Abstract
Purpose
We assessed the effect of age, health status and patient preferences on outcomes of surgery vs active surveillance for low risk prostate cancer.
Materials and Methods
We used Monte Carlo simulation of Markov models of the life courses of 200,000 men diagnosed with low risk prostate cancer and treated with surveillance or radical prostatectomy to calculate quality adjusted life expectancy, life expectancy, prostate cancer specific mortality and years of treatment side effects, with model parameters derived from the literature. We simulated outcomes for men 50 to 75 years old with poor, average or excellent health status (50%, 100% and 150% of average life expectancy, respectively). Sensitivity of outcomes to uncertainties in model parameters was tested.
Results
For 65-year-old men in average health, surgery resulted in 0.3 additional years of life expectancy, 1.6 additional years of impotence or incontinence and a 4.9% decrease in prostate cancer specific mortality compared to surveillance, for a net difference of 0.05 fewer quality adjusted life years. Increased age and poorer baseline health status favored surveillance. With greater than 95% probability, surveillance resulted in net benefits compared to surgery for age older than 74, 67 and 54 years for men in excellent, average and poor health, respectively. Patient preferences toward life under surveillance, biochemical recurrence of disease, treatment side effects and future discount rate affected optimal management choice.
Conclusions
Older men and men in poor health are likely to have better quality adjusted life expectancy with active surveillance. However, specific individual preferences impact optimal choices and should be a primary consideration in shared decision making.
Keywords: prostatic neoplasms, quality-adjusted life years, prostatectomy, watchful waiting, decision support techniques
The long natural history of screen detected prostate cancer and competing causes of death contribute to the overtreatment of prostate cancer, especially among older men. Active surveillance with selective delayed intervention for those patients with later evidence of disease progression is an alternative to immediate intervention that could reduce the overtreatment of prostate cancer, but is infrequently used in the United States.1 Watchful waiting for men older than 65 years with nonscreen detected prostate cancer has recently been shown to have a survival outcome equivalent to that of surgery during 15 years.2 Thus, we hypothesized that quality adjusted life years would be greater for older men with screen detected prostate cancers choosing active surveillance compared to surgery.
Decision models have been used to compare the effectiveness of treatment options for men with localized prostate cancer in the absence of long-term comparative data,3–8 but to our knowledge only 1 analysis to date has compared active surveillance to treatment.8 However, this study evaluated only men 65 years old in average health, thus limiting its clinical applicability to men of different ages with varying baseline health statuses.
Our goal was to examine the health outcomes of men at various ages and in different health states choosing active surveillance and surgery using a simulation model that 1) represents the clinical course of prostate cancer as experienced by patients; 2) incorporates the latest data available from prospective cohorts of patients diagnosed in the modern PSA era; and 3) examines the effect of patient age, health status and preferences in determining the optimal management option. We believe this evaluation is especially important given the increase in the surgical treatment of prostate cancer among older men for whom surveillance may be a better choice.9
MATERIALS AND METHODS
We conducted Monte Carlo simulations using Markov models of the life course of men with newly diagnosed low risk prostate cancer initially treated with curative intent or AS. For outcomes we took the patient perspective. To ensure we were not implicitly encoding a preference for surveillance, we biased our assumptions (and parameter estimates) to favor treatment over surveillance wherever such a choice needed to be made. If surveillance was deemed to result in better outcomes under these conditions, the conclusion would be considered robust.
Population and Base Case
Our model cohorts comprised men diagnosed with low risk prostate cancer (stage T1c–T2a, PSA less than 10 ng/ml and biopsy Gleason score less than 7)10 in the PSA era (since 1989) 50 to 75 years old who were eligible for surgery or surveillance. For each age we created separate models for men in excellent, average and poor baseline health, representing the top quartile, average and bottom quartile of health defined by life expectancies that are 1.5, 1.0 and 0.5 times the average life expectancy, respectively, following National Comprehensive Cancer Network clinical guidelines.11 To facilitate comparison with other studies our base case was 65-year-old men in average health.
Management Options
The most common options for managing newly diagnosed prostate cancer are RP or radiation therapy.1 In contrast, surveillance is an alternative that delays intervention and monitors disease progression. Triggers for intervention are not universally agreed upon but most clinicians recommending this approach use changes in biopsy grade (upgrading of Gleason score), PSA or digital rectal examination findings to reclassify patients into a higher risk category, prompting treatment. Upon reclassification of disease or patient choice, curative treatment is performed.
In light of the recent results from the Scandinavian Prostate Cancer Group Study Number 4 showing equivalent survival outcomes for watchful waiting and surgery for men older than 65 years,2 the increasing rates of surgery in elderly men who may benefit from surveillance,9 and the absence of long-term outcomes for AS in men with low risk prostate cancer, we believe it is important to focus on surgery and active surveillance. Thus, in this study we compared surgery and active surveillance.
Outcomes of Interest
Our primary outcome of interest was quality adjusted life expectancy, which accounts for the effect of disease status and treatment side effects on quality of life. Secondary outcomes were life expectancy, prostate cancer specific mortality and average years of treatment side effects.
Decision Analytic Model
Using decision analytic software (TreeAge Pro 2009 Suite v1.0.2) we constructed a core Markov model comparing 2 identical cohorts of men diagnosed with low risk prostate cancer undergoing initial management with surveillance or treated immediately with surgery. With time, men progress through a sequence of intermediate states representing prostate cancer disease states (following Fleming et al3) and eventually die of prostate cancer or another cause. This approach is analogous to a clinical trial of men randomized to surgery or surveillance with lifetime followup. Model structure and major assumptions are described elsewhere.
Literature Review and Model Parameters
Disease progression probabilities, treatment side effect rates, and patient utilities for disease states and side effects were determined through a literature search of Cochrane reviews and PubMed databases. We also reviewed sources found by an online meta-analysis.12 Relevant papers were read and their references reviewed. To the extent possible, we used data from PSA era cohorts of men with low risk disease.
Simulations
To estimate outcomes and minimize variations due to chance, we simulated the life courses of 200,000 men managed with surveillance and surgery per combination of age (50 to 75 years) and health status (excellent, average and poor as previously defined).
Sensitivity Analyses
We conducted several sensitivity analyses to determine 1) which model parameters and patient preferences had the greatest effect on the relative outcomes of surgery vs surveillance, and 2) the robustness of results to uncertainty in our model parameters (eg lack of data or variation in individual patient preferences). A probabilistic sensitivity analysis was used to generate confidence intervals for simulation outcomes.13–15
External Validation
To validate our models we compared prostate cancer mortality, metastasis risk and overall survival from existing cohorts with the corresponding outcomes of median or mean age matched cohorts in our simulations. We found that simulated model based outcomes were within a few percentage points of published observations.
RESULTS
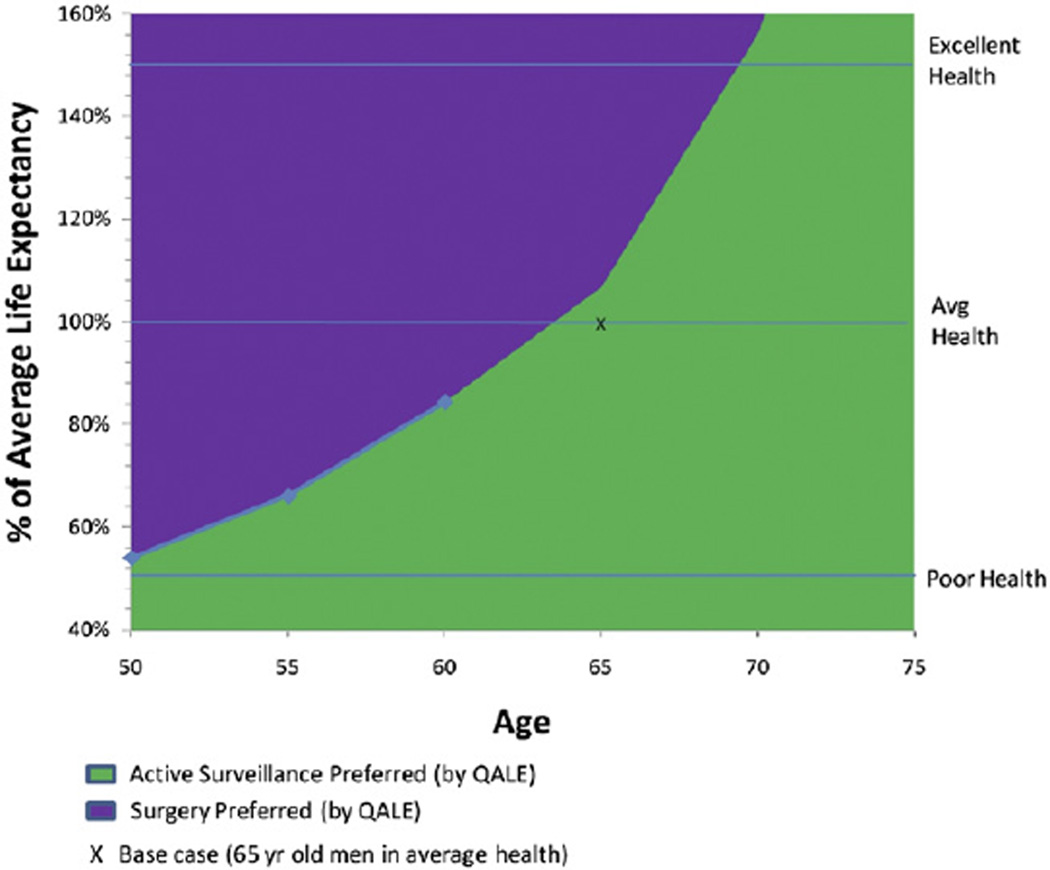
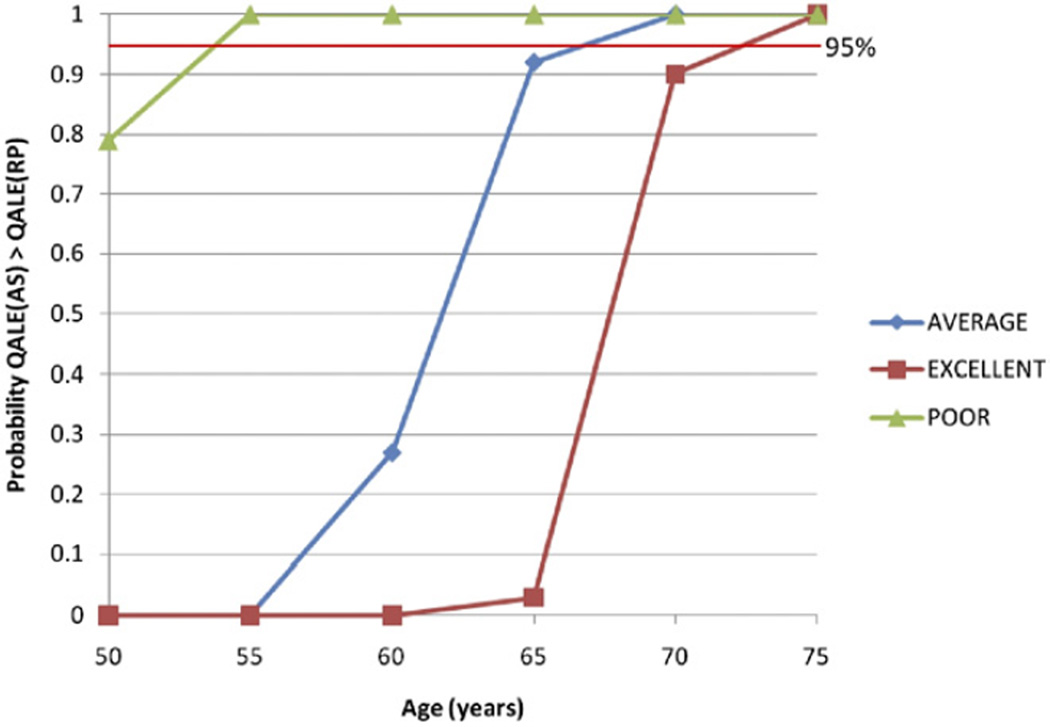
For 65-year-old men in average health (25th to 75th percentile), surgery resulted in 0.3 additional years of life expectancy, 1.6 additional years of impotence or incontinence and an absolute 4.9% decrease in PCSM compared to surveillance, for a net difference of 0.05 QALYs in favor of surveillance, with 92.8% of simulations in the probabilistic sensitivity analysis favoring surveillance compared to surgery (see table). Older age and worse baseline health status were associated with a smaller benefit in PCSM and life expectancy with surgery, and increased incremental years with treatment side effects, favoring surveillance with a higher QALE compared to surgery (fig. 1). In the probabilistic sensitivity analysis, the threshold ages at which surveillance had a higher QALE compared to surgery in more than 95% of simulations varying all model parameters were 54, 67 and 74 years for men in poor, average and excellent health, respectively (fig. 2).
Outcomes by age and health status
| Yrs Life Expectancy Without Ca |
Yrs Life Expectancy (95% CI) |
Yrs With ED or Incontinence (95% CI) |
QALE (95% CI) | % Ca Specific Mortality (95% CI) |
Incremental QALEs of RP vs AS (95% CI) |
Probability QALE (AS) Greater than QALE (RP)* |
|
|---|---|---|---|---|---|---|---|
| Excellent health | |||||||
| Age 50 yrs: | 43.8 | 1.58 (0.41–2.8) | 0.00 | ||||
| AS | 35.8 (32.5–39.9) | 7.3 (5.8–10.1) | 19.8 (15.8–24.3) | 34.5 (14.2–48.4) | |||
| RP | 40.8 (37.8–43.3) | 7.3 (6.0–9.6) | 21.4 (16.9–26.3) | 11.5 (4.7–20.9) | |||
| Age 55 yrs: | 37.6 | 1.11 (0.19–1.90) | 0.00 | ||||
| AS | 31.8 (29.1–35.3) | 7.9 (6.5–10.1) | 18.4 (15.0–22.6) | 28.7 (12.3–42.0) | |||
| RP | 35.3 (33.4–37.2) | 8.4 (7.3–10.5) | 19.5 (15.8–24.2) | 9.9 (4.6–18.0) | |||
| Age 60 yrs: | 31.6 | 0.72 (0.04–1.40) | 0.00 | ||||
| AS | 27.6 (25.6–30.1) | 8.0 (6.4–10.3) | 16.7 (14.3–20.1) | 22.9 (10.1–33.2) | |||
| RP | 30.0 (28.2–31.3) | 9.2 (7.6–11.8) | 17.5 (14.6–21.0) | 8.1 (3.6–14.2) | |||
| Age 65 yrs: | 26.1 | 0.41 (−0.16–0.90) | 0.04 | ||||
| AS | 23.5 (21.9–25.4) | 7.9 (6.1–10.2) | 14.9 (12.5–17.1) | 17.4 (7.5–24.9) | |||
| RP | 25.0 (23.8–26.3) | 9.4 (7.8–12.6) | 15.3 (12.7–17.8) | 6.4 (2.9–11.4) | |||
| Age 70 yrs: | 21.0 | −0.04 (−0.47–0.27) | 0.85 | ||||
| AS | 19.4 (18.3–20.6) | 7.2 (5.7–9.7) | 12.9 (11.2–14.7) | 12.2 (4.1–19.5) | |||
| RP | 20.3 (19.3–21.4) | 10.5 (8.7–14.2) | 12.8 (10.9–14.6) | 4.8 (1.9–8.5) | |||
| Age 75 yrs: | 16.4 | −0.42 (−0.80–−0.25) | 1.00 | ||||
| AS | 15.5 (14.6–16.2) | 5.7 (4.3–7.1) | 10.8 (9.5–12.0) | 8.1 (3.3–12.2) | |||
| RP | 16.0 (15.1–16.6) | 10.5 (8.9–13.4) | 10.3 (8.9–11.7) | 3.5 (1.1–6.4) | |||
| Av health | |||||||
| Age 50 yrs: | 29.2 | 0.70 (0.09–1.24) | 0.00 | ||||
| AS | 26.4 (25.0–28.3) | 4.7 (3.8–6.2) | 16.8 (14.1–20.0) | 21.3 (8.5–31.0) | |||
| RP | 28.0 (26.6–29.1) | 5.0 (4.2–6.3) | 17.5 (14.5–21.0) | 7.7 (3.1–13.8) | |||
| Age 55 yrs: | 25.0 | 0.39 (−0.02–0.81) | 0.01 | ||||
| AS | 23.2 (22.1–24.5) | 4.9 (4.0–6.4) | 15.3 (13.2–17.9) | 16.8 (5.7–24.3) | |||
| RP | 24.2 (23.2–25.0) | 5.8 (4.8–7.5) | 15.7 (13.5–18.2) | 6.4 (2.5–10.9) | |||
| Age 60 yrs: | 21.1 | 0.15 (−0.20–0.46) | 0.31 | ||||
| AS | 19.9 (19.2–20.8) | 4.9 (3.9–6.4) | 13.6 (11.7–15.6) | 12.3 (4.2–19.1) | |||
| RP | 20.5 (19.8–21.1) | 6.3 (5.3–8.3) | 13.8 (11.7–15.8) | 5.0 (2.0–9.0) | |||
| Age 65 yrs: | 17.4 | −0.05 (−0.37–0.13) | 0.93 | ||||
| AS | 16.7 (16.1–17.3) | 4.7 (3.9–5.7) | 11.9 (10.5–13.1) | 8.6 (3.2–13.9) | |||
| RP | 17.0 (16.3–17.5) | 6.3 (5.5–7.9) | 11.8 (10.4–13.0) | 3.7 (1.4–6.9) | |||
| Age 70 yrs: | 14.0 | −0.31 (−0.57–−0.20) | 1.00 | ||||
| AS | 13.6 (13.1–14.1) | 4.2 (3.1–5.3) | 10.1 (9.0–11.1) | 5.7 (1.9–9.1) | |||
| RP | 13.8 (13.3–14.2) | 7.1 (6.0–9.1) | 9.7 (8.6–10.7) | 2.6 (0.9–5.1) | |||
| Age 75 yrs: | 10.9 | −0.48 (−0.76–−0.44) | 1.00 | ||||
| AS | 10.7 (10.4–11.0) | 3.2 (2.2–3.9) | 8.2 (7.6–9.0) | 3.4 (1.0–5.8) | |||
| RP | 10.8 (10.4–11.1) | 7.0 (5.9–9.0) | 7.7 (6.9–8.5) | 1.7 (0.7–3.6) | |||
| Poor health | |||||||
| Age 50 yrs: | 14.6 | −0.01 (−0.18–0.14) | 0.79 | ||||
| AS | 14.1 (13.7–14.7) | 1.8 (1.3–2.3) | 10.7 (9.6–11.8) | 6.2 (2.1–10.1) | |||
| RP | 14.3 (13.9–14.8) | 2.5 (2.1–3.3) | 10.7 (9.5–11.8) | 2.9 (1.1–6.1) | |||
| Age 55 yrs: | 12.5 | −0.12 (−0.25–−0.03) | 1.00 | ||||
| AS | 12.2 (11.9–12.6) | 1.8 (1.3–2.4) | 9.5 (8.6–10.4) | 4.5 (1.3–7.3) | |||
| RP | 12.3 (12.0–12.7) | 2.9 (2.4–3.9) | 9.4 (8.5–10.2) | 2.2 (0.8–4.1) | |||
| Age 60 yrs: | 10.5 | −0.20 (−0.37–−0.14) | 1.00 | ||||
| AS | 10.4 (10.1–10.6) | 1.7 (1.2–2.2) | 8.2 (7.5–9.0) | 3.0 (0.9–5.0) | |||
| RP | 10.4 (10.1–10.7) | 3.1 (2.6–4.1) | 8.0 (7.3–8.7) | 1.6 (0.5–3.2) | |||
| Age 65 yrs: | 8.7 | −0.23 (−0.40–−0.21) | 1.00 | ||||
| AS | 8.6 (8.4–8.8) | 1.6 (1.1–2.0) | 7.0 (6.5–7.5) | 1.8 (0.6–3.3) | |||
| RP | 8.6 (8.4–8.8) | 3.2 (2.6–4.0) | 6.8 (6.2–7.2) | 1.1 (0.4–2.2) | |||
| Age 70 yrs: | 7.0 | −0.35 (−0.49–−0.29) | 1.00 | ||||
| AS | 6.9 (6.8–7.1) | 1.4 (1.0–1.8) | 5.8 (5.4–6.2) | 1.0 (0.2–1.9) | |||
| RP | 6.9 (6.8–7.1) | 3.5 (3.0–4.5) | 5.5 (5.1–5.8) | 0.7 (0.2–1.5) | |||
| Age 75 yrs: | 5.5 | −0.43 (−0.60–−0.38) | 1.00 | ||||
| AS | 5.5 (5.3–5.6) | 1.0 (0.7–1.3) | 4.7 (4.4–5.0) | 0.5 (0.1–0.9) | |||
| RP | 5.4 (5.3–5.6) | 3.5 (3.0–4.5) | 4.3 (4.0–4.5) | 0.4 (0.1–0.9) | |||
Proportion of simulations in the probabilistic sensitivity analysis varying all parameters where QALE (AS) is greater than QALE (RP).
Figure 1.
Optimal management strategy by age and health status. Analysis suggests that in base case of 65-year-old in average health, recommendation for surveillance over surgery may be sensitive to choices in model since corresponding point (X) is close to boundary curve.
Figure 2.
Probabilistic sensitivity analysis by age and health status. For each simulation, value is randomly sampled for each model parameter within its range. Then cohort of 10,000 men is simulated to undergo surgery and surveillance using sampled model values. For each age and health status combination 500 simulations were run, thereby generating range of outcomes from space of all possible model parameters. For each age and health status, y-axis indicates proportion of simulations which result in QALE (surveillance) greater than QALE (surgery). Threshold ages above which 95% of simulations favor surveillance are 54, 67 and 74 years old for men in poor, average and excellent health, respectively. This assumes no disutility to surveillance compared to posttreatment without side effects, eg no significant increased anxiety from living with untreated disease. All other parameters were allowed to vary within their ranges.
One-way sensitivity analyses in our base case showed that outcomes were particularly sensitive to variations in multiple utilities, namely the utility of living under surveillance, the utility of living with impotence and the utility of living with biochemical evidence of disease. Of these 3 the utility of living under surveillance was by far the most important, resulting in differences of up to 1.2 QALYs in favor of surgery when its value was varied down to 0.8. However, an increase in the utility of impotence (meaning that ED causes less patient distress) and the disutility of living with biochemical evidence of disease (eg PSA recurrence) also favored surgery. Uncertainty in model parameters associated with the increased risk of disease progression with delayed treatment (eg probability of disease progression, increased risk of metastatic progression with short biochemical recurrence-free survival) caused significant variation in the relative QALE of surgery and surveillance. Interestingly a low future discount rate (meaning that future outcomes are valued similarly to outcomes closer to the present) also favored surgery and was sufficient to make surgery the preferred option in our base case. Future discount rate, utility of life with biochemical evidence of disease and uncertainty in the risk of disease progression were more important for younger men, whereas the utility of treatment side effects was more important for older men.
DISCUSSION
Our simulation models show that age, health and patient preference are important determinants in the effectiveness of surveillance vs surgery after a diagnosis of low risk prostate cancer. Older age and poorer health status at diagnosis were associated with greater expected benefit for surveillance. These findings are not unexpected given the long natural history of most prostate cancers diagnosed with PSA screening and competing causes of death in older men.16 However, undertreatment and overtreatment are possible. A 70-year-old man in excellent health may still be a good candidate for surgery whereas a 55-year-old man in poor health may benefit from surveillance. The current study further defines and quantifies the range of ages and health states that may favor surveillance as a preferred option, which are likely robust given that our model was intentionally biased in favor of surgery. Furthermore, we identify important patient preferences and quantify the extent to which these preferences might influence decisions—a step toward shared decision making.
The results have important clinical implications. Our findings suggest that men older than 74, 67 and 54 years in excellent, average and poor health, respectively, will likely have higher quality adjusted life expectancy with surveillance than with surgery. Given that we biased our model against surveillance, these ages are likely to be conservative.
Furthermore, we identify important individual preferences. The ability to live with untreated disease without significant anxiety is a prerequisite for surveillance across all ages and health statuses, suggesting that this should be a key factor that physicians discuss with patients. The utility of life with posttreatment biochemical evidence of disease (ie asymptomatic PSA recurrence) can also affect decision making. Men with longer life expectancies who choose active surveillance are at greater risk for living a significant portion of their lives with asymptomatic PSA recurrence and the associated potential anxiety. We found that utilities for side effects are important factors in the choice of management option. In our model we use side effect rates that are roughly half the rate reported in settings where most prostate cancer surgeries are performed. Therefore, it is likely that in most settings utilities for side effects are even more important than our model suggests. We also found that a high future discount rate (ie valuing present life and quality of life more than those in the future) favors surveillance. Other utilities, including disutility for surgery and recovery, hormonal therapy or metastatic disease states had a minimal effect on model outcomes. A computer tool (web based or otherwise) using standardized instruments to directly assess individual utilities and calculate a personalized QALE based on age, health status and preferences might be helpful in clinical management.
To our knowledge only Hayes et al have examined the relative effectiveness of surveillance for low risk disease.8 Our study differs in certain key respects. Hayes et al assumed a fixed lifetime benefit to treatment (PCSM relative risk of 0.83). In the current study the simulated relative risk of PCSM with treatment varied from 0.33 to 0.83 depending on age and health status. Furthermore, Hayes et al assumed that life with surveillance has a higher utility than life after treatment without side effects, strongly favoring surveillance vs treatment. The current study assumes that they are equivalent. Overall our study demonstrated worse outcomes for surveillance relative to treatment, with a 0.05 QALY benefit relative to surveillance for a 65-year-old man in average health compared to a 0.5 QALY benefit in the Hayes et al model. It is important to note that our models differ in which treatment patients undergo after progression on surveillance. We modeled surgery whereas Hayes et al modeled radiation therapy, which they found has better outcomes. However, our results are consistent in finding that surveillance is an appropriate option for certain men with low risk disease.
The current analysis is not without limitations. 1) We did not consider radiation therapy (brachy-therapy or external beam radiation therapy). While there is no evidence that any treatment modality results in better cancer control or definitively better quality of life, surgery is the most common curative intervention for men diagnosed with prostate cancer, 1 and is increasingly performed in older men,9 thus making this study widely applicable. 2) We did not model the natural history of impotence and incontinence in an aging male population. However, the prevalence of severe incontinence which would not worsen with surgery is less than 10% in men 75 to 79 years old.17 Similarly the prevalence of severe ED (“not firm enough for sexual activity” or worse) that would not worsen with surgery was found to range from 4% for ages 50 to 59 years, to 57% in men 80 years old or older.18 Halving the probability of surgery induced ED in our base case cohort of men 65 years old (equivalent to assuming 50% of men have severe ED at baseline) increased the expected benefit of surgery by less than 0.1 QALY relative to surveillance (data not shown). 3) We did not account for adjuvant and salvage therapy or pre-metastatic androgen deprivation therapy because their incidence is low in men with low risk disease,19–22 and they have limited benefits.23,24 4) Expected outcome may not be the only measure of interest in individual decision making. Distribution of outcomes and individual risk tolerance are also important, and were not considered here. Nevertheless, we believe that quantification of expected outcomes, as we have done in this study, is important to inform individual decision making. 5) Our results only apply to men diagnosed with low risk disease, although this is the largest group of men diagnosed with prostate cancer today. Finally, our decision analysis simulates a randomized, controlled trial of active surveillance and surgery, but as with all simulations does not replace a true randomized, controlled trial and is limited by the quality of the existing data and accuracy of the model. However, we were able to validate our surgery and surveillance models against outcomes from modern cohorts. Furthermore, given the uncertainty of long-term AS outcomes, we simulated a worst case scenario for active surveillance vs surgery and, thus, believe that our conclusions about when surveillance is preferable are robust.
CONCLUSIONS
We have modeled and quantified the effectiveness of immediate surgery compared to surveillance with selective delayed intervention for men with low risk prostate cancer, and found that age, health status and patient preference significantly impact treatment choice. For older men and men in poorer health, active surveillance should be strongly considered as the preferred management option after a diagnosis of low risk prostate cancer. Furthermore, individual patient preference should be a primary consideration in shared decision making.
Supplementary Material
Acknowledgments
Supported by the Prostate Cancer Foundation and the Predoctoral Clinical Research Training Program (Johns Hopkins University, National Center for Research Resources, National Institutes of Health, Grant TL1 RR025007).
Abbreviations and Acronyms
- AS
active surveillance
- ED
erectile dysfunction
- PCSM
prostate cancer specific mortality
- PSA
prostate specific antigen
- QALE
quality adjusted life expectancy
- QALY
quality adjusted life-year
- RP
radical prostatectomy
Footnotes
Nothing to disclose.
Financial interest and/or other relationship with ITA Partners.
Supplementary material can be obtained at www.jurology.com.
REFERENCES
- 1.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bill-Axelson A, Holmberg L, Filén F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100:1144. doi: 10.1093/jnci/djn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleming C, Wasson JH, Albertsen PC, et al. A decision analysis of alternative treatment strategies for clinically localized prostate cancer. Prostate Patient Outcomes Research Team. JAMA. 1993;269:2650. [PubMed] [Google Scholar]
- 4.Beck JR, Kattan MW, Miles BJ. A critique of the decision analysis for clinically localized prostate cancer. J Urol. 1994;152:1894. doi: 10.1016/s0022-5347(17)32409-6. [DOI] [PubMed] [Google Scholar]
- 5.Kattan MW, Cowen ME, Miles BJ. A decision analysis for treatment of clinically localized prostate cancer. J Gen Intern Med. 1997;12:299. doi: 10.1046/j.1525-1497.1997.012005299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alibhai SM, Naglie G, Nam R, et al. Do older men benefit from curative therapy of localized prostate cancer? J Clin Oncol. 2003;21:3318. doi: 10.1200/JCO.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 7.Sommers BD, Beard CJ, D’Amico AV, et al. Decision analysis using individual patient preferences to determine optimal treatment for localized prostate cancer. Cancer. 2007;110:2210. doi: 10.1002/cncr.23028. [DOI] [PubMed] [Google Scholar]
- 8.Hayes JH, Ollendorf DA, Pearson SD, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. JAMA. 2010;304:2373. doi: 10.1001/jama.2010.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbash GI, Glied SA. New technology and health care costs–the case of robot-assisted surgery. N Engl J Med. 2010;363:701. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 10.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. [Accessed March 11, 2011];NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. 2011 Available at www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 12.Ollendorf DA, Hayes J, McMahon P, et al. Active surveillance and radical prostatectomy for clinically localized, low-risk prostate cancer. Institute for Clinical and Economic Review; [Accessed May 5, 2010]. Available at www.icer-review.org/index.php/as-rp.html. [Google Scholar]
- 13.Doubilet P, Begg CB, Weinstein MC, et al. Probabilistic sensitivity analysis using Monte Carlo simulation A practical approach. Med Decis Making. 1985;5:157. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 14.Briggs A, Sculpher M, Buxton M. Uncertainty in the economic evaluation of health care technologies: the role of sensitivity analysis. Health Econ. 1994;3:95. doi: 10.1002/hec.4730030206. [DOI] [PubMed] [Google Scholar]
- 15.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17:479. doi: 10.2165/00019053-200017050-00006. [DOI] [PubMed] [Google Scholar]
- 16.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302:1202. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson SO, Rashidkhani B, Karlberg L, et al. Prevalence of lower urinary tract symptoms in men aged 45–79 years: a population-based study of 40 000 Swedish men. BJU Int. 2004;94:327. doi: 10.1111/j.1464-410X.2004.04930.x. [DOI] [PubMed] [Google Scholar]
- 18.Bacon CG, Mittleman MA, Kawachi I, et al. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med. 2003;139:161. doi: 10.7326/0003-4819-139-3-200308050-00005. [DOI] [PubMed] [Google Scholar]
- 19.Louie-Johnsun M, Neill M, Treurnicht K, et al. Final outcomes of patients with low-risk prostate cancer suitable for active surveillance but treated surgically. BJU Int. 2009;104:1501. doi: 10.1111/j.1464-410X.2009.08597.x. [DOI] [PubMed] [Google Scholar]
- 20.Griffin CR, Yu X, Loeb S, et al. Pathological features after radical prostatectomy in potential candidates for active monitoring. J Urol. 2007;178:860. doi: 10.1016/j.juro.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Lu-Yao GL, Potosky AL, Albertsen PC, et al. Follow- up prostate cancer treatments after radical prostatectomy: a population-based study. J Natl Cancer Inst. 1996;88:166. doi: 10.1093/jnci/88.3-4.166. [DOI] [PubMed] [Google Scholar]
- 22.Grossfeld GD, Stier DM, Flanders SC, et al. Use of second treatment following definitive local therapy for prostate cancer: data from the CaPSURE database. J Urol. 1998;160:1398. [PubMed] [Google Scholar]
- 23.Moul JW, Wu H, Sun L, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2004;171:1141. doi: 10.1097/01.ju.0000113794.34810.d0. [DOI] [PubMed] [Google Scholar]
- 24.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.