Abstract
Objective
To evaluate measures of cardiac activity and reactivity as prospective biomarkers of treatment response to an empirically-supported behavioral intervention for attention-deficit/hyperactivity disorder (ADHD).
Method
Cardiac pre-ejection period (PEP), an index of sympathetic-linked cardiac activity, and respiratory sinus arrhythmia (RSA), and index of parasympathetic-linked cardiac activity, were assessed among 99 preschool children (ages 4–6 years) with ADHD both at rest and in response to behavioral challenge, before participants and their parents completed one of two versions of the Incredible Years parent and child interventions.
Results
Main effects of PEP activity and reactivity, and of RSA activity and reactivity were found. Although sample-wide improvements in behavior were observed at post treatment, those who exhibited lengthened cardiac PEP at rest and reduced PEP reactivity to incentives scored higher on measures of conduct problems and aggression both before and after treatment. In contrast, children who exhibited lower baseline RSA and greater RSA withdrawal scored lower on prosocial behavior before and after treatment. Finally, children who exhibited greater RSA withdrawal scored lower on emotion regulation before and after treatment.
Conclusions
We discuss these findings in terms of (a) individual differences in underlying neurobiological systems subserving appetitive (i.e., approach) motivation, emotion regulation, and social affiliation, and (b) the need to develop more intensive interventions targeting neurobiologically vulnerable children.
Keywords: ADHD, intervention, PEP, RSA, treatment response
Attention-deficit hyperactivity/disorder (ADHD) in preschool confers significant risk for more serious externalizing conditions—including oppositional defiant disorder (ODD), conduct disorder (CD), and delinquency—in middle childhood and adolescence (see Beauchaine, Hinshaw, & Pang, 2010; Campell, Shaw, & Gilliom, 2000). Furthermore, although prediction of later conduct problems from preschool ADHD is far from absolute, males who become delinquent as adults almost invariably traverse a developmental pathway that begins with hyperactivity/impulsivity in toddlerhood, followed by ODD in early childhood, early-onset CD in elementary school, substance use disorders (SUDs) in adolescence, and antisocial personality in adulthood (see Loeber & Hay, 1997; Lynam, 1996, 1998; Moffitt & Caspi, 2001). This developmental trajectory of externalizing conduct has been described in the literature for nearly 50 years (Robins, 1966). Given (a) the extraordinary costs associated with delinquency (Craig, Schumann, Petrunka, Khan, & Peters, 2011); (b) its resistance to treatment once canalized (Gatti, Tremblay, & Vitaro, 2009); and (c) the long term effectiveness of family, parent, and psychostimulant interventions when delivered early in life (Mannuzza et al., 2008; Piquero, Farrington, Welsh, Tremblay, & Jennings, 2009), identifying children with ADHD who are vulnerable to later delinquency and providing them with empirically supported treatments should be a national priority (Beauchaine, Klein, Crowell, Derbidge, & Gatzke-Kopp, 2009).
Hyperactive/impulsive males—both with and without comorbid conduct problems—exhibit structural and functional compromises in the central and peripheral nervous systems (see e.g., Beauchaine, Katkin, Strassberg, & Snarr, 2001; Crowell et al., 2006; Durston et al., 2003; Gatzke-Kopp et al., 2009; Nigg, in press; Sagvolden, Johansen, Aase, & Russell, 2005; Scheres, Milham, Knutson, & Castellanos, 2007; Sauder, Beauchaine, Gatzke-Kopp, Shannon, & Aylward, 2012; Castellanos et al., 2003; Shannon, Sauder, Beauchaine, & Gatzke-Kopp, 2009; Valera, Faraone, Murray, & Seidman, 2007). Accumulating evidence suggests that these neurobiological vulnerabilities interact with high risk and protective environments to either promote or inhibit progression along the externalizing trajectory outlined above (for reviews see Beauchaine & Gatzke-Kopp, 2012; Beauchaine & McNulty, in press; Beauchaine et al., 2009; 2010; Gatzke-Kopp, Gatzke-Kopp & Beauchaine, 2007). As a result, children who are impulsive are more likely to progress toward and/or engage in delinquent behaviors when reared in environments characterized by hostile and inconsistent parenting (Drabick, Gadow, & Sprafkin, 2006), maltreatment and neglect (De Sanctis et al., 2008), and/or neighborhood violence/criminality (Lynam et al., 2000; Meier, Slutske, Arndt, & Cadoret, 2008). Moreover, children who are impulsive are more likely than non-impulsive children to evoke aversive reactions from their caregivers, which may exacerbate their preexisting vulnerabilities (O’Connor, Deater-Deckard, Fulker, Rutter, & Plomin, 1998). Furthering our understanding of how biological vulnerabilities interact with high risk environments to promote progression of externalizing conduct—and how prevention and intervention programs can inhibit such progression—has therefore received considerable research attention in recent years (see Burnett & Cicchetti, 2012). In this paper, we examine whether cardiovascular biomarkers of trait impulsivity and emotion dysregulation predict response to an empirically supported treatment among 4–6-year-old children with ADHD.
Although full articulation of the neurobiological substrates of ADHD is well beyond the scope of this article, most theories of impulsivity focus at least in part on deficient responding in mesolimbic structures rich in dopamine (DA) neurons (see Volkow et al., 2009). The mesolimbic DA system comprises the nucleus accumbens, the caudate, and several other interconnected structures, which evolved to modulate appetitive (i.e., approach) behaviors. Midbrain DA neurons are less responsive to reward—including monetary incentives—among impulsive individuals than among controls (see Durston, 2003; Gatzke-Kopp & Beauchaine, 2007b; Sagvolden, Johansen, Aase, & Russell, 2005; Scheres et al., 2007). Consistent with theories of central and autonomic under-arousal (e.g., Gatzke-Kopp, Raine, Loeber, Stouthamer-Loeber, & Steinhauer, 2004), we have argued that those with ADHD engage in excessive reward-seeking behaviors—expressed as hyperactivity/impulsivity—in part to upregulate a persistently underactive mesolimbic DA system, which is experienced psychologically as an aversive, irritable mood state (Laakso et al., 2003). In high risk environments such as those outlined above, children who are impulsive often progress to more serious externalizing conduct given many opportunities to engage in maladaptive reward-seeking behavior (see Lynam et al., 2000).
Despite research indicating consistent group differences between children with ADHD and controls in mesolimbic responding to incentives, no studies have examined whether individual variation in reward responding among children with ADHD prospectively predicts treatment response. Identifying biomarkers of treatment outcome can be important for several reasons in addition to those alluded to above (Beauchaine, 2009a). In the present case, current interventions for ADHD—both pharmacological and behavioral—do not benefit all children (MTA Cooperative Group, 1999). Discovering biomarkers of treatment response may help identify which children are more likely to respond to current treatments, which children need additional forms of treatment, and which children should be assigned to different types of treatment (see Beauchaine et al., 2008). Identifying biomarkers of treatment outcome may also advance our basic understanding of heterogeneity in the ADHD phenotype (Castellanos & Tannock, 2002).
Assessing midbrain DA responding is difficult in young children—particularly those with ADHD. As noted above, neuroimaging experiments consistently reveal blunted mesolimbic reactivity to incentives among older children with ADHD and other externalizing behaviors (e.g., Durston, 2003; Durston et al., 2003; Scheres et al., 2007). However, preschoolers have difficulty tolerating lengthy imaging protocols. In an effort to circumvent this problem, we have used sympathetic nervous system (SNS)-linked cardiac reactivity as a proxy for central DA responding to incentives (see Beauchaine & Gatzke-Kopp, 2012). As detailed elsewhere, several sources of evidence suggest that cardiac pre-ejection period (PEP)—a sympathetic nervous system (SNS)-mediated systolic time interval spanning left ventricular depolarization to the onset of ejection of blood into the aorta—marks mesolimbic DA reactivity specifically during reward responding (Brenner & Beauchaine, 2011; Brenner, Beauchaine, & Sylvers, 2005)1. Cardiac PEP shortens through β-adrenergic mechanisms when the SNS is activated.
In a series of studies conducted over the past decade, we have found either reduced PEP reactivity or no PEP reactivity to incentives among preschoolers with ADHD (Crowell et al., 2006), and among both middle-schoolers and adolescents with ADHD and conduct problems (Beauchaine et al., 2001; see also Beauchaine, Gatzke-Kopp, & Mead, 2007). Furthermore, deficiencies in PEP reactivity to incentives among middle-schoolers prospectively predict initiation of alcohol and other substance use (Brenner & Beauchaine, 2011). Following from these findings, PEP reactivity to reward represents a plausible candidate biomarker of treatment response, particularly for interventions that rely heavily on operant principles of reinforcement to alter externalizing behaviors. We hypothesized that greater PEP reactivity to incentives would predict reductions in more severe externalizing conduct among preschoolers with ADHD who participated in a child- and parent-focused behavioral intervention, outlined below.
Independent of trait impulsivity, emotion regulation (ER) has received considerable attention as a likely moderator of vulnerability to psychopathology, including externalizing behaviors (e.g., Beauchaine et al., 2007; Cole & Hall, & Hajal, 2013). Emotion regulation comprises processes through which emotional experience and expression are shaped—whether volitionally or automatically—in the service of adaptive behavior (Thompson, 1990). Following from this definition, emotion dysregulation might best be described as a pattern of emotional experience and/or expression that interferes with adaptive functioning. In more severe forms of externalizing psychopathology, negative emotions including anger and rage are experienced either too intensely or too enduringly to be adaptive (Beauchaine et al., 2007; Beauchaine et al., 2009).
Much has been learned about the central nervous system (CNS) substrates of ER in the past decade. Neural structures that subserve ER include the amygdala, the septo-hippocampal system, and the ventromedial prefrontal cortex (see Beauchaine & Gatzke-Kopp, 2012; Beauchaine & McNulty, in press; Goldsmith & Davidson; Goldsmith, Pollak, & Davidson, 2008). The ventromedial prefrontal cortex (vmPFC) in particular inhibits amygdala activation when negative emotions are suppressed volitionally. Importantly, lesions to the vmPFC impair autonomic responses to emotional stimuli (Verbane & Owens, 1998). Rich theoretical models have also been articulated outlining modulatory functions of certain brainstem nuclei—particularly the nucleus ambiguus—on emotional experience and expression (see Porges, 1995, 2007). These nuclei serve as final common pathways—via the vagus nerve—from the central nervous system to the cardiovascular system.
It has become increasingly clear in recent years that ER capabilities are marked peripherally by parasympathetic (i.e., vagal) efference to the heart, which can be quantified by measuring respiratory sinus arrhythmia (RSA)—oscillatory increases and decreases in heart rate across the respiratory cycle (see Beauchaine, 2001; Porges, 1995, 2007). Under well controlled stimulus conditions, RSA indexes neural traffic through the vagus nerve (see Ritz, 2009), which provides a physiological mechanism for rapid acceleration and deceleration of cardiac output in response to environmental demands, including social engagement (e.g, Porges, 2007). Since publication of Porges’ (1995) Polyvagal Theory, a consistent body of research has emerged linking deficiencies in RSA to disrupted social affiliation, emotion dysregulation, and various forms of psychopathology (see e.g., Ahs, Sollers, Furmark, Fredrikson, & Thayer, 2009; Asmundson & Stein, 1994; Beauchaine, 2001, in press; Beauchaine et al., 2001, 2007; Crowell et al., 2005, 2006; Hastings et al., 2008; Porges, 2007; Rottenberg, 2007; Rottenberg, Salomon, Gross, & Gotlib, 2005; Rottenberg, Wilhelm, Gross, & Gotlib, 2002; Thayer, Friedman, & Borkovec, 1996; Vasilev, Crowell, Beauchaine, Mead, & Gatzke-Kopp, 2009). As reviewed elsewhere, low baseline RSA and/or excessive RSA withdrawal in response to emotionally evocative stimuli have been linked to conduct problems, trait hostility, eating disorders, anxiety disorders, depression, and panic—among other adverse outcomes (see Beauchaine, 2001).
In our own work, we have found consistent reductions in resting RSA and excessive RSA reactivity (i.e., withdrawal) to various social and emotional challenges among children and adolescents with ADHD and other externalizing behaviors (Beauchaine, 2002; Beauchaine, Hong, & Marsh, 2008; Marsh, Beauchaine, & Williams, 2008; Beauchaine et al., 2001, 2007). In contrast, numerous studies indicate that high resting RSA marks resilience to psychopathology in adverse rearing contexts, including those characterized by interparental conflict (Katz & Gottman, 1995), heavy parental drinking (El-Sheikh, 2005), divorce (El-Sheikh, Harger, & Whitson, 2001), and maternal depression (Shannon, Beauchaine, Brenner, Neuhaus, & Gatzke-Kopp, 2007). Children with low RSA and/or excessive RSA withdrawal might benefit less from treatment for at least three reasons. First, the experience of associated negative emotions such as anxiety and anger might interfere with learning and acquisition of alternative coping strategies. Second, low baseline RSA and excessive RSA withdrawal to behavioral challenge—both of which predict poor ER—are associated with sustained attention difficulties as well (see Beauchaine, 2001; Suess, Porges, & Plude, 1994), which might also interfere with learning and acquisition of alternative coping strategies. Third, low RSA and excessive RSA reactivity are associated with deficiencies in social affiliation (see Beauchaine et al., 2007; Porges, 1995), which might interfere with acquisition of prosocial skills, a target of many interventions. Accordingly, we hypothesized that higher resting RSA and less RSA withdrawal in response to behavioral challenge would predict greater improvement in ER and social competence following treatment among preschoolers with ADHD.
Method
In this study we describe links between patterns of cardiovascular responding and maternal reports of treatment outcome among 99 children, ages 4–6, who participated in a randomized controlled trial evaluating the effectiveness of the Incredible Years (IY) parent and child training programs for preschoolers with the hyperactive/impulsive and combined subtypes of ADHD. All study procedures were approved by the University of Washington Institutional Review Board, and parental consent was obtained. Immediate post-treatment and one-year follow-up outcomes for the intervention have been reported elsewhere (Webster-Stratton, Reid, & Beauchaine, 2011; 2012). In brief, significant intervention effects were observed immediately post treatment for both maternal and paternal reports of a wide range of externalizing behaviors, including hyperactivity, inattention, oppositionality, aggression, ER, and social competence. Improvements in externalizing behavior and social competence were also reported by teachers. Almost all of these effects were maintained at one-year follow-up. Below we evaluate whether baseline PEP, PEP reactivity to incentives, baseline RSA and/or RSA reactivity during behavioral challenge—all collected prior to the intervention—predict improvements in mother reports of externalizing behavior, emotion regulation, inattention, and/or hyperactivity.
Participants
Children enrolled in the intervention included 99 preschoolers, ages 4–6 years, who were diagnosed with either the hyperactive/impulsive or combined subtype of ADHD. Participants were assigned randomly to either (a) an immediate intervention condition (n=49) or (b) a delayed (waitlist control) intervention condition (n=50), described below. For purposes of this study, participants were aggregated into a single group for analysis. Between group comparisons of intervention and waitlist participants across 136 pre-to-post outcome measures yielded only 10 statistically significant differences (7%; about that expected by chance), with an average effect size of d=.04. Combining the groups was therefore justifiable.
Participants were recruited with the help of teachers and school counselors at local preschools and elementary schools, pediatricians, and mental health professionals, and through ads placed in family/parent-focused community publications. Parents were invited to call the lab if their child had been diagnosed with ADHD previously, or showed very high levels of hyperactive and/or impulsive behaviors. In an initial phone screen, a trained research assistant explained requirements of the study (e.g., random assignment to immediate treatment or waitlist condition, no medication for the duration of the study, length of intervention, no autism diagnosis, etc.). In total, 204 parents inquired. Among these, 156 felt their child might be eligible, and elected to continue with a detailed phone screen. These parents completed a structured telephone interview with a trained clinician, which included portions of the Child Symptom Inventory (CSI; Gadow & Sprafkin, 1997) and the Child Behavior Checklist (CBCL; Achenbach, & Edelbrock, 1991).
Among the 156 families who completed the phone screen, 103 had a child who met the following inclusion criteria: (1) scored ≥ 95th percentile on the CBCL attention problems scale, (2) met DSM–IV criteria for the hyperactive/impulsive or combined subtype of ADHD on the CSI, and (3) were not taking medication to treat ADHD. These families were scheduled for an initial clinic visit at which parents were administered the Diagnostic Interview Schedule for Children (DISC; Shaffer, Fisher, Lucas, Mina, & Schwab-Stone, 2000), ADHD module. Of the 103 parents interviewed with the DISC, 99 had a child who met criteria for the hyperactive-impulsive or combined subtype of ADHD and were enrolled in the study. Among these children, 48 also met criteria for oppositional defiant disorder (ODD). However, because no significant correlations were observed between continuous ODD symptom counts and any of the cardiac measures, all rs ≤ .09, all ps ≥ .55, ODD was not included as a covariate in any of the analyses.
Eighty (81%) of children provided scoreable psychophysiological data. Of the 19 participants who did not provide data, 9 did not show up for their psychophysiological assessment (described below), and 10 were lost due to problems with psychophysiological recording or excessive movement. These 19 participants did not differ at intake from the remainder of the sample on any of the psychopathology measures reported below, all Fs ≤ 0.47, all ps ≥ .50. Nevertheless, missing psychophysiological data were imputed in SPSS 20, following recommendations set forth by Graham (2009).
Interventions
Immediate intervention condition
Those enrolled in the immediate intervention condition received the IY parent and child training programs directly following baseline assessment. The IY parent program has proven effective in reducing conduct problems among children, both in the US and Europe (e.g., Beauchaine, Webster-Stratton, & Reid, 2005; Drugli & Larsson, 2006; Scott, Spender, Doolan, Jacobs, & Aspland, 2001; Reid, Webster-Stratton, & Beauchaine, 2001; Webster-Stratton & Reid, 2010). The IY Dinosaur school child program is effective in increasing children’s conflict management and cognitive problem solving, and in reducing their aggression in the classroom (Webster-Stratton & Hammond, 1997; Webster-Stratton, Reid, & Hammond, 2004). In the current study, the IY parent intervention included 20 weekly 2-hr sessions conducted with six families per group. The newest versions of the IY preschool parent and child curricula (2008 revision) were offered. This version includes new material focusing on academic persistence, social and emotional coaching, establishing predictable household routines and schedules, emotion regulation strategies, and teaching children to problem solve. The program includes vignettes showing children with ADHD in order to enhance parents’ understanding of (1) how to respond effectively to impulsive children, and (2) children’s developmental levels and temperaments. Additional sessions from the IY advance parent curriculum included problem solving between adults and teachers and strategies to build family interpersonal support, reduce depression, and manage anger.
The IY Dinosaur training program was held concurrently with the parent program. Topics included following group rules, identifying/expressing feelings, problem solving, anger management, friendship skills, and teamwork. Each 2-hr session consisted of 3 short circle times and 3–4 planned activities to reinforce concepts. Therapists used coaching methods during unstructured play times to encourage appropriate peer interactions and targeted social and emotional skills. See Webster-Stratton (2007), Webster-Stratton and Reid (2008), and Webster-Stratton et al. (2011) for more detailed information regarding the use of these two interventions for children with ADHD, and for details about fidelity monitoring.
Waitlist control condition
All waitlist control families received the IY intervention following a post-assessment comparison with the immediate treatment group. Parents in the waitlist condition received 10 sessions of treatment (about half the dose received by parents in the immediate condition). Children in both conditions received an equivalent treatment dose of about 40 hours (see Webster-Stratton, Reid, & Beauchaine, 2012). As noted above, IY and waitlist families were combined into a single group for all analyses. For both groups, baseline assessments were conducted before the immediate intervention condition. In contrast, post treatment assessments were conducted immediately after each group received their intervention (as opposed to before and after the immediate treatment group received their intervention). Although questions regarding potential Intervention Condition × Cardiac Psychophysiology × Time interaction effects in predicting treatment outcome may have been of interest, power to reliably detect three-way interactions requires very large sample sizes (e.g., Whisman & McClelland, 2005)—which is often overlooked in treatment-outcome research, resulting in high probabilities of Type II error (see Beauchaine et al., 2008). Furthermore, very few outcomes differentiated between groups (see above). Thus, we did not analyze intervention condition (IY vs. waitlist) effects. The same fidelity monitoring procedures were used for the waitlist group and the immediate treatment group (see Webster-Stratton et al., 2011).
Psychopathology Measures
Psychopathology measures included in this study comprise a subset of those reported in our analysis of post-treatment behavioral outcomes (see Webster-Stratton et al., 2011). Our choice of measures was driven by four considerations. First, we selected constructs, including aggression, emotion regulation, prosocial behavior, and hyperactivity, for which pre- to post-treatment improvement was observed. It would make little sense to examine physiological predictors of treatment outcome for behaviors that did not change following treatment. Second, we chose constructs that were linked theoretically to traits underlying aberrant reward responding (e.g., problem behaviors) and emotion regulation/dysregulation (e.g., prosocial behavior), consistent with the literature review presented above. Third, we restricted our analyses to six outcome measures in order to control—at least in part—for inflation of familywise Type I error. Finally, we included mother-reports only given substantially lower availability of father-report data (n = 58) and associated reduction in statistical power.
Child Symptom Inventory (CSI; Gadow & Sprafkin, 1997)
The CSI yields dimensional scores and diagnostic cutoffs for most Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev. [DSM–IV–TR]; American Psychiatric Association, 2000) internalizing and externalizing disorders. Both the hyperactive-impulsive and inattentive subscales were administered. In the most recent validation sample (Sprafkin, Gadow, Salisury, Schneider, & Loney, 2002), internal consistency (coefficient α) was .91 for ADHD. As outlined above, the CSI was used for screening purposes.
Child Behavior Checklist (CBCL; Achenbach, & Edelbrock, 1991)
The CBCL, which is validated for children ages 4–16, assesses broadband internalizing and externalizing symptoms, and a number of more specific behaviors. We used the attention problems subscale for screening purposes (see above), and the aggression subscale as an outcome measure of severe externalizing conduct. The CBCL has well-established norms, with coefficient αs of .91 and .84 for the aggression and attention problems subscales, respectively.
Eyberg Child Behavior Inventory (ECBI; Robinson, Eyberg, & Ross, 1980)
The ECBI is a commonly used measure for assessing conduct problems among children ages 2 to 16 years. We used the problem behavior subscale as a second measure of more severe externalizing symptoms. Coefficient α was .94.
Conners Parent Rating Scale–Revised (CPRS–R; Conners, Sitarenios, Parker, & Epstein, 1998)
The CPRS–R is a widely used instrument for assessing ADHD and comorbid psychopathology. For the present study, summary scores for both inattention and hyperactivity were used as outcome measures. Coefficient αs for both scales exceeded .90.
Social Competence Scale (SCS; Conduct Problems Prevention Research Group, 1999a, 1999b)
The SCS consists of 12 items that assess children’s prosocial behaviors (e.g., resolves peer problems, understands others, shares, is helpful, listens; α=.81), and ER (e.g., accepts things, copes with failure, thinks before acting, can calm down, controls temper; α=.80). We used the SCS as a broad index of ER capabilities2.
Cardiovascular Assessments
The psychophysiological protocol was similar to that used in our previous research assessing cardiovascular activity and reactivity among preschool children with ADHD (Crowell et al., 2006). This protocol is effective in eliciting both PEP reactivity to incentives and RSA reactivity to behavioral challenge in normal controls, and in eliciting group differences in baseline PEP and PEP reactivity among preschoolers with ADHD. Participant children and a parent (mothers in all but one case) visited the lab for a psychophysiological assessment roughly one week after their diagnostic interview (see above). Assessments were conducted in a separate lab session, before the intervention was delivered. The 30-min protocol was administered in a sound-attenuated room, which was monitored via microphone and closed-circuit video. Patterns of cardiac activity and reactivity were measured during a 5-min baseline, after which children played a simple matching game in which they viewed a shape (e.g., square, circle, star) projected on a large screen and pressed a corresponding button (among 5) on keyboard made specifically for preschool children. Correct responses were followed by an image of a smiley face and a tone, whereas incorrect responses were followed by a blank screen and contrasting tone. To increase the reward value of the game, we showed children a large container of toys valued at about $10 each and told them that they could choose their favorite toy if they earned enough smiley faces during the course of the game. Following completion of the task, all were given their chosen toy for “trying hard.” Parents waited in the adjacent room with the experimenter.
Next, children and their parent engaged in (a) 7 min of free play followed by (b) 7 min of a challenging block-building task that required parent-child cooperation and attentional focus. During free play, children and their parent were seated on the floor in the same room where the incentive task took place, and given a tub of assorted toys. This unstructured time was designed to encourage acclimation to the surroundings prior to block building. After the free play session, parents were instructed to (1) retrieve a box containing 32 large foam geometric shapes, which was stored in a corner of the room; (2) dump the contents of the box on the floor, and (3) instruct their child to build a series of four layers of blocks, in order, one on top of the other. Each layer required that multiple blocks of various shapes be arranged exactly as shown on a figure provided to the parent. Difficulty increased with each layer. Parents were told that they could provide only instructions, and that their child had to do the hands-on work. This task is difficult for 4–6-year-olds, and pulls for both parental and child frustration, as assessed by dyadic conflict, negative affective expression, and coercive parent-child exchanges (Beauchaine, Strassberg, Kees, & Drabick, 2002).
Cardiovascular Measures
Cardiac PEP
Sympathetic-linked (β-adrenergic) cardiac function was assessed by measuring PEP—the interval between the onset of left ventricular depolarization and ejection of blood into the aorta. Shorter intervals represent greater SNS effects (Sherwood et al., 1986, 1990). Electro- and impedance-cardiographic signals were obtained from a HIC 2004 impedance cardiograph (Chapel Hill, NC). Both waveforms were sampled at 1 kHz using the spot electrode configuration described by Qu, Zhang, Webster, and Tompkins (1986). Pre-ejection period values were extracted by ensemble-averaging data in 30-s epochs (see Kelsey & Guethlein, 1990) using Bio-Impedance Technology’s CopWin software system, version 5.06 (Chapel Hill, NC). All ensemble-averaged waveforms were inspected visually by trained research assistants to correctly locate the dZ/dt B-wave in cases where the computer algorithm failed to do so.
RSA
Parasympathetic-linked cardiac function was assessed using spectral analysis, which decomposes the heart rate time series into component frequencies through fast Fourier transformation. High-frequency heart rate variability (>0.15 Hz) was extracted to measure RSA. Spectral densities were calculated in 30-s epochs, roughly the minimum length required for reliable assessment (Berntson et al., 1997). As is customary, these values were normalized via natural log transformations using Nevrokard software. The validity of high frequency heart rate variability as an index of PNS-linked cardiac activity has been established via cholinergic blockade (see Berntson et al., 1997)
For purposes of this study, both PEP and RSA values were averaged across 30-s epochs within each condition (i.e., baseline, reward task, block building). Reactivity was computed by subtracting baseline averages from reward task and block building averages. Thus, negative values were associated with (1) PEP shortening, indicative of increased SNS activity, and (2) RSA withdrawal, indicative of reduced PNS activity.
Results
The average age of children, 76% of whom were male, was 64.3 months (SD=11.0). Pre- and post-intervention scores on measures of psychopathology are presented in Table 1. For outcomes reported in this study, pre-post effect sizes were generally large. Further details about the sample, including information about parental education, parental marital satisfaction, parents’ contacts with the criminal justice system, etc., are reported in Webster-Stratton et al. (2011). Mean PEP scores at baseline were 80.5 ms (SD=13.3), and mean PEP reactivity scores during the incentive condition were −0.8 ms (SD=6.9). Mean RSA scores at baseline were 6.6 ms2 (SD=1.0), and mean RSA reactivity scores during block building were 1.0 ms2 (SD=1.0). Intercorrelations among variables are reported in Table 2.
Table 1.
Descriptive Statistics
| Variable | Pre | Post | F | p | η2p |
|---|---|---|---|---|---|
| Severe Conduct | |||||
| CBCL aggression (T) | 66.6 (9.7) | 61.9 (9.4) | 29.1 | <.001 | .25 |
| ECBI problem behavior | 21.7 (6.6) | 14.5 (7.9) | 87.0 | <.001 | .50 |
| Emotion Regulation | |||||
| SCS emotion regulation | 1.9 (0.5) | 2.4 (0.6) | 66.7 | <.001 | .44 |
| SCS prosocial | 2.7 (0.6) | 3.2 (0.7) | 62.4 | <.001 | .42 |
| Inattention | |||||
| CPRS-R inattention (T) | 69.3 (13.1) | 64.9 (12.3) | 14.0 | <.001 | .14 |
| Hyperactivity | |||||
| CPRS-R hyperactive (T) | 74.5 (8.4) | 67.8 (10.7) | 39.7 | <.001 | .32 |
Notes. CBCL=Child Behavior Checklist (Achenbach & Edelbrock, 1991); CPRS–R=Conners’ Rating Scale–Revised (Conners, 1998; Conners, Sitarenios, Parker, & Epstein, 1998); ECBI=Eyberg Child Behavior Inventory (Robinson, Eyberg, & Ross, 1980). SCS=Social Competence Scale–Parent Report (Conduct Problems Prevention Research Group, 1999a, 1999b).
Table 2.
Intercorrelations among Pre-treatment Behavioral and Cardiac Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. RSA baseline | - | ||||||||
| 2. RSA change | .31** | - | |||||||
| 3. PEP baseline | .19 | .17 | - | ||||||
| 4. PEP change | .14 | .22 | .54** | - | |||||
| 5. CBCL aggression | −.04 | −.16 | −.12 | −.20 | - | ||||
| 6. ECBI problem behavior | −.03 | −.06 | −.25* | −.26* | .55** | - | |||
| 7. SCS emotion regulation | .29** | .11 | .00 | −.02 | −.37** | −.33** | - | ||
| 8. SCS prosocial | .19 | .07 | .11 | −.01 | −.32** | −.28** | .52** | - | |
| 9. CPRS-R inattentive | .01 | −.07 | −.01 | −.03 | .19 | .24* | −.13 | −.20* | - |
| 10. CPRS-R hyperactive | .03 | .03 | −.06 | −.11 | .40** | .32** | −.12 | −.17 | .40** |
Notes. CBCL=Child Behavior Checklist (Achenbach & Edelbrock, 1991); CPRS–R=Conners’ Rating Scale–Revised (Conners, 1998; Conners, Sitarenios, Parker, & Epstein, 1998); ECBI=Eyberg Child Behavior Inventory (Robinson, Eyberg, & Ross, 1980). SCS=Social Competence Scale–Parent Report (Conduct Problems Prevention Research Group, 1999a, 1999b).
p ≤ .05.
p ≤ .01.
Cardiac PEP
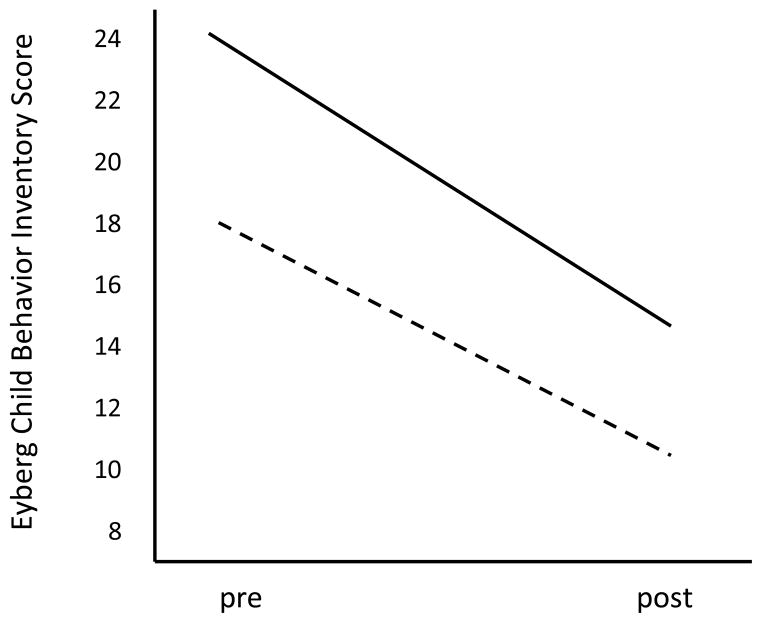
To assess prediction of treatment outcome from baseline PEP and PEP reactivity to incentives, ANOVAs were conducted in which pre-post scores on the behavioral outcome measures (e.g., CBCL aggression) served as repeated measures, and cardiovascular variables and age in months served as covariates. Including age as a covariate was necessary given normative developmental lengthening of PEP from ages 4–6. Missing psychophysiological data were imputed using SPSS 20. Fully conditional specification was used across 30 imputations. In these models, differential prediction of treatment response by cardiac function is carried in Behavioral Outcome × PEP interaction terms. These interactions are reported along with main effects in Table 3. Contrary to our hypotheses, significant interactions were not found for either PEP activity or reactivity in predicting aggression or problem behaviors. However, main effects of both PEP activity and reactivity on CBCL aggression and ECBI problem behavior were found, all Fs ≥ 5.87, all ps ≤ .18, all η2p ≥ .07. Thus, although all children tended to show improvement from pre to post treatment, those who exhibited lengthened cardiac PEP at rest and reduced PEP reactivity to incentives scored higher on measures of conduct problems and aggression both before and after treatment. The latter finding is illustrated in Figure 1, where we plot Eyberg problem behavior scores at pre treatmemnt and post treatment for those who scored > 1.0 SD above the sample mean on PEP reactivity, and those who scored > 1.0 SD below the sample mean on PEP reactivity.
Table 3.
Repeated Measures ANOVA Results with Cardiac Variables Predicting Mother Reports of Treatment Outcome
| Baseline PEP
|
PEP reactivity
|
Baseline RSA
|
RSA reactivity
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main effect | Baseline PEP × Outcome interaction | Main effect | PEP Reactivity × Outcome interaction | Main effect | Baseline RSA × Outcome interaction | Main effect | RSA Reactivity × Outcome interaction | |||||||||
|
| ||||||||||||||||
| Outcome | F | η2p | F | η2p | F | η2p | F | η2p | F | η2p | F | η2p | F | η2p | F | η2p |
| Severe conduct | ||||||||||||||||
| CBCL aggression | 6.11* | .07 | 1.54 | .02 | 6.47** | .08 | 0.55 | .01 | 1.89 | .02 | 0.15 | <.01 | 1.35 | .02 | 0.01 | <.01 |
| ECBI problem behavior | 5.87* | .07 | 0.12 | <.01 | 5.95* | .07 | 2.11 | .03 | 0.01 | <.01 | <0.01 | <.01 | 0.67 | .01 | 2.26 | .03 |
| Emotion regulation | ||||||||||||||||
| SCS emotion regulation | <0.01 | <.01 | 0.08 | <.01 | 0.00 | <.01 | 0.64 | .01 | 0.88 | .01 | 0.92 | .01 | 4.74* | .06 | 0.46 | .01 |
| Prosocial behavior | ||||||||||||||||
| SCS prosocial | 3.38 | .04 | 0.46 | .01 | 0.09 | <.01 | 0.42 | .01 | 4.16* | .05 | 0.69 | .01 | 4.16* | .05 | 0.67 | .01 |
| Inattention | ||||||||||||||||
| CPRS-R inattentive | 1.16 | .01 | 0.54 | .01 | 0.61 | .01 | 0.03 | <.01 | 0.91 | .01 | 0.89 | .01 | 0.98 | .01 | 5.15* | .06 |
| Hyperactivity | ||||||||||||||||
| CPRS-R hyperactive | 0.81 | .01 | 0.16 | <.01 | 2.33 | .03 | 2.22 | .03 | 0.48 | .01 | 0.87 | .01 | 0.88 | .01 | 0.92 | .01 |
Notes. CBCL=Child Behavior Checklist (Achenbach & Edelbrock, 1991); CPRS–R=Conners’ Rating Scale–Revised (Conners, 1998; Conners, Sitarenios, Parker, & Epstein, 1998); ECBI=Eyberg Child Behavior Inventory (Robinson, Eyberg, & Ross, 1980). SCS=Social Competence Scale–Parent Report (Conduct Problems Prevention Research Group, 1999a, 1999b).
p ≤ .05.
p ≤ .01.
Figure 1.
Eyberg Child Behavior Inventory scores at pre- to post-treatment for children who scored > 1.0 SD below the sample mean on PEP reactivity (solid line) and for children who scored > 1.0 SD above the sample median on PEP reactivity (dashed line). Although both groups improved, individual differences in problem behavior were maintained.
RSA
To assess prediction of treatment outcome from baseline RSA and RSA during block building, ANOVAs were conducted in which pre-post scores on the behavioral outcome measures (e.g., prosocial behavior) served as repeated measures, and cardiovascular variables and age in months served as covariates. Including age as a covariate was necessary given normative developmental increases RSA from ages 4–6. In these models, differential prediction of treatment response by cardiac function is carried in Behavioral Outcome × RSA interaction terms. These interactions are reported along with main effects in Table 3. Contrary to our hypotheses, significant interactions were not found for either RSA activity or reactivity in predicting ER or prosocial behavior. However, main of both RSA activity and reactivity on SCS prosocial behavior, and of RSA reactivity on ER, were found, all Fs ≥ 4.16, all ps ≤ .05, all η2p ≥ .05. These main effects indicate that despite sample-wide improvement in emotion regulation and prosocial behavior, (1) children who exhibited lower baseline RSA and greater RSA withdrawal scored lower on prosocial behavior both before and after treatment, and (2) children who exhibited greater RSA withdrawal scored lower on ER both before and after treatment.
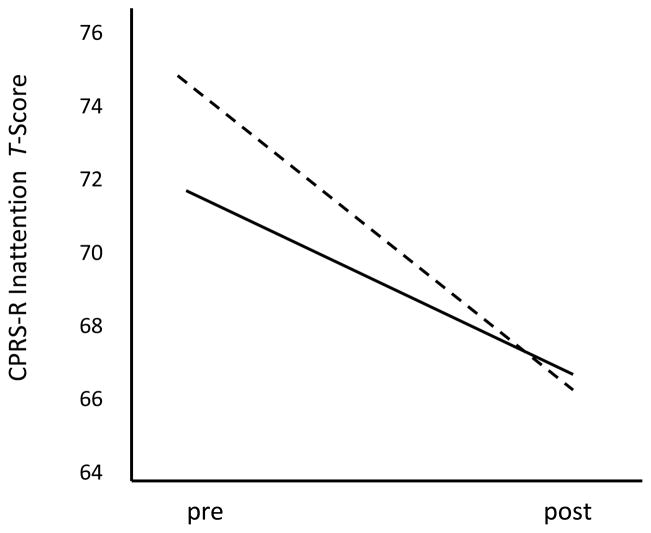
Finally, a significant RSA Reactivity × CPRS-R Inattention interaction was observed. As depicted in Figure 2, although greater RSA withdrawal during block building was associated with more attention problems at pre treatment, this difference disappeared by post treatment, when sample-wide improvement was observed.
Figure 2.
Conners’ Rating Scale—Revised inattention scale scores at pre- to post-treatment for children who scored > 1.0 SD below the sample mean on RSA withdrawal (solid line) and for children who scored > 1.0 SD above the sample median on RSA withdrawal (dashed line). Although greater RSA withdrawal was associated with more attention problems at pre treatment, this difference disappeared by post treatment.
Discussion
In this study, we evaluated SNS- and PNS-linked cardiac activity and reactivity as predictors of improvement in conduct problems and ER, respectively, among preschoolers who were treated for ADHD with an empirically-supported behavioral intervention. Contrary to our expectations, neither PEP at rest nor PEP reactivity to incentives predicted improvements in aggression or conduct problems. However, main effects of both PEP activity and reactivity indicated that children with lengthened PEP at baseline and less PEP reactivity to incentives scored higher on measures of aggression and problem behavior both at pretreatment and post treatment, even though they improved as much as their peers. On the one hand, this finding is encouraging since it indicates that current interventions are effective in reducing problem behaviors among preschool children who may be vulnerable to developing especially severe externalizing behaviors as they mature (see Beauchaine & Gatzke-Kopp, 2012). On the other hand, our findings present a treatment development challenge. As Brestan and Eyberg (1998) noted 15 years ago, we must address questions such as, “For whom does this treatment work?”, and “When is this treatment not enough?”. In the present case, the intervention we provided may not be enough for children with aberrant PEP responding, who continue to suffer from more behavioral difficulties than their peers following treatment.
Although individual differences in baseline PEP can arise from many sources (e.g., preload, afterload, total peripheral resistance) and are therefore difficult to interpret, PEP reactivity to behavioral challenge is effected almost exclusively through the SNS (Sherwood et al., 1990). As outlined above, reduced SNS reactivity to incentives likely marks deficient mesolimbic DA responding, which is observed consistently among older children with ADHD and other externalizing behaviors (Durston, 2003; Durston et al., 2003; Scheres et al., 2007). Children in our study were required to remain medication free throughout the intervention, which enabled us to attribute treatment effects—which were generally large—to the parent and child programs (see Webster-Stratton et al., 2011). However, despite these large effect sizes, individual variation in treatment response was observed. One question that emerges is whether children low in PEP activity and/or reactivity might have benefitted more from combined behavioral and medication management than from behavioral management alone. Psychostimulants normalize deficiencies in midbrain DA responding among children with ADHD (e.g., Rubia et al., 2011; Vaidya et al., 1998), and increase cardiac output through SNS mechanisms (see e.g., Gilden, 2012). Future research is required to test whether children with ADHD who are low in PEP and/or PEP reactivity may benefit more from combined forms of treatment.
We are the first research group to evaluate associations between treatment response and SNS-linked cardiac function among children at any age. Importantly, however, PEP reactivity to incentives also predicts initiation and escalation of alcohol and other substance use among middle-schoolers with conduct problems (Brenner & Beauchaine, 2011). Taken together, these findings suggest that PEP responding to reward may mark vulnerability to progression along the externalizing spectrum among children with ADHD, and may therefore be a useful biomarker in future research.
In addition to the PEP findings summarized above, children with low baseline RSA scored lower on prosocial behavior at both pre and post treatment, and children who exhibited RSA withdrawal during block building exhibited poorer ER and scored lower on prosocial behavior than their peers at both pre and post treatment. As with the PEP data, this occurred despite sample-wide improvements from pre to post treatment in prosocial behavior and ER. These findings add to a large body of research linking individual differences in RSA to competent ER and social affiliation (e.g., Beauchaine, 2001; Beauchaine et al., 2007, in press; Porges, 1995, 2007). As outlined above, high baseline RSA marks resilience to psychopathology in adverse rearing contexts, including those characterized by interparental conflict (Katz & Gottman, 1995), heavy parental drinking (El-Sheikh, 2005), divorce (El-Sheikh, Harger, & Whitson, 2001), and maternal depression (Shannon, Beauchaine, Brenner, Neuhaus, & Gatzke-Kopp, 2007). Children with higher resting RSA are also more empathic toward others and more socially competent than their peers (Eisenberg et al., 1995; Fabes, Eisenberg, Karbon, Troyer, & Switzer, 1994). Once again, however, the issue of “When is this treatment not enough?” arises.
In considering this question, several findings are worth noting. First, behavioral genetics studies indicate that ER is affected considerably by environmental influences (e.g., Kupper et al., 2004). Second, excessive RSA reactivity is usually observed among externalizing children who have progressed further along the externalizing spectrum than children in the current sample (e.g., Beauchaine et al., 2001; Beauchaine & McNulty, in press). Third, RSA reactivity appears to develop over time as emotional lability and associated ER deficiencies are socialized through repeated negative reinforcement processes within families that occur thousands of times across development (see Beauchaine et al., 2007, 2009; Crowell et al., 2006; 2012). This set of observations suggests that increasing the dose of ER training in our intervention might improve ER capabilities among children who exhibit low RSA and excessive RSA withdrawal while interacting with their parents, as they did in the block building task. Future research should address this question.
As in our previous research (see Beauchaine & Gatzke-Kopp, 2012; Beauchaine et al., 2007), associations of outcomes with PEP and RSA were specific to aggression/conduct problems and ER/prosocial behavior, respectively. Even though predictive effects were not found, this is consistent with theoretical expectations given the central and peripheral nervous system functions marked by each measure. Nevertheless, one limitation of our study is that we did not control for the inflated probability of Type I error associated with multiple statistical tests. Had we applied such a correction, none of our findings would have been significant. Yet if our findings were attributable to chance, it is highly unlikely that significant PEP and RSA effects would be specific to theoretically-relevant outcomes.
The only other significant finding was an interaction between RSA withdrawal and the intervention in predicting inattention scores. Although children who exhibited greater RSA withdrawal scored higher on inattention at pre test, no such difference was observed at post test. Interestingly, both resting RSA and RSA withdrawal have been linked to attentional capacity in previous studies (see Beauchaine 2001; Suess et al., 1994). Our findings suggest that the intervention was especially helpful in improving inattention among more vulnerable children. However, we are reluctant to interpret the interaction further given that it was not predicted.
Pre-ejection period and RSA were unrelated to any other changes in hyperactivity or inattention. For RSA, this is not surprising, since ER and impulsivity are separate constructs with distinct neural substrates, as reviewed above (see Beauchaine & Gatzke-Kopp, 2012; Beauchaine & McNulty, in press). In contrast, given our hypothesis that PEP reactivity to incentives marks central DA responding—a neural substrate of trait impulsivity—we might have expected to find links between PEP reactivity and improvements in hyperactivity/impulsivity. However, three considerations should be noted. First, previous studies have revealed stronger associations between deficient PEP responding and conduct problems than between deficient PEP responding and hyperactivity/impulsivity (Beauchaine et al., 2001). Second, participants were selected based on a very restricted range of hyperactivity/impulsivity scores, whereas aggression and conduct problem scores were free to vary. Thus, the sample was more homogeneous on hyperactivity/impulsivity than on aggression/conduct problems, which may have affected statistical power for tests involving the latter. Indeed, effect sizes for most measures of change in aggression/conduct problems were larger than effect sizes for hyperactivity/impulsivity (Webster-Stratton et al., 2011). Thus, there was a larger range of difference scores in aggression/conduct problems for the PEP variables to map onto. Smaller effect sizes for changes in hyperactivity/impulsivity are unsurprising given that ADHD is largely heritable (e.g., Willcutt, in press), whereas aggression and conduct problems are affected more by socialization mechanisms (see e.g., Beauchaine et al., 2009, 2010; Lynam, 2000; Patterson et al., 2000), and may therefore be more responsive to behavioral interventions.
In addition, all effect sizes for PEP and RSA in predicting treatment outcome were modest. Here it is important to note that all children in the sample met full criteria for the hyperactive/impulsive or combined subtype of ADHD on the DISC, and scored at or above the 95th percentile on attention problems on the CBCL. Such children consistently show reduced tonic PEP at baseline, lower PEP reactivity to incentives, and attenuated resting RSA compared to normal controls (e.g., Beauchaine et al., 2001, 2008; Crowell et al., 2006). Thus, even among a group of children likely characterized by restricted ranges on all cardiovascular measures, individual differences were related to theoretically-relevant and important functional outcomes. Yet another limitation concerns the role of parental behavior in affecting children’s physiological responses to the block building task. Coercive exchanges, for example, may have affected children’s autonomic functioning, yet we did not assess such exchanges in this paper.
We did not examine whether the intervention altered post-treatment cardiac function. Following from the rationale presented in the introduction of this paper, such a finding might be especially plausible for RSA, which shows sizable environmental effects in behavioral genetics studies (Kupper et al., 2004). Future research should determine whether relatively brief behavioral interventions are sufficient to effect such change—with associated improvements in ER—or whether parasympathetic functioning requires longer time intervals to be altered by familial and extra-familial influences.
Finally, our analyses were restricted to mother-reports. As noted above, the number of fathers who participated was much smaller than the number of mothers, so power was low. We did, however, collect teacher-report data across a number of outcomes. We did not include these data because teacher-reports on variables that are linked theoretically to the PEP (severe conduct) and RSA (ER, prosocial behavior) either did not show significant treatment effects in the original RCT (Webster-Stratton, Reid, & Beauchaine, 2011), or were not assessed. For example, we did not have teacher-report measures of ER or prosocial behavior that paralleled the SCS. Including variables for which treatment effects were not found, or variables that are not linked theoretically to psychophysiological responding makes little sense in the context of examining physiological predictors of treatment response. Notably, however, data from multiple informants are especially important when there is reason to suspect that reporting bias among one or more informants is affecting the validity of results. In the present case, mothers could not observe their child’s psychophysiological response patterns, so links between maternal reports of child behavior and psychophysiology cannot be attributable to response bias. This of course does not mean that rater discrepancies are uninteresting, yet response bias, at least in the traditional sense, is an unlikely explanation for our findings.
Limitations aside, this is the first study to evaluate associations between treatment outcome and cardiovascular measures among preschool children with ADHD. Our findings indicate that children who are hyperactive/impulsive and demonstrate lengthened PEP at rest, PEP non-reactivity to incentives, and/or low resting RSA score higher on several measures of psychopathology at pretreatment, and that even though they improve as much as their peers, they still score higher at post treatment. As noted elsewhere (Beauchaine et al., 2005; Brestan & Eyberg, 1998; Nock, 2003; Owens et al., 2003), developing effective treatments is not the only objective of intervention research. Once the efficacy of an intervention is established, mechanisms through which treatment effects are exerted must be elucidated, and factors that alter efficiency of the intervention within different subsamples must be identified if we wish to help the greatest number of children (see also Beauchaine et al., 2008). The research presented in this article represents a small step forward in identifying child-level variables that are associated with treatment outcome. We hope future treatment-outcome research will extend our findings by developing interventions that target children who appear to be neurobiologically vulnerable to continued behavioral difficulties following treatment.
Acknowledgments
Research reported in this article was supported by Grants MH67192 and MH63699 from the National Institute of Mental Health.
Footnotes
The argument that PEP shortening marks central DA reactivity to incentives is based on several considerations. First, approach behaviors characteristic of reward responding require energy mobilization, a metabolic function served by the SNS. Second, increases in cardiac output required for behavioral approach are controlled by SNS-mediated changes in the contractile force of the left ventricle (Sherwood, Allen, Obrist, & Langer, 1986). Third, infusions of DA agonists into mesolimbic structures produce SNS-induced increases in cardiac output (van den Buuse, 1998), similar to those observed when normal volunteers participate in reward tasks (see Brenner et al., 2005). Finally, PEP shortening among normal controls is not observed during extinction or mood induction in well controlled experiments and is therefore specific to reward (Brenner et al., 2005; Richter & Gendolla, 2009). Nevertheless, interpreting PEP reactivity as a biomarker of mesolimbic DA responding to incentives depends on careful selection of stimulus conditions (see Beauchaine, 2012; Beauchaine & Gatzke-Kopp, 2012). Some active coping tasks such as mental arithmetic also elicit PEP shortening (e.g., Kelsey, Soderlund, & Arthur, 2004). However, such tasks induce different motivational states including fear and anxiety. As a result, β-adrenergic cardiac reactivity, the peripheral mechanism of PEP change (see Sherwood et al., 1986), is likely effected through other brain systems depending on task type (see, e.g., Pribram & McGuinness, 1975). This illustrates why stimulus conditions must be chosen carefully if one wishes to interpret the neural origin(s) of reactivity in any peripheral or central nervous system measure.
As pointed out by an anonymous reviewer, the SCS is multifaceted, and includes items that assess impulsivity and compliance as well as those related more directly to ER. Nevertheless, the scale was conceptualized initially as a broad index of emotion regulation. Given our previous reports using the full SCS with this sample (Webster-Stratton et al., 2011, 2012), we continue to use all items to maintain consistency across reports.
Carolyn Webster-Stratton has disclosed a potential financial conflict of interest because she disseminates these treatments and stands to gain from favorable reports. Because of this, she has voluntarily agreed to distance herself from certain critical research activities, including recruitment, consenting, primary data handling, and data analysis. The University of Washington has approved these arrangements.
Contributor Information
Theodore P. Beauchaine, Washington State University
Lisa Gatzke-Kopp, Pennsylvania State University.
Emily Neuhaus, University of Washington.
Jane Chipman, University of British Columbia.
M. Jamila Reid, University of Washington.
Carolyn Webster-Stratton, University of Washington.
References
- Achenbach TM, Edelbrock CS. Manual for the Child Behavior Checklist and Revised Child Behavior Profile. Burlington, VT: University Associates in Psychiatry; 1991. [Google Scholar]
- Ahs F, Sollers JJ, III, Furmark T, Fredrikson M, Thayer JF. High-frequency heart rate variability and cortico-striatal activity in men and women with social phobia. Neuroimage. 2009;47:815–820. doi: 10.1016/j.neuroimage.2009.05.091. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, text revision. Washington, DC: Author; 2000. (DSM–IV–TR) [Google Scholar]
- Asmundson GJ, Stein MB. Vagal attenuation in panic disorder: An assessment of parasympathetic nervous system function and subjective reactivity to respiratory manipulations. Psychosomatic Medicine. 1994;56:187–193. doi: 10.1097/00006842-199405000-00002. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Autonomic substrates of heart rate reactivity in adolescent males with conduct disorder and/or attention-deficit/hyperactivity disorder. In: Shohov SP, editor. Advances in psychology research. Vol. 18. New York: Nova; 2002. pp. 83–95. [Google Scholar]
- Beauchaine TP. Role of biomarkers and endophenotypes in prevention and treatment of psychopathological disorders. Biomarkers in Medicine. 2009a;3:1–3. doi: 10.2217/17520363.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP. Some difficulties in interpreting psychophysiological research with children. Monographs of the Society for Research in Child Development. 2009b;509:80–88. doi: 10.1111/j.1540-5834.2009.00509.x. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Physiological markers of emotion and behavior dysregulation in externalizing psychopathology. Monographs of the Society for Research in Child Development. 2012;77:79–86. doi: 10.1111/j.1540-5834.2011.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp LM. Instantiating the multiple levels of analysis perspective into a program of study on the development of antisocial behavior. Development and Psychopathology. 2012;24:1003–1018. doi: 10.1017/S0954579412000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Hinshaw SP, Pang KC. Comorbidity of attention-deficit/hyperactivity disorder and conduct disorder: Biological, environmental, and developmental mechanisms. Clinical Psychology: Science and Practice. 2010;17:327–336. [Google Scholar]
- Beauchaine TP, Hong J, Marsh P. Sex differences in autonomic correlates of conduct problems and aggression. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:788–796. doi: 10.1097/CHI.0b013e318172ef4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, McNulty T. Comorbidities and continuities as ontogenic processes: toward a developmental spectrum model of externalizing psychopathology. Development and Psychopathology. doi: 10.1017/S0954579413000746. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Neuhaus E, Brenner SL, Gatzke-Kopp L. Ten good reasons to consider biological variables in prevention and intervention research. Development and Psychopathology. 2008;20:745–774. doi: 10.1017/S0954579408000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Katkin ES, Strassberg Z, Snarr J. Disinhibitory psychopathology in male adolescents: Discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. Journal of Abnormal Psychology. 2001;110:610–624. doi: 10.1037//0021-843x.110.4.610. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Klein DN, Crowell SE, Derbidge C, Gatzke-Kopp LM. Multifinality in the development of personality disorders: A Biology × Sex × Environment interaction model of antisocial and borderline traits. Development and Psychopathology. 2009;21:735–770. doi: 10.1017/S0954579409000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Strassberg Z, Kees MR, Drabick DAG. Cognitive response repertoires to child noncompliance by mothers of aggressive boys. Journal of Abnormal Child Psychology. 2002;30:89–101. doi: 10.1023/a:1014287217012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Webster-Stratton C, Reid MJ. Mediators, moderators, and predictors of one-year outcomes among children treated for early-onset conduct problems: A latent growth curve analysis. Journal of Consulting and Clinical Psychology. 2005;73:371–388. doi: 10.1037/0022-006X.73.3.371. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger T, Eckberg DL, Grossman P, Kaufman PG, Malik M, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Brenner SL, Beauchaine TP. Cardiac pre-ejection period reactivity and psychiatric comorbidity prospectively predict substance use initiation among middle-schoolers: A pilot study. Psychophysiology. 2011;48:1587–1595. doi: 10.1111/j.1469-8986.2011.01230.x. [DOI] [PubMed] [Google Scholar]
- Brenner SL, Beauchaine TP, Sylvers PD. A comparison of psychophysiological and self-report measures of BAS and BIS activation. Psychophysiology. 2005;42:108–115. doi: 10.1111/j.1469-8986.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- Brestan EV, Eyberg SM. Effective psychosocial treatments of conduct-disordered children and adolescents: 29 years, 82 studies, and 5,272 kids. Journal of Clinical Child Psychology. 1998;27:180–189. doi: 10.1207/s15374424jccp2702_5. [DOI] [PubMed] [Google Scholar]
- Burnette ML, Cicchetti D, editors. Multiple-level approaches toward Understanding Antisocial Behavior: Current Research and Future Directions [Special Issue] Development and Psychopathology. 2012;24:703–704. doi: 10.1017/S0954579412000314. [DOI] [PubMed] [Google Scholar]
- Campbell SB, Shaw DS, Gilliom M. Early externalizing behavior problems: Toddlers and preschoolers at risk for later maladjustment. Development and Psychopathology. 2000;12:467–488. doi: 10.1017/s0954579400003114. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sharp WS, Gottesman RF, Greenstein DK, Giedd JN, Rapoport JL. Anatomic brain abnormalities in monozygotic twins discordant for attention deficit hyperactivity disorder. American Journal of Psychiatry. 2003;160:1693–1696. doi: 10.1176/appi.ajp.160.9.1693. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nature Reviews Neuroscience. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Cole PM, Hall SE. Emotion dysregulation as a risk factor for psychopathology. In: Beauchaine TP, Hinshaw SP, editors. Child and adolescent psychopathology. Hoboken, NJ: Wiley; 2008. pp. 265–300. [Google Scholar]
- Cole PM, Hall SE, Hajal NJ. Emotion dysregulation as a risk factor for psychopathology. In: Beauchaine TP, Hinshaw SP, editors. Child and adolescent psychopathology. 2. Hoboken, NJ: Wiley; 2013. pp. 341–373. [Google Scholar]
- Conduct Problems Prevention Research Group. Initial impact of the Fast Track prevention trial for conduct problems: I. The high-risk sample. Journal of Consulting and Clinical Psychology. 1999a;67:631–647. [PMC free article] [PubMed] [Google Scholar]
- Conduct Problems Prevention Research Group. Technical reports for the Fast Track assessment battery. 1999b. Unpublished manuscript. [Google Scholar]
- Conners CK. Revision and restandardization of the Conners’ Teacher Rating Scale (CTRS–R): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26:279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JDA, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS–R): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Craig W, Schumann L, Petrunka K, Khan S, Peters R. Government costs associated with delinquent trajectories. International Journal of Child Youth and Family Studies. 2011;2:263–293. [Google Scholar]
- Crowell S, Beauchaine TP, Gatzke-Kopp L, Sylvers P, Mead H, Chipman-Chacon J. Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. Journal of Abnormal Psychology. 2006;115:174–178. doi: 10.1037/0021-843X.115.1.174. [DOI] [PubMed] [Google Scholar]
- Crowell S, Beauchaine TP, McCauley E, Smith C, Stevens AL, Sylvers P. Psychological, autonomic, and serotonergic correlates of parasuicidal behavior in adolescent girls. Development and Psychopathology. 2005;17:1105–1127. doi: 10.1017/s0954579405050522. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Potapova NV, McCauley E, Fittleson M, Barth H, Linhoff-Harpham Beauchaine TP. Manuscript submitted for publication. 2012. Mechanisms of contextual risk for adolescent self injury: Emotion invalidation and conflict escalation in mother-child interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sanctis VA, Trampush JW, Harty SC, Marks DJ, Newcorn JH, Miller CJ, Halperin JM, et al. Childhood maltreatment and conduct disorder: Independent predictors of adolescent substance use disorders in youth with attention-deficit / hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology. 2008;37:785–793. doi: 10.1080/15374410802359650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabick DAG, Gadow KD, Sprafkin J. Co-occurrence of conduct disorder and depression in a clinic-based sample of boys with ADHD. Journal of Child Psychology and Psychiatry. 2006;47:766–774. doi: 10.1111/j.1469-7610.2006.01625.x. [DOI] [PubMed] [Google Scholar]
- Drugli MB, Larsson B. Children aged 4–8 years treated with parent training and child therapy because of conduct problems: Generalisation effects to day-care and school settings. European Child and Adolescent Psychiatry. 2006;15:392–399. doi: 10.1007/s00787-006-0546-3. [DOI] [PubMed] [Google Scholar]
- Durston S. A review of the biological bases of ADHD: What have we learned from neuroimaging studies? Mental Retardation and Developmental Disabilities Research Reviews. 2003;9:184–195. doi: 10.1002/mrdd.10079. [DOI] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IE, Yang Y, Casey BJ. Differential patterns of striatal activation in young children with and without ADHD. Biological Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Murphy B, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children’s social functioning: A longitudinal study. Child Development. 1995;66:1360–1384. [PubMed] [Google Scholar]
- El-Sheikh M. Does poor vagal tone exacerbate child maladjustment in the context of parental problem drinking? A longitudinal examination. Journal of Abnormal Psychology. 2005;114:735–741. doi: 10.1037/0021-843X.114.4.735. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Keller P, Cummings EM, Staton L. Marital conflict and children’s externalizing behavior: Interactions between parasympathetic and sympathetic nervous system activity. Monographs of the Society for Research in Child Development. 2009;509 doi: 10.1111/j.1540-5834.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, Whitson SM. Exposure to interparental conflict and children’s adjustment and physical health: The moderating role of vagal tone. Child Development. 2001;72:1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N, Karbon N, Troyer D, Switzer G. The relations of children’s emotion regulation to their vicarious emotional responses and comforting behaviors. Child Development. 1994;65:1678–1693. doi: 10.1111/j.1467-8624.1994.tb00842.x. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J. Child symptom inventories norms manual. Stony Brook, NY: Checkmate Plus; 1997. [Google Scholar]
- Gatzke-Kopp LM. The canary in the coalmine: The sensitivity of mesolimbic dopamine to environmental adversity during development. Neuroscience and Biobehavioral Reviews. 2011;35:794–803. doi: 10.1016/j.neubiorev.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp L, Beauchaine TP. Central nervous system substrates of impulsivity: Implications for the development of attention-deficit/hyperactivity disorder and conduct disorder. In: Coch D, Dawson G, Fischer K, editors. Human behavior, learning, and the developing brain: Atypical development. New York: Guilford Press; 2007. pp. 239–263. [Google Scholar]
- Gatzke-Kopp LM, Beauchaine TP, Shannon KE, Chipman J, Fleming AP, Crowell SE, Aylward E. Neurological correlates of reward responding in adolescents with and without externalizing behavior disorders. Journal of Abnormal Psychology. 2009;118:203–213. doi: 10.1037/a0014378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti U, Tremblay RE, Vitaro F. Iatrogenic effects of juvenile justice. Journal of Child psychology and Psychiatry. 2009;50:991–998. doi: 10.1111/j.1469-7610.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Raine A, Loeber R, Stouthamer-Loeber M, Steinhauer SR. Serious delinquent behavior, sensation seeking, and electrodermal arousal. Journal of Abnormal Child Psychology. 2004;30:477–486. doi: 10.1023/a:1019816930615. [DOI] [PubMed] [Google Scholar]
- Gilden JL. Midodrine, adrenergic agonists and antagonists. In: Roberton D, Biaggiono I, Burnstock G, Low PA, Paton JFR, editors. Primer on the autonomic nervous system. 3. San Diego, CA: Academic Press; 2012. pp. 621–625. [Google Scholar]
- Goldsmith HH, Davidson RJ. Disambiguating the components of emotion regulation. Child Development. 2004;75:361–365. doi: 10.1111/j.1467-8624.2004.00678.x. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Pollak SD, Davidson RJ. Developmental neuroscience perspectives on emotion regulation. Child Development Perspectives. 2008;2:132–140. doi: 10.1111/j.1750-8606.2008.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JW. Missing data analysis: Making it work in the real world. Annual Review of Psychology. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Hastings PD, Nuselovici JN, Utendale WT, Coutya J, McShane KE, Sullivan C. Applying the polyvagal theory to children’s emotion regulation: Social context, socialization, and adjustment. Biological Psychology. 2008;79:229–306. doi: 10.1016/j.biopsycho.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Katz LF, Gottman JM. Vagal tone protects children from marital conflict. Development and Psychopathology. 1995;7:83–92. [Google Scholar]
- Kelsey RM, Guethlein W. An evaluation of the ensemble averaged impedance cardiogram. Psychophysiology. 1990;27:24–33. doi: 10.1111/j.1469-8986.1990.tb02173.x. [DOI] [PubMed] [Google Scholar]
- Kelsey RM, Soderlund K, Arthur CM. Cardiovascular reactivity and adaptation to recurrent psychological stress: Replication and extension. Psychophysiology. 2004;41:924–934. doi: 10.1111/j.1469-8986.2004.00245.x. [DOI] [PubMed] [Google Scholar]
- Kupper NHM, Willemsen G, van den Berg M, de Boer D, Posthuma D, Boomsma DI, de Geus EJC. Heritability of ambulatory heart rate variability. Circulation. 2004;110:2792–2796. doi: 10.1161/01.CIR.0000146334.96820.6E. [DOI] [PubMed] [Google Scholar]
- Laakso A, Wallius E, Kajander J, Bergman J, Eskola O, Solin O, Hietala J. Personality traits and striatal dopamine synthesis capacity in healthy subjects. American Journal of Psychiatry. 2003;160:904–910. doi: 10.1176/appi.ajp.160.5.904. [DOI] [PubMed] [Google Scholar]
- Loeber R, Hay D. Key issues in the development of aggression and violence from childhood to early adulthood. Annual Review of Psychology. 1997;48:371–410. doi: 10.1146/annurev.psych.48.1.371. [DOI] [PubMed] [Google Scholar]
- Lynam DR. The early identification of chronic offenders: Who is the fledgling psychopath? Psychological Bulletin. 1996;120:209–234. doi: 10.1037/0033-2909.120.2.209. [DOI] [PubMed] [Google Scholar]
- Lynam DR. Early identification of the fledgling psychopath: Locating the psychopathic child in the current nomenclature. Journal of Abnormal Psychology. 1998;107:566–575. doi: 10.1037//0021-843x.107.4.566. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Caspi A, Moffitt TE, Wikström PH, Loeber R, Novak S. The interaction between impulsivity and neighborhood context in offending: The effects of impulsivity are stronger in poorer neighborhoods. Journal of Abnormal Psychology. 2000;109:563–574. doi: 10.1037//0021-843x.109.4.563. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Truong NL, Moulton JL, Roizen ER, Howell KH, Castellanos FX. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: Prospective follow-up into adulthood. American Journal of Psychiatry. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh P, Beauchaine TP, Williams B. Dissociation of sad facial expressions and autonomic nervous system responding in boys with disruptive behavior disorders. Psychophysiology. 2008;45:100–110. doi: 10.1111/j.1469-8986.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Slutske WS, Arndt S, Cadoret RJ. Impulsive and callous traits are more strongly associated with delinquent behavior in higher risk neighborhoods among boys and girls. Journal of Consulting and Clinical Psychology. 2008;117:377–385. doi: 10.1037/0021-843X.117.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A. Childhood predictors differentiate life-course persistent and adolescence-limited antisocial pathways among males and females. Development and Psychopathology. 2001;13:355–375. doi: 10.1017/s0954579401002097. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Attention-deficit/hyperactivity disorder. In: Beauchaine TP, Hinshaw SP, editors. Child and adolescent psychopathology. 2. Hoboken, NJ: Wiley; in press. [Google Scholar]
- Nock MK. Progress review of the psychosocial treatment of child conduct problems. Clinical Psychology Science and Practice. 2003;10:1–28. [Google Scholar]
- O’Connor TG, Deater-Deckard K, Fulker D, Rutter M, Plomin R. Genotype-environment correlations in late childhood and adolescence: Antisocial behavior problems and coercive parenting. Developmental Psychology. 1998;34:970–981. doi: 10.1037//0012-1649.34.5.970. [DOI] [PubMed] [Google Scholar]
- Owens EB, Hinshaw SP, Kraemer HC, Arnold LE, Abikoff HB, Cantwell DP, Wigal T. Which treatment for whom for ADHD? Moderators of treatment response in the MTA. Journal of Consulting and Clinical Psychology. 2003;71:540–552. doi: 10.1037/0022-006x.71.3.540. [DOI] [PubMed] [Google Scholar]
- Piquero AR, Farrington DP, Welsh BC, Tremblay R, Jennings WG. Effects of early family/parent training programs on antisocial behavior and delinquency. Journal of Experimental Criminology. 2009;5:83–120. [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage: A polyvagal perspective. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribram KH, McGuinness D. Arousal, activation, and effort in the control of attention. Psychological Review. 1975;82:116–149. doi: 10.1037/h0076780. [DOI] [PubMed] [Google Scholar]
- Qu M, Zhang Y, Webster JG, Tompkins WJ. Motion artifact from spot and band electrodes during impedance cardiography. Transactions on Biomedical Engineering. 1986;11:1029–1036. doi: 10.1109/TBME.1986.325869. [DOI] [PubMed] [Google Scholar]
- Reid MJ, Webster-Stratton C, Beauchaine TP. Parent training in Head Start: A Comparison of program response among African American, Asian American, Caucasian, and Hispanic mothers. Prevention Science. 2001;2:209–227. doi: 10.1023/a:1013618309070. [DOI] [PubMed] [Google Scholar]
- Ritz T. Studying non-invasive indices of vagal control: The need for respiratory control and the problem of target specificity. Biological Psychology. 2009;80:158–168. doi: 10.1016/j.biopsycho.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Robins LN. Deviant children grown up. Baltimore, MD: Williams & Wilkins; 1966. [Google Scholar]
- Robinson EA, Eyberg SM, Ross AW. The standardization of an inventory of child conduct problem behaviors. Journal of Clinical Child Psychology. 1980;9:22–28. [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: A critical analysis. Biological Psychology. 2007;74:200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Salomon K, Gross JJ, Gotlib IH. Vagal withdrawal to a sad film predicts recovery from depression. Psychophysiology. 2005;42:277–281. doi: 10.1111/j.1469-8986.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Wilhelm FH, Gross JJ, Gotlib IH. Respiratory sinus arrhythmia as a predictor of outcome in major depressive disorder. Journal of Affective Disorders. 2002;71:265–272. doi: 10.1016/s0165-0327(01)00406-2. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Smith AB, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalizes fronto-striatal underactivation during interference inhibition in medication-naïve boys with attention-deficit/hyperactivity disorder. Neuropsychopharmacology. 2011;36:1575–1586. doi: 10.1038/npp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Johansen E, Aase H, Russell V. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioural and Brain Sciences. 2005;28:397–468. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Sauder C, Beauchaine TP, Gatzke-Kopp LM, Shannon KE, Aylward E. Neuroanatomical correlates of heterotypic comorbidity in externalizing youth. Journal of Clinical Child and Adolescent Psychology. 2012;41:346–352. doi: 10.1080/15374416.2012.658612. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Scott S, Spender Q, Doolan M, Jacobs B, Aspland H. Multicentre controlled trial of parenting groups for child antisocial behaviour in clinical practice. British Medical Journal. 2001;323:1–5. doi: 10.1136/bmj.323.7306.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Mina K, Schwab-Stone ME. NIMH diagnostic interview schedule for children version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shannon KE, Beauchaine TP, Brenner SL, Neuhaus E, Gatzke-Kopp L. Familial and temperamental predictors of resilience in children at risk for conduct disorder and depression. Development and Psychopathology. 2007;19:701–727. doi: 10.1017/S0954579407000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon KE, Sauder C, Beauchaine TP, Gatzke-Kopp L. Disrupted effective connectivity between the medial frontal cortex and the caudate in adolescent boys with externalizing behavior disorders. Criminal Justice and Behavior. 2009;36:1141–1157. [Google Scholar]
- Sherwood A, Allen MT, Fahrenbert J, Kelsey RM, Lovallo WR, van Doornen LJP. Committee report: Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Obrist PA, Langer AW. Evaluation of beta-adrenergic influences on cardiovascular and metabolic adjustments to physical and psychological stress. Psychophysiology. 1986;23:89–104. doi: 10.1111/j.1469-8986.1986.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Sprafkin J, Gadow KD, Salisury H, Schneider J, Loney J. Further evidence of reliability and validity of the child Symptom Inventory–4: Parent checklist in clinically referred boys. Journal of Clinical Child and Adolescent Psychology. 2002;31:513–524. doi: 10.1207/S15374424JCCP3104_10. [DOI] [PubMed] [Google Scholar]
- Suess PA, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Thompson RA. Emotion and self-regulation. In: Thompson RA, editor. Socioemotional development (Nebraska Symposium on Motivation) Vol. 36. Lincoln: University of Nebraska Press; 1990. [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD. Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proceedings of the National Academy of Sciences. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- van den Buuse M. Role of the mesolimbic dopamine system in cardiovascular homeostasis: Stimulation of the ventral tegmental area modulates the effect of vasopressin in conscious rats. Clinical Experimental Pharmacology and Physiology. 1998;25:661–668. doi: 10.1111/j.1440-1681.1998.tb02273.x. [DOI] [PubMed] [Google Scholar]
- Vasilev CA, Crowell SE, Beauchaine TP, Mead HK, Gatzke-Kopp LM. Correspondence between physiological and self-report measures of emotion dysregulation: A longitudinal investigation of youth with and without psychopathology. Journal of Child Psychology and Psychiatry. 2009;50:1357–1364. doi: 10.1111/j.1469-7610.2009.02172.x. [DOI] [PubMed] [Google Scholar]
- Verbane AJ, Owens NC. Cortical modulation of the cardiovascular system. Progress in Neurobiology. 1998;54:149–168. doi: 10.1016/s0301-0082(97)00056-7. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Swanson J. Evaluating dopamine reward pathway in ADHD. Journal of the American Medical Association. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster-Stratton C. Tailoring the Incredible Years parenting program according to children’s developmental needs and family risk factors. In: Briesmeister JM, Schaefer CE, editors. Handbook of parent training. Hoboken, NJ: John Wiley & Sons; 2007. pp. 305–344. [Google Scholar]
- Webster-Stratton C, Hammond M. Treating children with early-onset conduct problems: A comparison of child and parent training interventions. Journal of Consulting and Clinical Psychology. 1997;65:93–109. doi: 10.1037//0022-006x.65.1.93. [DOI] [PubMed] [Google Scholar]
- Webster-Stratton C, Reid MJ. Adapting the Incredible Years child dinosaur social, emotional, and problem-solving intervention to address comorbid diagnoses. Journal of Children’s Services. 2008;3:17–30. [Google Scholar]
- Webster-Stratton C, Reid MJ. The Incredible Years Parents, Teachers and Children Training Series: A multifaceted treatment approach for young children with conduct problems. In: Weisz J, Kazdin A, editors. Evidence-based psychotherapies for children and adolescents. 2. New York: Guilford; 2010. pp. 194–210. [Google Scholar]
- Webster-Stratton C, Reid MJ, Beauchaine TP. Combining parent and child training for young children with attention-deficit/hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology. 2011;40:191–203. doi: 10.1080/15374416.2011.546044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster-Stratton C, Reid MJ, Beauchaine TP. Combining parent and child training for young children with ADHD: 1-year follow-up. Manuscript submitted for publication. 2012 doi: 10.1080/15374416.2011.546044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster-Stratton C, Reid MJ, Hammond M. Treating children with early-onset conduct problems: Intervention outcomes for parent, child, and teacher training. Journal of Clinical Child and Adolescent Psychology. 2004;33:105–124. doi: 10.1207/S15374424JCCP3301_11. [DOI] [PubMed] [Google Scholar]
- Whisman MA, McClelland GH. Designing, testing, and interpreting interactions and moderator effects in family research. Journal of Family Psychology. 2005;19:111–120. doi: 10.1037/0893-3200.19.1.111. [DOI] [PubMed] [Google Scholar]
- Willcutt EG. Genetics of ADHD. In: Barch D, editor. Cognitive and affective neuroscience of psychopathology. New York: Oxford University Press; in press. [Google Scholar]