Abstract
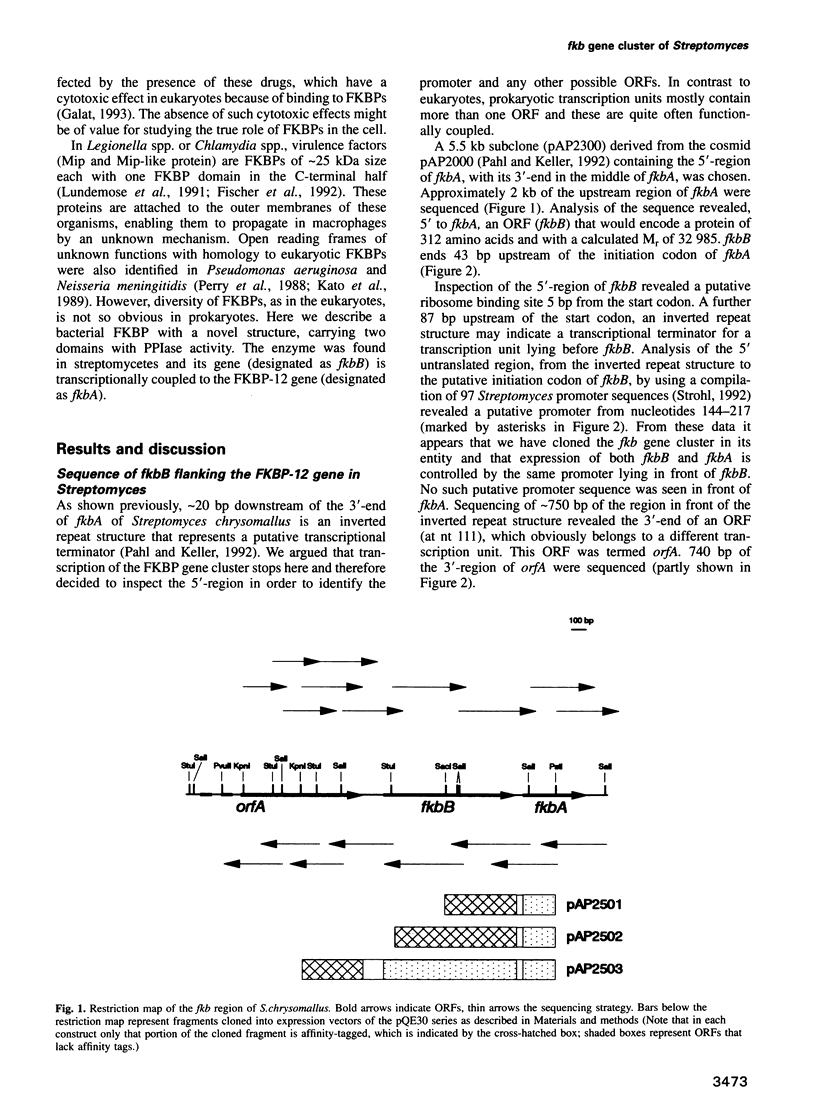
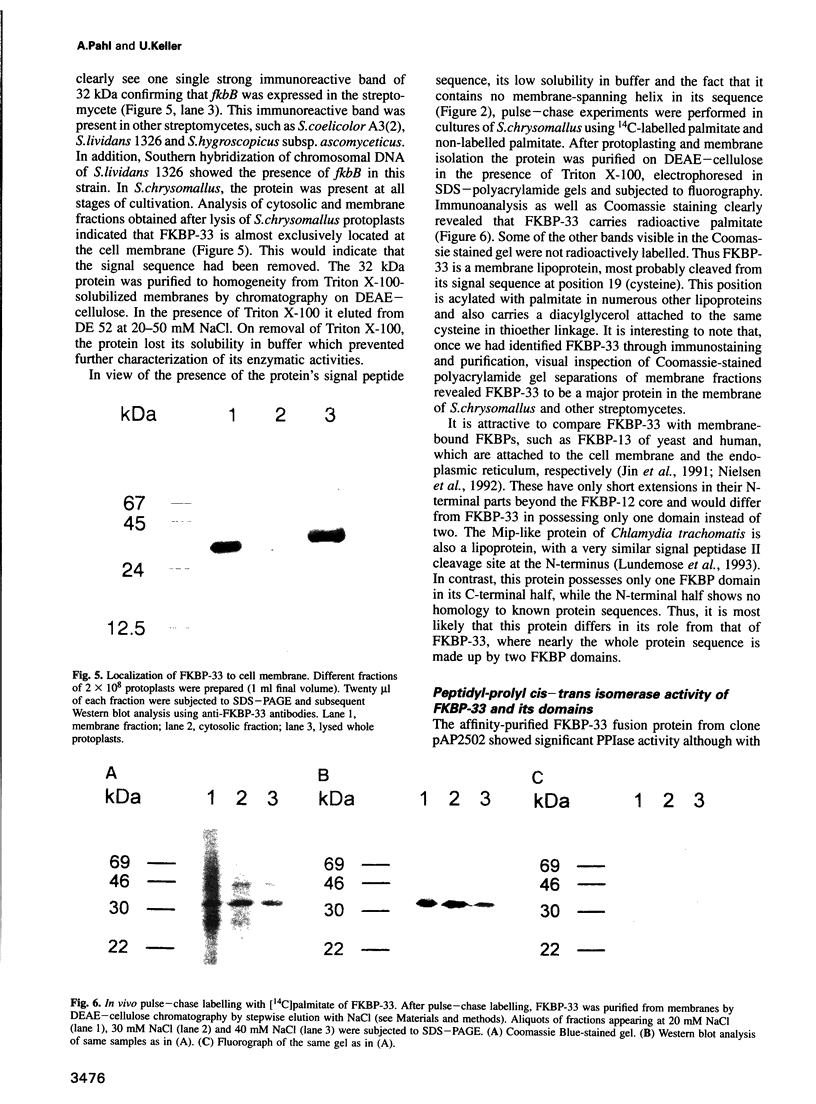
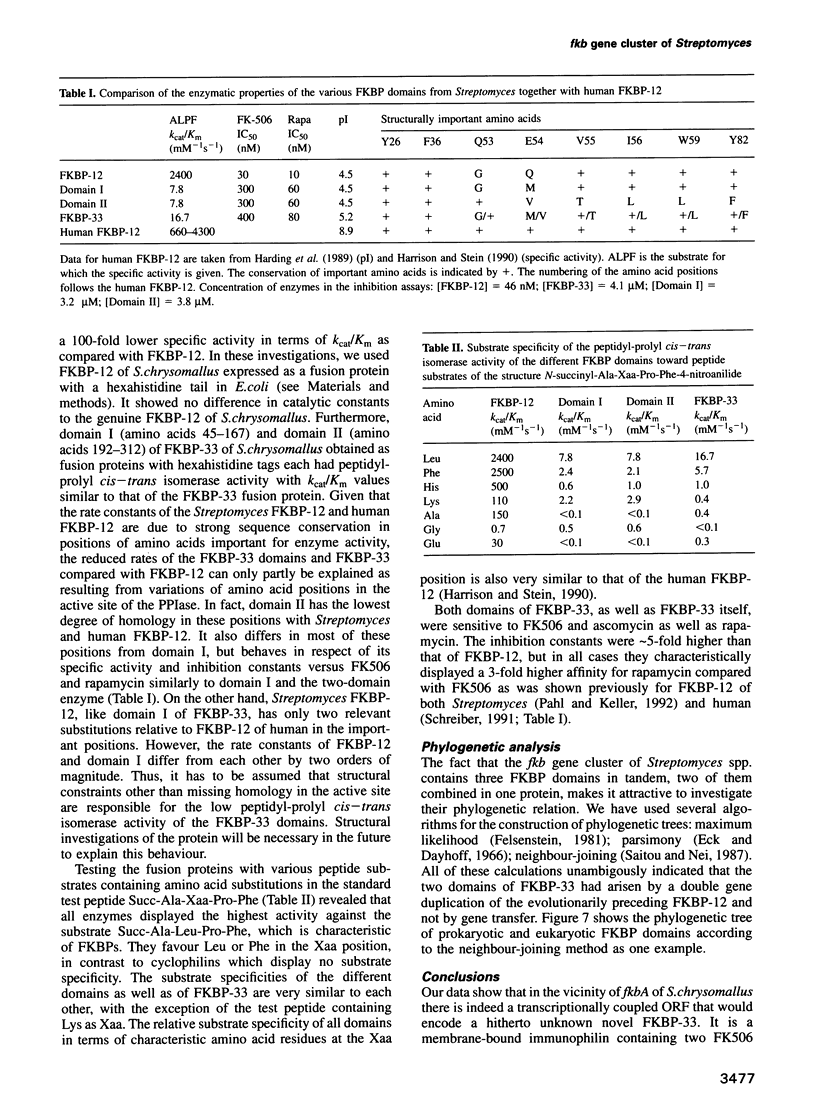
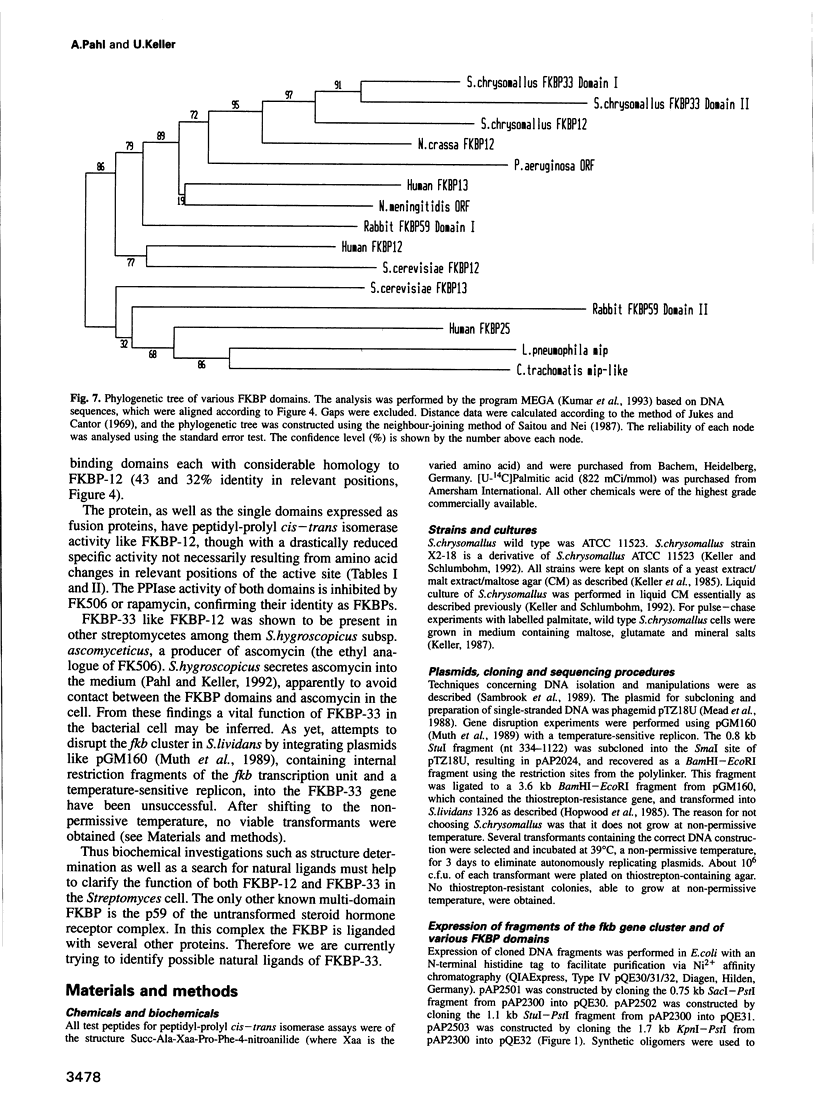
The nucleotide sequence of the region 5' to the fkbA gene, encoding the Streptomyces chrysomallus FK506 binding protein (FKBP-12), revealed an open reading frame (fkbB) encoding a protein of 312 amino acids, with an M(r) of approximately 33,000. FkbB and fkbA appear to be co-transcribed under the control of a promoter upstream of fkbB. The presumptive protein encoded by fkbB would be an FKBP (designated FKBP-33) consisting of two FK506 binding domains with 43 and 32% sequence identity to FKBP-12 and a signal peptide sequence characteristic of bacterial membrane lipoproteins. The portion of the gene comprising the two FKBP domains, as well as each individual domain, were expressed as fusion proteins in Escherichia coli and purified. Each expressed domain, as well as FKBP-33 itself, possesses peptidyl-prolyl cis-trans isomerase activity, though with much lower specific activities than FKBP-12. FKBP-33 is located in the cell membrane of S.chrysomallus and of other streptomycetes, as predicted from the presence of the signal peptide sequence. Pulse-chase experiments with radioactive palmitate in whole cells revealed significant labelling of FKBP-33, which probably carries palmitate at its N-terminus and an additional diacylglycerol residue attached to the N-terminal cysteine in thioether linkage. The two domains of FKBP-33 showed considerable homology with numerous eukaryotic and prokaryotic FKB domains. Calculations of phylogenetic relationships indicate with high probability that the two domains of the protein have arisen by a double gene duplication of fkbA lying in tandem to fkbB.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Callebaut I., Renoir J. M., Lebeau M. C., Massol N., Burny A., Baulieu E. E., Mornon J. P. An immunophilin that binds M(r) 90,000 heat shock protein: main structural features of a mammalian p59 protein. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6270–6274. doi: 10.1073/pnas.89.14.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciotto N. P., Eisenstein B. I., Mody C. H., Toews G. B., Engleberg N. C. A Legionella pneumophila gene encoding a species-specific surface protein potentiates initiation of intracellular infection. Infect Immun. 1989 Apr;57(4):1255–1262. doi: 10.1128/iai.57.4.1255-1262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17(6):368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- Fischer G., Bang H., Ludwig B., Mann K., Hacker J. Mip protein of Legionella pneumophila exhibits peptidyl-prolyl-cis/trans isomerase (PPlase) activity. Mol Microbiol. 1992 May;6(10):1375–1383. doi: 10.1111/j.1365-2958.1992.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Fischer G., Schmid F. X. The mechanism of protein folding. Implications of in vitro refolding models for de novo protein folding and translocation in the cell. Biochemistry. 1990 Mar 6;29(9):2205–2212. doi: 10.1021/bi00461a001. [DOI] [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Galat A., Lane W. S., Standaert R. F., Schreiber S. L. A rapamycin-selective 25-kDa immunophilin. Biochemistry. 1992 Mar 3;31(8):2427–2434. doi: 10.1021/bi00123a031. [DOI] [PubMed] [Google Scholar]
- Galat A. Peptidylproline cis-trans-isomerases: immunophilins. Eur J Biochem. 1993 Sep 15;216(3):689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- Gill S. C., von Hippel P. H. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989 Nov 1;182(2):319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- Gilson E., Alloing G., Schmidt T., Claverys J. P., Dudler R., Hofnung M. Evidence for high affinity binding-protein dependent transport systems in gram-positive bacteria and in Mycoplasma. EMBO J. 1988 Dec 1;7(12):3971–3974. doi: 10.1002/j.1460-2075.1988.tb03284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Galat A., Uehling D. E., Schreiber S. L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989 Oct 26;341(6244):758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- Harrison R. K., Stein R. L. Substrate specificities of the peptidyl prolyl cis-trans isomerase activities of cyclophilin and FK-506 binding protein: evidence for the existence of a family of distinct enzymes. Biochemistry. 1990 Apr 24;29(16):3813–3816. doi: 10.1021/bi00468a001. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Wu H. C. Lipoproteins in bacteria. J Bioenerg Biomembr. 1990 Jun;22(3):451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- Jin Y. J., Albers M. W., Lane W. S., Bierer B. E., Schreiber S. L., Burakoff S. J. Molecular cloning of a membrane-associated human FK506- and rapamycin-binding protein, FKBP-13. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6677–6681. doi: 10.1073/pnas.88.15.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Chu L., Kitano K., DeVault J. D., Kimbara K., Chakrabarty A. M., Misra T. K. Nucleotide sequence of a regulatory region controlling alginate synthesis in Pseudomonas aeruginosa: characterization of the algR2 gene. Gene. 1989 Dec 7;84(1):31–38. doi: 10.1016/0378-1119(89)90136-4. [DOI] [PubMed] [Google Scholar]
- Keller U. Actinomycin synthetases. Multifunctional enzymes responsible for the synthesis of the peptide chains of actinomycin. J Biol Chem. 1987 Apr 25;262(12):5852–5856. [PubMed] [Google Scholar]
- Keller U., Krengel U., Haese A. Genetic analysis in Streptomyces chrysomallus. J Gen Microbiol. 1985 May;131(5):1181–1191. doi: 10.1099/00221287-131-5-1181. [DOI] [PubMed] [Google Scholar]
- Keller U., Schlumbohm W. Purification and characterization of actinomycin synthetase I, a 4-methyl-3-hydroxyanthranilic acid-AMP ligase from Streptomyces chrysomallus. J Biol Chem. 1992 Jun 15;267(17):11745–11752. [PubMed] [Google Scholar]
- Koltin Y., Faucette L., Bergsma D. J., Levy M. A., Cafferkey R., Koser P. L., Johnson R. K., Livi G. P. Rapamycin sensitivity in Saccharomyces cerevisiae is mediated by a peptidyl-prolyl cis-trans isomerase related to human FK506-binding protein. Mol Cell Biol. 1991 Mar;11(3):1718–1723. doi: 10.1128/mcb.11.3.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebeau M. C., Massol N., Herrick J., Faber L. E., Renoir J. M., Radanyi C., Baulieu E. E. P59, an hsp 90-binding protein. Cloning and sequencing of its cDNA and preparation of a peptide-directed polyclonal antibody. J Biol Chem. 1992 Mar 5;267(7):4281–4284. [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Lundemose A. G., Birkelund S., Fey S. J., Larsen P. M., Christiansen G. Chlamydia trachomatis contains a protein similar to the Legionella pneumophila mip gene product. Mol Microbiol. 1991 Jan;5(1):109–115. doi: 10.1111/j.1365-2958.1991.tb01831.x. [DOI] [PubMed] [Google Scholar]
- Lundemose A. G., Rouch D. A., Penn C. W., Pearce J. H. The Chlamydia trachomatis Mip-like protein is a lipoprotein. J Bacteriol. 1993 Jun;175(11):3669–3671. doi: 10.1128/jb.175.11.3669-3671.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massol N., Lebeau M. C., Renoir J. M., Faber L. E., Baulieu E. E. Rabbit FKBP59-heat shock protein binding immunophillin (HBI) is a calmodulin binding protein. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1330–1335. doi: 10.1016/0006-291x(92)90448-t. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Inouye M. DNA sequence of the gene for the outer membrane lipoprotein of E. coli: an extremely AT-rich promoter. Cell. 1979 Dec;18(4):1109–1117. doi: 10.1016/0092-8674(79)90224-1. [DOI] [PubMed] [Google Scholar]
- Neugebauer K., Sprengel R., Schaller H. Penicillinase from Bacillus licheniformis: nucleotide sequence of the gene and implications for the biosynthesis of a secretory protein in a Gram-positive bacterium. Nucleic Acids Res. 1981 Jun 11;9(11):2577–2588. doi: 10.1093/nar/9.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Foor F., Siekierka J. J., Hsu M. J., Ramadan N., Morin N., Shafiee A., Dahl A. M., Brizuela L., Chrebet G. Yeast FKBP-13 is a membrane-associated FK506-binding protein encoded by the nonessential gene FKB2. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7471–7475. doi: 10.1073/pnas.89.16.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl A., Keller U. FK-506-binding proteins from streptomycetes producing immunosuppressive macrolactones of the FK-506 type. J Bacteriol. 1992 Sep;174(18):5888–5894. doi: 10.1128/jb.174.18.5888-5894.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A. C., Nicolson I. J., Saunders J. R. Neisseria meningitidis C114 contains silent, truncated pilin genes that are homologous to Neisseria gonorrhoeae pil sequences. J Bacteriol. 1988 Apr;170(4):1691–1697. doi: 10.1128/jb.170.4.1691-1697.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt W. B. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem. 1993 Oct 15;268(29):21455–21458. [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991 Jan 18;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L. Immunophilin-sensitive protein phosphatase action in cell signaling pathways. Cell. 1992 Aug 7;70(3):365–368. doi: 10.1016/0092-8674(92)90158-9. [DOI] [PubMed] [Google Scholar]
- Siekierka J. J., Hung S. H., Poe M., Lin C. S., Sigal N. H. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature. 1989 Oct 26;341(6244):755–757. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- Standaert R. F., Galat A., Verdine G. L., Schreiber S. L. Molecular cloning and overexpression of the human FK506-binding protein FKBP. Nature. 1990 Aug 16;346(6285):671–674. doi: 10.1038/346671a0. [DOI] [PubMed] [Google Scholar]
- Strohl W. R. Compilation and analysis of DNA sequences associated with apparent streptomycete promoters. Nucleic Acids Res. 1992 Mar 11;20(5):961–974. doi: 10.1093/nar/20.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropschug M., Wachter E., Mayer S., Schönbrunner E. R., Schmid F. X. Isolation and sequence of an FK506-binding protein from N. crassa which catalyses protein folding. Nature. 1990 Aug 16;346(6285):674–677. doi: 10.1038/346674a0. [DOI] [PubMed] [Google Scholar]
- Wiederrecht G., Martin M. M., Sigal N. H., Siekierka J. J. Isolation of a human cDNA encoding a 25 kDa FK-506 and rapamycin binding protein. Biochem Biophys Res Commun. 1992 May 29;185(1):298–303. doi: 10.1016/s0006-291x(05)80990-8. [DOI] [PubMed] [Google Scholar]
- von Heijne G. The structure of signal peptides from bacterial lipoproteins. Protein Eng. 1989 May;2(7):531–534. doi: 10.1093/protein/2.7.531. [DOI] [PubMed] [Google Scholar]