Abstract
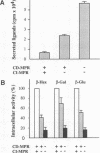
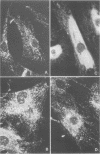
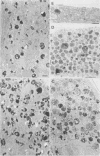
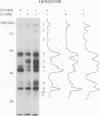
In higher eukaryotes, the transport of soluble lysosomal enzymes involves the recognition of their mannose 6-phosphate signal by two receptors: the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor (CI-MPR) and the cation-dependent mannose 6-phosphate receptor (CD-MPR). It is not known why these two different proteins are present in most cell types. To investigate their relative function in lysosomal enzyme targeting, we created cell lines that lack either or both MPRs. This was accomplished by mating CD-MPR-deficient mice with Thp mice that carry a CI-MPR deleted allele. Fibroblasts prepared from embryos that lack the two receptors exhibit a massive missorting of multiple lysosomal enzymes and accumulate undigested material in their endocytic compartments. Fibroblasts that lack the CI-MPR, like those lacking the CD-MPR, exhibit a milder phenotype and are only partially impaired in sorting. This demonstrates that both receptors are required for efficient intracellular targeting of lysosomal enzymes. More importantly, comparison of the phosphorylated proteins secreted by the different cell types indicates that the two receptors may interact in vivo with different subgroups of hydrolases. This observation may provide a rational explanation for the existence of two distinct mannose 6-phosphate binding proteins in mammalian cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock D. S., Bove K. E., Hug G., Dignan P. S., Soukup S., Warren N. S. Fetal mucolipidosis II (I-cell disease): radiologic and pathologic correlation. Pediatr Radiol. 1986;16(1):32–39. doi: 10.1007/BF02387502. [DOI] [PubMed] [Google Scholar]
- Barlow D. P., Stöger R., Herrmann B. G., Saito K., Schweifer N. The mouse insulin-like growth factor type-2 receptor is imprinted and closely linked to the Tme locus. Nature. 1991 Jan 3;349(6304):84–87. doi: 10.1038/349084a0. [DOI] [PubMed] [Google Scholar]
- Cantor A. B., Baranski T. J., Kornfeld S. Lysosomal enzyme phosphorylation. II. Protein recognition determinants in either lobe of procathepsin D are sufficient for phosphorylation of both the amino and carboxyl lobe oligosaccharides. J Biol Chem. 1992 Nov 15;267(32):23349–23356. [PubMed] [Google Scholar]
- Chen J. W., Murphy T. L., Willingham M. C., Pastan I., August J. T. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985 Jul;101(1):85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel C. A., Goldberg D. E., Kornfeld S. Identification and characterization of cells deficient in the mannose 6-phosphate receptor: evidence for an alternate pathway for lysosomal enzyme targeting. Proc Natl Acad Sci U S A. 1983 Feb;80(3):775–779. doi: 10.1073/pnas.80.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman J. N., Kornfeld S. Mannose 6-phosphate-independent targeting of lysosomal enzymes in I-cell disease B lymphoblasts. J Cell Biol. 1993 Oct;123(1):99–108. doi: 10.1083/jcb.123.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Waheed A., von Figura K. Enzymatic phosphorylation of lysosomal enzymes in the presence of UDP-N-acetylglucosamine. Absence of the activity in I-cell fibroblasts. Biochem Biophys Res Commun. 1981 Feb 12;98(3):761–767. doi: 10.1016/0006-291x(81)91177-3. [DOI] [PubMed] [Google Scholar]
- Hoflack B., Fujimoto K., Kornfeld S. The interaction of phosphorylated oligosaccharides and lysosomal enzymes with bovine liver cation-dependent mannose 6-phosphate receptor. J Biol Chem. 1987 Jan 5;262(1):123–129. [PubMed] [Google Scholar]
- Hoflack B., Kornfeld S. Purification and characterization of a cation-dependent mannose 6-phosphate receptor from murine P388D1 macrophages and bovine liver. J Biol Chem. 1985 Oct 5;260(22):12008–12014. [PubMed] [Google Scholar]
- JOHNSON D. R. Hairpin-tail: a case of post-reductional gene action in the mouse egg. Genetics. 1974 Apr;76(4):795–805. doi: 10.1093/genetics/76.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- Köster A., Saftig P., Matzner U., von Figura K., Peters C., Pohlmann R. Targeted disruption of the M(r) 46,000 mannose 6-phosphate receptor gene in mice results in misrouting of lysosomal proteins. EMBO J. 1993 Dec 15;12(13):5219–5223. doi: 10.1002/j.1460-2075.1993.tb06217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T., Ovitt C. E., Bauer U., Hollinshead M., Remmler J., Lobel P., Rüther U., Hoflack B. Targeted disruption of the mouse cation-dependent mannose 6-phosphate receptor results in partial missorting of multiple lysosomal enzymes. EMBO J. 1993 Dec 15;12(13):5225–5235. doi: 10.1002/j.1460-2075.1993.tb06218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld E. F. Lysosomal storage diseases. Annu Rev Biochem. 1991;60:257–280. doi: 10.1146/annurev.bi.60.070191.001353. [DOI] [PubMed] [Google Scholar]
- Owada M., Neufeld E. F. Is there a mechanism for introducing acid hydrolases into liver lysosomes that is independent of mannose 6-phosphate recognition? Evidence from I-cell disease. Biochem Biophys Res Commun. 1982 Apr 14;105(3):814–820. doi: 10.1016/0006-291x(82)91042-7. [DOI] [PubMed] [Google Scholar]
- Rijnboutt S., Kal A. J., Geuze H. J., Aerts H., Strous G. J. Mannose 6-phosphate-independent targeting of cathepsin D to lysosomes in HepG2 cells. J Biol Chem. 1991 Dec 15;266(35):23586–23592. [PubMed] [Google Scholar]
- Tong P. Y., Gregory W., Kornfeld S. Ligand interactions of the cation-independent mannose 6-phosphate receptor. The stoichiometry of mannose 6-phosphate binding. J Biol Chem. 1989 May 15;264(14):7962–7969. [PubMed] [Google Scholar]
- Tong P. Y., Kornfeld S. Ligand interactions of the cation-dependent mannose 6-phosphate receptor. Comparison with the cation-independent mannose 6-phosphate receptor. J Biol Chem. 1989 May 15;264(14):7970–7975. [PubMed] [Google Scholar]
- Varki A. P., Reitman M. L., Kornfeld S. Identification of a variant of mucolipidosis III (pseudo-Hurler polydystrophy): a catalytically active N-acetylglucosaminylphosphotransferase that fails to phosphorylate lysosomal enzymes. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7773–7777. doi: 10.1073/pnas.78.12.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A., Pohlmann R., Hasilik A., von Figura K., van Elsen A., Leroy J. G. Deficiency of UDP-N-acetylglucosamine:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase in organs of I-cell patients. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1052–1058. doi: 10.1016/0006-291x(82)91076-2. [DOI] [PubMed] [Google Scholar]
- von Figura K. Molecular recognition and targeting of lysosomal proteins. Curr Opin Cell Biol. 1991 Aug;3(4):642–646. doi: 10.1016/0955-0674(91)90035-w. [DOI] [PubMed] [Google Scholar]