Abstract
The burden of depressive disorders and the frequent inadequacy of their current pharmacological treatments are well established. The anaesthetic and hallucinogenic drug ketamine has provoked much interest over the past decade or so as an extremely rapidly acting antidepressant that does not modify ‘classical’ monoaminergic receptors. Current evidence has shown several ways through which it might exert therapeutic antidepressant actions: blockade of glutamatergic NMDA receptors and relative upregulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) subtypes may alter cortical connectivity patterns; through intracellular changes in protein expression, including the proteins mammalian target of rapamycin (mTOR) and brain-derived neurotrophic factor (BDNF); and alteration of intracellular signalling cascades. The clinical evidence demonstrates rapid improvements in mood and suicidal thinking in most participants, although study numbers have generally been small and many trials are unblinded and methodologically weak. There is a small body of work to suggest ketamine might also augment electroconvulsive therapy and potentially have a role as a surgical anaesthetic in depressed patients. A major problem is that the effects of ketamine appear temporary, disappearing after days to weeks (although longer benefits have been sustained in some), and attempts to circumvent this through pharmacological augmentation have been disappointing thus far. These exciting data are providing new insights into neurobiological models of depression, and potentially opening up a new class of antidepressants, but there are significant practical and ethical issues about any future mainstream clinical role it might have.
Keywords: antidepressant, glutamatergic, ketamine
Introduction
Major depressive disorders (MDDs) and bipolar affective disorders (BPADs) are frequently persistent, disabling psychiatric illnesses [Baune et al. 2007; Kessler et al. 2006]. Lifetime prevalence of MDDs stands at approximately 16% [Kessler et al. 2003], and BPADs at 1–4% [Grant et al. 2005; Merikangas et al. 2007]: although diagnosed by the presence of pathological highs, depressive episodes (so-called bipolar depression) constitute the majority of illness in BPADs [Lloyd et al. 2011]. Our recent review [Penn and Tracy, 2012] highlighted the limited efficacy of traditional antidepressants and the lack of a robust evidence base to guide the management of patients with treatment-resistant depression (TRD). There is a considerable need to develop novel and efficacious antidepressants.
Hallucinogenic drugs produce alterations in consciousness, perception, thought and emotion and have been used recreationally and entheogenically for millennia. So-called ‘classical’ psychedelic drugs such as lysergic acid diethylamide (LSD), psilocybin, dimethyltryptamine (DMT) and mescaline are thought to exert their effects through agonism at the 5-HT2A receptors [Nichols, 2004]. Dissociative hallucinogens including ketamine, phencyclidine (PCP) and dextromethorphan (DXM) act primarily as N-methyl-D-aspartate (NMDA) glutamate (Glu) receptor antagonists [Krystal et al. 1994].
There has been growing interest in the observation that ketamine has a rapid positive effect on depressive symptoms. Ketamine is used in medicine for inducing and maintaining anaesthesia, and illicitly for its hallucinogenic and dissociative effects. The fact that ketamine does not work through the ‘conventional’ antidepressant monoaminergic targets of serotonin and noradrenaline has provoked excitement: understanding its effects could provide novel insights into the pathophysiology of depression and open up a new class of medications. In this paper we will consider how ketamine might produce antidepressant effects, systematically review the evidence base for its efficacy and discuss the clinical utility of this novel compound.
The pharmacokinetics and pharmacodynamics of ketamine
Pharmacokinetics
Owing to the water and lipid solubility of ketamine, it can be administered by a variety of routes, including intravenous (IV), intramuscular (IM), intranasal (IN) and oral. The bioavailability of ketamine is approximately 90% when given IV or IM, compared with 16% orally, although peak effects occur rapidly with all methods [Craven, 2007].
Whilst oral administration is inevitably more convenient for both patients and staff, to date, the majority of research on the antidepressant effects of ketamine has used IV administration. IN and IM administration of ketamine have been far less explored in the treatment of depression. IN is reasonably easily administered, and has been shown to provide benefit in a trial of analgesic-refractory chronic pain patients [Carr et al. 2004]: there are currently two trials underway with IN administration, but as yet no data to support IN use in depression [aan het Rot, 2012]. To date, two case studies have investigated the efficacy of IM administration with promising results, but with a total number of three participants it is hard to infer efficacy at this time [Goforth and Holsinger, 2007; Glue et al. 2011].
Psychotomimetic effects
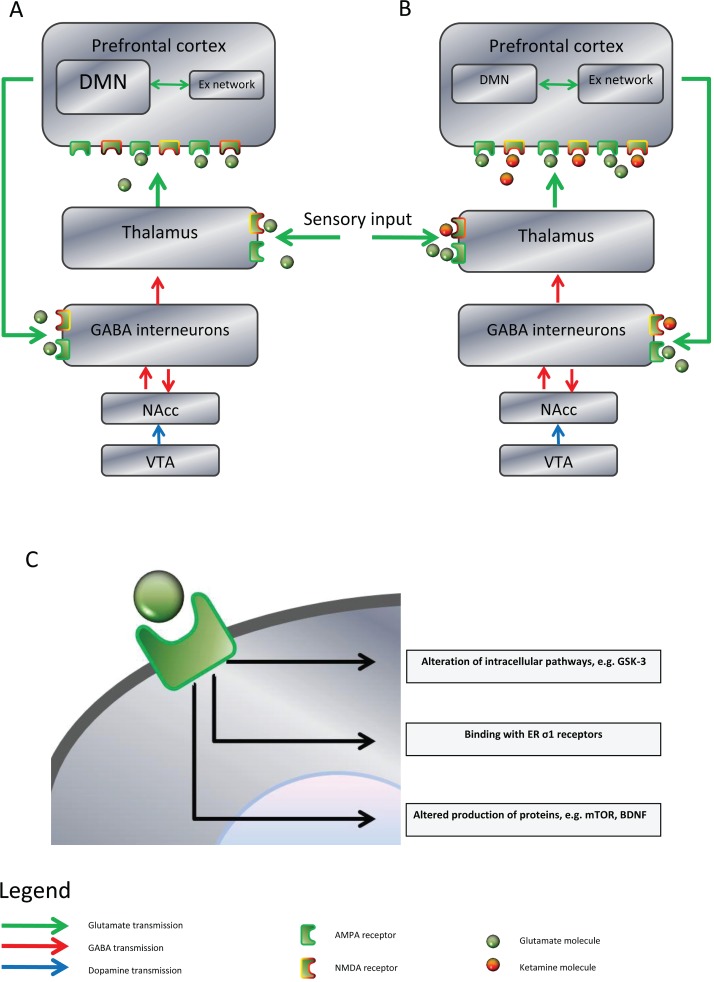
The prefrontal cortex (PFC) homeostatically limits its own input via a cortico–striatal–thalamic–cortical loop: glutamatergic neurons feedback to GABAergic interneurons that provide a tonic inhibition to ascending thalamic pyramidal neurons. Mesolimbic dopaminergic activity between the ventral tegmental area (VTA) and the striatal nucleus accumbens (NAcc) disinhibits the GABAergic interneurons, increasing stimuli that reach the PFC (Figure 1). Amongst the accepted neuropathological changes that occur in schizophrenia there is evidence for reduction in the PFC feedback and mesolimbic hyperdopaminergia leading to increased input to the PFC and cortical dysconnectivity.
Figure 1.
Schematic illustration of the effects of ketamine. (A) Normal and pathological physiology: the prefrontal cortex (PFC) homeostatically limits input via a feedback loop to GABAergic interneurons. The mesolimbic pathway can increase such input through dopaminergic modulation from the ventral tegmental area (VTA) to the nucleus accumbens (NAcc). There is evidence for dysregulation of PFC connectivity patterns in depression, particularly between the default mode network (DMN) and the extrinsic network (Ex network). In schizophrenia there is evidence for both underactive PFC glutamatergic feedback to GABA interneurons and overactive dopaminergic activity in the mesolimbic system, both of which serve to pathologically dysregulate PFC activity. (B) The effects of ketamine: ketamine is an antagonist at the glutamatergic NMDA receptor, but not the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, increasing the relative activation at the latter. Effects of ketamine binding are dose dependent. At lower doses it appears to alter PFC connectivity from a ‘depressive pattern’ of excess DMN activation to a more ‘normal’ pattern. Ketamine can be psychotomimetic: its antagonism at GABA interneurons reduces thalamic inhibition mimicking psychotic pathology without involving the dopaminergic system. (C) Through relative increase in activation, intracellular changes from AMPA receptors are affected. Evidence for several types of change have been discovered, including altering intracellular signalling pathways, binding with endoplasmic reticulum (ER) sigma-one (σ1) receptors, and changing production of cellular proteins such as mammalian target of rapamycin (mTOR) and brain-derived neurotrophic factor (BDNF).
Ketamine appears to produce its psychotomimetic effects through a parallel disinhibitory process, acting as a noncompetitive and nonselective high-affinity NMDA antagonist [Krystal et al. 1994] on the GABAergic interneurons, increasing PFC input. Ketamine-induced psychosis has thus been shown to be independent of stimulation of mesolimbic dopaminergic D2 receptors. This model is incomplete insofar as it would predict that benzodiazepines, through facilitation of GABAergic activity, should ameliorate both the effects of ketamine and psychosis more generally [Moghaddam and Krystal, 2012]. Largely based upon early pharmacological work on ketamine, there is much interest in Glu as a target for a new generation of antipsychotic drugs [Sendt et al. 2012; Papanastasiou et al. 2013].
Modulation of cortical networks
Linking these well-established actions with a mechanism to explain putative antidepressant effects has proven more difficult. At a more global cortical level, data from healthy subjects have demonstrated the concept of two large anticorrelated cortical networks. The so-called default mode network (DMN) is an intrinsic functionally dominant non-goal-orientated resting state, whilst the extrinsic attentional network is involved in goal-driven behaviour, and the connections between these modular hubs can dysfunction in mental illnesses [Raichle et al. 2001; Tracy and Shergill, 2013]. In depression a so-called ‘dorsal nexus’ comprising the bilateral dorsal medial PFC has been shown to have marked increased functional connectivity with the DMN [Sheline et al. 2010]. This greater activation of the resting-state non-goal-directed network is associated with introspection and self-reflective processes that can pathologically increase in depression, and the degree of DMN dominance has been demonstrated to be correlated with the degree of depressive rumination [Hamilton et al. 2011]. Scheidegger and colleagues showed that in healthy individuals ketamine decreased the connectivity of the DMN to the dorsal nexus and the medial PFC, and the authors argue that the antidepressant effects of ketamine might therefore be due to re-regulating illness-induced dysfunctional connectivity, particularly in the limbic–cortico–striato–pallido–thalamic circuits involved in mood [Scheidegger et al 2012].
Effects on neurotransmitters
The dominant, albeit incomplete, pharmacological model of depression focuses upon the monoaminergic neurotransmitters serotonin and noradrenaline (and to a far lesser extent dopamine). The therapeutic actions of current antidepressants are highly likely to involve complex intracellular enzymatic chains downstream of changes to monoamines, with alterations in neuronal gene transcription [Brown and Tracy, 2013; Penn and Tracy, 2012]. Far less work has explored the role of the ubiquitous excitatory neurotransmitter Glu in depressive disorders: there is reasonably strong evidence to support dysfunction, though not attribute clear causality (for a review, see Sanacora and colleagues [Sanacora et al. 2012]). Extracellular levels of Glu are tightly controlled, as in excess in the synapse it is excitotoxic: after neuronal release it is recycled through glial support cells and enzymatically converted by glutamine synthetase to glutamine, which is then re-uptaken by neurons and hydrolysed back into Glu. Proton magnetic resonance spectroscopy ([1H]-MRS) work by Salvadore and colleagues administered ketamine to 14 individuals with MDDs and demonstrated an association between a lower pretreatment Glx:Glu ratio (Glx is a composite peak of Glu and glutamine), which is taken as a surrogate marker of glutamine levels, and a greater clinical response to drug treatment [Salvadore et al. 2012]. Glutamine is primarily found in glial cells, and these results indirectly implicate such support cells and Glu synthesis as a potential pathological process behind depressive symptomatology, and one possibly therapeutically facilitated by ketamine administration. However, another 1H-MRS study, by Valentine and colleagues, failed to show any association between ketamine administration in ten participants with MDD and alterations to amino acid neurotransmitter content [Valentine et al. 2011].
Effects on intracellular protein expression and function
Antagonism of inhibitory GABA interneuron NMDA receptors and subsequent disinhibitory increases in Glu release also increases the relative activation of other glutamatergic receptors, particularly α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. Although ionotropic receptors and responsible for the majority of the brain’s fast acting excitatory communication, activation of post-synaptic AMPA receptors also results in changes in protein expression in the post-synaptic cell, including brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR). These proteins are associated with neuronal growth, differentiation, synaptogenesis, and general functioning of the neuron: lower serum [Duman and Voleti, 2012], hippocampal and cortical levels are associated with depression [Duman, 2004] and have been shown to return to normal levels with antidepressant treatment [Sen et al. 2008; Shimizu et al. 2003]. Ketamine has been demonstrated to increase expression of mTOR, VEGF and BDNF [Kinsler and Dunman, 2008; Jernigan et al. 2011; Yang et al. 2013].
A recent rat study showed increases in mTOR phosphorylation and activation within half an hour of ketamine administration, followed by rapid increases in the density, maturation and function of prefrontal pyramidal neuron spines [Li et al. 2010]. Interestingly this animal depression model study also showed that blockade of mTOR signalling, through a selective AMPA receptor inhibitor, inhibited ketamine-induced synaptogenesis and behavioural improvement, providing further evidence for the importance both of mTOR and functional AMPA receptors. Increased PFC synapse growth has the neurophysiological attraction as a putative model as it could also provide a mechanism to reverse known atrophic brain changes, cell loss and altered glutamatergic neurotransmission from chronic stress–depression paradigms [Popoli et al. 2012].
Autry and colleagues showed that ketamine promptly reduced depression-like behaviour in mouse controls, but not in BDNF knockout mice [Autry et al. 2011]. Their data further suggest that inhibition of spontaneous miniature NMDA-receptor mediated currents by ketamine leads to deactivation of the kinase eukaryotic elongation factor 2 (eEF2) with subsequent rapid (within half an hour) and transient increases in BDNF translation, and that longer-term effects are due to secondary changes in synaptic plasticity. This work did not detect any changes in mTOR regulation, although analysis of the brain fractionates occurred earlier (30 minutes) than in the studies that showed changes in mTOR. This study also showed that drug effects were due to enhanced plasticity occurring in tonic resting glutamatergic neurons’ spontaneous neurotransmission and could not be elicited by evoked neurotransmission. The authors posited that this supports the hypothesis that spontaneous and evoked forms of glutamatergic signalling are segregated.
The ubiquitous protein kinase glycogen synthase kinase 3 (GSK-3) has been identified as a regulator of a diverse range of signalling pathways and has a key role in a number of cellular functions including inflammatory responses. Modulation of GSK-3 is held as one of the mechanisms by which lithium exerts its effects [Brown and Tracy, 2013]. Beurel and colleagues demonstrated that ketamine administration to mice rapidly inhibited GSK-3, and in this study such action was necessary for its rapid antidepressant effects [Beurel et al. 2011].
Effects on circadian patterns
Many depressive disorders have established diurnal patterns of mood change and dysregulated sleep. The therapeutic role of ameliorating pathological sleep and circadian patterns has received renewed interest in recent times through evaluation of the novel antidepressant agomelatine. This melatonergic analogue acts as a melatonin MT1 and MT2 agonist, as well as a 5-HT2C antagonist and has been shown to be efficacious as an antidepressant [Pompili et al. 2013]. The melatonergic system has been implicated in depressive disorders [De Berardis et al. 2013] and some of the effects of agomelatine appear to be through the resynchronization of circadian rhythms [Grassi-Zucconi et al. 1996]. Ketamine has been shown in animal studies to change NMDA and AMPA circadian rhythmicity [Colwell and Menaker, 1992], and inhibit light induction in the suprachiasmatic nucleus [Abe et al. 1992], a centre for temporal patterns of gene transcription and neuroendocrine function. Work by Bellet and colleagues showed that ketamine induced a dose-dependent reduction in the circadian transcription of genes driven by the key CLOCK:BMAL-1 heterodimeric complex, and that such action was attenuated by administration of the GSK-3B antagonist SB21673 [Bellet et al. 2011]. The authors argue that the rapid effects of ketamine might at least in part be accounted for by changes to clock gene expression. However, a study by Ma and colleagues found that whilst single-dose ketamine produced antidepressant effects in mice, sustained up to the 8-day study cut-off, the GSK-3 inhibitor SB216763 did not, challenging the role of GSK-3 as part of the effect of ketamine, and thus the therapeutic role if any for modulation of this pathway by ketamine remains uncertain [Ma et al. 2013].
Effects on endoplasmic reticulae σ1 receptors
Intracellular σ1 receptors are primarily located at endoplasmic reticulae (ER) membranes and are involved in regulation of intracellular calcium signalling through interaction with voltage-gated channels. They interact with other signalling pathways and receptors such as inositol-1, 4, 5 triphosphate (IP3), phospholipase C (PLC)-gamma, protein kinase C (PKC) and the Ras/Raf/MAPK pathways. The roles of σ1 include: promoting correct folding of proteins and facilitating transfer of degraded proteins to proteasomes for lysis to prevent toxic protein accumulation [Nishimura et al. 2008]; promoting neuronal plasticity, neurite growth and synaptogenesis [Hayashi et al. 2011]; and by activating antioxidant responses [Pal et al. 2012]. Reduced levels have been reported in various neurological and psychiatric disorders [Hayashi and Su, 2008], and ketamine [Stahl, 2008], fluvoxamine and donepezil [Albayrak and Hashimoto, 2012], and methylphenidate [Zhang et al. 2012] have been shown to also be σ1 agonists. There are interesting preliminary data from the NMDA GluN2B subunit antagonist ifenprodil, which has generally been used a cerebral vasodilator. This drug has pharmacological similarities to ketamine, although ex vivo receptor occupancy data in mice hippocampi has demonstrated differential NMDA binding profiles [Lord et al. 2013]. Work by Ishima and Hashimoto showed that ifenprodil potentiated concentration-dependent nerve growth factor-induced neurite outgrowth in cell cultures, and that such effects were blocked by concomitant administration of a specific σ1 or IP3 but not an σ2 antagonist [Ishima and Hashimoto, 2012]. Forced swim depression models in mice have been shown to respond to ifenprodil when co-administered with NMDA partial or full antagonists, with ifenprodil’s effects blocked by NMDA agonists [Poleszak et al. 2013]. Clinically nascent data has shown ifenprodil to be effective in managing so-called emotional incontinence in vascular dementia [Kishimoto et al. 2013], and in reducing flashbacks in female sufferers of post-traumatic stress disorder (PTSD) [Kishimoto et al. 2012]. Whether or not ketamine produces some of its antidepressive actions through similar mechanisms remains unclear at this time.
Effects on adipokines
Epidemiological studies have linked obesity and depressive disorders through psychosocial factors [Luppino et al. 2010], although there is growing interest in the potential role of the neuroendocrinologically active adipokines secreted by adipose tissue such as leptin, adiponectin and resistin [Taylor and MacQueen, 2010]. These hormones have roles in homeostatic feedback loops to hypothalamic satiety centres: their expression is stimulated by glucocorticoids, and hence altered in stress states, and they promote cytokine inflammatory responses [Wozniak et al. 2009]. Both excessive and depleted leptin levels have been linked with depressive disorders [Yamada et al. 2011]. Leptin levels have been shown to be reduced in rodents subjected to chronic stress, and administration of leptin in animal studies has been shown to have antidepressant effects, although the mechanisms of action and clinical implications are unclear [Liu et al. 2010; Lu et al. 2006]. Guo and colleagues showed that mice without the long form of the leptin receptor (Lepr), which are selectively distributed in the PFC and hippocampus, demonstrated normal growth and body weight but depression-like behaviour and NMDA-induced hippocampal long-term synaptic depression [Guo et al. 2012]. These mice were very sensitive to antidepressant-like effects of the selective NMDA receptor GluN2B (NR2B) antagonist Ro25-6981 but resistant to leptin. The authors argue that defective Lepr signalling in Glu neurons may play a role in the pathogenesis of depressive disorders and long-term synapse depression mediated through NMDA GluN2B receptors; further, the therapeutic actions of NMDA antagonists might be through facilitation of normal leptin-Glu functioning.
A systematic review of the efficacy of ketamine as an antidepressant
Materials and methods
Data acquisition
We attempted to identify all randomized controlled trials (RCTs) and non-RCTs available to review up to January 2013, in which the potential efficacy of hallucinogen drugs in the treatment of depression was analysed.
Search strategy
References were retrieved through searching electronic databases and manual searches through reference lists of identified literature. The following data sources were searched: PSYCINFO (1806 to 26 June 2013), MEDLINE (1946 to 26 June 2013), EMBASE (1980 to 26 June 2013). Although the primary aim was to explore the efficacy of ketamine, to ensure the fullest data collection the search criteria were as follows: “hallucinogen” OR “lsd” OR “lysergic acid diethylamide” OR “ketamine” OR “mescaline” OR “psilocybin” OR “magic mushroom” OR “mdma” OR “ecstasy” OR “psychedelic” OR “dissociative” OR “phenethylamine” OR “phencyclidine” combined with AND “antidepressant” OR “depression*” OR “mood disorder” OR “bipolar” OR “depressive-disorder” OR “unipolar”.
Eligibility criteria
The following inclusion and exclusion criteria were established prior to the literature search.
Participants
Studies that looked at adult populations (≥18 years old) with a diagnosis of a MDD or BPAD based on a structured diagnostic interview (DSM or ICD) were included.
Interventions
All designs evaluating the effect of ketamine on depressed mood were included. Investigations on addiction, ketamine misuse or specifically looking at psychedelic effects of ketamine were excluded.
Comparators
No comparators were required for inclusion in this review.
Outcomes
Studies investigating the effect of ketamine on mood symptoms and/or suicidality were included. Studies that failed to use a validated assessment scale for the evaluation of mood changes were excluded.
Study design
Only journal articles were included for review, with books, letters, comments, editorial and poster presentations excluded. Case studies documenting fewer than three participants were excluded.
Study selection
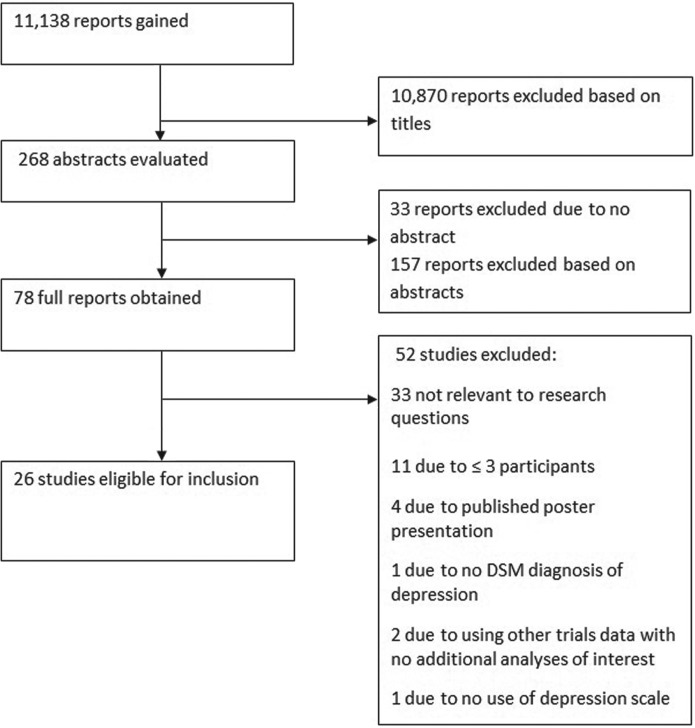
Using the stated search strategy 11,138 reports were identified. The search and process of identification is summarized in Figure 2. In total, 26 studies were ultimately identified as fulfilling criteria, with a total of 629 participants.
Figure 2.
Flow diagram demonstrating the process of inclusion of studies for review.
Data extraction
In order to collate relevant information from each article the following data were extracted from each: characteristics of participants (age, gender, length of illness, length of current episode, method of diagnosis); trial inclusion and exclusion criteria; type of intervention (type, dose, duration, design); response criteria; type of outcome measure (depression scale, response rates, remission rates, follow up). Several different and quite diverse themes emerged during the data extraction and the sample was thus divided into the following three categories to allow better clarification and interpretation of results: ketamine only; ketamine plus a second drug; ketamine and electroconvulsive therapy (ECT) or surgery.
Data analysis
For each of the subcategories of theme identified a table is presented with the characteristics of the included studies.
Hamilton depression scale ratings data from the five studies with control groups [Berman et al. 2000; DiazGranados et al. 2010b; Valentine et al. 2011; Zarate et al. 2006, 2012] were subjected to two cross-study meta-analyses using OpenMeta[Analyst], Brown Education software (see http://www.cebm.brown.edu/open_meta). For the first meta-analysis, the effects of ketamine versus placebo on depressive ratings were assessed at baseline, for the second 60–80 minutes post-infusion, and for the third 210–230 minutes post-infusion. Given that the included studies did not coherently report the means and standard deviations for each group at each time point, values were read off the available graphs in each paper.
Ketamine only
Studies with no control group
There were 11 studies that administered ketamine to all participants with no control condition: their characteristics are detailed in Table 1 and results are given in Table 2. Six studies evaluated single-dose ketamine administration on depressive symptoms, three multiple-dose schedules and two primarily evaluated changes to suicidal ideation. Trial size varied from 11 to 33 participants, and recorded follow up from 230 minutes to 83 days post-ketamine administration. A total of 206 participants, all with major depressive episodes (MDEs; diagnosed using DSM), completed these trials, and all were undertaken within the past 5 years. Ketamine was administered at 0.5 mg/kg in all trials except one [Larkin and Beautrais, 2011]. All studies adopted the Montgomery–Asberg Depression Rating Scale (MADRS) as the primary outcome measure: response was defined as ≥50% reduction in scores throughout, and remission a score of < 10. Outcomes affected by ketamine in these studies were very strong, although this must be contextualized by their open-label and loosely controlled design.
Table 1.
Demographic and clinical characteristics of included studies.
| Study | Age (years) | Gender | Length of Illness (years) | Length of current episode (months) | Lifetime failed antidepressant trials (SD) | Primary outcome measure(s) | Psychotropic washout period | Concurrent medication | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| Murrough et al. [2013] | 48.1 (±13.0) | 62.5% Female | Not stated | 217.2 (±201.6) | In this episode 6.1 (±3.3) | MADRS | Yes | Nil | MDD, TR |
| Salvadore et al. [2009] | 43.8 (±15.2) | 40% Female | Not stated | Not stated | Not stated | MADRS | Yes | Nil | MDD, TR |
| Salvadore et al. [2010] | 50.5 (±13.1) | Not stated | Not stated | Not stated | Not stated | MADRS | Yes | Nil | MDD, TR |
| Salvadore et al. [2012] | 50.1 (±10.4) | 36% Female | 28.9 (±14.2) | 158.1 (±179.0) | Not stated | MADRS | Yes | Nil | MDD |
| Price et al. [2009] | 48.2 (±11.8) | 39% Female | Not stated | 100% > 24 | In this episode 6.0 (±4.1) | MADRS | Yes | Nil | MDD, TR |
| Larkin and Beautrais [2011] | 31.1 (±3.4) | 50% Female | Not stated | Not stated | Not stated | MADRS, SSI | Not stated | Nil | MDD |
| aan het Rot et al. [2010] | 51.4 (±14.6) | 50% Female | Not stated | 260.4 (±223.2) | 8.2 (±3.4) | MADRS | Yes | Nil | MDD, TR |
| Rasmussen et al. [2013] | 47.2 (±15) | 60% Female | Not stated | Not stated | Not stated | MADRS | No | Depressed patients continued on existing antidepressant | MDE (BPAD II/MDD), TR |
| Valentine et al. [2011] | 41.7 (±12) | 60% Female | 21.2 (±17.4) | 39.6 (±51.6) | 2.2 (±1.9) | HDRS | Yes | Nil | MDD |
| Berman et al. [2000] | 37 (±10) | 55.55% Female | Not stated | Not stated | Not stated | HDRS | Yes | Nil | MDE (BPAD/MDD) |
| Zarate et al. [2006] | 46.7 (±10.4) | 66.67% Female | 23.7 (±12.5) | 33.6 (±37.4) | 5.7 (±3.4) | HDRS | Yes | Nil | MDD, TR |
| Zarate et al. [2012] | 46.7 (±10.4) | 53.33% Female | 30.6 (±11.2) | 20.9 (±27.5) | 9.7 (±4.3) | MADRS | Yes | Lithium or Valproate | BPAD |
| DiazGrandos et al. [2010b] | 47.9 (±13.1) | 66.67% Female | 27.6 (±11.2) | 15.1 (±13.3) | 7.2 (±4.0) | MADRS | Yes | Lithium or Valproate | BPAD, TR |
| Ibrahim et al. [2012] | 47.2 (±13.0) | 38% Female | 26.4 (±12.8) | 110.0 (±152.0) | Not stated | MADRS | Yes | Nil | MDD |
| Duncan et al. [2012] | 48.06 (±2.34) | 33.33% Female | Not stated | Not stated | Not stated | MADRS | Yes | Nil | MDD, TR |
| Mathew et al. [2010] | 48.2 (±11.8) | 39% Female | 291.6 (±195.6) | Not stated | 6.0 (±4.1) | MADRS, QIDS-SR | Yes | Nil | MDD, TR |
| Machado-Vieira et al. [2009] | 43.9 (±13.9) | 39.13% Female | 24.8 (±12.4) | 80.7 (±108.5) | Not stated | MADRS | Yes | Nil | MDD, TR |
| Phelps et al. [2009] | 43.5 (±14.1) | 39% Female | 23.7 (±12.5) | 82.1 (±103.5) | Not stated | MADRS | Yes | Nil | MDD, TR |
| DiazGrandos et al. [2010a] | 49.3 (±13.4) | 40% Female | 28.8 (±11.7) | 92.0 (±123.4) | Not Stated | MADRS | Yes | Nil | MDD |
| Ibrahim et al. [2011] | 46.5 (±10.8) | 41% Female | 25.4 (±10.2) | 99.1 (±108.8) | Not stated | MADRS | Yes | Nil | MDD, TR |
| Loo et al. [2012] | Ketamine: 45.2 (±15.6) Placebo: 41.4 (±12.0) | Ketamine: 50% Female Placebo: 70.83% Female | Not stated | Ketamine: 35.8weeks (±35.8) Placebo: 52.9weeks (±84.0) | In this episode Ketamine: 1.8 (±1.7) Placebo: 2.0 (±1.8) | MADRS | No | 22 remained on antidepressant | MDE, TR |
| Okamoto et al. [2010] | Ketamine: 59.3 (±13.5) Propofol: 55.1 (±15.4) | Ketamine: 55% Female Propofol: 50% Female | Not stated | Ketamine: 33.6 (±25.2) Propofol: 32.4 (±24.0) | Ketamine: 6.5 (±2.7) Propofol: 6.7 (±2.2) | HDRS | Not stated | Nil | MDD, TR |
| Kranaster et al. [2011] | Ketamine: 65.4 (±14.8) Thiopental: 63.8 (±12.4) | Not stated | Not stated | Not stated | Not stated | HAMD | Not stated | Nil | MDD, TR |
| Abdallah et al. [2012] | Ketamine: 47.8 (±5.0) Control: 46.5 (±3.3) | Ketamine: 37% Female Control: 50% Female | Not stated | Not stated | Not stated | HDRS, BDI | Not stated | Nil | MDD/ BPAD |
| Wang et al. [2012] | Ketamine: 56.2 (±11.5) Propofol: 53.8 (±15.2) Propofol + Ketamine: 58.6 (±16.3) | Ketamine: 50% Female Propofol: 58% Female Propofol + Ketamine: 42% Female | Not stated | Ketamine: 2.3 (±1.6) Propofol: 2.6 (±1.2) Propofol + Ketamine: 2.7 (±1.5) | Not stated | HDRS | Not stated | Nil | MDD |
| Kudoh et al. [2002] | Propofol + Ketamine + Fentanyl: 46.9 (±8.8) Propofol + Fentanyl: 48.2 (±7.4) Control: 46.2 (±10.3) | Not stated | Not stated | Not stated | Not stated | HDRS | No | Depressed patients continued on existing antidepressant | MDD |
BDI, Beck Depression Inventory; BPAD, bipolar affective disorder; HAMD, Hamilton Rating Scale for Depression; HDRS, Hamilton Depression Rating Scale; IV, intravenous administration; MADRS, Montgomery–Asberg Depression Rating Scale; MDD, major depressive disorder; MDE, major depressive episode; QIDS-SR, Quick Inventory of Depressive Symptomatology—Self-Report; SSI, Scale for Suicide Ideation; TR, treatment resistant.
Table 2.
Results of included studies addressing the use of ketamine only.
| Study | Subjects | Intervention | Control | Blinded/randomized | Time to significant improvement | Response (%) | Remission (%) | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Salvadore et al. [2012] | 14 | Ketamine IV 0.5 mg/kg | Nil | N/A | 230 min | Not stated | Not stated | Rapid effect of ketamine on depressive symptoms, significant at 230 min to 1 day |
| Machado-Vieira et al. [2009] | 23 | Ketamine IV 0.5 mg/kg | Nil | N/A | 40 min | Not stated | Not stated | Significant and rapid anti-depressant response to ketamine from 40 min to 240 min |
| Salvadore et al. [2009] | 11 | Ketamine IV 0.5 mg/kg | Nil | N/A | 230 min | Not stated | Not stated | Significant effect on depressive and anxiety symptoms at 230 min |
| Salvadore et al. [2010] | 15 | Ketamine IV 0.5 mg/kg | Nil | N/A | 230 min | Not stated | Not stated | Significant effect on depressive and anxiety symptoms at 230 min |
| Larkin and Beautrais [2011] | 14 | Ketamine IV 0.2 mg/kg | Nil | N/A | 240 min | 92.3 | Not stated | Rapid remission of depression and suicidal ideation, significant 40 min to 10 days |
| Phelps et al. [2009] | 26 | Ketamine IV 0.5 mg/kg | Nil | N/A | 120 min | 43 | 26 | Significant improvements in MADRS scores post-ketamine |
| aan het Rot et al. [2010] | 9 | Ketamine IV 0.5 mg/kg | Nil | N/A | Not stated | After 6 further infusions = 100 | After 6 further infusions = 88.89 | Responders generally maintain response if subsequent infusions |
| Murrough et al. [2013] | 24 | Ketamine IV 0.5 mg/kg | Nil | N/A | 120 min | 70.8 | Not stated | Rapid effect of ketamine on depressive symptoms, response to first infusion predicts response to subsequent infusions |
| Rasmussen et al. [2013] | 10 | Ketamine IV 0.5 mg/kg | Nil | N/A | Not stated | 80 | 50 | 50% met remission, 40% of remitters sustained improvement to 4 weeks. May be some advantage to serial infusions as opposed to single |
| Price et al. [2009] | 26 | Ketamine IV 0.5 mg/kg | Nil | N/A | 24 hours- first time point | Not stated | Not stated | Rapid effects on suicidal cognition, sustained through repeated infusions |
| DiazGranados et al. [2010a] | 33 | Ketamine IV 0.5 mg/kg | Nil | N/A | 40 min | Not stated | Not stated | Rapid and sustained significant reduction in MADRS scores up to 230 min |
| Valentine et al. [2011] | 10 | Ketamine IV 0.5 mg/kg | Placebo | Yes- Single/ Non-Counterbalanced | 60 min | Not stated | Not stated | Rapid antidepressant effect of ketamine within 60 min to 7 days |
| Berman et al. [2000] | 8 | Ketamine IV 0.5 mg/kg | Placebo | Yes- Double/Yes | 240 min | 50 | 26 | Robust decreases in depressive symptoms through to 3 day follow up |
| Zarate et al. [2006] | 17 | Ketamine IV 0.5 mg/kg | Placebo | Yes- Double/Yes | 110 min | 71 | 29 | Robust, rapid response sustained to 1 week follow up |
| Zarate et al. [2012] | 14 | Ketamine IV 0.5 mg/kg | Placebo | Yes- Double/Yes | 40 min | 79 | At 24 hrs: 29 | Rapid improvement in depressive symptoms and suicidal ideation |
| DiazGranados et al. [2010b] | 17 | Ketamine IV 0.5 mg/kg | Placebo | Yes- Double/Yes | 40 min | 71 | At 24 hrs:21 | Robust and rapid antidepressant effect of ketamine across 4 scales |
IV, intravenous administration.
Of the six open-label studies assessing response to single-dose ketamine in MDD, two were primarily evaluating postulated drug-induced changes to cortical proteins via 1H-MRS [Salvadore et al. 2012] (n = 14) and serum analysis [Machado-Vieira et al. 2009] (n = 23), and two investigated anterior cingulate cortex activity with regards to drug response [Salvadore et al. 2009, 2010] (n = 11, 15, respectively), but all also reported clinical responses as secondary measures. These four studies showed statistically significant improvement in mood at 230-minute post-infusion time points (p = 0.005 in Salvadore et al. [2009]; p = 0.001 in Salvadore et al. [2010]; p = 0.006 in Salvadore et al. [2012]; p < 0.001 in Machado-Vieira et al. [2009]). A somewhat different model was undertaken in open-label work by Larkin and Beautrais who trialled the feasibility of undertaking single-dose ketamine administration, administered, somewhat atypically for ketamine studies, as a 0.2mg/kg single bolus over 1–2 minutes, in 14 participants with MDD and suicidal ideation in an emergency department [Larkin and Beautrais, 2011]. A primary aim with this work was to evaluate a key conceptual concern about the viability of ketamine use in such ‘real-world’ scenarios. Fitting with previous data they found rapid antidepressive effects within 240 minutes, with 13 (92.3%) meeting response criteria and mean scores falling from a baseline MADRS score of 40.4 (standard error of the mean [SEM] = 1.8) to 11.5 (SEM = 2.2).
There are high rates of comorbidity between depressive disorders and substance misuse [Davis et al. 2007]. A family history of alcohol dependency has been shown to result in altered responses to ketamine in healthy participants, and changes to the glutamatergic system and NMDA binding has been implicated in both disorders [Petrakis et al. 2004]. Phelps and colleagues used linear mixed models to evaluate differential response in 26 MDD participants with and without a (self-reported) family history of alcohol dependency to open-label ketamine administration [Phelps et al. 2009]. Those with positive family histories showed a statistically significant improvement over those who did not in MADRS, Hamilton Depression Rating Scale (HDRS) and Beck Depression Inventory (BDI) scores within 230 minutes of ketamine infusion. Individual past alcohol dependency and past family history of depression were not correlated with outcome. The authors note that alcohol acts on NMDA receptors (amongst others) and genetic data have shown inheritable variations of these receptors associated with alcohol dependence: they speculate that both clinical histories and genetic markers may in future act as predictors of drug response.
Three open-label studies evaluated multiple-dosing ketamine. aan het Rot enrolled 10 participants from an earlier trial of the effects of ketamine on suicidality [Price et al. 2009] who had an initial depressive symptom severity ≥32 on the Inventory for Depressive Symptoms (IDS-C30) and who had demonstrated a clinical response to this single ketamine infusion without significant side effects [aan het Rot et al. 2010]. Participants were given 6 infusions over 12 days: 90% of participants responded to the first infusion, and by the end of the trial 100% met response criteria and 88.89% met remission criteria. The same research group undertook a larger study [Murrough et al. 2013] of 24 individuals, including the 10 participants in the previous work, who, after a wash-out period from their antidepressants, each received thrice weekly injections of ketamine over 12 days. There was a large, statistically significant (p < 0.01) decrease in MADRS scores 2 hours after the initial infusion (mean decrease 18.9, standard deviation [SD] 6.6). A total of 21 participants completed the full 6-infusion schedule and the response rate at the study’s conclusion was 70.8% (17 of 24). Response to ketamine 4 hours after the initial infusion was strongly associated with the response by the study’s end, with 94% of those responding doing so 4 hours after the first infusion. A more recent study conducted by Rasmussen and colleagues administered 10 participants in a MDE (BPAD II/MDD) with up to 4 ketamine infusions at 0.5 mg/kg over 100 minutes [Rasmussen et al. 2013]. A total of 80% of the participants demonstrated response to ketamine, defined as at least a 50% reduction in MADRS scores, and furthermore 50% met remission, defined as a MADRS score of 9 and below. Of the 5 participants who met remission, 2 still met remission criteria at the 4-week follow up. Rasmussen and colleagues further documented the effect of ketamine on suicidal ideation, reporting significant improvements in the Scale for Suicide Ideation (SSI; p = 0.007) and the Suicide Status Form (SSF; p = 0.026). In addition, this study reported a significant correlation between SSI/SSF scores and MADRS (p < 0.01), suggesting the observed decrease in suicidality occurred in unison with that of depression scores.
Two studies, both single-dosing, looked primarily at the effects of ketamine on suicidal ideation. Price and colleagues tested changes in 26 patients with TRD [Price et al. 2009]: 24 hours post-infusion the average reduction in the six-point MADRS Suicidality Item (SI) subscale score across all participants was 2.08 (p < 0.001), with 81% scoring of 0 or 1. Of the 13 patients with clinically significant baseline suicidal ideation (MADRS-SI ≥4) 8 (62%) scored zero or one at the 24-hour follow up. Similarly positive results were reported by DiazGranados and colleagues [DiazGranados et al. 2010a]. Suicidal ideation, in the context of MDD, improved within 40 minutes of infusion in the 33 participants, with benefits sustained by the 4-hour final assessment (p < 0.001). Some of the earlier studies also reported positive effects on suicidal ideation, and Larkin and Beautrais recorded suicidal ideation completely resolved in all participants (n = 14) within 240 minutes of infusion [Larkin and Beautrais, 2011], from a baseline mean MADRS-SI of 3.9 (SEM = 0.4) to a mean of 0.6 (0.1), maintained at day 10 (0.7 (0.2)). Interestingly, the work by Murrough and colleagues demonstrated that even non-responders to the anti-depressant effects showed statistically significant improvements in suicidal ideation [Murrough et al. 2013].
Most of these trials were designed with only brief follow up of participants, particularly in the single-dose studies. Salvadore and colleagues noted that improvements in MADRS scores remained significant (p = 0.01) at 24 hours [Salvadore et al. 2012], whilst Larkin and Beautrais record that clinical gains were maintained for the 13 participants who completed the study to the end-point at day 10 (mean MADRS 9.2 (SEM = 1.7)) [Larkin and Beautrais, 2011]. The two multiple-dosing trials had longer follow ups recorded. Relapse occurred on average 19 (SD 13) days following final infusion, though one participant remained asymptomatic for three months, in the work of aan hen Rot and colleagues [aan hen Rot et al. 2010]; whilst in the study by Murrough and colleagues the median time to relapse was 18 days (interquartile range 11–27 days) after the final infusion [Murrough et al. 2013].
Overall ketamine appeared well tolerated though there were frequent reports of mild psychotomimetic symptoms, as measured with the Brief Psychiatric Rating Scale (BPRS) or Clinician Administered Dissociative States Scale (CADSS), primarily an unpleasant dissociative effect, typically occurring and resolving within an hour of administration. Fitting with its known anaesthetic profile transient hypertension and tachycardia were also recorded. In the studies that so-reported, no difference in side-effect profile was noted between depression/suicidal-ideation responders and nonresponders.
Studies with a control group
Five studies compared the effect of ketamine (0.5 mg/kg) with a placebo saline infusion treatment: their characteristics are detailed in Table 1 and results are given in Table 2. Two trials studied patients with MDD [Valentine et al. 2011; Zarate et al. 2006]; one trial used a sample of both bipolar depression BPAD and MDD patients [Berman et al. 2000]; and two trials evaluated the effects on patients with bipolar depression [DiazGranados et al. 2010b; Zarate et al. 2012], in all cases having diagnoses made via DSM criteria. All studies utilized a cross-over design, with participants each receiving both the active and placebo treatments in a randomized and double-blinded treatment order. Overall the results are as impressive as the open-label trials, although a commonly noted issue was the difficulty in truly blinding participants as the short-term dissociative effects of ketamine are common and distinctive.
Regarding the two studies only assessing effects on MDD, Zarate and colleagues undertook a cross-over RCT on 17 participants off psychotropics for 2 weeks who received either ketamine or placebo saline infusion 1 week apart [Zarate et al. 2006]. Ketamine demonstrated a significant improvement over placebo at 24 hours (effect size for drug difference d = 1.46; 95% confidence interval [CI] 0.91–2.01) and at 1 week (d = 0.68; 95% CI 0.13–1.23). A total of 71% of those administered ketamine met response criteria and 29% met remission criteria, measured on the HAMD, at 24 hours. Valentine and colleagues had a similar design, although in a smaller cohort (n = 10) and with a primary aim of evaluating changes in occipital amino acid neurotransmitters (discussed earlier in this paper) [Valentine et al. 2010]. They similarly found rapid antidepressant effects in the active group that were statistically significantly greater than that of the placebo group (HDRS score main effect of treatment (F(1,131) = 11.84, p < 0.0008). An earlier, but methodologically similar, study by Berman and colleagues included individuals with bipolar depression, although of the nine participants, only one had a bipolar depression, with the rest having a history of MDD [Berman et al. 2000]. At the time point 230 minutes post-ketamine infusion HAMD and HDRS scores displayed statistically significant improvements over placebo and 72 hours post-ketamine infusion HAMD scores were reduced by an average of 48%.
The two studies investigating the effect of ketamine in bipolar depression yielded similar positive results [DiazGranados et al. 2010b; Zarate et al. 2012]. Within 40 minutes of ketamine infusion, depressive symptoms significantly improved compared with placebo administration, remaining significant to day 3 in both studies (p < 0.001). DiazGranados and colleagues found an effect size of d = 0.52 (95% CI 0.28–0.76) at 40 minutes, d = 0.67 (95% CI 0.42–0.91) at 1 day and d = 0.22 (95% CI −0.03 to 0.48) at day 14 [DiazGranados et al. 2010b]. The largest effect size recorded by DiazGranados and colleagues was at 2 days post-infusion, d = 0.80 (95% CI 0.55–1.04). The work by Zarate and colleagues was with 14 subjects with treatment-resistant bipolar depression already stabilized on either lithium or lamotrigine who received either ketamine or saline infusions on two test days a fortnight apart [Zarate et al. 2012]. The authors reported a moderate to large drug effect size of d = 0.89 (95% CI 0.61–1.16) at 40 minutes through to 230 minutes (d = 0.85; 95% CI 0.57–1.14), at day 1 (d = 0.70; 95% CI 0.42–0.98) and at day 2 (d = 0.65; 95% CI 0.37–0.93), whilst the placebo showed no significant change in symptomatology. The largest effect size recorded by Zarate and colleagues was at 40 minutes post-infusion. Response rates were comparable at between 71% and 79%, as were remission rates of between 29% and 31%.
As with the single-dose studies the effects of ketamine waned after a reasonably short period. Of the 79% who attained remission criteria at 24 hours in the work by Zarate and colleagues [Zarate et al. 2006], 35% were reported as maintaining this at 1 week, whilst Valentine and colleagues [Valentine et al. 2010] described the statistically significant improvement of the active treatment over the placebo group was sustained for a week. In the bipolar depression studies the mean time to relapse was reported as 4.5 days by Zarate and colleagues [Zarate et al. 2012], and although this figure was not documented by DiazGranados and colleagues [DiazGranados et al. 2010b], comparisons from baseline at days 7, 10 and 14 were no longer significant.
The side effects recorded in the controlled studies were similar in nature and frequency to those of the open-label trials, with the active drug generally well tolerated, although distinctive transient dissociative and perceptual disturbances were noted in all trials, and likely to have affected study blinding.
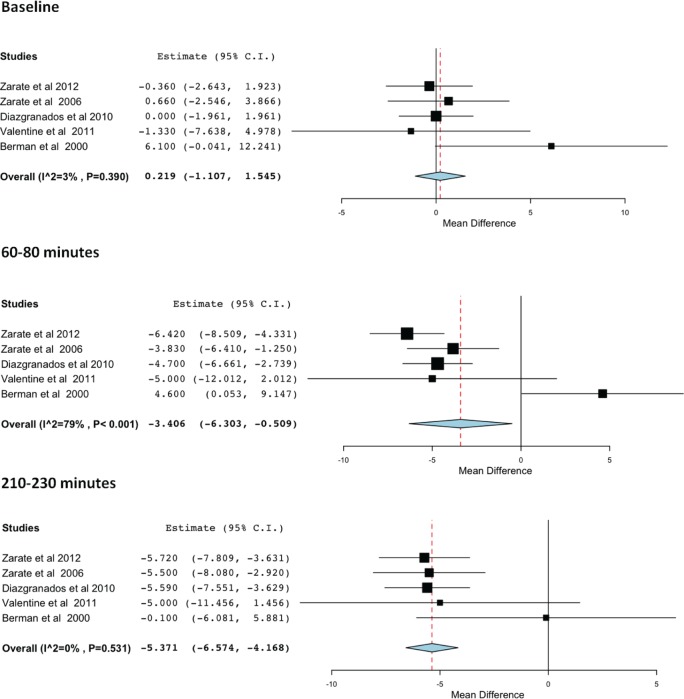
Meta-analysis of the double-blind RCTs of ketamine
Meta-analysis of the double-blind RCTs supports the antidepressant efficacy of ketamine. Figure 3 shows the difference between ketamine and placebo at baseline (top), 60–80 minutes (middle) and 210–230 minutes (bottom). At 60–80 minutes the mean difference in depression scores on the HDRS was −3.406 (p < 0.001; 95% CI −6.303 to −0.509) and at 210–230 minutes the mean difference was −5.371 (p < 0.001; 95% CI −6.574 to −4.168).
Figure 3.
Forest plots for the efficacy of ketamine compared with placebo at baseline (top), 60–80 minutes (middle) and 210–230 minutes (bottom).
Three studies [DiazGranados et al. 2010b; Valentine et al. 2011; Zarate et al. 2012] reported significant reductions in depression scores between 60 and 80 minutes, whilst the change was not statistically significant in two studies [Zarate et al. 2006; Berman et al. 2000]. Four studies report the change in depression scores at the 210–230 minute time point as statistically significant [Berman et al. 2000; DiazGranados et al. 2010b; Zarate et al. 2006, 2012], whilst one study [Valentine et al. 2011] reports the change in depression scores as not statistically significant.
Berman and colleagues [Berman et al. 2000] reported a large disparity between baseline depression scores in the placebo and ketamine condition, but this difference was deemed not statistically significant through a paired t test (p = 0.10). Thus, although the Forest plots reveal the mean difference between scores in the placebo and ketamine condition as relatively small, this is owing to the differences recorded at baseline. This disparity led to a heterogeneity p = 0.531 in the meta-analysis for 210–230 minutes, but the model showed a statistically significant effect of p < 0.001. The high heterogeneity evident at 60–80 minutes (I2 = 79%) is likely due to the figures reported by Berman and colleagues [Berman et al. 2000].
Ketamine and a second drug
There has been work to see whether augmentation of ketamine could support or enhance the initial improvement and avert the rapid relapse seen in the single-drug studies. Three studies have evaluated ketamine (0.5 mg/kg in both) augmentation with an anticonvulsant, either lamotrigine or riluzole, and unfortunately their results have been disappointing: study characteristics are detailed in Table 1 and results are given in Table 3. Both of these chosen augmenting drugs are ‘neuroprotective’, inhibiting Na+ channels and glutamate exocytosis, blocking NMDA receptor activation and enhancing AMPA, GluR1 and 2 receptor membrane expression, as well as having demonstrated efficacy in bipolar depression [Du et al. 2007].
Table 3.
Results of included studies addressing the use of ketamine and a second drug.
| Study | Subjects | Primary intervention |
Secondary intervention |
Time to significant improvement | Response (%) | Remission (%) | Conclusion | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Blinded/randomized | Intervention | Control | Blinded/randomized | ||||||
| Ibrahim et al. [2012] | 42 | Ketamine IV 0.5 mg/kg | Nil | N/A | Riluzole | Placebo | Yes- Double/Yes | 24 h | 62 | Not stated | Significantly lower MADRS scores from 1 day to 28-day follow up |
| Duncan et al. [2012] | 30 | Ketamine IV 0.5 mg/kg | Nil | N/A | Riluzole | Placebo | Yes- Double/Yes | 230 min | 43.33 | Not stated | Significant antidepressant response to ketamine from 230 min to 2 days |
| Mathew et al. [2010] | 26 | Lamotrigine + ketamine IV 0.5 mg/kg | Placebo + ketamine IV 0.5 mg/kg | Yes- Double/Yes | If met response criteria 72 h post-infusion, riluozole | Placebo | Yes- Double/Yes | 120 min | At 24 h: 65 | At 24 h: 50 | Ketamine has rapid and sustained antidepressant properties |
IV, intravenous administration.
Ibrahim and colleagues [Ibrahim et al. 2012] found no difference in time to relapse between 42 participants with MDD randomized to either riluzole (100–200 mg/day, n = 21) or placebo (n = 21) after an initial ketamine infusion (0.5mg/kg) in a 4-week follow-up study. A significant improvement over baseline MADRS scores was seen (p < 0.001), with mean time to relapse, across groups, of 13.2 days. Interestingly 27% of responders had not relapsed by the study’s 4-week end point. A substudy conducted by Duncan and colleagues [Duncan et al. 2012] randomized patients to receive either double-blind placebo (N = 11) or riluzole (N = 19), 4–6 hours following ketamine infusion (0.5 mg/kg). Results indicated a significant improvement in MADRS scores 230 minutes post-ketamine infusion (p < 0.00001), maintained at 1-day post-infusion (p < 0.00001) and 2 days post-infusion (p < 0.00001). However, no significant effect was reported for drug (p = 0.93), suggesting no difference in depression scores between the riluzole and placebo group. An earlier study by Mathew and colleagues [Mathew et al. 2010] used a two-stage methodology incorporating both lamotrigine and riluzole with hypothesized differing roles for each drug. In the first stage of this study 26 medication-free participants with TRD received open-label ketamine and either lamotrigine 300 mg or a placebo 2 hours prior to this to test whether lamotrigine could both limit any psychotomimetic side effects and potentially augment the antidepressive effects of ketamine. Those who met response criteria of a ≥50% decrease in MADRS scores72 hours post-infusion were then entered into a second, double-blind randomized controlled stage, that consisted of a 32-day trial of receiving flexible-dose riluzole (100–200 mg/day) or a placebo to test whether the active drug, which has some pharmacological similarities to ketamine, would limit post-ketamine relapse. In the first stage of the study the authors found a significant mean reduction in MADRS (60 ± 32%; d = 2.11; 95% CI 1.25–2.97) and Quick Inventory of Depressive Symptomatology—Self-Report (QIDS-SR; 62 ± 28%) scores at 24 hours, with response rates of 65% (n = 17) and remission rates of 50%; this dropped to 54% (n = 14, who therefore proceeded to stage 2) response rates at 72 hours. Lamotrigine was ineffective in either limiting side effects or augmenting ketamine efficacy and the two treatment groups did not differ in MADRS scores at any point (p = 0.36). In the second stage no difference between the groups (log-rank χ2 = 0.17, degrees of freedom = 1, p = 0.68) in time to relapse, which for riluzole was a mean of 24.4 days (95% CI 15.9–33.0) and for placebo was 22.0 days (95% CI 14.9–29.1).
Ketamine as an antidepressant in ECT or surgery
There are clear parallels between ketamine and ECT insofar as both have rapid actions and their effects are typically short-lived. In addition ketamine has been used as an anaesthetic, including induction prior to ECT, for decades, although its propensity to raise blood pressure through systemic catecholamine release and to cause aversive dissociative experiences generally makes it a second-line drug. It is thus not surprising that some studies have explored their combined use, especially as ketamine may also, by attenuating Glu release, moderate neurotoxic and cognitive impairment from ECT-induced cortical hyperexcitability [MacPherson and Loo, 2008]. Furthermore, unlike most anaesthetics ketamine is proconvulsive, which might facilitate ECT. Studies have evaluated augmenting ECT with a subanaesthetic dose of ketamine, using ketamine as the anaesthetic agent and one study looked at the use of ketamine as an anaesthetic agent in general orthopaedic surgery in depressed patients. The results shows promising potential for ketamine, with most work showing additional, albeit brief, benefits from its use, although not all research showed positive outcomes. The characteristics of these studies are detailed in Table 1 and results are given in Table 4.
Table 4.
Results of included studies addressing the use of ketamine as an antidepressant in ECT or surgery.
| Study | Subjects | Intervention | Control | Blinded/Randomised | Time to significant improvement | Response (%) | Remission (%) | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Abdallah et al. [2012] | 18 | Ketamine IV 0.5 mg/kg+ thiopental + ECT | Thiopental + ECT | Yes-Single/Unclear | Sixth ECT session | 13 | Not stated | Ketamine did not improve depressive symptoms following ECT |
| Loo et al. [2012] | 51 | Ketamine IV 0.5 mg/kg + ECT | Placebo + ECT | Yes- Double/Yes | Not stated | Ketamine: 100 Placebo: 63.64 |
Ketamine: 57.14 Placebo: 27.27 |
Small but significant increase in efficacy with ketamine over first week, not latter part of treatment course |
| Wang et al. [2012] | 48 | Ketamine IV 0.8 mg/kg+ ECT versus propofol + ECT versus ketamine IV 0.8 mg/kg + propofol + ECT | Nil | Yes-Single/Yes | 24 hrs | Not stated | Not stated | Ketamine + propofol or ketamine alone significant improvement in depressive symptoms following ECT treatment |
| Okamoto et al. [2010] | 31 | Ketamine IV 0.8 mg/kg + ECT | Propofol + ECT | No/No | Second ECT session | Not stated | Not stated | Ketamine anaesthesia early antidepressant effect in ECT, superior to propofol |
| Kranaster et al. [2011] | 42 | Ketamine + ECT | Thiopental + ECT | No/No | Not stated | Not stated | Not stated | HAMD scores significantly lower in ketamine group, needed significantly fewer ECT sessions |
| Kudoh et al. [2002] | 70 | Ketamine IV 1.0 mg/kg + propofol + fentanyl versus propofol + fentanyl | Nondepressed patients ketamine 1.0 mg/kg + propofol + fentanyl | Not stated/Yes | 24 h | Not stated | Not stated | Depressive state of depressed patients improved post-surgery with ketamine |
HAMD, Hamilton Rating Scale for Depression; ECT, electroconvulsive therapy; IV, intravenous administration.
ECT augmented with subanaesthetic ketamine
Two recent studies have evaluated augmentation of ECT with ketamine, one showing initial, although not sustained, positive results [Loo et al. 2012], the other [Abdallah et al. 2012] reporting no benefit. Loo and colleagues undertook a RCT of 51 participants with TRD undergoing a course of ultrabrief pulse-width right unilateral ECT randomized to augmentation with either a subanaesthetic dose of ketamine (0.5 mg/kg) or saline placebo in addition to standard thiopentone anaesthetic [Loo et al. 2012]. ECT was given three times a week, with ketamine or placebo given after induction of anaesthesia in all sessions. No group differences in neuropsychometric testing, measured on a battery of tests, were observed at any time point, although the study was only powered to detect large changes, and ketamine had no effect on seizure duration. The ketamine group did show a small but statistically significant clinical improvement in depressive symptoms during the first week of treatment and the 1 week follow-up measurement, but this had disappeared by the end of the treatment course (F(1, 40) = 0.921, p = 0.343; η2 = 0.22). No psychotomimetic problems were noted in the ketamine group, although these typically brief and self-limiting phenomena might be masked by post-anaesthetic recovery.
The work by Abdallah and colleagues had a similar design, although it included participants with bipolar depression, and ECT could be unilateral or bilateral for six sessions over 2 weeks [Abdallah et al. 2012]. The number of participants evaluated (n = 18) was smaller than originally planned as the trial was prematurely terminated due to a lack of between-group clinical differences (measured on the HDRS) in improvement of depressive symptoms at 24 or 72 hours after the first ECT session, or after the final (sixth) one. This result is interesting in that the very commonly seen initial positive response to ketamine was not demonstrated. The authors postulate that the known GABAergic potentiation and AMPA blocking effects of the barbiturate anaesthetic might have pharmacologically countered the actions of ketamine.
Use of ketamine as an anaesthetic in ECT
Three papers explored the effect of ketamine use as the anaesthetic agent in ECT compared with a common anaesthesia. The methodology was quite different in each, with two prospective studies, one evaluating single-session ECT [Wang et al. 2012] and the other an eight-session protocol [Okamoto et al. 2010], as well as one retrospective case-note study [Kranaster et al. 2011]. All demonstrated significantly improved depression scores in the ketamine groups, although benefits were short-lived.
The single session ECT study by Wang and colleagues had an interesting methodology in that 48 patients with MDD were randomized into three equal-sized (n = 16) groups, each receiving a differing ECT anaesthesia protocol [Wang et al. 2012]: ‘standard’ propofol, ketamine (0.8 mg/kg) and a third group that received combined ketamine (0.8 mg/kg) and propofol anaesthesia. This allowed the authors to test dual hypotheses of the clinical superiority of ketamine in treating depressive symptoms as well evaluating whether the combination might result in propofol ameliorating any ketamine-induced cardiovascular excitement. Patients were clinically assessed 1 day before and 1, 2, 3 and 7 days post-single-session bilateral ECT with the HDRS in a double-blinded paradigm. HDRS scores improved earlier (up to and including day 3 post-ECT) in the two ketamine groups compared with the propofol-alone group (p < 0.01), but this difference was lost by day 7 (p > 0.05). The combination anaesthesia group showed fewer physical (hypertension, p = 0.037) and psychological (post-anaesthetic hallucinations, p = 0.33) adverse effects than the ketamine-alone group.
The longer prospective study [Okamoto et al. 2010] undertook an open-label trial of 31 inpatients with TRD who were given either ketamine (n = 11) or propofol (n = 20) before each of eight ECT sessions over 4 weeks. Drug allocation was according to patient preference in discussion with the anaesthetist. Those anaesthetized with ketamine showed statistically significantly improvements in HDRS scores compared with those who received propofol after the second (p < 0.001) and fourth sessions (p < 0.001) but not after the sixth (p = 0.086) or eighth (p = 0.360) sessions.
The retrospective study by Kranaster and colleagues evaluated the records of 42 patients with TRD who had ECT anaesthetised with either ketamine (n = 16) or thiopental (n = 26), and those treated with ketamine needed significantly fewer ECT sessions (p = 0.015), had lower HAMD scores (p = 0.015) and, contrary to the negative (although more thorough assessment battery) neuropsychological tests noted in the study by Loo and colleagues [Loo et al. 2012], had higher Mini Mental State Examination (MMSE) scores (p = 0.025) [Kranaster et al. 2011]. Depression severity was similar in both groups prior to treatment, and needing fewer ECT sessions was taken as an implicit marker of positive outcome, although with the obvious caveats in interpreting retrospective data collection. However, the ketamine group also needed more anaesthetic interventions to manage raised blood pressure.
Surgical use of ketamine as an anaesthetic in depressed patients
A single study [Kudoh et al. 2002] evaluated the antidepressant effects of ketamine when used as a general anaesthetic (combined with propofol and fentanyl, n = 35) compared with combined propofol and fentanyl (n = 35) on 70 depressed patients undergoing orthopaedic surgery. A control group of 25 nondepressed patients received the three-drug combination. In the depressed cohort those receiving ketamine as part of their anaesthesia showed statistically significant improvement in their mood measured by the HDRS (p < 0.05) one day after surgery; interestingly the ketamine group also showed a significant reduction in post-operative pain scores (p < 0.05), which the authors highlight is a noted complication of surgery in depressed patients. The control group showed no change in their mental state.
Discussion
Ketamine’s efficacy as an antidepressant
In total, 22 RCTs and non-RCTs were identified that investigated the potential role of ketamine as an antidepressant in MDD and BPAD, totalling 629 participants. Ketamine infusion resulted in a rapid antidepressant effect in the vast majority of the presented studies, either administered alone, with an augmenter, or in combination with ECT. Furthermore, several recent studies demonstrated a rapid antisuicidal effect that was independent of antidepressant response. High response rates were documented in many studies, with three of the included RCTs recording a 71–79% response rate at 24 hours post-ketamine infusion [DiazGranados et al. 2010b; Zarate et al. 2006, 2012]. This is a considerable response rate to be observed at this early time point, certainly when compared with traditional monoaminergic antidepressants wherein response rates of 65% following 6–8 weeks of treatment are notable [Entsuah et al. 2001; Thase et al. 2005].
The parallels between ketamine and ECT are obvious and there is interesting work in this area, although not all studies showed positive results. The role of ketamine as an anaesthetic is generally as a second-line drug these days due to its side-effect profile. However, given its mood enhancing effects consideration of its use in both ECT and surgery for depressed patients is an interesting question. The preliminary data presented in this study are very interesting, but clearly more work is needed. Perhaps the most disappointing aspect of the existing research is the loss of improvements within days to weeks, although some studies had individuals maintaining gains for months. Inadequate work has explored the augmentation of ketamine, and the few studies that do exist do not have positive results. Interestingly to the best of our knowledge no one has yet looked at commencing a traditional antidepressant with ketamine.
Most studies reported dissociative and psychotomimetic effects following ketamine infusion, typically peaking at 40 minutes, but returning to normal around 80 minutes post-infusion [Ibrahim et al. 2011; Zarate et al. 2012]. The most commonly reported side effects included perceptual disturbances, confusion, drowsiness, elevated blood pressure, elevated pulse and dizziness. Studies demonstrated no significant correlations between change in depression scores and dissociative and psychotomimetic effects [DiazGranados et al. 2010b], indicating psychotomimetic effects were not related to the documented rapid antidepressant effect of ketamine. Adverse effects were not followed up in any of the identified studies, with only short-term effects recorded.
Methodologically, many of the discussed studies in this review are severely limited in regard to their sample size, a problem that continues to hinder many pharmacological studies more generally [Tracy et al. 2013]. A sample size of 102, 51 in each group, would be required within RCT methodology to detect a moderate effect size of 0.5, with a power of 80% and 0.05 significance [Stern et al. 1997]. However, none of the included studies included a sample size in this region, with the highest sample provided in a non-RCT design of 70. Only six studies adopted the gold standard randomized, double-blind, placebo-controlled design [Berman et al. 2000; DiazGranados et al. 2010b; Loo et al. 2012; Valentine et al. 2011; Zarate et al. 2006, 2012]. Caution must therefore be taken in interpreting and applying these results, although several authors identified the difficulties in blinding the administration of ketamine.
The potential utility of ketamine in clinical settings
The very fast antidepressant effects after single dosing in even treatment-resistant cohorts pose tantalizing possibilities in the treatment of MDD and bipolar depression. Such rapid improvement could have enormous benefits in the care of depressed (and especially acutely suicidal) patients, including allowing time to get other treatments and services in place. It seems self-evident that this could be literally life-saving.
The practical issues of IV ketamine administration with anaesthetic support are considerable. This is hardly a common, or even possible, practice for many psychiatric units, and the hurdles in terms of the necessary staff, training equipment, and potentially attitudes are formidable. The work by Larkin and colleagues [Larkin and Beautrais, 2011] was intentionally undertaken in an accident and emergency department to test the viability of emergency care, so there are preliminary results to potentially support this, but the practical challenges are clearly immense. However, in support of the current use of intravenous ketamine it has been argued [Krystal et al. 2013] that at this time when the drug’s effects and risk are not fully understood, that IV affords a convenient mechanism to: more accurately determine the lowest effective dose; see whether there is a dose–response relationship; evaluate whether or not any higher antidepressant dosing overlaps with significant perceptual or psychotomimetic symptoms; and to rapidly terminate treatment if problematic side effects arise. A further factor influencing the mode of administration is that there is currently less evidence for sustained efficacy from repeated dosing and thus there has been potentially less pressure to devise more patient-tolerable regimens. Finally concerns have been raised about the abuse potential of ketamine and that easier access to the drug (in oral preparations) increase risks of misappropriation of the medication.
Suicidal ideation is very common in many crisis presentations, many of which are not depressive disorders. The efficacy of ketamine in such situations is unknown, and ethics challengeable, although ketamine has been shown to lessen suicidal thinking independent of effects on depressive symptoms. Further, such emergency presentations are often outside normal working hours and at times when services are provided by more junior and inexperienced staff. Protocols on who would or should make a decision on the provision of such treatment, and which patients might be excluded, for example those with histories of current or past substance misuse or psychoses, would need defining.
The counter-argument is that there is almost overwhelming clinical evidence to support the acute efficacy of ketamine in severely unwell populations; and there is an uncalculated opportunity cost for admissions to psychiatric hospitals, the use of crisis teams [Carpenter et al. 2013], compulsory detention under section of the Mental Health Act, and the sometimes atherapeutic or undesired aspects of hospital admission. This is without consideration of the incalculable costs of suicide in personal and societal terms. An argument can be made that it is unethical to withhold such treatment. The practical, ethical and longer-term efficacy arguments remain unresolved. Hypothetically could a suicidal patient be administered ketamine against their wishes either, in the UK, under the Mental Health Act where their life was in danger from a mental illness, or under the Mental Capacity Act where they lacked the ability to make decisions about their care?
The future of ketamine: prototype for a new class of antidepressant?
The longer-term role of ketamine in the management of depression is unclear. Optimal dosing and longer-term data on relapse prevention and tolerability are lacking. Although most studies administered ketamine at a dose of 0.5 mg/kg in a saline drip over about 40 minutes, this was not the only schedule, with for example a bolus administration of 0.2 mg/kg over 1–2 minutes. Most studies utilized participants with treatment-resistant MDDs: on the one hand this adds to the clinical appeal of a therapy that works on those who have failed to respond to standard treatment; on the other hand it leaves open the question of the effects of ketamine on mild, moderate or treatment-naïve depressive disorders. There is no current consensus whether those who are treatment refractory and fail to respond to traditional antidepressants have a neurobiologically distinct form of the illness. All studies have methodologically appropriate inclusion and exclusion criteria that nevertheless might hinder the wider generalizability of the data. Whilst used in some individuals with bipolar depression it is not clear if the side effects would differ in those who had previous psychotic episodes as part of their BPAD.
Ketamine is well established as a psychotomimetic: occurrences of psychotic symptoms were not common in the trials, but these were short-term studies in controlled environments, and excluded those with psychotic illnesses and histories of drug dependency. The potential for harm and the broader use of this drug has not been satisfactorily answered, and MRI data on longer-term illicit use of the drug has shown it can cause cortical atrophy [Wang et al. 2013].
The very strong efficacy data but the practical administration and side-effect problems may terminally limit the use of ketamine in general psychiatric practice, although undoubtedly larger longer follow-up RCTs are needed. Ketamine data have provided new neurobiological evidence to both support aspects of the monoaminergic hypothesis of depression, and also offering novel insights into this illness. There are current trials on selective NMDA receptor subunit 2B (NR2B) antagonists such as Ro 25-6981 to see whether they can elicit the therapeutic responses seen with ketamine without the major potential problems of psychosis and dependency [Maeng et al. 2008; Preskorn et al. 2008]. It currently seems that the primary role of ketamine in depression may turn out to be as a prototype for the development of future glutamatergic antidepressants, and in furthering our understanding of the neuropathology of depression.
Abbreviations used in this article
BDI, Beck Depression Inventory; BPAD, bipolar affective disorder; ECT, electroconvulsive therapy; HAMD, Hamilton Rating Scale for Depression; HDRS, Hamilton Depression Rating Scale; IV, intravenous administration; MADRS-SI, Montgomery–Asberg Depression Rating Scale—Suicidality Item; MDD, major depressive disorder; MDE, major depressive episode; QIDS-SR, Quick Inventory of Depressive Symptomatology—Self-Report; SSF, Suicide Status Form; SSI, Scale for Suicide Ideation; TR, treatment resistant.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
Conflict of interest statement: Derek Tracy has received honoraria for educational talks from Lilly UK and Roche UK. Sukhwinder Shergill has received grant support from clinical trials from GlaxoSmithKline, Roche, Abbvie and Envivo, and has served as consultant, scientific advisor and had speaking engagements for Sunovion, Roche and Dainippon Sumitomo Pharma.
Contributor Information
Caroline Caddy, Cognition Schizophrenia and Imaging Laboratory, Department of Psychosis Studies, the Institute of Psychiatry, King’s College London, UK.
Giovanni Giaroli, Cognition Schizophrenia and Imaging Laboratory, Department of Psychosis Studies, the Institute of Psychiatry, King’s College London, UK and North East London NHS Foundation Trust, London, UK.
Thomas P. White, Cognition Schizophrenia and Imaging Laboratory, Department of Psychosis Studies, the Institute of Psychiatry, King’s College London, UK
Sukhwinder S. Shergill, Cognition Schizophrenia and Imaging Laboratory, Department of Psychosis Studies, the Institute of Psychiatry, King’s College London, UK and South London and Maudsley NHS Foundation Trust, London, UK
Derek K. Tracy, Consultant Psychiatrist, Oxleas NHS Foundation Trust, Princess Royal University Hospital, Orpington, BR6 8NY, UK and Cognition Schizophrenia and Imaging Laboratory, Department of Psychosis Studies, the Institute of Psychiatry, King’s College London, UK
References
- aan het Rot M., Collins K., Murrough J., Perez A., Reich D., Charney D., et al. (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67: 139–145 [DOI] [PubMed] [Google Scholar]
- Abdallah C., Fasula M., Kelmendi B., Sanacora G., Ostroff R. (2012) Rapid antidepressant effect of ketamine in the electroconvulsive therapy setting. J ECT 28: 157–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H., Rusak B., Robertson H. (1992) NMDA and non-NMDA receptor antagonists inhibit photic induction of Fos protein in the hamster suprachiasmatic nucleus. Brain Res Bull 28: 831–835 [DOI] [PubMed] [Google Scholar]
- Albayrak Y., Hashimoto K. (2012) Beneficial effects of sigma-1 agonist fluvoxamine for tardive dyskinesia in patients with postpsychotic depressive disorder of schizophrenia: report of 5 cases. Prim Care Companion CND Disord 14: PCC.12br01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry A., Adachi M., Nosyreva E., Na E., Los M., Cheng P., et al. (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475: 91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune B., Adrian I., Jacobi F. (2007) Medical disorders affect health outcome and general functioning depending on comorbid major depression in the general population. J Psychosom Res 62: 109–118 [DOI] [PubMed] [Google Scholar]
- Bellet M., Vawter M., Bunney B., Bunnet W., Sassone-Corsi P. (2011) Ketamine influenced CLOCK:BMAL1 function leading to altered circadian gene expression. PLoS One 6: e23982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman R., Cappiello A., Anand A., Oren D., Heninger G., Charney D., et al. (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351–354 [DOI] [PubMed] [Google Scholar]
- Beurel E., Song L., Jope R. (2011) Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry 16: 1068–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K., Tracy D. (2013) Lithium: the pharmacodynamics actions of the amazing ion. Ther Adv Psychopharmacol 3: 163–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter R., Falkenburg J., White T., Tracy D. (2013) Crisis teams: systematic review of their effectiveness in practice. Psychiatrist 37: 232–237 [Google Scholar]
- Carr D.B., Goudas L.C., Denman W.T., Brookoff D., Lavin P.T., Staats P.S. (2004) Safety and efficacy of intranasal ketamine in a mixed population with chronic pain. Pain 110: 762–764 [DOI] [PubMed] [Google Scholar]
- Colwell C., Menaker M. (1992) NMDA as well as non-NMDA receptor antagonists can prevent the phase-shifting effects of light on the circadian system of the golden hamster. J Biol Rhythms 7: 125–136 [DOI] [PubMed] [Google Scholar]
- Craven R. (2007) Ketamine. Anaesthesia 62: S48–53 [DOI] [PubMed] [Google Scholar]
- Davis L., Frazier E., Gaynes B., Trivedi M., Wisniewski S., Fava M., et al. (2007) Are depressed outpatients with and without a family history of substance use disorder different? A baseline analysis of the STAR*D cohort. J Clin Psychiatry 68: 1931–1938 [DOI] [PubMed] [Google Scholar]
- De Berardis D., Marini S., Fornaro M., Srinivasan V., Iasevoli F., Tomasetti C., et al. (2013) The melantonergic system in mood and anxiety disorders and the role of agomelatine: implications for clinical practice. Int J Mol Sci 14: 12458–12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N., Ibrahim L., Brutsche N., Ameli R., Henter I., Luckenbaugh D., et al. (2010a) Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychol 71: 1605–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N., Ibrahim L., Brutsche N., Newberg A., Kronstein P., Khalife S., et al. (2010b) Randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment resistant bipolar depression. Arch Gen Psychiatry 67: 793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Suzuki K., Wei Y., Wang Y., Blumenthal R., Chen Z., et al. (2007) The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology 32: 793–802 [DOI] [PubMed] [Google Scholar]
- Duman R. (2004) Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromol Med 5: 11–25 [DOI] [PubMed] [Google Scholar]
- Duman R., Voleti B. (2012) Signalling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid acting agents. Trends Neurosci 35: 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan W., Sarasso S., Ferrarelli F., Selter J., Riedner B., Hejazi N., et al. (2012) Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Intl J Neuropsychopharmacol 16: 301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entsuah A.R., Huang H., Thase M.E. (2001) Response and remission rates in different subpopulations with major depressive disorder administered venlafaxine, selective serotonin reuptake inhibitors, or placebo. J Clin Psychiatry 62: 869–77 [DOI] [PubMed] [Google Scholar]
- Glue P., Gulati A., Le Nedelec M., Duffull S. (2011) Dose- and exposure-response to ketamine in depression. Biol Psychiatry 70: e9–10 [DOI] [PubMed] [Google Scholar]
- Goforth H.W., Holsinger T. (2007) Rapid relief of severe major depressive disorder by use of preoperative ketamine and electroconvulsive therapy. J ECT 23: 23–5 [DOI] [PubMed] [Google Scholar]
- Grant B., Stinson F., Hasin D., Dawson D., Chou S., Ruan W., et al. (2005) Prevalence, correlates, and comorbidity of bipolar I disorder and axis I and II disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychol 66: 1205–1215 [DOI] [PubMed] [Google Scholar]
- Grassi-Zucconi G., Semprevivo M., Mocaer E., Kristensson K., Bentivoglio M. (1996) Melatonin and its new agonist S-20098 restore synchronised sleep fragmented by experimental trypanosome infection in the rat. Brain Res Bull 39:63–68 [DOI] [PubMed] [Google Scholar]
- Guo M., Lu Y., Garza J., Li Y., Chua S., Zhang W., et al. (2012) Forebrain glutamatergic neurons mediate leptin action on depression-like behaviours and synaptic depression. Translational Psychiatry 2: e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J., Furman D., Chang C., Thomason M., Dennis E., Gotlib I. (2011) Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biol Psychiatry 70: 327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Su T. (2008) An update on the development of drugs for neuropsychiatric disorders: focusing on the sigma 1 receptor ligand. Exp Opin Ther Targets 12: 45–58 [DOI] [PubMed] [Google Scholar]
- Hayashi T., Tsai S., Mori T., Fujimoto M., Su T. (2011) Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Exp Opin Ther Drugs 15: 557–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L., DiazGranados N., Luckenbaugh D., Machado-Vieira R., Baumann J., Mallinger A., et al. (2011) Rapid decrease in depressive symptoms with an N-methyl-D-aspartate antagonist in ECT-resistant major depression. Prog Neuro-Psychopharmacol Biol Psychiatry 35: 1155–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L., DiazGranados N., Franco-Chavos J., Brutsche N., Henter I., Kronstein P., et al. (2012) Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology 37: 1526–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishima T., Hashimoto K. (2012) Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by ifenprodil; the role of sigma-1 and IP3 receptors. PLoS One 7: e37989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan C., Goswami D., Austin M., Iyo A., Chandran A., Stockmeier C., et al. (2011) The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuro-Psychopharmacology Biol Psychiatry 35: 1774. –1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R., Akiskal H., Ames M., Birnbaum H., Greenberg P., Hirschfeld R., et al. (2006) Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. Am J Psychiatry 163(9); 1561–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R., Berglund P., Demler O., Jin R., Koretz D., Merkangas K., et al. (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). J Am Med Assoc 289: 3095–3105 [DOI] [PubMed] [Google Scholar]
- Kinsler R., Dunman R. (2008) Acute ketamine administration increases VEGF expression in the hippocampus: potential role in the rapid antidepressant effects of ketamine. In: Society for Neuroscience Meeting, Washington, DC, 2003, Abstract #56.14. [Google Scholar]
- Kishimoto A., Kaneko M., Gotoh Y., Hashimoto K. (2012) Ifenprodil for the treatment of flashbacks in female posttraumatic stress disorder patients with a history of childhood sexual abuse. Biol Psychiatry 71: e7-e8 [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Yatomi K., Yokoyama Y., Nakatsu N., Fujita K., Hashimoto K. (2013) Ifenprodil for emotional incontinence in patients with vascular dementia. J Clin Psychopharmacol 33: 143–145 [DOI] [PubMed] [Google Scholar]
- Kranaster L., Kammerer-Ciernioch J., Hoyer C., Sartorius A. (2011) Clinically favourable effects of ketamine as an anaesthetic for electroconvulsive therapy: a retrospective study. Eur Arch Psychiatry Clin Neurosci 261: 575–582 [DOI] [PubMed] [Google Scholar]
- Krystal J., Karper L., Seibyl J., Freeman G., Delaney R., Bremmer D., et al. (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Arch Gen Psychiatry 51: 199–214 [DOI] [PubMed] [Google Scholar]
- Krystal J.H., Sanacora G., Duman R.S. (2013) Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 73: 1133–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh A., Takahira Y., Katagai H., Takazawa T. (2002) Small-dose ketamine improves the postoperative state of depressed patients. Anesthesia Analgesia 95: 114–118 [DOI] [PubMed] [Google Scholar]
- Larkin G., Beautrais A. (2011) A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol 14: 1127–1131 [DOI] [PubMed] [Google Scholar]
- Li N., Lee B., Liu R., Bansr M., Dwyer J., Iwata M., et al. (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329: 959–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Garza J., Bronner J., Kim C., Zhang W., Lu X. (2010) Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology 207: 535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd L., Giaroli G., Taylor D, Tracy D. (2011) Bipolar depression: clinically missed, pharmacologically mismanaged. Ther Adv Psychopharmacol 1: 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo C., Katalinic N., Garfield J., Sainsbury K., Hadzi-Pavlovic D., MacPherson R. (2012) Neuropsychological and mood effects of ketamine in electroconvulsive therapy: a randomised controlled trial. J Affect Disord 142: 233–240 [DOI] [PubMed] [Google Scholar]
- Lord B., Wintmolders C., Langlois X., Nguyen L., Lovenberg T., Bonaventure P. (2013) Comparison of the ex vivo receptor occupancy profile of ketamine to several NMDA receptor antagonists in mouse hippocampus. Eur J Pharmacol, in press. DOI: 10.1016/j.ejphar.2013.06.028. [DOI] [PubMed] [Google Scholar]
- Lu X., Kim C., Frazer A., Zhang W. (2006) Leptin: a potential novel antidepressant. Proc Natl Acad Sci USA 103: 1593–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino F., de Wit L., Bouvy P., Stijnen T., Cuijpers P., Penninx B., et al. (2010) Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 67: 220–229 [DOI] [PubMed] [Google Scholar]
- Ma X., Dang Y., Jia M., Ma R., Wang F., Wu J., et al. (2013) Long-lasting antidepressant action of ketamine, but not glycogen synthase kinase-3 inhibitor SB216763, in the chronic mild stress model of mice. PLoS One 8: e56053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R., Salvadore G., Luckenbaugh D., Manji H., Zarate C. (2009) Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. J Clin Psychology 69: 946–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson R., Loo C. (2008) Cognitive impairment following electroconvulsive therapy-does the choice of anesthetic agent make a difference? J ECT 24: 52–56 [DOI] [PubMed] [Google Scholar]
- Maeng S., Zarate C., Du J., Schloesser R., McCammon J., Chen G., et al. (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydorxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63: 349–352 [DOI] [PubMed] [Google Scholar]
- Mathew S., Murrough J., aan het Rot M., Collins K., Reich D., Charney D. (2010) Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol 13: 71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas K., Akiskal H., Angst J., Greenberg P., Hirschfeld R., Petukhova M., et al. (2007) Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 64: 543552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B., Krystal J. (2012) Capturing the angel in “angel dust”: Twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophrenia Bull 38: 942–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough J., Perez A., Pillemer S., Stern J., Parides M., aan het Rot M., et al. (2013) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D. (2004) Hallucinogens. Pharmacol Therapeutics 101: 131–181 [DOI] [PubMed] [Google Scholar]
- Nishimura T., Ishima T., Iyo M., Hashimoto K. (2008) Potentiation of nerve growth factor-induced neurite outgrowth by fluvoxamine: role of sigma-1 receptors, IP3 receptors and cellular signalling pathways. PLoS One 3: e2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N., Nakai T., Sakamoto K., Nagafusa Y., Higuchi T., Nishikawa T. (2010) Rapid antidepressant effect of ketamine anaesthesia during electroconvulsive therapy of treatment-resistant depression. J ECT 26: 223–227 [DOI] [PubMed] [Google Scholar]
- Pal A., Fontanilla D., Gopalakrishnan A., Chae Y., Markley J., Ruoho A. (2012) The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur J Pharmacol 5: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanastasiou E., Stone J., Shergill S. (2013) When the drugs don’t work: the potential of glutamatergic antipsychotics in schizophrenia. Br J Psychiatry 202: 91–93 [DOI] [PubMed] [Google Scholar]
- Penn E., Tracy D. (2012) The drugs don’t work? Antidepressants and the current and future pharmacological management of depression. Ther Adv Psychopharmacol 2: 179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis I., Limoncelli D., Gueorguieva R., Jatlow P., Boutros N., Trevisan L., et al. (2004) Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry 161: 1776–1782 [DOI] [PubMed] [Google Scholar]
- Phelps L., Brutsche N., Moral J., Luckenbaugh D., Manki H., Zarate C. (2009) Family history of alcohol dependence and initial antidepressant response to an NMDA antagonist. Biol Psychiatry 65: 181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleszak E., Wośko Serefko A., Szopa A., Wlaź A., Szewczyk B., Nowak G., et al. (2013) Effects of ifenprodil on the antidepressant-like activity of NMDA ligands in the forced swim test in mice. Prog Neuro-Psychopharmacol Biol Psychiatry 46: 29–35 [DOI] [PubMed] [Google Scholar]
- Pompili M., Serafina G., Innamorati M., Venturini P., Fusar-Poli P., Sher L., et al. (2013) Agomelatine, a novel intriguing antidepressant option enhancing neuroplasticity: a critical review. World J Biol Psychiatry, in press. DOI: 10.3109/15622975.2013.765593 [DOI] [PubMed] [Google Scholar]
- Popoli M., Yan Z., McEwen B., Sanacora G. (2012) The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13: 22–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn S., Baker B., Kolluri S., Menniti F., Krams M., Landen J. (2008) An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 28: 631–637 [DOI] [PubMed] [Google Scholar]
- Price R., Nock M., Charney D., Mathew S. (2009) Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry 66: 522–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M., MacLeod A., Snyder A., Powers W., Gusnard D., Shulman G. (2001) A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K., Lineberry T., Galardy C., Kung S., Lapid M., Palmer B., et al. (2013) Serial infusions of low-dose ketamine for major depression. J Psychopharmacol 27: 444–450 [DOI] [PubMed] [Google Scholar]
- Salvadore G., Cornwell B., Colon-Roasario V., Coppola R., Grillon C., Zarate C., et al. (2009) Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry 65: 289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G., Cornwell B., Sambataro F., Latov D., Colon-Rosario V., Carver F., et al. (2010) Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology 35: 1415–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G., van der Veen J., Zhang Y., Marenco S., Machado-Vieira R., Baumann J., et al. (2012) An investigation of amino-acid neurotransmitters as potential predictors of clinical improvement to ketamine in depression. Int J Neuropsychopharmacol 15: 1063–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G., Treccani G., Popoli M. (2012) Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders Neuropharmacology 62: 63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger M., Walter M., Lehmann M., Metzger C., Grimm S., Heinz B., et al. (2012) Ketamine decreases restung state functional network connectivity in healthy subjects: Implications for antidepressant action. PloS One 7: e44799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S., Duman R., Sanacora G. (2008) Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry 64: 527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendt K., Giaroli G., Tracy D. (2012) Beyond dopamine: glutamate as a target for future antipsychotics. ISRN Pharmacol. DOI: 10.5402/2012/427267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y., Price J., Yan Z., Mintun M. (2010) Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci 107: 11020–11025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E., Hashimoto K., Okamura N., Koike K., Komatsu N., Kumakiri C., et al. (2003) Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry 54: 70–75 [DOI] [PubMed] [Google Scholar]
- Stahl S. (2008) The sigma enigma: can sigma receptors provide a novel target for disorders of mood and cognition? J Clin Psychiatry 69: 1673–1634 [DOI] [PubMed] [Google Scholar]
- Stern R., Schmeidler J., Davidson M. (1997) Limitations of controlled augmentation trails in schizophrenia. Biol Psychiatry 42: 138–143 [DOI] [PubMed] [Google Scholar]
- Taylor V., MacQueen G. (2010) The role of adipokines in understanding the associations between obesity and depression. J Obesity. DOI: 10.1155/2010/748048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase M.E., Haight B.R., Richard N., Rockett C.B., Mitton M., Modell J.G., et al. (2005) Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: a meta-analysis of original data from 7 randomised controlled trials. J Clin Psychiatry 66: 974–81 [DOI] [PubMed] [Google Scholar]
- Tracy D., Sendt K., Shergill S. (2013) Antipsychotic polypharmacy: still dirty, hardly a secret. A systematic review and clinical guide. Curr Psychopharmacol 2: 1–29 [Google Scholar]
- Tracy D., Shergill S. (2013) Mechanisms underlying auditory hallucinations – understanding perception without stimulus. Brain Sci 3: 642–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine G., Mason G., Gomex R., Fasula M., Watzl J., Pittman B., et al. (2011) The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [1H]-MRS. Psychiatry Res 191: 122–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zheng D., Xu J., Lam W., Yew D. (2013) Brain damages in ketamine addicts as revealed by magnetic resonance imaging. Front Neuroanat 7: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Chen Y., Zhou X., Liu F., Zhang T., Zhang C. (2012) Effects of propofol and ketamine as combined anaesthesia for electroconvulsive therapy in patients with depressive disorder. J ECT 28: 128–132 [DOI] [PubMed] [Google Scholar]
- Wozniak S., Gee L., Wachtel M., Frezza E. (2009) Adipose tissue: the new endocrine organ? A review article. Digest Dis Sci 54: 1847–1856 [DOI] [PubMed] [Google Scholar]
- Yamada N., Katsuura G., Ochi Y., Ebihara K., Kusakabe T., Hosoda K., et al. (2011) Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology 152: 2634–2643 [DOI] [PubMed] [Google Scholar]
- Yang C., Hu Y., Zhou Z., Zhang G., Yang J. (2013) Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Upsala J Med Sci 118: 3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate C., Brutsche N., Ibrahim L., Franco-Chaves J., DiazGranados N., Cravchik A., et al. (2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71: 939–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate C., Singh J., Carlson P., Brutsche N., Ameli B., Luckenbaugh D., et al. (2006) A randomized trials of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63: 856–864 [DOI] [PubMed] [Google Scholar]
- Zhang C., Feng Z., Liu Y., Ji X., Peng J., Zhang X., et al. (2012) Methylphenidate enhances NMDA-receptor response in medial prefrontal cortex via sigma-1 receptor: a novel mechanism for methylphenidate action. PLoS One 7: e51910. [DOI] [PMC free article] [PubMed] [Google Scholar]