Abstract
Background:
Data about adherence of antidepressants during pregnancy are lacking. However, it is important to gain insight into adherence in this population to reduce perinatal risks for relapse of depression.
Objective:
The objective of this study was to search for an inexpensive and easy method to implement daily for assessing medication adherence during pregnancy.
Methods:
An observational study was conducted to measure adherence by comparing pill count, Beliefs about Medicine questionnaire (BMQ) and blood level monitoring against the standard, the Medication Event Monitoring System (MEMS). We used a logistic regression model to determine potential predictors for poor adherence (age, marital class, highest level of education, monthly net income, employment, smoking, alcohol use and type of antidepressant).
Results:
From January 2010 until January 2012, 41 women were included within the first trimester of pregnancy; data could be evaluated in 29 women. Using MEMS, 86% of the women took in more than 80% of all prescribed doses on time and could be classified as adherent. Pill counts showed good agreement with MEMS. We did not find predictors for poor adherence in our study population.
Conclusion:
Adherence of antidepressants during pregnancy using MEMS is 86%. There was a good agreement between MEMS and pill counts. This method may serve as a good alternative that can be easily implemented into daily practice.
Keywords: Antidepressants, adherence, pregnancy, MEMS, pill count, BMQ, TDM
Introduction
Major depressive disorder (MDD) during pregnancy is relatively common. Up to 12.7% of women reported a depression or signs of depression during pregnancy [Grote et al. 2010]. Pregnancy may be a trigger for the recurrence of depressive symptoms in vulnerable women [Bennett et al. 2004] and depression during pregnancy predicts for more than 50% of post-partum depression [Milgrom et al. 2008; Robertson et al. 2004].
The consequences of depression during pregnancy for the mother include difficulties in performing usual activities, failure to seek prenatal care, inadequate maternal nutrition and weight loss, the use of tobacco and alcohol, an increased risk for pre-eclampsia and risk of self-harm or suicide [Bennett et al. 2004; Stewart, 2011]. In addition to the negative effects for the mother, the foetus may also be affected by maternal depression. This will lead to premature delivery and lower birth weight [Bennett et al. 2004; Weinstock, 2005; Eberhard-Gran et al. 2006; Boyd et al. 2006; Howard et al. 2007], more emotional problems, hyperactivity and attention deficit hyperactivity disorder (ADHD) at a later age [Weinstock, 2005]. The latter two outcomes are associated with more morbidity and increased mortality [Bennett et al. 2004].
On average 83.1% of women use some kind of medication at some stage during pregnancy [Sawicki et al. 2011]. Approximately 13% worldwide [Stewart, 2011] and about 2% of women in the Netherlands use antidepressants during pregnancy [Ververs et al. 2006]. Adherence to prescribed medication is of major importance for successful treatment. The adherence rate of antidepressants in a nonpregnant population is between 20% and 60% within the first 6 weeks of treatment [WHO, 2012; Muzina et al. 2011]. This is a risk for relapse of depression [WHO, 2012; Muzina et al. 2011; Akerblad et al. 2006; Lee et al. 2010; Cohen et al. 2006; Geddes et al. 2003]. Data about adherence of antidepressants during pregnancy are lacking. For chronic medication such as anti-anaemics, medication for chronic airway conditions and antidiabetics among pregnant women, adherence is about 59% [Sawicki et al. 2011].
To reduce perinatal risks for relapse of depression it is of importance to gain insight into poor adherence in the pregnant population. Therefore, we conducted this study using the golden standard [Medication Event Monitoring System (MEMS)] [Claxton et al. 2001]. We compared this method with three other methods to test adherence. We also tested for potential predictors for poor adherence.
Methods
Setting
This observational study was performed in an outpatient population of a large teaching 1000-bed hospital in the middle of the Netherlands, in the period January 2010 until January 2012. The study was approved by the Medical Ethics Committee (NL 27726.075.09).
A Pregnancy Consultation Service (PCS) team, providing collaborative care with medical specialists and other healthcare professionals including gynaecology, psychiatry, paediatrics, specialized nurses, physiotherapy, mental-health workers, clinical psychology and clinical pharmacology, developed a specific psycho–obstetric–paediatric (POP) protocol for the treatment of pregnant women with psychiatric diseases. Midwives, general practitioners and mental-health care workers, in and outside the region of our hospital, refer pregnant women with psychiatric diseases to the PCS professionals. Furthermore, PCS professionals refer their patients to each other if necessary. So treatment of this patient category is tailor-made.
The POP protocol comprises extended ultrasounds, easily accessible telephone consultations by a psychiatrist, physiotherapy for relaxation and pelvic exercises. For all women included in the POP protocol, a detailed specific birth plan is available as a result of consultations from specialized nurses and sometimes clinical psychologists.
The psychiatrist gives extensive information and explains in an open and honest way in the (pre)conceptual phase about the advantages and disadvantages of (dis)continuing antidepressants during pregnancy. It has been emphasized that antidepressants are not contraindicated during pregnancy, although use of paroxetine is not preferable [Stewart, 2011; Koren and Nordeng, 2012]. To avoid feelings of guilt women, have to make their own decision to (dis)continue the antidepressant, together with their partner.
In consultation with the physician, patients can consider the possibilities of discontinuing or decreasing the dose in late pregnancy. This to mitigate the risk of persistent pulmonary hypertension of the newborn infant (PPHN) or withdrawal syndrome [Koren and Nordeng, 2012].
Women and their newborns included in the POP protocol were observed for at least the first 48 hours after birth.
Study population
Women ≥18 years were included during the first trimester of pregnancy after signing informed consent if it was to be expected that antidepressant medication would be used throughout all trimesters. Patients who were incapable of following the study protocol according to the attending specialist from the POP protocol were excluded.
Adherence assessment
To assess a daily practice method compared to MEMS, we used three different adherence methods: pill count, Beliefs about Medicine questionnaire (BMQ) and blood level monitoring.
Medication Event Monitoring System
The bottles were filled with the antidepressants with a MEMS 6 TrackCap 38 mm (Aardex Group Ltd, Switzerland) for 4 periods of 3 months. The MEMS bottles together with a diary to record deviations in intake of their antidepressant were supplied after inclusion. Patients were instructed to open the MEMS bottle only when they intended to take their medication.
The patients were made aware of the MEMS cap function prior to the start of the study.
After the first supply, the patient was responsible during the rest of the study period for collecting the antidepressant at the hospital pharmacy. Primary investigators were not involved in dispensing study medication to avoid triggers for adherence. MEMS packages were analysed at the end of the study period with Powerview® (Aardex Group Ltd, Switzerland). Additional events, such as opening for refill or by accident, were removed from the MEMS data prior to analysis, according to dispensing protocols and notes from diaries.
As an indicator to measure the adherence with MEMS we used dose-time, defined as the percentage of doses taken on schedule within 25% of the expected time interval (e.g. one-daily dose should be taken 24 ± 6 hours apart) [Claxton et al. 2001]. The cut-off point for MEMS for good adherence was ≥80% [Brook et al. 2006].
Pill count
Pill count was calculated at the end of the study program and the adherence was measured as: % Pill count = (number of prescribed pills − number of pills left in the bottle)/(number of days between dispensing date and return date of pill bottle) × 100. The cut-off point for good adherence was ≥90% [van Onzenoort et al. 2011].
Beliefs about Medicine questionnaire
The self-reported questionnaire BMQ was evaluated at the end of the study period and was used to measure the perception of the use of antidepressant. The BMQ comprises two scales: (1) assessing patient’s belief about the necessity of using medication for maintaining present and future health (necessity scale); (2) assessing patient’s concerns about the potential adverse consequences of using antidepressants (concerns scale). The necessity and concerns framework was used according to Horne and Weinman [Horne and Weinman, 1999] to define four subgroups representing different attitudes towards medication; sceptical (low necessity, high concerns), indifferent (low necessity, low concerns), ambivalent (high necessity, high concerns) and accepting (high necessity, low concerns). Each woman was categorized into one of four groups.
To calculate the adherence we dichotomized the results. The women in the accepting and ambivalent groups were classified to be adherent and the sceptical and indifferent group as poor adherent [Menckeberg et al. 2008; Clatworthy et al. 2009].
Blood level monitoring
Every trimester (3, 6 and 9 months) and 2–3 months post-partum, the blood concentration of the antidepressant was measured for possible relationships with adherence.
The therapeutic ranges of the AGNP guidelines for Therapeutic Drug monitoring in Psychiatry were used [Hiemke et al. 2011]. A plasma concentration level outside the 75–125% range of the therapeutic window was defined as poor adherence.
Plasma concentrations of fluoxetine, fluvoxamine paroxetine, sertraline and venlafaxine were analysed using a modified straight phase high-performance liquid chromatography with ultraviolet detection (HPLC-UV). Plasma concentrations of citalopram, escitalopram and clomipramine were analysed using liquid chromatography–tandem mass spectrometry (LC-MS/MS). The overall intra- and inter-assay coefficients of variation were <10% with a recovery of at least 85%. The calibration for (nor)fluoxetine was linear over the range of 62.5–812.5 μg/l, fluvoxamine over the range 10–300 μg/l, paroxetine over the range 10–200 μg/l, (desm)venlafaxine over the range 50–1000 μg/l, (es)citalopram over the range 10–300 μg/l, desmethylcitalopram over the range 10–160 μg/l and clomipramine over the range 20–400 μg/l.
Data analyses
All analyses were performed with assistant PASW statistics 18 (release 18.0.1 SPSS, Inc., Chicago, IL, USA). For continuous variables the mean and standard deviation were calculated and for categorical variables the frequencies and percentages were calculated. To measure the agreement between MEMS and the other adherence methods, pill count, blood level monitoring and BMQ, we used the Cohen’s kappa coefficient with five classes of agreement: poor (less than 0.20), fair (0.21–0.40), moderate (0.41–0.60), good (0.61–0.80) and very good (0.81–1.00).
To evaluate potential predictors which contribute to poor adherence between MEMS and the other methods a logistic regression model was used. The following sociodemographic characteristics were classified as potential predictors: age, marital class, highest level of education, monthly net income, employment, smoking, alcohol use. Odds ratios and 95% confidence intervals were calculated for the relevant factors. The significance level was set at α of 0.05.
Results
A total of 41 women were screened and established to be eligible for inclusion, of which 29 women completed the study program. Within the first trimester of pregnancy 4 women had an abortion, 1 woman delivered a child with trisomie 13 (which passed away after childbirth), 4 women lost their bottles and 3 women refused participation.
The main patient characteristics of the 29 women who completed the study program are summarized in Table 1.
Table 1.
Sociodemographic characteristics.
| (N = 29) | |
|---|---|
| Age mean ± SD (years) | 32 ± 5 |
| Marital class (%) married/living together | 100% |
| Highest education level (%) ≥ bachelor/master | 45% |
| Employed (%) | 66% |
| Monthly net income, euro’s (%) | |
| < €1100 | 0% |
| €1100–€2100 | 17% |
| €2100–€3100 | 31% |
| > €3100 | 52% |
| Planned (%) | 83% |
| Partus n (%) | |
| 1 | 31% |
| 2 | 45% |
| >3 | 24% |
| Antidepressant (%) | |
| clomipramine | 4% |
| paroxetine | 35% |
| (es)citalopram | 10% |
| fluoxetine | 10% |
| fluvoxamine | 7% |
| sertraline | 10% |
| venlafaxine | 24% |
| Smoking (%) during pregnancy | 14% |
| Alcohol use (%) during pregnancy | 14% |
SD, standard deviation.
More than 80% of all pregnancies were planned and most women (70%) were pregnant with their second or third child. Paroxetine was the most frequently used antidepressant in this population (32%) followed by venlafaxine (26%).
All women were consulted by the psychiatrist with a mean of six visits per pregnancy. More than 90% of all women were consulted by the gynaecologist with a mean of nine consultations. Almost half of the women had physiotherapy for relaxation or pelvic exercises (Table 2).
Table 2.
Consultations by PCS professionals during the pregnancy.
| Gynaecologist | Psychiatrist | Mental-health worker | Physiotherapist | Clinical psychologist | |
|---|---|---|---|---|---|
| Number of patients (%) | 28 (97%) | 29 (100%) | 2 (7%) | 12 (41%) | 2 (7%) |
| Number of consultations (mean ± SD) |
9 ± 7 | 6 ± 4 | 5 ± 1 | 5 ± 2 | 9 ± 11 |
PCS, Pregnancy Consultation Service; SD, standard deviation; MEMS, Medication Event Monitoring System.
Adherence: MEMS
According to the MEMS reports 86% of all women took more than 80% of all doses on schedule (Figure 1). According to our definitions, four women were poorly adherent and took the antidepressant on schedule less than 80% of the time. The median percentage of doses taken on schedule was 96% with a range of 48.0–100.0% (data not shown).
Figure 1.
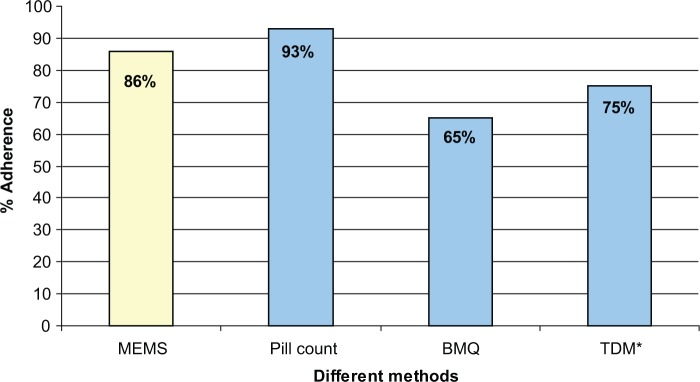
Adherence to antidepressants measured by MEMS data, pill count, TDM and BMQ.
*TDM, there are no statistical differences between trimesters of pregnancy, therefore mean is chosen.
MEMS, Medication Event Monitoring System; Therapeutic Drug Monitoring (TDM), BMQ, Beliefs about Medicine questionnaire.
Adherence: pill count
According to the pill count more than 90% of all women can be classified as adherent.
The median percentage of pills taken was 99% with a range of 72.2%–103.9% (data not shown). Six women are regarded as over-adherent.
Adherence: blood level monitoring
The number of evaluable samples was 110. Overall, for blood level monitoring, 75% of all samples were within the therapeutic range and can be classified as adherent. At 3 months of pregnancy approximately 69% of all blood samples were within the therapeutic range. At 6 months of pregnancy the percentage was increased to 75%: seven women had raised their doses in concordance with the psychiatrist. At 9 months of pregnancy the percentage of samples within the therapeutic range decreased to 64%. At 2–3 months after childbirth, more than 82% of the blood samples were within the range.
Adherence: BMQ
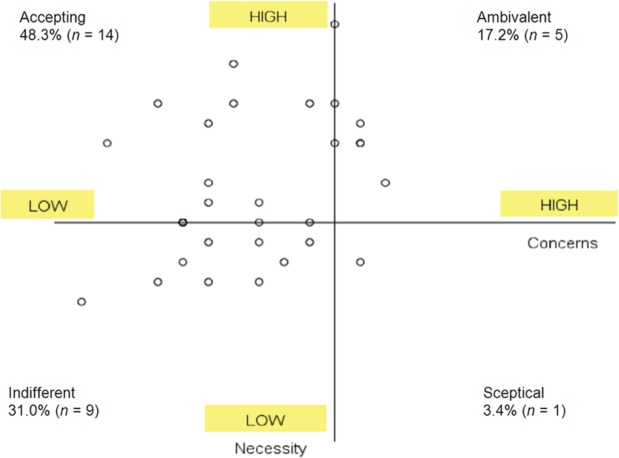
According to the concern and necessity framework of all 29 women, 48.3% can be classified as acceptors, 17.2% as ambivalent, 31.0% as indifferent and 3.4% as sceptical. The framework is shown in Figure 2.
Figure 2.
Beliefs about Medicine questionnaire defined in a necessity and concerns framework.
Table 3 shows the agreement, calculated with Cohen’s kappa coefficient between MEMS and the other adherence methods. Only pill count can be established to have good agreement with MEMS. For the BMQ and the blood level monitoring there is poor agreement between the method and MEMS, κ = 0.110 and 0.129, respectively.
Table 3.
The agreement between MEMS and other adherence methods.
| MEMS (κ) | Agreement | |
|---|---|---|
| Pill count | 0.633 | Good |
| BMQ | 0.110 | Poor |
| Blood level monitoring | 0.129 | Poor |
BMQ, Beliefs about Medicine questionnaire; MEMS, Medication Event Monitoring System
After logistic regression analyses, there were no potential predictors found for poor adherence for none of the applied methods.
Discussion
In this study we found that adherence in our PCS guided population, defined as ≥80% of doses taken on schedule, was 86% with MEMS. Compared with MEMS, only pill count had good agreement for adherence. TDM and BMQ were not associated with MEMS. It seems that in daily practice, pill counts can be used instead of MEMS.
The results of our study compared with previous research show that adherence of antidepressants during pregnancy is relatively high, compared with data from nonpregnant women with chronic medication use or the general population with antidepressants [Sawicki et al. 2011; WHO, 2012; Muzina et al. 2011].
Although pill count is a direct and relatively inexpensive way to measure adherence, data may be unreliable because patients can discard pills before visits in order to appear to be following the regime [Osterberg and Blaschke, 2005]. Compared with MEMS, we found that with using pill counts 93% of our patients were adherent. The value of pill counts in pregnant women in relation to good compliance needs further evaluation in larger studies.
The BMQ has only been validated in studies with antidepressants and chronic medications and not during pregnancy [Horne and Weinman, 1999; Menckeberg et al. 2008; Phatak and Thomas, 2006]. Using the BMQ we defined poor adherence for women categorized in the sceptical and indifferent group, according to Menckeberg and colleagues and Clatworthy and coworkers [Menckeberg et al. 2008; Clatworthy et al. 2009]. We found that 65% of pregnant women are classified as adherent. It may be that using a dichotomized value, as we did for our study population, does not reflect the method as developed by Horne and Weinman [Horne and Weinman, 1999]. For practical reasons we used an easy method in our population for measuring the adherence with BMQ.
The results for the adherence using BMQ compared with MEMS, however showed that the agreement between these methods was poor. This might be because of the dichotomized distribution of the BMQ results. In a general population the BMQ can be an appropriate method to measure the adherence, but unfortunately this was not the case in our population. For healthcare professionals, it may be important to know the beliefs about the antidepressant use, so that they can adjust therapy if necessary.
We found poor agreement between blood level concentration and MEMS. The reasons for this are possibly the large inter-individual blood concentration levels of antidepressants in general [Hiemke et al. 2011]. During pregnancy, there are changes in pharmacokinetic parameters such as increased plasma volume, decreased concentration of plasma albumin, changes in hepatic metabolism of some drugs (induction of CYP2D6 and CYP3A4) and gastrointestinal changes in absorbance [Ververs et al. 2009; Freeman et al. 2008; Altshuler and Hendrick, 1996; Sit et al. 2008]. Another problem is the interpretation of blood levels against clinical efficacy, because clear relationships are lacking, especially for selective serotonin reuptake inhibitors (SSRIs) [Rasmussen and Brosen, 2000]. Therefore, in this population, blood level concentration as a method to measure adherence does not seem to be a good alternative for MEMS.
Despite the possibility of predictors for poor adherence in our data with logistic regression analyses, our results are in line with ten Doesschate and colleagues [ten Doesschate et al. 2009].
The strength of our study is that we compared different methods in a specific population. We found that pill count was a good alternative to measure the adherence in our population and that this method can be easily implemented into daily practice by the community pharmacist or specialized pharmacists in our situation.
Our study has some limitations. First, women were aware of the MEMS cap function and needed to be instructed, which may have influenced the adherence rate in a positive way; however, from other studies we know that this method has no impact on adherence over a longer period [Hugen et al. 2002; Kastrissios et al. 1998].
Second, our study population was small. Despite the high number of patients visiting our clinic, we could not include most women because the time of inclusion was set at less than 3 months of pregnancy. They had their first visit at the PCS professional within the second or third trimester of pregnancy.
Third, in our setting the PCS team delivers high standards of care, which may not be representative of other clinics. It is possible that high standard of care and the frequent visits (see Table 2) leads to higher adherence in general. In a pilot experiment, using Medication Possession Ratio we found a lower number of adherent women, where adherence decreased from 62% to 46% during pregnancy [home-PW, 2012]. These results are in contrast to the findings presented here. In our earlier retrospective study, women were not aware of participation at time of inclusion. It could be that participating in a trial leads to higher adherence [van Onzenoort et al. 2011]. This has to be further evaluated in larger studies.
Conclusion
Adherence to antidepressant use during pregnancy using MEMS is 86%. Compared with MEMS, pill counts show good agreement. Therefore, this method may serve as a good alternative that can be easily implemented into daily practice. Owing to the small number of patients in our study, further investigation is mandatory.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors have no conflicts of interest to declare.
Contributor Information
Judith Bosman, Department of Clinical Pharmacy, Isala Clinics, Dr. van Heesweg 2, Zwolle, The Netherlands.
Peter G.J. ter Horst, Department of Clinical Chemistry, Isala Clinics, Zwolle, The Netherlands
Jan Pieter Smit, Department of Psychiatry, Isala Clinics, Zwolle, The Netherlands.
Jeroen R. Dijkstra, Department of Gynaecology and Obstetrics, Isala Clinics, Zwolle, The Netherlands
Hans R. Beekhuis, Department of Gynaecology and Obstetrics, Isala Clinics, Zwolle, The Netherlands
Robbert J. Slingersland, Department of Clinical Chemistry, Isala Clinics, Zwolle, The Netherlands
Wobbe Hospes, Department of Clinical Pharmacy, Isala Clinics, Zwolle, The Netherlands.
References
- Akerblad A., Bengtsson F., von Knorring L., Ekselius L. (2006) Response, remission and relapse in relation to adherence in primary care treatment of depression: a 2-year outcome study. Int Clin Psychopharmacol 21: 117–124 [DOI] [PubMed] [Google Scholar]
- Altshuler L., Hendrick V. (1991) Pregnancy and psychotropic medication: changes in blood levels. J Clin Psychopharmacol 16: 78–80 [DOI] [PubMed] [Google Scholar]
- Bennett H., Einarson A., Taddio A., Koren G., Einarson T. (2004) Depression during pregnancy: overview of clinical factors. Clin Drug Investig 24: 157–179 [DOI] [PubMed] [Google Scholar]
- Boyd R., Zayas L., McKee M. (2006) Mother-infant interaction, life events and prenatal and postpartum depressive symptoms among urban minority women in primary care. Matern Child Health J 10: 139–148 [DOI] [PubMed] [Google Scholar]
- Brook O., van Hout H., Stalman W., de Haan M. (2006) Nontricyclic antidepressants: predictors of nonadherence. J Clin Psychopharmacol 26: 643–647 [DOI] [PubMed] [Google Scholar]
- Clatworthy J., Bowskill R., Parham R., Rank T., Scott J., Horne R. (2009) Understanding medication non-adherence in bipolar disorders using a Necessity-Concerns Framework. J Affect Disord 116: 51–55 [DOI] [PubMed] [Google Scholar]
- Claxton A., Cramer J., Pierce C. (2001) A systematic review of the associations between dose regimens and medication compliance. Clin Ther 23: 1296–1310 [DOI] [PubMed] [Google Scholar]
- Cohen L., Altshuler L., Harlow B., Nonacs R., Newport D., Viguera A., et al. (2006) Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. J Am Med Assoc 295: 499–507 [DOI] [PubMed] [Google Scholar]
- Eberhard-Gran M., Eskild A., Opjordsmoen S. (2006) Use of psychotropic medications in treating mood disorders during lactation: practical recommendations. CNS Drugs 20: 187–198 [DOI] [PubMed] [Google Scholar]
- Freeman M., Nolan P., Jr, Davis M., Anthony M., Fried K., Fankhauser M., et al. (2008) Pharmacokinetics of sertraline across pregnancy and postpartum. J Clin Psychopharmacol 28: 646–653 [DOI] [PubMed] [Google Scholar]
- Geddes J., Carney S., Davies C., Furukawa T., Kupfer D., Frank E., et al. (2003) Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet 361: 653–661 [DOI] [PubMed] [Google Scholar]
- Grote N., Bridge J., Gavin A., Melville J., Iyengar S., Katon W. (2010) A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry 67: 1012–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemke C., Baumann P., Bergemann N., Conca A., Dietmaier O., Egberts K., et al. (2011) AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry 44: 195–235 [DOI] [PubMed] [Google Scholar]
- Horne R., Weinman J. (1999) Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 47: 555–567 [DOI] [PubMed] [Google Scholar]
- Howard L., Kirkwood G., Latinovic R. (2007) Sudden infant death syndrome and maternal depression. J Clin Psychiatry 68: 1279–1283 [DOI] [PubMed] [Google Scholar]
- Hugen P., Langebeek N., Burger D., Zomer B., van Leusen R., Schuurman R., et al. (2002) Assessment of adherence to HIV protease inhibitors: comparison and combination of various methods, including MEMS (electronic monitoring), patient and nurse report, and therapeutic drug monitoring. J Acquir Immune Defic Syndr 30: 324–334 [DOI] [PubMed] [Google Scholar]
- Kastrissios H., Suarez J., Katzenstein D., Girard P., Sheiner L., Blaschke T. (1998) Characterizing patterns of drug-taking behavior with a multiple drug regimen in an AIDS clinical trial. AIDS 12: 2295–2303 [DOI] [PubMed] [Google Scholar]
- Koren G., Nordeng H. (2012) Antidepressant use during pregnancy: the benefit-risk ratio. Am J Obstet Gynecol 207: 157–163 [DOI] [PubMed] [Google Scholar]
- Lee M., Lee H., Kang S., Yang J., Ahn H., Rhee M., et al. (2010) Variables influencing antidepressant medication adherence for treating outpatients with depressive disorders. J Affect Disord 123: 216–221 [DOI] [PubMed] [Google Scholar]
- Menckeberg T., Bouvy M., Bracke M., Kaptein A., Leufkens H., Raaijmakers J., et al. (2008) Beliefs about medicines predict refill adherence to inhaled corticosteroids. J Psychosom Res 64: 47–54 [DOI] [PubMed] [Google Scholar]
- Milgrom J., Gemmill A., Bilszta J., Hayes B., Barnett B., Brooks J., et al. (2008) Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord 108: 147–157 [DOI] [PubMed] [Google Scholar]
- Muzina D., Malone D., Bhandari I., Lulic R., Baudisch R., Keene M. (2011) Rate of non-adherence prior to upward dose titration in previously stable antidepressant users. J Affect Disord 130: 46–52 [DOI] [PubMed] [Google Scholar]
- Osterberg L., Blaschke T. (2005) Adherence to medication. N Engl J Med 353:487–497 [DOI] [PubMed] [Google Scholar]
- Phatak H., Thomas J., III (2006) Relationships between beliefs about medications and nonadherence to prescribed chronic medications. Ann Pharmacother 40: 1737–1742 [DOI] [PubMed] [Google Scholar]
- Rasmussen B., Brosen K. (2000) Is therapeutic drug monitoring a case for optimizing clinical outcome and avoiding interactions of the selective serotonin reuptake inhibitors? Ther Drug Monit 22: 143–154 [DOI] [PubMed] [Google Scholar]
- Robertson E., Grace S., Wallington T., Stewart D. (2004) Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry 26: 289–295 [DOI] [PubMed] [Google Scholar]
- Sawicki E., Stewart K., Wong S., Leung L., Paul E., George J. (2011) Medication use for chronic health conditions by pregnant women attending an Australian maternity hospital. Aust N Z J Obstet Gynaecol 51: 333–338 [DOI] [PubMed] [Google Scholar]
- Sit D., Perel J., Helsel J., Wisner K. (2008) Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J Clin Psychiatry 69: 652–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart D. (2011) Clinical practice. Depression during pregnancy. N Engl J Med 365: 1605–1611 [DOI] [PubMed] [Google Scholar]
- ten Doesschate M., Bockting C., Koeter M., Schene A. (2009) Predictors of nonadherence to continuation and maintenance antidepressant medication in patients with remitted recurrent depression. J Clin Psychiatry 70: 63–69 [DOI] [PubMed] [Google Scholar]
- ter Horst P.G.J., Wilffert B., Smit J.P., den Boon J., Hospes W., de Jong-van den Berg L.T.W. (2011) Therapy adherence to antidepressive agents before and during pregnancy determined with the medication possession ratio. PW Wetenschappelijk Platform 5:a1148 [Google Scholar]
- van Onzenoort H., Menger F., Neef C., Verberk W., Kroon A., de Leeuw P., et al. (2011) Participation in a clinical trial enhances adherence and persistence to treatment: a retrospective cohort study. Hypertension 58: 573–578 [DOI] [PubMed] [Google Scholar]
- Ververs F., Voorbij H., Zwarts P., Belitser S., Egberts T., Visser G., et al. (2009) Effect of cytochrome P450 2D6 genotype on maternal paroxetine plasma concentrations during pregnancy. Clin Pharmacokinet 48: 677–683 [DOI] [PubMed] [Google Scholar]
- Ververs T., Kaasenbrood H., Visser G., Schobben F., de Jong-van den Berg L., Egberts T. (2006) Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol 62: 863–870 [DOI] [PubMed] [Google Scholar]
- Weinstock M. (2005) The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun 19:296–308 [DOI] [PubMed] [Google Scholar]
- WHO (2012) Adherence to long-term therapies: evidence for action. Available at: http://www.who.int/chp/knowledge/publications/adherence_report/en/ (accessed 20 February 2012).