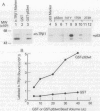
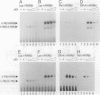
Abstract
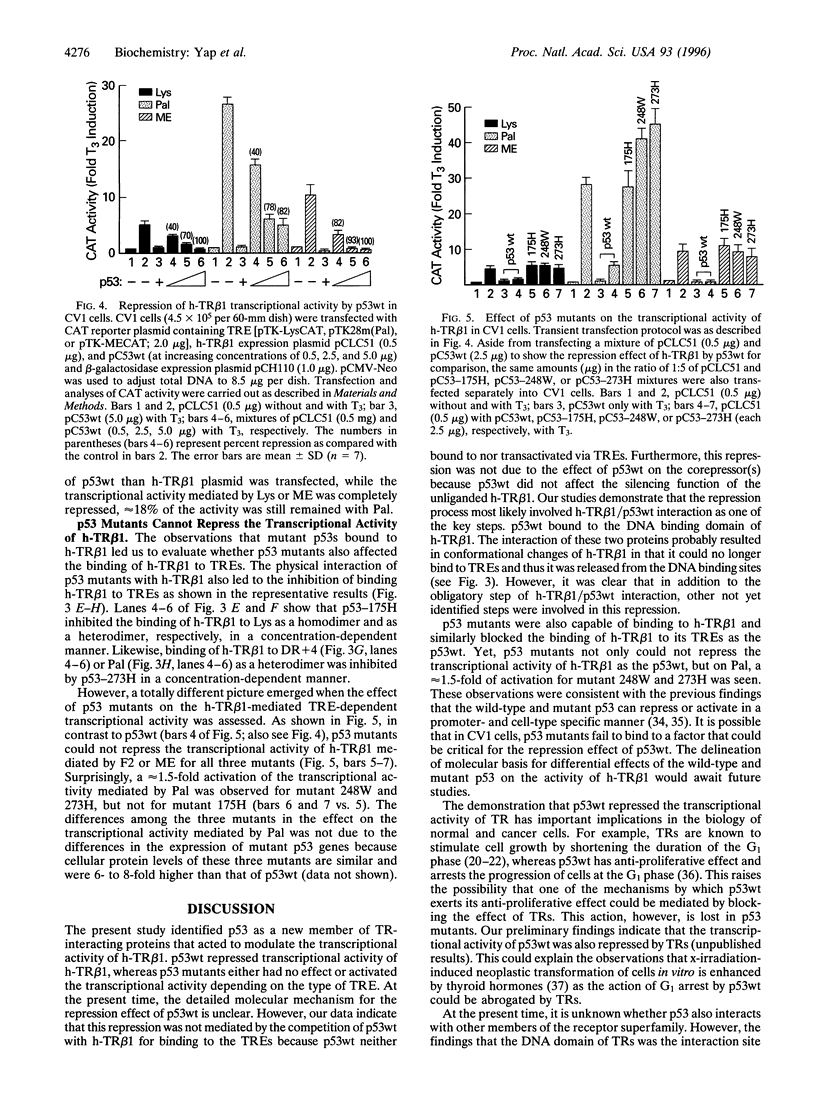
Thyroid hormone nuclear receptors (TRs) are ligand-dependent transcriptional factors that regulate growth, differentiation, and development. The molecular mechanisms by which TRs mediate these effects are unclear. One prevailing hypothesis suggests that TRs may cooperate with other transcriptional factors to mediate their biological effects. In this study, we tested this hypothesis by examining whether the activity of TRs is modulated by the tumor suppressor p53. p53 is a nuclear protein that regulates gene expression via sequence-specific DNA binding and/or direct protein-protein interaction. We found that the human TR subtype beta 1 (h-TR beta 1) physically interacted with p53 via its DNA binding domain. As a result of this physical interaction, binding of h-TR beta 1 to its hormone response elements either as homodimer or as a heterodimer with the retinoic X receptor was inhibited by p53 in a concentration-dependent manner. In transfected cells, wild-type p53 repressed the hormone-dependent transcriptional activation of h-TR beta 1. In contrast, mutant p53 either had no effect or activated the transcriptional activity of h-TR beta 1 depending on the type of hormone response elements. These results indicate the gene regulating activity of TRs was modulated by p53, suggesting that the cross talk between these two transcriptional factors may play an important role in the biology of normal and cancer cells.
Full text
PDF
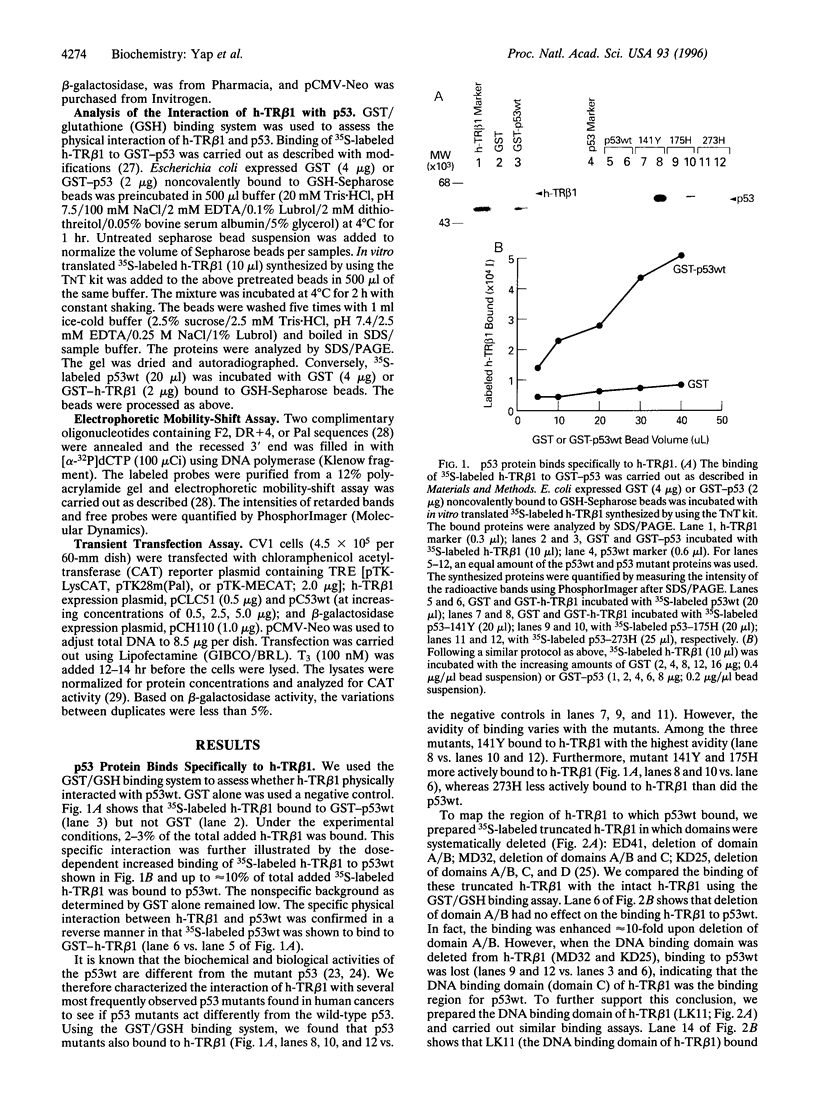
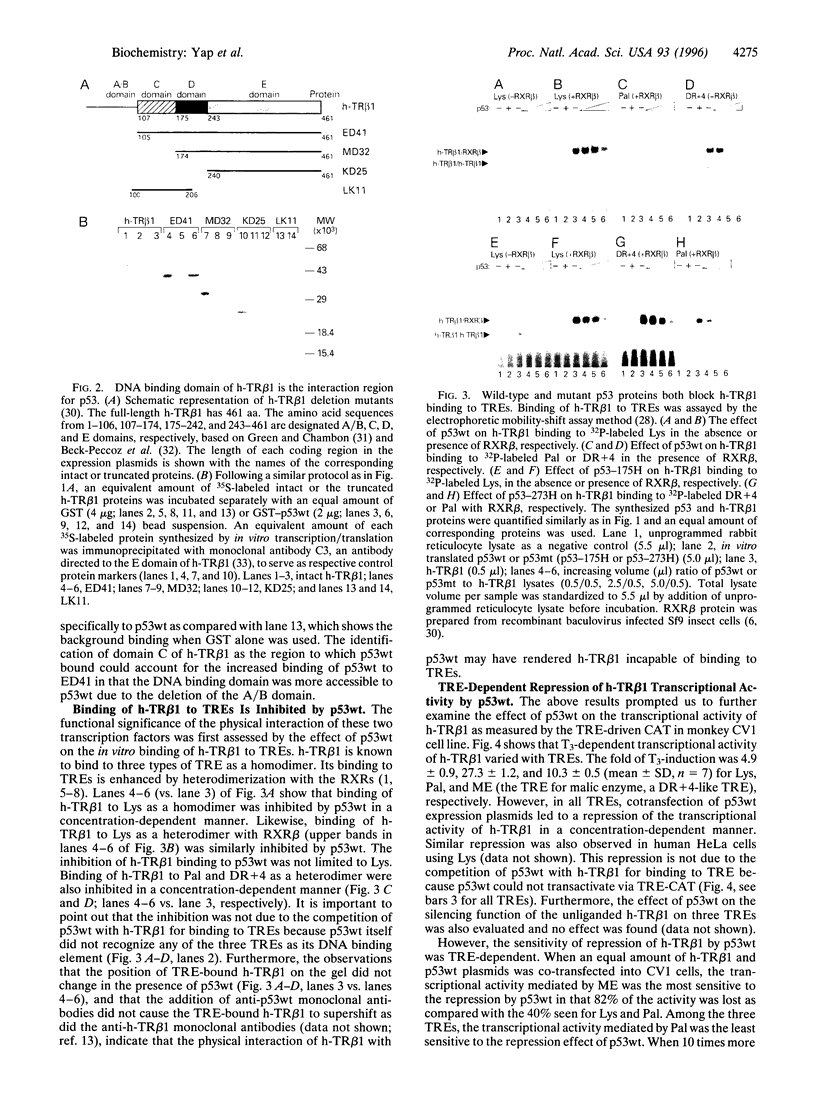

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashizawa K., Cheng S. Y. Regulation of thyroid hormone receptor-mediated transcription by a cytosol protein. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9277–9281. doi: 10.1073/pnas.89.19.9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck-Peccoz P., Chatterjee V. K., Chin W. W., DeGroot L. J., Jameson J. L., Nakamura H., Refetoff S., Usala S. J., Weintraub B. D. Nomenclature of thyroid hormone receptor beta gene mutations in resistance to thyroid hormone. First workshop on thyroid hormone resistance, July 10-11, 1993, Cambridge, U.K. J Endocrinol Invest. 1994 Apr;17(4):283–287. doi: 10.1007/BF03348977. [DOI] [PubMed] [Google Scholar]
- Bhat M. K., Ashizawa K., Cheng S. Y. Phosphorylation enhances the target gene sequence-dependent dimerization of thyroid hormone receptor with retinoid X receptor. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):7927–7931. doi: 10.1073/pnas.91.17.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat M. K., McPhie P., Cheng S. Y. Interaction of thyroid hormone nuclear receptor with antibody: characterization of the thyroid hormone binding site. Biochem Biophys Res Commun. 1995 May 16;210(2):464–471. doi: 10.1006/bbrc.1995.1683. [DOI] [PubMed] [Google Scholar]
- Bogazzi F., Hudson L. D., Nikodem V. M. A novel heterodimerization partner for thyroid hormone receptor. Peroxisome proliferator-activated receptor. J Biol Chem. 1994 Apr 22;269(16):11683–11686. [PubMed] [Google Scholar]
- Chen J., Marechal V., Levine A. J. Mapping of the p53 and mdm-2 interaction domains. Mol Cell Biol. 1993 Jul;13(7):4107–4114. doi: 10.1128/mcb.13.7.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Sy S.-y. New Insights into the Structure and Function of the Thyroid Hormone Receptor. J Biomed Sci. 1995 Apr;2(2):77–89. doi: 10.1007/BF02253060. [DOI] [PubMed] [Google Scholar]
- Chin K. V., Ueda K., Pastan I., Gottesman M. M. Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science. 1992 Jan 24;255(5043):459–462. doi: 10.1126/science.1346476. [DOI] [PubMed] [Google Scholar]
- DeFesi C. R., Fels E. C., Surks M. I. L-Triiodothyronine (T3) stimulates growth of cultured GC cells by action early in the G1 period: evidence for mediation by the nuclear T3 receptor. Endocrinology. 1985 May;116(5):2062–2069. doi: 10.1210/endo-116-5-2062. [DOI] [PubMed] [Google Scholar]
- DeFesi C. R., Fels E. C., Surks M. I. Triiodothyronine stimulates growth of cultured GC cells by action early in the G1 period. Endocrinology. 1984 Jan;114(1):293–295. doi: 10.1210/endo-114-1-293. [DOI] [PubMed] [Google Scholar]
- DeFesi C. R., Surks M. I. 3,5,3'-Triiodothyronine effects on the growth rate and cell cycle of cultured GC cells. Endocrinology. 1981 Jan;108(1):259–267. doi: 10.1210/endo-108-1-259. [DOI] [PubMed] [Google Scholar]
- Farmer G., Bargonetti J., Zhu H., Friedman P., Prywes R., Prives C. Wild-type p53 activates transcription in vitro. Nature. 1992 Jul 2;358(6381):83–86. doi: 10.1038/358083a0. [DOI] [PubMed] [Google Scholar]
- Ginsberg D., Mechta F., Yaniv M., Oren M. Wild-type p53 can down-modulate the activity of various promoters. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9979–9983. doi: 10.1073/pnas.88.22.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Chambon P. A superfamily of potentially oncogenic hormone receptors. Nature. 1986 Dec 18;324(6098):615–617. doi: 10.1038/324615a0. [DOI] [PubMed] [Google Scholar]
- Guernsey D. L., Fisher P. B. Thyroid hormone and neoplastic transformation. Crit Rev Oncog. 1990;1(4):389–408. [PubMed] [Google Scholar]
- Hallenbeck P. L., Phyillaier M., Nikodem V. M. Divergent effects of 9-cis-retinoic acid receptor on positive and negative thyroid hormone receptor-dependent gene expression. J Biol Chem. 1993 Feb 25;268(6):3825–3828. [PubMed] [Google Scholar]
- Huibregtse J. M., Scheffner M., Howley P. M. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991 Dec;10(13):4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S. E., Pietenpol J. A., Thiagalingam S., Seymour A., Kinzler K. W., Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992 May 8;256(5058):827–830. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- Lazar M. A. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993 Apr;14(2):184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- Lee J. W., Choi H. S., Gyuris J., Brent R., Moore D. D. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol Endocrinol. 1995 Feb;9(2):243–254. doi: 10.1210/mend.9.2.7776974. [DOI] [PubMed] [Google Scholar]
- Lee J. W., Ryan F., Swaffield J. C., Johnston S. A., Moore D. D. Interaction of thyroid-hormone receptor with a conserved transcriptional mediator. Nature. 1995 Mar 2;374(6517):91–94. doi: 10.1038/374091a0. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Levine A. J. The tumor suppressor genes. Annu Rev Biochem. 1993;62:623–651. doi: 10.1146/annurev.bi.62.070193.003203. [DOI] [PubMed] [Google Scholar]
- Lin K. H., Ashizawa K., Cheng S. Y. Phosphorylation stimulates the transcriptional activity of the human beta 1 thyroid hormone nuclear receptor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7737–7741. doi: 10.1073/pnas.89.16.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K. H., Fukuda T., Cheng S. Y. Hormone and DNA binding activity of a purified human thyroid hormone nuclear receptor expressed in Escherichia coli. J Biol Chem. 1990 Mar 25;265(9):5161–5165. [PubMed] [Google Scholar]
- Lin K. H., Parkison C., McPhie P., Cheng S. Y. An essential role of domain D in the hormone-binding activity of human beta 1 thyroid hormone nuclear receptor. Mol Endocrinol. 1991 Apr;5(4):485–492. doi: 10.1210/mend-5-4-485. [DOI] [PubMed] [Google Scholar]
- Marks M. S., Hallenbeck P. L., Nagata T., Segars J. H., Appella E., Nikodem V. M., Ozato K. H-2RIIBP (RXR beta) heterodimerization provides a mechanism for combinatorial diversity in the regulation of retinoic acid and thyroid hormone responsive genes. EMBO J. 1992 Apr;11(4):1419–1435. doi: 10.1002/j.1460-2075.1992.tb05187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S. A., Roth J. A. Posttranslational regulation of p53 tumor suppressor protein function. Crit Rev Oncog. 1994;5(1):23–57. doi: 10.1615/critrevoncog.v5.i1.20. [DOI] [PubMed] [Google Scholar]
- Meier C. A., Parkison C., Chen A., Ashizawa K., Meier-Heusler S. C., Muchmore P., Cheng S. Y., Weintraub B. D. Interaction of human beta 1 thyroid hormone receptor and its mutants with DNA and retinoid X receptor beta. T3 response element-dependent dominant negative potency. J Clin Invest. 1993 Oct;92(4):1986–1993. doi: 10.1172/JCI116793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Strait K. A. Thyroid hormone action 1994: the plot thickens. Eur J Endocrinol. 1994 Jan;130(1):15–24. doi: 10.1530/eje.0.1300015. [DOI] [PubMed] [Google Scholar]
- Pietenpol J. A., Vogelstein B. Tumour suppressor genes. No room at the p53 inn. Nature. 1993 Sep 2;365(6441):17–18. doi: 10.1038/365017a0. [DOI] [PubMed] [Google Scholar]
- Prives C. How loops, beta sheets, and alpha helices help us to understand p53. Cell. 1994 Aug 26;78(4):543–546. doi: 10.1016/0092-8674(94)90519-3. [DOI] [PubMed] [Google Scholar]
- Rosen E. D., O'Donnell A. L., Koenig R. J. Ligand-dependent synergy of thyroid hormone and retinoid X receptors. J Biol Chem. 1992 Nov 5;267(31):22010–22013. [PubMed] [Google Scholar]
- Santhanam U., Ray A., Sehgal P. B. Repression of the interleukin 6 gene promoter by p53 and the retinoblastoma susceptibility gene product. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7605–7609. doi: 10.1073/pnas.88.17.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaufele F., West B. L., Baxter J. D. Synergistic activation of the rat growth hormone promoter by Pit-1 and the thyroid hormone receptor. Mol Endocrinol. 1992 Apr;6(4):656–665. doi: 10.1210/mend.6.4.1584227. [DOI] [PubMed] [Google Scholar]
- Schräder M., Müller K. M., Nayeri S., Kahlen J. P., Carlberg C. Vitamin D3-thyroid hormone receptor heterodimer polarity directs ligand sensitivity of transactivation. Nature. 1994 Aug 4;370(6488):382–386. doi: 10.1038/370382a0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Wong R., Zhu X. G., Pineda M. A., Cheng S. Y., Weintraub B. D. Cell type-dependent modulation of the dominant negative action of human mutant thyroid hormone beta 1 receptors. Mol Med. 1995 Mar;1(3):306–319. [PMC free article] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Wills K. N., Husmann M., Hermann T., Pfahl M. Novel pathway for thyroid hormone receptor action through interaction with jun and fos oncogene activities. Mol Cell Biol. 1991 Dec;11(12):6016–6025. doi: 10.1128/mcb.11.12.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]