cm) in two patients (10%) and thus was managed conservatively. In three patients (15%) perforation of the staple line of the intrathoracic gastric conduit was found and managed by reoperation. Bigger anastomotic leak (>1 cm) occurred in 15 patients (75%) and was treated with an esophageal stent (Hanaro stent, DIAGMED healthcare, Thirsk, YO7 3TD, United Kingdom). Functional sealing of anastomoses after stent placement could be achieved in 10 patients (67%). Stent-related morbidity developed in five patients (33%). These included unsuccessful functional sealing of the leak demonstrated by CT esophagogram 10 days after stent placement with migration of the stent in three patients and serious tracheoesophageal fistulas in two. Two patients underwent replacement of new stents and two patients had resolution clips. All stents were smoothly removed three weeks after discharge without residual leak. Hospital stay from time of stent placement to discharge ranged between 16 and 137 days with a mean of 25 days. There was only one death (6.6%) due to sepsis and multiple organ failure. The clinical characteristics of the patients are shown in Table 1.
Table 1.
Patient demographics

Table 2 displays the details of surgical procedures performed prior to esophageal stent placement. The most common procedure was thoracotomy and laparoscopy, n=109 (52.4%), whereas the least was thoracoscopy and laparoscopy, n= 20 (9.5%). Table 3 shows the details of management of postesophagectomy leak.
Table 2.
Surgical procedures performed prior to esophageal stent placement

Table 3.
Details of management of postesophagectomy leak
DISCUSSION
Esophagectomy is still the only potentially curative treatment for cancer of esophagus and cadria.[6,7] Esophageal and gastric leakage from postoperative anastomoses dehiscence, staple-line dehiscence, or iatrogenic perforation can be a devastating event and have significant impact on patient's morbidity, mortality and quality of life.[2,3,4]
The rate of post-esophagectomy anastomotic leak as shown by a recent review of the Society of General Thoracic Surgery Database is 11%;[7] hence our rate (9.5%) is lower and it is in the range reported by Blewett et al (5–20%).[8]
Previous reports found a hospital mortality of 35.7% in patients with anastomotic leak versus 4.2% for those without a leak, whereas anastomotic leak was also a negative predictor of long-term survival.[1]
Conservative management with effective drainage of the mediastinum and pleural space, antibiotic therapy, selective decontamination of the gastrointestinal tract, and restriction of oral intake is a well-accepted management for small leaks.[9] We achieved a successful outcome in two patients with small anastomotic leak (>1 cm).
Recently, endoscopic stent placement has emerged as an effective treatment for esophageal ruptures and postoperative leaks. A favourable outcome with low morbidity and mortality has been reported.[1,2,3,4,5,9]
Surgical repair or esophageal diversion with subsequent complex reconstruction is necessary for large leaks or following unsuccessful conservative management of small ones. This has long been considered to be the “Gold standard” management.[3] Survival improvements have been reported with early operative policy, although it is associated with a high mortality rate (30%) after a late diagnosis.[8]
Functional sealing of the leak (tested by CT-esophagography after 10 days) was achieved in 10 patients (75%). This is in accordance with previous studies reporting successful sealing rates of 48-100%.[1,5,9]
Stent-related morbidity in this study was found in five patients (33%), which is relatively low compared with other series (46–72%).[10,11] Three patients had unsuccessful functional sealing with stent migration and two had serious tracheoesophageal fistulae. It is not clear whether T.E. fistula occurred due to stent erosion through esophagus or anastomotic defect, or because of mediastinal abscess and necrosis of the trachea. Reoperation with stent removal, disruption of esophagogastric continuity, direct repair of the trachea with esophageal diversion was undertaken in one patient with good clinical outcome. The other patient died because of sepsis and multiorgan failure syndrome after many futile interventions. (The mortality in this study was 6.6%; within the range of 0–28% reported by other studies).[5]
The rate of additional interventions was high in this study, mainly because of stent migration in three patients. Migration is the predominant problem with esophageal stents.[3] Migration rate of 6–18% has been reported for the use of stents in patients with inoperable esophageal strictures.[12] This is relatively lower than migration rate of stents used for anastomotic leaks. In palliative stent placement of esophageal strictures, the luminal stenoses act as anchors for the stent minimizing the risk of migration, whereas in anastomotic leaks, such luminal stenoses are absent and the direct contact of the stent with the mucosa is the only brace for it. This raises the question of the role of additional measures (clips, temporary sutures, and pexy techniques) to anchor the stent and prevent migration.[3]
This study did not show correlation between onset of leak and time of performing intervention. Previous studies concluded that the time between onset of leak and intervention is the most critical prognostic factor; increasing delay between leaks and intervention is associated with a worse prognosis due to high occurrence of septic complications.[13]
Immediate reoperation and repair of staple line leak in the intrathoracic gastric tube was needed in three patients with good clinical outcome. Currently, the standard treatment of gastric staple line dehiscence is surgical exploration,[14] although there are reports of successful therapy with endoscopic stent placement.[15]
In conclusion, this study demonstrates the value of using endoluminal stent placement in treating patients with postesophagectomy leak with acceptable morbidity and mortality. The procedure is a good and efficient alternative to reoperation, esophageal diversion, and subsequent reconstruction.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Moyes LH, Mackay CK, Forshaw MJ. The use of self-expanding plastic stents in the management of esophageal leaks and spontaneous esophageal perforations. Diagn Ther Endosc 2011. 2011 doi: 10.1155/2011/418103. 418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schubert D, Pross M, Nestler G, Ptok H, Scheidbach H, Fahlke J, et al. Endoscopic treatment of mediastinal anastomotic leaks. Zentralbl Chir. 2006;131:369–75. doi: 10.1055/s-2006-949532. [DOI] [PubMed] [Google Scholar]
- 3.Blackmon SH, Santora R, Schwarz P, Barroso A, Dunkin BJ. Utility of removable esophageal covered self-expanding metal stents for leak and fistula management. Ann Thorac Surg. 2010;89:931–7. doi: 10.1016/j.athoracsur.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 4.Van Boeckel PG, Sijbring A, Vleggaar FP, Siersema PD. Systematic review: Temporary stent placement for benign rupture or anastomotic leak of the esophagus. Aliment Pharmacol Ther. 2011;33:1292–301. doi: 10.1111/j.1365-2036.2011.04663.x. [DOI] [PubMed] [Google Scholar]
- 5.Tuebergen D, Rijcken E, Mennigen R, Hopkins AM, Senninger N, Bruewer M. Treatment of thoracic esophageal anastomotic leaks and esophageal perforations with endoluminal stents: Efficacy and current limitations. J Gastrointest Surg. 2008;12:1168–76. doi: 10.1007/s11605-008-0500-4. [DOI] [PubMed] [Google Scholar]
- 6.Viklund P, Lindblad M, Lu M, Ye W, Johansson J, Lagergren J. Risk factors for complications after esophageal cancer resection: A prospective population-based study in Sweden. Ann Surg. 2006;243:204–11. doi: 10.1097/01.sla.0000197698.17794.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gockel I, Exner C, Junginger T. Morbidity and mortality after esophagectomy for esophageal carcinoma: A risk analysis. World J Surg Oncol. 2005;3:37. doi: 10.1186/1477-7819-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blewett CJ, Miller JD, Young JE, Bennett WF, Urschel JD. Anastomotic leaks after esophagectomy for esophageal cancer: A comparison of thoracic and cervical anastomoses. Ann Thorac Cardiovasc Surg. 2001;7:75–8. [PubMed] [Google Scholar]
- 9.Freeman RK, Vyverberg A, Ascioti AJ. Esophageal stent placement for the treatment of acute intrathoracic anastomotic leak after esophagectomy. Ann Thorac Surg. 2011;92:204–8. doi: 10.1016/j.athoracsur.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Sharma P, Kozarek R and the Practice Parameters Committee of the American College of Gastroenterology. Role of Esophageal Stents in Benign and Malignant Diseases. Am J Gastroenterol. 2010;105:258–73. doi: 10.1038/ajg.2009.684. [DOI] [PubMed] [Google Scholar]
- 11.Roy-Choudhury SH, Nicholson AA, Wedgwood KR, Mannion RA, Sedman PC, Royston CM, et al. Symptomatic malignant gastroesophageal anastomotic leak: Management with covered metallic esophageal stents. AJR Am J Roentgenol. 2001;176:161–5. doi: 10.2214/ajr.176.1.1760161. [DOI] [PubMed] [Google Scholar]
- 12.Petersen JM. The use of a self-expandable plastic stent for an iatrogenic esophageal perforation. Gastroenterol Hepatol. 2010;6:389–91. [PMC free article] [PubMed] [Google Scholar]
- 13.van Boeckel PG, Dua KS, Weusten BL, Schmits RJ, Surapaneni N, Timmer R, et al. Fully covered self-expandable metal stents (SEMS), partially covered SEMS and self-expandable plastic stents for the treatment of benign esophageal ruptures and anastomotic leaks. BMC Gastroenterol. 2012;12:19. doi: 10.1186/1471-230X-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allum WH, Griffin SM, Watson A, Colin-Jones D. Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland; British Society of Gastroenterology; British Association of Surgical Oncology. Guidelines for the management of esophageal and gastric cancer. Gut. 2002;50(Suppl 5):v1–23. doi: 10.1136/gut.50.90005.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abraham A, Rizvon K, Singh J, Siddiqui G, Prasad A, Rashid S, et al. Successful management of a gastric sleeve leak with an endoscopic stent. Case Rep Gastrointest Med 2012. 2012 doi: 10.1155/2012/205979. 205979. [DOI] [PMC free article] [PubMed] [Google Scholar]


