Abstract
Extracellular nucleotides regulate critical liver functions via the activation of specific transmembrane receptors. The hepatic levels of extracellular nucleotides, and therefore the related downstream signaling cascades, are modulated by cell-surface enzymes called ectonucleotidases, including nucleoside triphosphate diphosphohydrolase-1 (NTPDase1/CD39), NTPDase2/CD39L1, and ecto-5'-nucleotidase/CD73. The goal of this study was to determine the molecular identity of the canalicular ecto-ATPase/ATPDase that we hypothesized to correspond to the recently cloned NTPDase8. Human and rat NTPDase8 cDNAs were cloned, and the genes were located on chromosome loci 9q34 and 3p13, respectively. The recombinant proteins, expressed in COS-7 and HEK293T cells, were biochemically characterized. NTPDase8 was also purified from rat liver by Triton X-100 solubilization, followed by DEAE, Affigel Blue, and concanavalin A chromatographies. Importantly, NTPDase8 was responsible for the major ectonucleotidase activity in liver. The ion requirement, apparent Km values, nucleotide hydrolysis profile, and preference as well as the resistance to azide were similar for recombinant NTPDase8s and both purified rat NTPDase8 and porcine canalicular ecto-ATPase/ATPDase. The partial NH2-terminal amino acid sequences of all NTPDase8s share high identity with the purified liver canalicular ecto-ATPase/ATPDase. Histochemical analysis showed high ectonucleotidase activities in bile canaliculi and large blood vessels of rat liver, in agreement with the immunolocalization of NTPDase1, 2, and 8 with antibodies developed for this study. No NTPDase3 expression could be detected in liver. In conclusion, NTPDase8 is the canalicular ecto-ATPase/ATPDase and is responsible for the main hepatic NTPDase activity. The canalicular localization of this enzyme suggests its involvement in the regulation of bile secretion and/or nucleoside salvage.
Keywords: ecto-ATPDase, P1/P2 receptors, ATP, adenosine, nucleoside transporters
in liver, extracellular nucleotides are critical regulators of biological functions such as regulation of cell volume and proliferation (11, 27, 46, 54). These effects are mediated by specific nucleotide P2 receptors (8) that are subdivided into P2X1–7 (ion-gated channels) and P2Y1,2,4,6,11–14 (G-protein coupled receptors) (16, 19). The levels of P2 receptor agonists are regulated by various enzymes expressed at the cell surface called ectonucleotidases. This group of enzymes includes members of the ectonucleoside triphosphate diphosphohydrolase (E-NTPDase) family, which is composed of eight proteins (NTPDase1–8). NTPDases hydrolyze tri- and diphosphonucleosides into their monophosphate counterparts with different ability and specificity (44, 56).
Among all tissues, the liver has one of the highest ATPase and ADPase activities (37). Histochemical studies showed that most of liver ectonucleotidase activity was associated with the canalicular domain of hepatocytes (18). It was originally believed that this activity, which was different from a classical ATPase activity, was due to a protein structurally similar to the cell adhesion molecule C-CAM 105 (39). However, this protein was later shown to be an embryocarcinogenic antigen that could not explain the high ATPase activity in this tissue (30). Further studies revealed that NTPDase1 is expressed by Kupffer cells and vascular endothelial cells (52) whereas NTPDase2 is produced by portal fibroblasts and activated hepatic stellate cells (14, 17). Yet activity assays indicated that the combined activity of these two NTPDases could not account for the major ectonucleotidase activity observed in the liver (52). Thereafter, the major ectonucleotidase activity in porcine liver was shown to bear the basic properties of an NTPDase (36, 52). Interestingly, this enzyme called hepatic ATPDase was localized in bile canaliculi membrane (52) and was proposed to be responsible for the high ectonucleotidase activity previously detected in liver (18, 40). This ectonucleotidase represents the oldest canalicular marker and has been of great importance to liver cell biologists (18, 41).
We have recently cloned a novel member of the ENTPDase family in mouse and designated it NTPDase8 (4). In the present work, we report the cloning and biochemical characterization of NTPDase8 in human and rat species and its identification as the hepatic canalicular ecto-ATPase/ATPDase.
MATERIALS AND METHODS
Materials
Culture cell media were obtained from Invitrogen (Burlington, ON, Canada). Agarose, aprotinin, PMSF, nucleotides, tetrabutylammonium hydrogen sulfate, EGTA, and sodium deoxycholate were provided by Sigma-Aldrich (Oakville, ON, Canada); sodium azide by Fisher Scientific (Fair Lawn, NJ), Triton X-100 by Roche Diagnostics (Mannheim, Germany); bis(2-hydroxyethyl)amino-tris(hydroxymethyl) methane (Bis-Tris), Tris, and EDTA by EMD Chemicals (Gibbstown, NJ). TOPRO-3 dye was purchased from Molecular Probes (Eugene, OR) and hematoxylin from Biomeda (Foster City, CA).
Animals and Antibody Production
Sprague-Dawley rats, Hartley guinea pigs, and New Zealand rabbits were obtained from Charles River Laboratories (Québec, QC, Canada). Genetic immunization protocol was carried out with plasmids (pcDNA3.1) encoding each protein, in New Zealand rabbits for antibodies against rat NTPDase1, and Hartley guinea pigs for rat NTPDase8 antibodies. All procedures were approved by the Canadian Council on Animal Care and the Université Laval Animal Welfare Committee.
Plasmids
Cloning
The plasmid encoding rat NTPDase1 (GenBank accession no. NM_022587) has been described in a published report (26). The complementary DNAs (cDNAs) encoding human NTPDase8 (AY430414) or rat NTPDase8 (AY536920) were cloned as follows. Total RNA was isolated from rat liver with Trizol reagent (Invitrogen) or obtained from AMBION (Austin, TX) (kindly donated by Dr. C. Guillemette, Centre de Recherche du Centre Hospitalier de l’Université Laval). The cDNA was synthesized with SuperScriptII (Invitrogen) with oligo(dT)18 as the primer, in accordance with manufacturer’s instructions. For amplification, 10% of the reverse transcription reaction was used as template in a reaction mixture containing 0.6 μM primer, 400 μM dNTP, and 3.5 U Expand High Fidelity PCR System (Roche, Laval, Canada) with Mg2+ concentrations of 1.5 mM for rat or 2.5 mM for human. Amplification was done with primers designed from human or rat expressed sequence tags (ESTs; XM_231041 and AI535212, respectively) that revealed highest homology to mouse NTPDase8 (AY364442): one human forward sequence 5′-CCA-GTA-CCA-CCT-GCA-CCA-3′ and two reverse sequences 5′-GCA-GAA-AGG-CAC-CTA-CGG3′, 5′-GGG-GTC-CCT-GCT-GTG-TTC-3′; rat sequences, forward 5′-TCA-GCC-CCTCCC-ACC-ATG-AGA-CTT-3′ and reverse 5′-TGT-TTC-TAT-CCC-TGG-GGC-AAC-T-3′. Amplification for human NTPDase8 (or rat) was started by an incubation of 2 min at 94°C, followed by 30 cycles of 30 s (rat: 15 s) denaturation at 94°C, 15-s annealing at 55.5°C (rat: 30 s at 60°C) and 2-min primer extension at 72°C, and ended with 10-min incubation at 72°C. The PCR products of ~1.5 kb were purified on 1% agarose gel using the QIAEX II gel extraction kit (Qiagen, Mississauga, ON, Canada) and ligated into the expression vector pcDNA3.1/V5-His (Invitrogen). Plasmids were purified with QIAprep Spin Miniprep kit (Qiagen), and orientation of the inserts was determined by restriction-enzyme mapping. One clone obtained with each set of primers was amplified and fully sequenced in one direction. These plasmids were used for transfection and generation of antisera.
Characterization of genomic sequences
The human and rat NTPDase8 cDNA sequences were used to blast the National Center for Biotechnology Information (NCBI) genome database. The sequences identified as NT_024000.16 and NW_001084810.1 showed 100% homology with human and rat NTPDase8 cDNAs, respectively. The genomic sequences and exon/intron junctions were analyzed with NCBI BLAST programs.
Purification of Rat Liver NTPDase8
The isolation of rat liver membrane fractions and protein solubilization were performed as previously described (51). Membrane proteins (6 mg/ml) were mixed with an equal volume of 0.6% Triton X-100. Further purification of solubilized NTPDase8 was done by column chromatography using a Pharmacia FPLC system. Unless indicated otherwise, chromatographies were carried out at the flow rate of 1 ml/min and 2-ml fractions were collected. Briefly, Triton-solubilized proteins (500 mg) were applied to a DEAE Sepharose column (DEAE fast flow, 10 cm × 2.5 cm, Amersham Biosciences, Uppsala, Sweden) and resolved in the presence of 0.1% Triton X-100, 7.5% glycerol, and 10 mM Tris, pH 8.0, with a NaCl gradient: no salt for 0–50 min, 0.03 M NaCl for 50–150 min, 0.03–0.13 M NaCl for 150–650 min, and 0.13 M NaCl for 650–750 min. Samples containing NTPDase8, as determined by activity assay and immunoblotting, were pooled, reequilibrated by ultrafiltration in an Amicon stirred cell in 0.1% Triton X-100 and 25 mM Tris/His, pH 5.95 (Millipore PBTK membrane, MWWL 30,000) and applied to an Affigel Blue column (20 cm × 1 cm, Bio-Rad, Hercules, CA). Affigel Blue unbound material was washed out with 20 ml of reequilibrating buffer, and the elution of retained proteins was done by a gradient developed from 0.1% Triton X-100, 7.5% glycerol, and 10 mM Tris, pH 6.8 (buffer A) and 0.1% Triton X-100, 7.5% glycerol, 1 M NaCl, and 10 mM Tris, pH 7.5 (buffer B). The following chromatography program was used: gradient A→B from 0% to 100% for the first 120 min and 100% B for the following 150 min. NTPDase8-enriched fractions were pooled and reequilibrated in concanavalin A (ConA) buffer (1 M NaCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM MnCl2, 0.1% Triton X-100, and 20 mM Pipes/OH, pH 6.8) and loaded on a ConA Sepharose column (Amersham Biosciences, 5 ml) at gravity flow. Unbound proteins were washed out with 6X bed volume of ConA buffer. NTPDase8 and other remaining glycosylated proteins were desorbed with 0.5 M methyl α-D-mannopyranoside (Sigma-Aldrich, St. Louis, MO) in ConA buffer. For the biochemical characterization, mannopyranoside removal and enzyme concentration (to ~1 mg/ml) were done by ultrafiltration with Centurion [YM-30] filter devices (Amicon).
Cell Transfection and Nucleotidase Activity Assays
African green monkey COS-7 or human embryonic kidney 293T cell transfection and preparation of cell lysates were carried out as previously described (34). Enzyme activity tests and HPLC profiles of substrate hydrolysis were performed as previously described (34). NTPDase activity was measured at 37°C in the following incubation medium: 5 mM CaCl2 (or 5 mM MgCl2) and 80 mM Tris, pH 7.4. Intact transfected cells were assayed in 24-well plates in the same incubation medium plus 150 mM NaCl. Where indicated, cell lysates were incubated with sodium azide or sodium deoxycholate salts. In assays using sodium deoxycholate, reaction mixtures (100 μl) were added to 1 M perchloric acid 1:1 to stop the reaction and precipitate deoxycholate as a sodium salt before phosphate estimation. Ecto-5′-nucleotidase activity was evaluated in 5 mM MgCl2 and 80 mM Tris, pH 7.4, with AMP as the substrate. The released phosphate was measured by the malachite green assay (1). In all assays, less than 10% of substrate was hydrolyzed.
PAGE and Immunoblotting Procedures
Subcellular membrane fractions of rat hepatocytes were prepared following the method of Kipp and Arias (29). Protein concentration was estimated by the Bradford microplate assay using BSA as a standard (6). Protein samples were resuspended in 2% (wt/vol) lithium dodecyl sulfate sample buffer and fractionated by PAGE under non-reducing conditions according to Laemmli (35). Proteins were transferred to Immobilon-P membrane (Millipore, Bedford, MA) by electroblotting. After incubation with primary antibodies, proteins of interest were visualized with the appropriate horseradish peroxidase-conjugated secondary antibody (anti-rabbit, Amersham Biosciences, Boston, MA; anti-guinea pig, Santa Cruz Biotechnology, Santa Cruz, CA) and the Chemiluminescent Reagent Plus (Perkin-Elmer, Boston, MA), as recommended.
Immunohistochemistry, Immunofluorescence, and Enzyme Histochemistry
For immunohistochemistry, sections of snap-frozen rat liver (6 μm) or cultured cells (105 per coverslip) were fixed in cold acetone (Fisher) mixed with 10% phosphate-buffered formalin (Fisher) and were incubated with rabbit polyclonal sera to rat NTPDase1 (rN1-6L), NTPDase2 (BZ3-4F) (14), or a guinea pig polyclonal serum to rat NTPDase8 (rN8-8c), as described previously (53). Immunofluorescence was performed with polyclonal rN8-8c serum and monoclonal anti-multidrug resistance related protein-2 (Mrp-2) antibody (kindly donated by Dr. C. Soroka, Yale Liver Center), as previously described (14). Localization of ectonucleotidase activities was determined using the Wachstein/Meisel lead phosphate method (7).
RESULTS
Given that the presence of NTPDase1 and NTPDase2 has already been demonstrated in the liver and could not account for the canalicular ectonucleotidase activity, and that there are low levels of NTPDase3 mRNA in this tissue, we hypothesized that NTPDase8 was responsible for this activity. To address this assumption, we first searched for the existence of NTPDase8 in human and rat and cloned the respective cDNAs.
Cloning and Characterization of Human ENTPD8 and Rat Entpd8 cDNAs
The sequence of human NTPDase8 includes an open reading frame of 1,485 nucleotides (rat: 1,482), which is translated into a protein of 495 amino acid residues (rat: 494) with a predicted molecular mass of 53,888 Da (rat: 54,330) and a calculated isoelectric point of 5.19 (rat: 5.83). The deduced amino acid sequence contains seven (rat: eight) potential N-glycosylation sites, five apyrase conserved regions featured by all NTPDases, and various potential phosphorylation sites. Interestingly, there is a conserved potential phosphorylation site for both protein kinase C and casein kinase II on the fourth amino acid of mouse (4), human, and rat NTPDase8s. Hydrophobicity analysis of human and rat NTPDase8s predicts two transmembrane domains, one near the NH2 terminus (amino acids 9–30, for both enzymes) and one near the COOH terminus (amino acids 472–488 for human and 466–488 for rat). Both human and rat cDNAs were deposited in the database (Table 1). Sequence analysis on the website http://www.ncbi.nlm.nih.gov/genome/seq/ localized the genes to chromosome 9q34 (entry NT_024000.16) for human and chromosome 3p13 (NW_ 001084810.1) for rat. Alignment of the full cDNA with the human genomic sequence reveals that ENTPD8 covers 3,631 bases and is organized into nine exons (data not shown). The analysis with this recent sequence indicates that the rat gene would be organized similarly to both human ENTPD8 and mouse Entpd8.
Table 1.
Alignment of amino acid sequences of the purified porcine canalicular ecto-ATPase/ATPDase with NTPDase8s and chicken ATPDases
| Amino Acid Identity, % |
||||
|---|---|---|---|---|
| Enzyme Name | GenBank Accession Number or Reference* |
NH2-Terminal Amino Acid Sequence | NH2 Terminal | cDNA-Based Homology |
| Porcine canalicular ecto-ATPase/ATPDase | Sévigny et al. (52) | -GQTRKDRVFTALLAAA | 100 | n/a |
| Porcine EST | BF192072/BGS94445 | MGQTRKDRVFTALLWAA | 85.3 | 100 |
| Human NTPDase8 | AY430414 | MGLSRKEQVFLALLGAS | 60.0 | 81.0 |
| Mouse NTPDase8 | AY364442 | MGLSWKERVFMALLGVA | 54.9 | 79.5 |
| Rat NTPDase8 | AY536920 | MRLSWKERVFMVLLGVA | 42.8 | 77.7 |
| Chicken ATPDase | AF426405 | MEYKGKVVAGLLTATCV | 28.2 | 61.9 |
| Chicken stomach ecto-apyrase/ATPDase | Lewis-Carl and Kirley (38) | MEYKGKVVAGLLTATWV | 28.2 | n/a |
Alignment of the NH2-terminal amino acid sequence of the purified porcine canalicular ecto-ATPase/ATPDase was done with various NTPDase8s, chicken ATPDase, and purified chicken stomach ecto-apyrase/ATPDase. The identical amino acids between the different sequences are underlined. The indicated percentage of amino acid identity was calculated by using the sequence of the porcine canalicular ecto-ATPase/ATPDase as template and determined by pairwise alignment. Once translated into amino acids the 5′ ends of 2 porcine liver ESTs revealed to be nearly identical to this enzyme. The cDNA-based homology was calculated by comparing the first 260 amino acid sequence deduced from the open reading frame obtained from these overlapping porcine expressed sequence tags (ESTs) with the corresponding sequence of all NTPDase8s and determined by pairwise alignment. All forms of NTPDase8 have high homology with the porcine liver canalicular ecto-ATPase/ATPDase, suggesting that all these proteins are orthologs.
References are provided for partial amino acid sequences obtained from purified proteins and accession numbers are given for cDNA sequences used to deduce the amino acid sequence.
The NH2-terminal amino acid sequence deduced from the coding sequence of ENTPD8 shows high homology (60%) with the NH2-terminal sequence of the canalicular ecto-AT-Pase/ATPDase purified from porcine liver (Table 1). Two porcine ESTs corresponding to this purified enzyme were obtained from the database. The comparison of the partial sequence of the 260 amino acids derived from these overlapping ESTs revealed over 75% identity with the corresponding sequence of the other mammalian NTPDase8s (Table 1). These data suggest that NTPDase8 and the hepatic canalicular ecto-ATPase/ATPDase are orthologs.
Cellular Distribution of NTPDases in Rat Liver
To further characterize hepatic NTPDases, antibodies were developed and their specificity was verified by immunocytochemistry and immunoblotting (Fig. 1). Antibodies to NTPDase1, 2, and 8 were all specific, as verified by cross-reaction experiments in transfected COS-7 cells (data not shown). These antibodies also stained different cells in liver tissue (Fig. 2), confirming that the antibodies do not cross-react.
Fig. 1.
Specificity of antibodies to rat NTPDases. The specificity of polyclonal sera to rat NTPDase8 (rN8-8c) and NTPDase1 (rN1-6l) was tested in A by immunocytochemistry and in B by immunoblotting. A: immunocytochemistry on mock or transfected COS-7 cells with plasmids encoding rat NTPDase8 (a) or rat NTPDase1 (b). Transfected cells expressing rat NTPDase8 displayed a high staining with rN8-8c (a, bottom right), whereas untransfected cells (mock) were devoid of immunoreactivity (a, top right) as well as when the preimmune serum was incubated with these cells (a, left). Similar results were obtained with sera to rat NTPDase1, rN1-6l (b). Scale bar = 20 μm. B: immunoblotting of protein extracts from mock (ctl) or transfected COS-7 cells with plasmids encoding rat NTPDase8 (N8) or rat NTPDase1 (N1) and protein fractions from rat and mouse tissues. Protein samples of 6 μg for cell lysates (recombinant proteins) or 10 μg for murine tissues were loaded in each well. Each serum is specific for its antigen, as demonstrated by immunoblotting with transfected cell lysates (left and middle). When tested on protein extracts from murine tissues, rN8-8C detected NTPDase8 in liver (L; highest expression), kidney (K), and jejunum (J) from rat species, but not from mouse (right). Subcellular membrane fractionation of rat liver showed a much higher intensity of the NTPDase8 immunoreactive band in the canalicular membrane vesicle fraction (CMVs) than in the total liver membrane fraction (L0). Molecular weights are indicated in kDa.
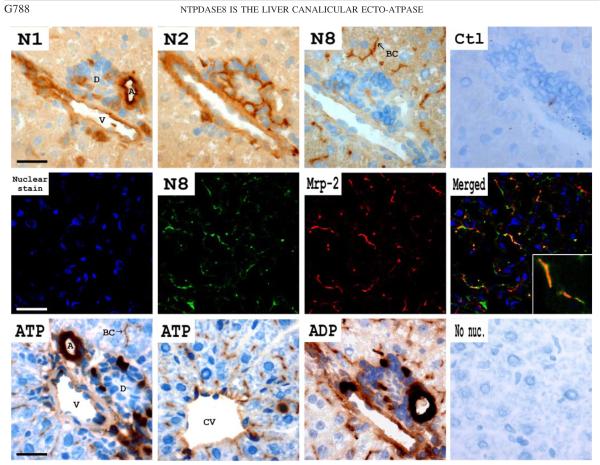
Fig. 2.
Cellular localization of ectonucleotidases in rat liver. Top: distribution of rat NTPDases was determined in consecutive rat liver sections by immunohistochemistry, as described in MATERIALS AND METHODS. Counterstaining of nuclei in blue was performed with aqueous hematoxylin. No staining is observed in control sections incubated with preimmune sera (Ctl). Although NTPDase1 is detected at the luminal surface of large blood vessels (N1), NTPDase2 is present at the external face of large blood vessels and in periductular area (N2). Rat NTPDase8 staining is restricted to the canalicular domain of hepatocytes (N8). A, portal artery; V, portal vein; D, duct; BC, bile canaliculus. Scale bar = 20 μm. Middle: to confirm the canalicular localization of NTPDase8, double immunofluorescence staining was performed with a monoclonal anti-multidrug resistance related protein-2 (Mrp-2) antibody as canalicular marker. Nuclear staining was performed with TOPRO-3 dye (blue). NTPDase8 (green) colocalizes with Mrp-2 (red; merged). Inset: ×4.5 magnification of canalicular colocalization of NTPDase8 and Mrp-2. Scale bar = 40 μm. Bottom: ectonucleotidase activity was located after incubation (1 h) with various nucleotides (1 mM) as substrates. In all assays, tissue sections were incubated with tetramisole (5 mM) to block alkaline phosphatase activity. A portal track area is shown for ATP and ADP (bottom, 1st and 3rd from left) and a central vein for ATP (bottom, 2nd from left). ATPase and ADPase activities are mainly detected at the bile canaliculi level and in large blood vessels. Hydrolysis of ATP is also detected in periductular regions, in the hepatic triad, but not in cholangiocytes. The control assay without any nucleotide (no nuc.) showed no reaction. CV, central vein. Scale bar = 20 μm.
Expression of NTPDase8 was evaluated in murine particulate fractions by immunoblotting with the serum rN8 – 8c. A strong protein band with molecular weight of ~75 kDa was detected in rat liver whereas weaker bands were observed in kidney and jejunum (Fig. 1B, right). No signal could be detected in tissues devoid of NTPDase8 mRNA expression (4) such as spleen (data not shown). Interestingly, a very intense NTPDase8 immunoreactive band was detected in a canalicular membrane vesicle preparation, suggesting the presence of NTPDase8 in this cellular compartment (Fig. 1B, right). Additionally, and in agreement with this observation, NTPDase8 expression was limited to the canalicular domain of hepatocytes (Fig. 2, top). This result was confirmed by the colocalization of NTPDase8 with the canalicular transport protein Mrp-2 (Fig. 2, middle). No NTPDase8 immunostaining was detected neither in other compartments of the biliary tree, such as small and large bile ducts nor the portal space area. Immunostaining of NTPDase1 on rat liver sections showed its expression at the surface of vascular endothelium, Kupffer cells, and smooth muscle cells (Fig. 2). NTPDase2 was detected at the external surface of blood vessels and periductular area. No staining was observed with an antiserum to NTPDase3 (data not shown). The combined immunohistological expression pattern of NTPDase1, 2, and 8 observed here in rat liver correlates with histochemical data with ATP, UTP, ADP, and UDP (Fig. 2, bottom, and data not shown). Indeed, the hydrolysis of all nucleotides was detected mainly at the bile canaliculi and at the luminal surface of large blood vessels. In bile canaliculi, the hydrolysis of diphosphonucleosides was weaker than the one of triphosphonucleosides, in agreement with the biochemical properties of NTPDase8 (Biochemical Characterization of NTPDase8s).
Purification of NTPDase8 from Rat Liver
To evaluate the relative contribution of each ectonucleotidase in the liver, we purified ectonucleotidase activities from rat liver and identified the enzymes involved with an emphasis on NTPDase8. DEAE was chosen for the separation of NTPDase1, 2, and 8 because these enzymes are characterized by different predicted isoelectric points of 7.5, 8.8, and 5.83, respectively. This separation resulted in three ATP- and ADP-hydrolyzing fractions (Fig. 3A and data not shown). Immunoblotting revealed that peaks 2 and 3 corresponded to NTPDase1 and NTPDase8, respectively, as expected from their isoelectric points. The first peak of activity could be attributed to NTPDase2 because of its isoelectric point and also by the comparison with the chromatograms from previous purifications (2). A qualitative assessment of surface areas of activity in Fig. 3A suggests that the main nucleotidase activity in the liver is attributable to NTPDase8. Particular attention was taken to exclude fractions containing NTPDase1 in the NTPDase8 pooled fractions that were further separated on an Affigel Blue column. One peak of ATPase and ADPase activities was obtained that did not contain NTPDase1, as verified by immunoblotting (Fig. 3B and data not shown). To exclude contamination with ecto-5′-nucleotidase, fewer fractions with NTPDase8 activity were pooled and applied to a ConA affinity column, as indicated in the figure. One peak of activity corresponding to NTPDase8 was observed, as confirmed by immunoblotting (data not shown). During the purification process, a clear increase in NTPDase8 contents could be detected (Fig. 3C).
Fig. 3.
Purification of NTPDase8 from rat liver. The purification procedure is detailed in materials and methods. A: DEAE column. Liver membrane proteins were solubilized with Triton X-100, loaded on a DEAE column, and eluted by a NaCl gradient from fractions 1 to 281. Three peaks of ATPase activity are distinguishable, and the 3rd and highest peak of activity corresponds to NTPDase8 (N8), as determined by immunoblotting (data not shown). A significant AMPase activity was also associated with the peaks corresponding to NTPDase2 (N2) and NTPDase8. Fractions containing NTPDase8 activity were collected (pooled fractions) and applied to an Affigel Blue column. Note that we took care to avoid NTPDase1 activity in the selected pooled fractions. B: Affigel Blue column. After washing the column and eluting the retained proteins, 1 peak of ATPase activity corresponding to NTPDase8 as determined by immunoblotting (data not shown) was collected (pooled fractions) and further purified on a concanavalin A (ConA) column. Note that we chose to avoid most AMPase activity in the selected pooled fractions. C: protein samples from the pooled fractions of each purification step were monitored for NTPDase8 content by SDS-PAGE and immunoblotting. NTPDase8 levels increase with each purification step: N8, NTPDase8 transfected COS-7 cell lysate (4 μg); lane 1, total liver homogenate (5 μg); lane 2, liver particulate fraction (5 μg); lane 3, DEAE chromatography pooled fractions (5 μg = 220 pmol Pi/min); lane 4, Affigel Blue chromatography pooled fractions (5 μg = 435 pmol Pi/min); lane 5, ConA chromatography pooled fractions (5 μg = 2,270 pmol Pi/min). Molecular weights are indicated in kDa.
Note that the purification factor of NTPDase8 shown in Table 2 is under evaluated as there are no specific substrates for NTPDase8. Phosphatases, classical ATPases, nucleotide pyrophosphatase/phosphodiesterases (NPPs), and other NTPDases, including intracellular NTPDase4–7, also hydrolyze the same substrates. Many of these enzymes are present in the liver homogenate and most likely eliminated during the steps of purification. In addition, as highlighted above, to avoid contamination with other ectonucleotidases, some fractions displaying high NTPDase8 activity were discarded during the purification procedure. This resulted in an important loss of NTPDase8 and a much lower purification factor (Table 2). Most importantly, the ConA-purified NTPDase8 fraction was almost completely devoid of any other nucleotidases, allowing its biochemical characterization.
Table 2.
Table summarizing the purification of rat liver NTPDase8
| Total Activity, μmol Pi · min−1 |
Specific Activity, nmol Pi · min −1 · mg−1 |
Purification Fold |
Yield, % |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Step | Total Protein, mg | ATP | ADP | AMP | ATP | ADP | AMP | ATP | ADP | ATP | ADP |
| Homogenate | 19,000 | 1067 | 615 | 461 | 56 | 32 | 24 | 1 | 1 | 100 | 100 |
| Particulate fraction | 998 | 187 | 76 | 27 | 187 | 76 | 27 | 3.3 | 2.4 | 17.5 | 12.4 |
| Particulate fraction + Triton X-100 | 998 | 153 | 112 | 231 | 153 | 112 | 231 | 2.7 | 3.5 | 14.3 | 18.2 |
| Solubilized proteins | 918 | 55 | 62 | 108 | 60 | 68 | 118 | 1.1 | 2.1 | 5.1 | 10 |
| DEAE | 160 | 7 | 11 | 20 | 44 | 69 | 22 | 0.8 | 2.1 | 0.7 | 1.8 |
| Affigel | 9.2 | 0.80 | 1.40 | 0.70 | 87 | 152 | 76 | 1.5 | 4.8 | 0.07 | 0.2 |
| ConA | 0.86 | 0.39 | 0.40 | 0.10 | 454 | 465 | 116 | 8 | 15 | 0.04 | 0.07 |
ConA, concanavalin A.
Biochemical Characterization of NTPDase8s
The biochemical properties of human and rat recombinant NTPDase8s were determined with cell lysates and with intact COS-7 and 293T cells transiently transfected with an expression vector (pcDNA3.1) encoding either human or rat NTPDase8. Activity assays were carried out for 12–20 min to ascertain the linearity of the reaction (data not shown). Recombinant human and rat NTPDase8s, as well as purified rat NTPDase8, required Ca2+ or Mg2+ for catalytic activity with a preference for Ca2+ with all nucleotides tested. Therefore the following assays for NTPDase activities were carried out in the presence of Ca2+. Both recombinant NTPDase8s hydrolyzed ATP, UTP, ADP, and UDP and preferred the triphosphonucleosides. In these assays, we observed some variation in the ratio of hydrolysis of ATP/ADP between intact cells and cell lysates as well as between the cell lines transfected (Fig. 4A). Neither recombinant human nor rat NTPDase8 hydrolyzed AMP (data not shown). The purified enzyme from rat liver hydrolyzed tri- and diphosphonucleosides with a ratio ATP/ADP of 0.94 and UTP/UDP of 0.98. The NTPDase8 ConA purified fraction hydrolyzed AMP at the rate of 16% of ATP, which was most likely due to the presence of some remaining ecto-5′-nucleotidase (see Fig. 3B).
Fig. 4.
Catalytic properties of human and rat NTPDase8s, effect of inhibitors. ATPase and ADPase activities were measured in COS-7 and 293T intact cells and cell lysates by the malachite green method, as described in MATERIALS AND METHODS. Residual activity of untransfected cells ranged from 3 to 13% of the activity detected in transfected cells and was subtracted. A: activities with ATP (open bars) and ADP (solid bars) as substrates were measured on intact COS-7 and HEK293T cells transfected with recombinant human NTPDase1 (hN1) or human and rat NTPDase8 (hN8, rN8), or using the indicated cell lysates. Overall, NTPDase8 transfected cells or lysates showed both ATPase and ADPase activities. The activity measured for each cell lysate was higher in transfected 293T cells compared with transfected COS-7 cells. Data are shown as means ± SD of 3 independent experiments, each performed in triplicate. B: effect of 10 mM sodium azide on the activity of recombinant human, mouse and rat NTPDase1s (solid bars), NTPDase8s (shaded bars), or purified NTPDase8s (hatched bars) from rat and porcine hepatic ATPDase was tested. ADP was used as substrate for the hydrolysis assay. Although azide only slightly inhibited recombinant NTPDase8 activities, it blocked NTPDase1 activities by ~50%. Solubilized NTPDase8s were totally resistant to azide. Data show mean values ± SD of 1 (porcine hepatic ATPDase) or 3 independent experiments (other enzymes), each performed in triplicate. *Statistically significant differences as evaluated by Student’s t-test analysis (P values < 0.01). C: the effect of sodium deoxycholate (0.01–1 mM) on the activity of recombinant human, mouse, and rat NTPDase 1s (solid bars) or NTPDase8s (shaded bars) was tested. ATP was used as substrate for this hydrolysis assay. No significant differences were seen between the inhibition of NTPDase1 and NTPDase8 by deoxycholate. Data are shown as means ± SD of at least 2 independent experiments, each performed in triplicate.
Next, we tested whether azide, an inhibitor of NTPDase1 (32), could affect the activity of recombinant and purified NTPDase8s. ATPase and ADPase activities of NTPDase8s were less affected by 10 mM sodium azide than the recombinant NTPDase1s (Fig. 4B and data not shown). Since the purified enzymes were solubilized in Triton X-100, the effect of this detergent was tested. NTPDase1 has previously been reported to be highly sensitive to azide inhibition after solubilization in Triton X-100 (52). Once solubilized in this detergent, recombinant rat NTPDase8 was totally resistant to azide inhibition, as for the purified enzymes.
We also compared the activity of NTPDase1 and NTPDase8 in the presence of a common bile salt, sodium deoxycholate, which has been reported to affect differently bovine spleen NTPDases (dominantly NTPDase1) and porcine liver ATPDase activities (36). ATPase and ADPase activities of these recombinant NTPDases were affected similarly by deoxycholate (Fig. 4C and data not shown), suggesting that the canalicular microenvironment, rather than the NTPDase, is responsible for the enzyme resistance to deoxycholate.
The apparent Km and Vmax values of recombinant human NTPDase8 are summarized in Table 3. In agreement with its kinetic constants, NTPDase8 hydrolyzed ATP and UTP first to their respective diphosphonucleoside and then to the monophosphonucleoside with the distinction that ADP was dephosphorylated to AMP much faster than UDP to UMP (Fig. 5). This resulted in an accumulation of UDP. Simultaneous hydrolysis of ATP and UTP confirmed that human NTPDase8 preferred adenine over uracil nucleotides as substrates, as expected from the kinetic parameters. Importantly, purified rat NTPDase8 displayed similar nucleotide preferences and hydrolysis profiles compared with its recombinant isoform (data not shown).
Table 3.
Kinetic parameters of human recombinant NTPDase8
| Substrate | Km, μM | Vmax, nmol Pi·min−1 · mg−1 |
|---|---|---|
| ATP | 81±7 | 790±35 |
| ADP | 137±13 | 163±9 |
| UTP | 480±32 | 1100±50 |
| UDP | 241±17 | 110±4 |
Human NTPDase8 exhibited Michaelis-Menten kinetics for the hydrolysis of ATP, ADP, UTP, and UDP. Apparent Km and Vmax values were estimated from Eadie-Hofstee plots created with GraphPad Prism software (GraphPad Software, San Diego, CA). Results are expressed as means ± SE of 3 separate experiments, each performed in triplicate.
Fig. 5.
Nucleotide hydrolysis and product formation by human recombinant NTPDase8. The nucleotide hydrolysis pattern was determined by HPLC, as described in MATERIALS AND METHODS. Human NTPDase8 was incubated with ATP and/or UTP (500 μM), and the formation of nucleotide derivatives was followed over a period of 1 h. In A and B, 24 nmol/min of enzymatic activity were used. This amount was doubled for C. A: ATP hydrolysis: ATP (■), ADP (◆), AMP (▲). Human NTPDase8 dephosphorylates ATP to ADP, which is then hydrolyzed to AMP. No adenosine production was detected. B: UTP hydrolysis. UTP (□), UDP (◇), UMP (△). The profile of UTP hydrolysis (UTP → UDP → UMP) is similar to that observed for ATP, with the difference that the conversion of UDP to UMP is slower than that of ADP to AMP. No uridine production was detected. C: concurrent hydrolysis of ATP and UTP: ■, ATP; ◆, ADP; ▲, AMP; □, UTP; ◇, UDP; △, UMP. NTPDase8 prefers adenine nucleotides over uracil nucleotides as substrates. Notice that AMP accumulates more rapidly than UMP.
DISCUSSION
By 1) molecular cloning, 2) biochemical approaches, and 3) immunolocalization, we have demonstrated that the newly cloned ectonucleotidase NTPDase8 accounts for the high ectonucleotidase activity observed in the bile canaliculi. Thus the molecular identity of the long-known hepatic canalicular ecto-ATPase is NTPDase8.
1) The existence of NTPDase8 in human and rat liver was established by cDNA cloning based on a sequence homology with the mouse NTPDase8 (4). Importantly, the transfection of COS-7 and HEK293T cells with an expression vector encoding human or rat NTPDase8 led to the appearance of nucleotidase activity at the cell surface, demonstrating that these enzymes are ectonucleotidases. Genomic analysis with these cDNAs revealed that ENTPD8 is located on chromosome 9q34 and rat Entpd8 on chromosome 3p13. The distinct localization of both ENTPD8 and rat Entpd8 confirms that the products of these genes are different from the other previously characterized NTPDases (34). Analysis of the deduced amino acid sequence of each of these NTPDase8s, as well as of chicken ecto-ATPDase (33, 38), with the canalicular ecto-ATPase/ATPDase purified from porcine liver showed high identity, suggesting that these enzymes are orthologs.
2) Our purification procedure combined with immunoblotting experiments showed that NTPDase8 is responsible for the main ectonucleotidase activity in liver. In rat liver, the combined ecto-ATPase activities of the two other ectonucleotidases NTPDase1 and NTPDase2 were far less than that of NTPDase8 alone. In further support for this notion, ectonucleotidase activity and histochemistry performed in Entpd1−/− mice showed that, despite the absence of NTPDase1, the liver of these mice retained most ATPase and ADPase activities (O. Guckelberger and J. Sévigny, personal observations). Recombinant human and rat NTPDase8s as well as the purified rat NTPDase8 favored hydrolysis of triphosphonucleosides over diphosphonucleosides also with a preference for adenine over uracil nucleotides. All NTPDase8s analyzed preferred Ca2+ over Mg2+ for nucleoside triphosphatase and diphosphatase activities and were resistant to sodium azide. Similar properties have previously been described for recombinant mouse NTPDase8 (4) and purified porcine hepatic ATPDase (52). We have also observed that the solubilization of rat NTPDase8 with Triton X-100 affected the biochemical properties of the enzyme. For example, the addition of Triton to rat recombinant NTPDase8 made this nucleotidase completely resistant to azide. This effect may be attributable to the transmembrane domains of the enzyme, as shown by Knowles et al. with a mutant of human NTPDase8 lacking both hydrophobic domains (31). In general, the human recombinant enzyme cloned by the latter group showed similar biochemical characteristics with the ones presented here with some differences for deoxycholate and azide inhibition that may be due to different buffer compositions.
3) Histochemical data showed that the ectonucleotidase activity in the liver matched the immunolocalization pattern of NTPDase1, 2, and 8. NTPDase8 represented the ectonucleotidase activity of the bile canaliculi. NTPDase1 and NTPDase2 were responsible for the ectonucleotidase activity in blood vessels; NTPDase1 was present on endothelial cells and smooth muscle cells whereas NTPDase2 was on adventitial cells. NTPDase2 was also detected on portal fibroblasts, in the vicinity of bile ducts. These observations for NTPDase1 and NTPDase2 are in agreement with previous reports (14, 52).
Other hepatic ectonucleotidases have been previously reported in the liver and their contribution to nucleotide hydrolysis needs to be considered as well. The expression of NPP1 and NPP3 has been described in rat liver (21, 22, 50, 55). The products of triphosphonucleoside hydrolysis by NPPs are a monophosphonucleoside and a pyrophosphate group, both undetectable by the malachite green method used in this work to estimate ectonucleotidase activity (5, 56). The latter enzymes are meanwhile more active in alkaline pHs than at physiological pH (50). Another class of ectonucleotidases expressed in the liver is the alkaline phosphatase family (56). However, their contribution to the ectonucleotidase activity in the liver may be considered negligible in our experiments, as the hydrolysis of tri-, di-, and monophosphonucleosides was not significantly reduced in the presence of tetramisole, an inhibitor of this enzyme.
Taken together, these data indicate that NTPDase8 is the canalicular ecto-ATPase/ATPDase and, moreover, accounts for the major liver ectonucleotidase activity. The highly specific localization and biochemical properties of NTPDase8 suggest potential functions in the liver. NTPDase8, which efficiently hydrolyzes ATP and UTP, may attenuate and/or terminate the activation of receptors for these two nucleotides (P2X1–7 and P2Y2,4,11) but may also favor the activation of ADP and UDP specific receptors (P2Y1,6,12,13), as it produces a transient accumulation of diphosphonucleosides. As a result, NTPDase8 would modulate the activity of P2 receptors present in the hepatocyte membrane bordering the bile canaliculi upstream, but also along the epithelium lining the intrahepatic biliary tree downstream. Interestingly, the expression of multiple P2 receptors (P2X2– 4,6 and P2Y1,2,4,6,13) mRNAs has been reported in hepatocytes (12) and cholangiocytes (13, 15, 20). The activation of these nucleotide receptors is involved in the regulation of various functions like cell volume regulation, ATP release, ion secretion, and bile formation (13, 15, 24, 28, 45– 48, 57). A potential function of NTPDase2 in the physiology of cholangiocytes has recently been demonstrated in vitro (25). In the latter study, NTPDase2 expressed by portal fibroblasts blocked the mitogenic response of neighboring cholangiocytes, through the modulation of their basolateral P2Y receptors.
The coexpression of NTPDase8 with ecto-5′-nucleotidase/CD73 (data not shown and Ref. 49) in the bile canaliculi suggests that the monophosphonucleosides (e.g., AMP) eventually produced by NTPDase8 are further dephosphorylated to the nucleosides (e.g., adenosine), also responsible for various biological functions in liver. For example, adenosine has been shown to protect the liver against inflammation-induced injury via specific A2A receptor activation (3, 42, 43). By their enzymatic activities, NTPDase8 and ecto-5′-nucleotidase would control the levels of nucleotide and/or nucleosides present in the bile flow and possibly their reuptake and salvage by hepatocytes. Of great importance for such a function, nucleoside transporters are also highly expressed in the canalicular domain of hepatocytes (10, 11, 23). There are evidences that nucleotides are released from hepatocytes and bile duct cells in vitro; the concentration of adenine nucleotides in canalicular effluents was estimated in the order of 0.1 to 5 μM (9, 19). Because liver represents the major source of purines for tissues incapable of de novo nucleotide synthesis such as brain, intestinal mucosa, muscles, and bone marrow, adequate nucleotide hydrolysis and nucleoside reuptake in the bile canaliculi may be essential for maintenance of purine balance at the extrahepatic level.
In summary, the canalicular ecto-ATPase is NTPDase8. The coexpression of NTPDase8, CD73, and nucleoside transporters in bile canaliculi suggests the importance of extracellular nucleotide signaling in the control of bile secretion and in nucleoside-nucleotide balance and reuptake.
ACKNOWLEDGMENTS
The authors thank Dr. J. L. Boyer (Yale University School of Medicine) for drafting suggestions and Dr. R. Lemmens (Limburgs Universitair Centrum, Belgium) for donation of a purified porcine liver ATPDase sample.
GRANTS
This work was supported by the Canadian Institutes of Health Research (CIHR; MOP-49460 to J. Sévigny) and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-02379 (to J. A. Dranoff). M. Fausther is a recipient of a scholarship from the government of Gabon, F. Kukulski of a fellowship from CIHR/Wyeth, S. A. Lévesque from Fonds de la Recherche en Santé du Québec, and J. Sévigny of a New Investigator award from the CIHR.
REFERENCES
- 1.Baykov AA, Evtushenko OA, Avaeva SM. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem. 1988;171:266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- 2.Beaudoin AR, Seévigny J, Grondin G, Daoud S, Levesque FP. Purification, characterization, and localization of two ATP diphosphohydrolase isoforms in bovine heart. Am J Physiol Heart Circ Physiol. 1997;273:H673–H681. doi: 10.1152/ajpheart.1997.273.2.H673. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Ari Z, Pappo O, Sulkes J, Cheporko Y, Vidne BA, Hochhauser E. Effect of adenosine A2A receptor agonist (CGS) on ischemia/reperfusion injury in isolated rat liver. Apoptosis. 2005;10:955–962. doi: 10.1007/s10495-005-0440-3. [DOI] [PubMed] [Google Scholar]
- 4.Bigonnesse F, Leévesque SA, Kukulski F, Lecka J, Robson SC, Fernandes MJG, Seévigny J. Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-8. Biochemistry. 2004;43:5511–5519. doi: 10.1021/bi0362222. [DOI] [PubMed] [Google Scholar]
- 5.Bollen M, Gijsbers R, Ceulemans H, Stalmans W, Stefan C. Nucleotide pyrophosphatases/phosphodiesterases on the move. Crit Rev Biochem Mol Biol. 2000;35:393–432. doi: 10.1080/10409230091169249. [DOI] [PubMed] [Google Scholar]
- 6.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Braun N, Seévigny J, Mishra SK, Robson SC, Barth SW, Gerstberger R, Hammer K, Zimmermann H. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur J Neurosci. 2003;17:1355–1364. doi: 10.1046/j.1460-9568.2003.02567.x. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 9.Chari RS, Schutz SM, Haebig JE, Shimokura GH, Cotton PB, Fitz JG, Meyers WC. Adenosine nucleotides in bile. Am J Physiol Gastrointest Liver Physiol. 1996;270:G246–G252. doi: 10.1152/ajpgi.1996.270.2.G246. [DOI] [PubMed] [Google Scholar]
- 10.Che M, Nishida T, Gatmaitan Z, Arias IM. A nucleoside transporter is functionally linked to ectonucleotidases in rat liver canalicular membrane. J Biol Chem. 1992;267:9684–9688. [PubMed] [Google Scholar]
- 11.Che MX, Gatmaitan Z, Arias IM. Ectonucleotidases, purine nucleoside transporter, and function of the bile canalicular plasma membrane of the hepatocyte. FASEB J. 1997;11:101–108. doi: 10.1096/fasebj.11.2.9039951. [DOI] [PubMed] [Google Scholar]
- 12.Dixon CJ, Woods NM, Webb TE, Green AK. Evidence that rat hepatocytes co-express functional P2Y1 and P2Y2 receptors. Br J Pharmacol. 2000;129:764–770. doi: 10.1038/sj.bjp.0703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doctor RB, Matzakos T, McWilliams R, Johnson S, Feranchak AP, Fitz JG. Purinergic regulation of cholangiocyte secretion: identification of a novel role for P2X receptors. Am J Physiol Gastrointest Liver Physiol. 2005;288:G779–G786. doi: 10.1152/ajpgi.00325.2004. [DOI] [PubMed] [Google Scholar]
- 14.Dranoff JA, Kruglov EA, Robson SC, Braun N, Zimmermann H, Seévigny J. The ecto-nucleoside triphosphate diphosphohydrolase NTPDase2/CD39L1 is expressed in a novel functional compartment within the liver. Hepatology. 2002;36:1135–1144. doi: 10.1053/jhep.2002.36823. [DOI] [PubMed] [Google Scholar]
- 15.Dranoff JA, Masyuk AI, Kruglov EA, LaRusso NF, Nathanson MH. Polarized expression and function of P2Y ATP receptors in rat bile duct epithelia. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1059–G1067. doi: 10.1152/ajpgi.2001.281.4.G1059. [DOI] [PubMed] [Google Scholar]
- 16.Dranoff JA, Nathanson MH. It’s swell to have ATP in the liver. J Hepatol. 2000;33:323–325. doi: 10.1016/s0168-8278(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 17.Dranoff JA, Ogawa M, Kruglov EA, Gaca MD, Seévigny J, Robson SC, Wells RG. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G417–G424. doi: 10.1152/ajpgi.00294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Essner E, Novikoff AB, Masek B. Adenosinetriphosphatase and 5-nucleotidase activities in the plasma membrane of liver cells as revealed by electron microscopy. J Biophys Biochem Cytol. 1958;4:711–716. doi: 10.1083/jcb.4.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feranchak AP, Fitz JG. Adenosine triphosphate release and purinergic regulation of cholangiocyte transport. Semin Liver Dis. 2002;22:251–262. doi: 10.1055/s-2002-34503. [DOI] [PubMed] [Google Scholar]
- 20.Fumagalli M, Trincavelli L, Lecca D, Martini C, Ciana P, Abbracchio MP. Cloning, pharmacological characterisation and distribution of the rat G-protein-coupled P2Y (13) receptor. Biochem Pharmacol. 2004;68:113–124. doi: 10.1016/j.bcp.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 21.Goding JW, Grobben B, Slegers H. Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim Biophys Acta. 2003;1638:1–19. doi: 10.1016/s0925-4439(03)00058-9. [DOI] [PubMed] [Google Scholar]
- 22.Goding JW, Terkeltaub R, Maurice M, Deterre P, Sali A, Belli SI. Ecto-phosphodiesterase/pyrophosphatase of lymphocytes and non-lymphoid cells: structure and function of the PC-1 family. Immunol Rev. 1998;161:11–26. doi: 10.1111/j.1600-065x.1998.tb01568.x. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths M, Beaumont N, Yao SY, Sundaram M, Boumah CE, Davies A, Kwong FY, Coe I, Cass CE, Young JD, Baldwin SA. Cloning of a human nucleoside transporter implicated in the cellular uptake of adenosine and chemotherapeutic drugs. Nat Med. 1997;3:89–93. doi: 10.1038/nm0197-89. [DOI] [PubMed] [Google Scholar]
- 24.Haussinger D, Stehle T, Gerok W. Actions of extracellular UTP and ATP in perfused rat liver. A comparative study. Eur J Biochem. 1987;167:65–71. doi: 10.1111/j.1432-1033.1987.tb13304.x. [DOI] [PubMed] [Google Scholar]
- 25.Jhandier MN, Kruglov EA, Lavoie EG, Seévigny J, Dranoff JA. Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J Biol Chem. 2005;280:22986–22992. doi: 10.1074/jbc.M412371200. [DOI] [PubMed] [Google Scholar]
- 26.Kegel B, Braun N, Heine P, Maliszewski CR, Zimmermann H. An ecto-ATPase and an ecto-ATP diphosphohydrolase are expressed in rat brain. Neuropharmacology. 1997;36:1189–1200. doi: 10.1016/s0028-3908(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 27.Keppens S, Vandekerckhove A, De Wulf H. Characterization of purinoceptors present on human liver plasma membranes. FEBS Lett. 1989;248:137–140. doi: 10.1016/0014-5793(89)80448-x. [DOI] [PubMed] [Google Scholar]
- 28.Keppens S, Vandekerckhove A, De Wulf H. Extracellular ATP and UTP exert similar effects on rat isolated hepatocytes. Br J Pharmacol. 1992;105:475–479. doi: 10.1111/j.1476-5381.1992.tb14278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kipp H, Arias IM. Newly synthesized canalicular ABC transporters are directly targeted from the Golgi to the hepatocyte apical domain in rat liver. J Biol Chem. 2000;275:15917–15925. doi: 10.1074/jbc.M909875199. [DOI] [PubMed] [Google Scholar]
- 30.Knowles AF. The rat liver ecto-ATPase/C-CAM cDNA detects induction of carcinoembryonic antigen but not the mercurial-insensitive ecto-ATPase in human hepatoma Li-7A cells treated by epidermal growth factor and cholera toxin. Biochem Biophys Res Commun. 1995;207:529–535. doi: 10.1006/bbrc.1995.1220. [DOI] [PubMed] [Google Scholar]
- 31.Knowles AF, Li C. Molecular cloning and characterization of expressed human ecto-nucleoside triphosphate diphosphohydrolase 8 (E-NTPDase 8) and its soluble extracellular domain. Biochemistry. 2006;45:7323–7333. doi: 10.1021/bi052268e. [DOI] [PubMed] [Google Scholar]
- 32.Knowles AF, Nagy AK. Inhibition of an ecto-ATP-diphosphohydrolase by azide. Eur J Biochem. 1999;262:349–357. doi: 10.1046/j.1432-1327.1999.00389.x. [DOI] [PubMed] [Google Scholar]
- 33.Knowles AF, Nagy AK, Strobel RS, Wu-Weis M. Purification, characterization, cloning, and expression of the chicken liver ecto-ATP-diphosphohydrolase. Eur J Biochem. 2002;269:2373–2382. doi: 10.1046/j.1432-1033.2002.02898.x. [DOI] [PubMed] [Google Scholar]
- 34.Kukulski F, Leévesque SA, Lavoie EG, Lecka J, Bigonnesse F, Knowles AF, Robson SC, Kirley TL, Seévigny J. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal . 2005;1:193–204. doi: 10.1007/s11302-005-6217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laemmli UK, Beguin F, Gujer-Kellenberger G. A factor preventing the major head protein of bacteriophage T4 from random aggregation. J Mol Biol. 1970;47:69–85. doi: 10.1016/0022-2836(70)90402-x. [DOI] [PubMed] [Google Scholar]
- 36.Leclerc MC, Grondin G, Gendron FP, Seévigny J, Beaudoin AR. Identification, characterization, and immunolocalization of a nucleoside triphosphate diphosphohydrolase in pig liver. Arch Biochem Biophys. 2000;377:372–378. doi: 10.1006/abbi.2000.1800. [DOI] [PubMed] [Google Scholar]
- 37.Lemmens R, Vanduffel L, Kittel A, Beaudoin AR, Benrezzak O, Seévigny J. Distribution, cloning, and characterization of porcine nucleo-side triphosphate diphosphohydrolase-1. Eur J Biochem. 2000;267:4106–4114. doi: 10.1046/j.1432-1327.2000.01462.x. [DOI] [PubMed] [Google Scholar]
- 38.Lewis-Carl S, Kirley TL. Immunolocalization of the ecto-ATPase and ecto-apyrase in chicken gizzard and stomach. Purification and N-terminal sequence of the stomach ecto-apyrase. J Biol Chem. 1997;272:23645–23652. doi: 10.1074/jbc.272.38.23645. [DOI] [PubMed] [Google Scholar]
- 39.Lin SH, Guidotti G. Cloning and expression of a cDNA coding for a rat liver plasma membrane ecto-ATPase. The primary structure of the ecto-ATPase is similar to that of the human biliary glycoprotein I. J Biol Chem. 1989;264:14408–14414. [PubMed] [Google Scholar]
- 40.Novikoff AB, Hausman DH, Podber E. The localization of adenosine triphosphatase in liver: in situ staining and cell fractionation studies. J Histochem Cytochem. 1958;6:61–71. doi: 10.1177/6.1.61. [DOI] [PubMed] [Google Scholar]
- 41.Novikoff AB, Hecht L, Podber E, Ryan J. Phosphatases of rat liver. I. The dephosphorylation of adenosinetriphosphate. J Biol Chem. 1952;194:153–170. [PubMed] [Google Scholar]
- 42.Odashima M, Otaka M, Jin M, Komatsu K, Wada I, Matsuhashi T, Horikawa Y, Hatakeyama N, Oyake J, Ohba R, Linden J, Watanabe S. Selective A2A adenosine agonist ATL-146e attenuates acute lethal liver injury in mice. J Gastroenterol. 2005;40:526–529. doi: 10.1007/s00535-005-1609-9. [DOI] [PubMed] [Google Scholar]
- 43.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 44.Robson SC, Seévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure, function, relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roman RM, Feranchak AP, Salter KD, Wang Y, Fitz JG. Endogenous ATP release regulates Cl− secretion in cultured human and rat biliary epithelial cells. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1391–G1400. doi: 10.1152/ajpgi.1999.276.6.G1391. [DOI] [PubMed] [Google Scholar]
- 46.Roman RM, Fitz JG. Emerging roles of purinergic signaling in gastrointestinal epithelial secretion and hepatobiliary function. Gastroenterology. 1999;116:964–979. doi: 10.1016/s0016-5085(99)70081-8. [DOI] [PubMed] [Google Scholar]
- 47.Schlenker T, Romac JM, Sharara AI, Roman RM, Kim SJ, LaRusso N, Liddle RA, Fitz JG. Regulation of biliary secretion through apical purinergic receptors in cultured rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 1997;273:G1108–G1117. doi: 10.1152/ajpgi.1997.273.5.G1108. [DOI] [PubMed] [Google Scholar]
- 48.Schlosser SF, Burgstahler AD, Nathanson MH. Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleo-tides. Proc Natl Acad Sci USA. 1996;93:9948–9953. doi: 10.1073/pnas.93.18.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmid TC, Loffing J, Le Hir M, Kaissling B. Distribution of ecto-5′-nucleotidase in the rat liver: effect of anaemia. Histochemistry. 1994;101:439–447. doi: 10.1007/BF00269494. [DOI] [PubMed] [Google Scholar]
- 50.Scott LJ, Delautier D, Meerson NR, Trugnan G, Goding JW, Maurice M. Biochemical and molecular identification of distinct forms of alkaline phosphodiesterase I expressed on the apical and basolateral plasma membrane surfaces of rat hepatocytes. Hepatology. 1997;25:995–1002. doi: 10.1002/hep.510250434. [DOI] [PubMed] [Google Scholar]
- 51.Seévigny J, Levesque FP, Grondin G, Beaudoin AR. Purification of the blood vessel ATP diphosphohydrolase, identification and localization by immunological techniques. Biochim Biophys Acta. 1997;1334:73–88. doi: 10.1016/s0304-4165(96)00079-7. [DOI] [PubMed] [Google Scholar]
- 52.Seévigny J, Robson SC, Waelkens E, Csizmadia E, Smith RN, Lemmens R. Identification and characterization of a novel hepatic canalicular ATP diphosphohydrolase. J Biol Chem. 2000;275:5640–5647. doi: 10.1074/jbc.275.8.5640. [DOI] [PubMed] [Google Scholar]
- 53.Seévigny J, Sundberg C, Braun N, Guckelberger O, Csizmadia E, Qawi I, Imai M, Zimmermann H, Robson SC. Differential catalytic properties and vascular topography of murine nucleoside triphosphate diphosphohydrolase 1 (NTPDase1) and NTPDase2 have implications for thromboregulation. Blood. 2002;99:2801–2809. doi: 10.1182/blood.v99.8.2801. [DOI] [PubMed] [Google Scholar]
- 54.Thevananther S, Sun H, Li D, Arjunan V, Awad SS, Wyllie S, Zimmerman TL, Goss JA, Karpen SJ. Extracellular ATP activates c-jun N-terminal kinase signaling and cell cycle progression in hepatocytes. Hepatology. 2004;39:393–402. doi: 10.1002/hep.20075. [DOI] [PubMed] [Google Scholar]
- 55.Yano Y, Hayashi Y, Sano K, Nagano H, Nakaji M, Seo Y, Ninomiya T, Yoon S, Yokozaki H, Kasuga M. Expression and localization of ecto-nucleotide pyrophosphatase/phosphodiesterase I-1 (E-NPP1/PC-1) and −3 (E-NPP3/CD203c/PD-Ibeta/B10/gp130 (RB13– 6)) in inflammatory and neoplastic bile duct diseases. Cancer Lett. 2004;207:139–147. doi: 10.1016/j.canlet.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 57.Zsembery A, Spirli C, Granato A, LaRusso NF, Okolicsanyi L, Crepaldi G, Strazzabosco M. Purinergic regulation of acid/base transport in human and rat biliary epithelial cell lines. Hepatology. 1998;28:914–920. doi: 10.1002/hep.510280403. [DOI] [PubMed] [Google Scholar]