Abstract
Background: Endothelial dysfunction measured via flow-mediated dilation (FMD) is associated with greater risk of future hypertension and cardiovascular events in postmenopausal women. Aerobic exercise training has been shown to improve endothelial function in Caucasian populations, but has not been evaluated specifically in African Americans. This has clinical importance due to the increased prevalence of cardiovascular disease in African Americans.
Methods: In the present pilot study, 8 African American (age: 55.8±1.7 years, peak oxygen uptake [VO2 peak]: 21.0±3.9 mL/kg/minute, body mass index [BMI]: 30.1± 6.3 kg/m2) and 16 Caucasian (age: 57.2±5.9 years, VO2 peak: 21.8±3.7 mL/kg/minute, BMI: 29.3±5.2 kg/m2) sedentary postmenopausal women underwent brachial artery FMD measurements before and after 12 weeks of aerobic exercise training. FMD was quantified by comparing B-mode ultrasound images of the brachial artery at rest and following reactive hyperemia after 5 minutes of forearm occlusion. Participants performed aerobic exercise training 4 days per week for 12 weeks.
Results: Despite improvements in fitness in both groups, aerobic exercise training did not significantly improve FMD in African American (5.8% to 5.7%, p=0.950) or Caucasian postmenopausal women (5.7% to 6.6%, p=0.267). In women with the greatest impairment in endothelial function at baseline (FMD<4.5%), a significant improvement in FMD was observed, independent of race, following exercise training (2.2% to 6.2%, p=0.007).
Conclusion: The benefits of aerobic exercise training on endothelial function in postmenopausal women are most pronounced in women with endothelial dysfunction prior to training and do not appear to be affected by race.
Introduction
African American women are at a substantially greater risk of cardiovascular disease (CVD) compared to their Caucasian counterparts.1 Specifically, the overall prevalence of CVD in the United States for African American women and Caucasian women are 47.3% and 33.8%, respectively.1 Similar to the epidemiologic data showing health disparities in CVD prevalence, several studies have shown that African Americans have a greater risk of impaired endothelial function compared with Caucasians, which has been reported in a variety of populations,2–5 including postmenopausal women.4 Endothelial dysfunction is a subclinical event in atherogenesis that precedes the development of overt CVD6 and has been shown to be predictive of future hypertension7 and cardiovascular events in postmenopausal women.8 Potential etiologies for these racial differences include higher levels of oxidative stress,9 endothelin-1,2 and asymmetric dimethylarginine10 in African Americans compared with Caucasians. Specifically, Loehr et al.4 found lower brachial artery flow-mediated dilation (FMD) in African American (2.9%) compared with Caucasian (3.5%) postmenopausal women in the Cardiovascular Health Study. Therefore, improving endothelial function should have a beneficial effect on overall cardiovascular risk in African Americans.
Aerobic exercise training has been shown to improve endothelial function, especially in populations with CVD risk.6,11,12 However, to our knowledge no data exist specifically investigating the effect of aerobic exercise training on endothelial function in African American women, which has clinical importance due to the increased risk of CVD in African Americans13 and postmenopausal women in general.14 Thus, the purpose of the present pilot study was to examine the effect of 12 weeks of aerobic exercise training on endothelial function in sedentary postmenopausal African American and Caucasian women. As many physiological responses to exercise training are affected by baseline levels and because African American postmenopausal women have a greater risk of impaired endothelial function,4 we hypothesized that both Caucasian and African American women would improve endothelial function with exercise training, with greater improvements observed in African American women.
Methods
Study design
Prior to intervention, participants underwent a maximal incremental treadmill protocol and body composition assessment. On a separate occasion, endothelial function was assessed by FMD. Following completion of baseline measurements, all participants began a 12-week supervised aerobic exercise training program that consisted of walking/jogging on an indoor track or treadmill. Following the completion of exercise training, participants repeated the maximal incremental treadmill protocol, body composition, and endothelial function measurements from baseline. The primary dependent variable of the study was the change in FMD following exercise training in African American and Caucasian women. Additionally, we evaluated changes in peak oxygen uptake (VO2 peak), weight, percent body fat, and waist circumference. This study was approved by the Institutional Review Board for Health Sciences Research at the University of Virginia.
Participants
After providing written informed consent, each participant underwent an outpatient history, physical examination, and vital signs, height, and weight measurements. Blood was drawn for biochemical screening tests of hepatic, renal, hematological, endocrine, and metabolic function. In addition, follicle-stimulating hormone was measured to confirm postmenopausal status. Exclusion criteria included taking ace inhibitors, cholesterol lowering medications, or estrogen replacement medications; participating in aerobic exercise more than two times per week; having type 1 or 2 diabetes; being premenopausal; or having other medical conditions that contraindicated exercise training.
Body composition and anthropometry
Body density was measured using air displacement plethysmography (Bod-Pod, Life Measurement Instruments) corrected for thoracic gas volume as described previously.15 In Caucasian participants, the Siri equation16 was used to determine percent body fat from body volume measurements. For African American women, a race specific equation was used to determine percent body fat.17 Weight was evaluated on a calibrated scale. Waist circumference measurements were evaluated at the level of the umbilicus and the natural waist (narrowest region of the waist between the lower rib and the iliac crest). At each site, we evaluated waist circumferences in triplicate, and the average of these measurements was used for data analysis.
Exercise testing
Aerobic fitness was measured using an incremental maximal treadmill test. Each woman began walking at an initial velocity of 60 meters per minute and the treadmill velocity was increased by 10 meters per minute every 3 minutes (the duration of each stage) until volitional exhaustion. Pulmonary ventilation and gas exchange were measured with standard open-circuit spirometry (VMax 29 series, Cardinal Health). Peak oxygen uptake (VO2 peak) was defined as the highest 1-minute oxygen uptake obtained prior to volitional exhaustion.
Endothelial function
Women were tested after an overnight fast at the University of Virginia General Clinical Research Center in the morning (between 7:00 and 9:00 am) in a quiet, temperature controlled room. In addition, participants were asked not to consume caffeine during this fasting period and not to exercise 24 hours prior to measurements. Brachial artery assessments were obtained using 2D and Doppler ultrasound measurements (HDI 5000, Philips Ultrasound). Brachial artery diameter was assessed using Brachial Analyzer Software (Brachial Analyzer, Medical Imaging Applications), and the brachial artery mean blood velocity was measured using a pulse-wave Doppler with online angle correction and analysis software. All imaging was performed in the long axis approximately 4 cm proximal of the antecubital fold in the anterior/medial plane, and were triggered to the R wave of the cardiac cycle. Arterial diameters (mm) were calculated as the mean distance between the anterior and posterior wall at the vessel–blood interface. Flow mediated dilation measurements were defined as the percent change in vessel diameter from rest to peak dilation after treatment.
Prior to baseline measurements, participants were asked to rest in a supine position for 15 minutes. After the baseline measurements were performed, a blood pressure cuff was placed 4–5 cm distal of the antecubital fold and inflated to 50 mm Hg above resting systolic blood pressure for 5 minutes. The cuff was then rapidly deflated (reactive hyperemia) and measurements were obtained at peak dilation (60 to 90 seconds after cuff release).
All brachial artery images were captured and stored to an optical disk and recorded on a super-VHS videotape to allow for data analysis by using a custom designed edge-detection and wall-tracking software (Brachial Analyzer, Medical Imaging Applications). This software significantly minimizes investigator bias and has a mean reported intraobserver coefficient of variation of 6.7% for repeated measures of FMD, which is significantly lower than the traditional manual, online caliper method.18 The digital images were acquired and analyzed in accordance with the guidelines offered by the International Brachial Artery Reactivity Task Force.19 The same blinded investigator analyzed the data from all FMD measurements after the completion of the study. In our lab, the intraobserver variability is 2.93% [CV′=SDΔ/(100+μ); μ=mean absolute change in diameter. The coefficient of variation of our laboratory for FMD measurements is 27.5%.
At baseline and follow-up, FMD measurements were separated from exercise testing measurements by at least 24 hours. At follow-up, the FMD measurement was performed between 24 and 72 hours after the last exercise session.
Exercise intervention
Women participated in a 12-week supervised exercise intervention (walking/jogging). Each participant was asked to complete four exercise sessions (days) per week at alternating exercise intensities; two at moderate intensity (rating of perceived exertion [RPE] ∼ 10–12), and two at high intensity (RPE ∼ 15–17). The duration/distance of each exercise session was adjusted based on each participant's individual VO2–velocity relationship so that each participant would expend approximately 250 kcal per training session during weeks 1–2, 300 kcal per training session during weeks 3–4, and 350 kcal per training session during weeks 5–12. As each participant's fitness improved and the velocity required to maintain the assigned RPE increased, exercise duration/distance was readjusted to maintain kcal requirement. Participant's weight was measured every 2 weeks in order to adjust exercise duration/distance to the correct caloric expenditure. All sessions were supervised by the research team. Training compliance was calculated by dividing the number of attended sessions by the total number of scheduled sessions.
Statistical analysis
Baseline subject characteristics and exercise training compliance were compared between the African American and Caucasian study groups using Wilcoxon rank sum test. The baseline to post-intervention changes in VO2 peak and waist circumference were analyzed using repeated measures analysis of covariance (ANCOVA), with participant race (African American, Caucasian) functioning as the ANCOVA model factor, and baseline value functioning as the ANCOVA model covariate.
The FMD data were analyzed using repeated measures ANCOVA. The response measurements denoted the change in FMD from baseline to follow-up. Two sources of variation in the response measurements were examined: participant race (African American, Caucasian), and study phase (baseline, follow-up). The baseline response measurements were utilized in the analysis as covariate adjustment information. With regard to hypothesis testing, linear-contrasts of the ANOVA least squared means were utilized to estimate the means for the baseline to post-intervention changes in FMD, and to estimate the means for between-phase and between-race difference in the changes in FMD.
Analyses were also performed to evaluate the change in FMD in participants categorized with impaired FMD at baseline (<4.5%) and participants with normal FMD (>4.5%) at baseline. We selected these criteria based on data published by Rossi et al.,8 which found that FMD values below 4.5% were associated with the highest relative risk of cardiovascular events in postmenopausal women.8 An ANOVA of repeated measures was used to determine the change in FMD from baseline to follow-up. For confirmation purposes, a similar analysis was performed for participants in the lower 50th percentile (impaired) and the upper 50th percentile (not impaired) in baseline FMD (median split).The median of our sample for baseline FMD was 5.7%. Baseline demographic characteristics of women classified as impaired and not impaired (for 4.5% and the median split criteria) were compared with a Wilcoxon rank sum test for continuous variables. A Fisher's exact test was used to compare the proportion of African Americans compared to Caucasian who were classified as impaired at baseline for 4.5% and the median split criteria. For each statistical test, a p value≤0.05 was the criterion for rejecting the null hypothesis.
Results
Participants
Demographic information is displayed in Table 1. At baseline, no significant differences were observed for age, VO2 peak, BMI, percent fat, or body weight between African American and Caucasian participants (all p values>0.05). No significant differences were observed in exercise training compliance (p=0.690) between the African American (83.3%) and Caucasian (86.6%) women.
Table 1.
Participant Baseline Characteristics
| African American (n=8) | Caucasian (n=16) | |
|---|---|---|
| Age (years) | 55.9±1.7 | 57.2±5.9 |
| Height (cm) | 162.7±3.5 | 162.8±6.8 |
| Weight (kg) | 79.5±16.6 | 78.0±15.4 |
| BMI (kg/m2) | 30.1±6.3 | 29.3±5.2 |
| VO2 peak (mL/kg/minute) | 22.0±3.9 | 23.8±3.7 |
| Body fat (%) | 43.0±8.0 | 44.0±3.8 |
Values are presented as mean±standard deviation (SD).
BMI, body mass index; VO2 peak, peak oxygen uptake.
Exercise training
VO2 peak
Significant improvements in VO2 peak were observed in both African American (21.0 to 23.8 mL/kg/minute, p=0.05) and Caucasian participants (21.8 to 25.8 mL/kg/min, p<0.001, Table 2). However, no significant differences in the change in VO2 peak between African American (13.5%) and Caucasian (18.3%) women were observed (p=0.445) (Table 2).
Table 2.
The Effect of Exercise Training on Fitness and Body Composition in African American and Caucasian Postmenopausal Women
| Baseline | Follow-up | Change | p-value | |
|---|---|---|---|---|
| African Americans (n=8) | ||||
| VO2 peak (mL/kg/minute) | 21.0±3.9 | 23.8±8.0 | +2.8 | 0.05 |
| Weight (kg) | 79.5±16.5 | 79.0±15.2 | −0.4 | NS |
| BMI (kg/m2) | 30.0±6.3 | 29.9±5.8 | −0.2 | NS |
| Umbilicus waist circumference (mm) | 98.1±14.9 | 95.7±12.6 | −2.4 | NS |
| Natural waist circumference (mm) | 93.9±13.5 | 90.4±9.1 | −3.6 | NS |
| Percent fat (%) | 42.9±8.0 | 41.2±8.7 | −1.7 | NS |
| Caucasians (n=16) | ||||
| VO2 Peak (mL/kg/min) | 21.8±3.7 | 25.8±5.2 | +4.0 | <0.001 |
| Weight (kg) | 78.0±15.4 | 76.8±14.8 | −1.2 | NS |
| BMI (kg/m2) | 29.3±5.2 | 28.8±4.8 | −0.6 | NS |
| Umbilicus waist circumference (mm) | 102.0±12.5 | 101.5±13.8 | −0.4 | NS |
| Natural waist circumference (mm) | 93.9±13.5 | 92.3±13.0 | −1.5 | NS |
| Percent fat (%) | 44.0±3.9 | 43.4±4.6 | −0.6 | NS |
Values are presented as mean±SD.
NS, not significant.
Body composition
No significant within- or between-group changes in BMI, body weight, percent body fat, or waist circumference were observed (Table 2).
FMD.
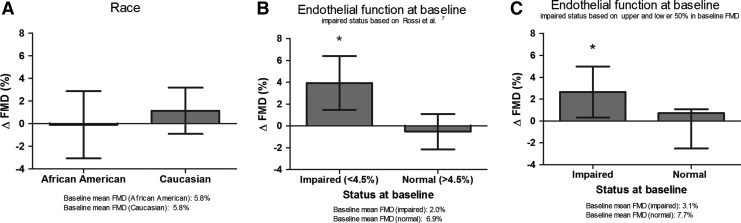
Baseline FMD was not significantly different between African American and Caucasian participants (5.77% vs. 5.75%, p=0.695). Similarly, no significant changes in FMD were observed following aerobic training in African American or Caucasian women (all p values>0.05) (Fig. 1A).
FIG. 1.
Change in flow-mediated dilation (FMD) following aerobic exercise training in (A) African American and Caucassian participants; (B) participants with impaired (<4.5%) and not impaired (>4.5%) FMD at baseline; (C) participants with impaired FMD (lower 50th percentile) and not impaired (upper 50th percentile) based on median split. Changes in FMD are expressed as adjusted least square means±95% confidence intervals. *Significantly greater change in FMD in women who had impaired FMD at baseline compared to those that had normal FMD levels at baseline.
Training effects on endothelial function in participants with impaired FMD at baseline
We evaluated the effect of exercise training on endothelial function in participants with impaired FMD prior to training in order to determine the efficacy of exercise training in women with the greatest CVD risk.8 Participants with baseline FMD <4.5% (n=8) were classified as having impaired endothelial function. The remaining participants (n=16) were classified as not impaired. Results for baseline FMD before and after exercise training are presented in Fig. 1B. In participants with impaired baseline FMD, ANOVA revealed a significant improvement in FMD following exercise training (2.2% to 6.2%, p=0.007). Participants with normal FMD at baseline did not improve FMD after training (7.8% to 6.7%, p=0.508) (Fig. 1B).
We performed a similar analysis for participants with baseline FMD <50th percentile (n=12) and >50th percentile (n=12) of our study sample (Fig. 1C). The 50th percentile for the baseline FMD in the present study was 5.7%. Similarly, we found that participants with FMD<50th percentile increased FMD significantly following exercise training (3.2% to 5.9%, p=0.029), whereas participants above the 50th percentile did not improve (7.6% to 6.9%, p=0.399). No significant differences were observed in demographic baseline characteristics of the women categorized as impaired and not impaired (by both the 4.5% and the median criteria) shown in Table 3. In addition, no significant differences were observed in the proportion of African Americans or Caucasians who were classified as impaired by the 4.5 criteria (p=1.00) or median split criteria (p=0.715).
Table 3.
Baseline Characteristics of Participants with Impaired FMD at Baseline Based on the 4.5% Criteria and Median Split Criteria
| Impaired criteria: FMD<4.5% | Not impaired (n=16) | Impaired (n=8) | p-value |
|---|---|---|---|
| African American/Caucasian (n) | 6/10 | 2/6 | |
| Age (years) | 55.7 (3.9) | 58.0 (4.9) | 0.31 |
| BMI (kg/m2) | 28.6 (5.7) | 31.8 (3.5) | 0.19 |
| Body fat (%) | 42.8 (5.8) | 45.3 (4.8) | 0.28 |
| VO2 peak (mL/kg/minute) | 22.1 (4.5) | 21.0 (1.9) | 0.42 |
| Waist circumference (umbilicus) | 98.0 (12.3) | 105.9 (12.4) | 0.23 |
| Waist circumference (natural waist) | 90.6 (11.3) | 99.1 (13.0) | 0.17 |
| FMD (%) | 7.0 (1.7) | 2.2 (2.3) | <0.001 |
| Impaired criteria: lower 50th percentile | Not impaired (n=12) | Impaired (n=12) | p-value |
|---|---|---|---|
| African American/Caucasian (n) | 5/7 | 3/9 | |
| Age (years) | 55.3 (3.9) | 57.6 (4.6) | 0.25 |
| BMI (kg/m2) | 29.8 (6.1) | 29.5 (5.9) | 0.75 |
| Body fat (%) | 43.5 (6.2) | 43.8 (4.9) | 0.90 |
| VO2 peak (mL/kg/minute) | 21.8 (5.2) | 21.6 (1.9) | 0.91 |
| Waist circumference (umbilicus) | 100.1 (13.3) | 100.1 (12.3) | 0.99 |
| Waist circumference (natural waist) | 92.5 (12.3) | 94.0 (12.7) | 0.78 |
| FMD (%) | 7.6 (1.5) | 3.2 (2.3) | <0.001 |
FMD, flow-mediated dilation.
Discussion
The primary finding of the present pilot study was that aerobic exercise training did not improve endothelial function in African American or Caucasian postmenopausal women. However, we observed a significant improvement in FMD in women with the greatest degree of endothelial dysfunction prior to training. The clinical implications are that aerobic exercise training improves endothelial function in individuals with heightened CVD risk (e.g., individuals with impaired endothelial function), but may not further increase FMD in participants with normal endothelial function prior to training. Future studies should evaluate African Americans and Caucasians with greater levels of cardiovascular risk or endothelial dysfunction.
Lack of racial differences in baseline endothelial function prior to exercise training
In contrast to previous studies,3,4,9 we observed no significant differences in baseline FMD between African Americans and Caucasians women. Specifically, Loehr et al.4 observed impaired endothelial function in postmenopausal African Americans (2.9%) compared with Caucasians (3.4%) with CVD or suspected CVD. However, Gokce et al.20 reported no differences in FMD between healthy African American and Caucasian participants, but a racial difference in hypertensive subjects, suggesting that racial differences in endothelial function become more apparent in higher risk populations.
A potential limitation of previous studies observing racial differences between African Americans and Caucasians in endothelial function is that fitness/physical activity levels were not measured.3,4,9 This may represent an important confounder as fitness level in some studies has been shown to be positively associated with endothelial function,21–23 and fitness differences exist between African Americans and Caucasians.24,25 Our findings suggest that in postmenopausal women without cardiovascular disease and similar fitness levels, no racial differences in baseline endothelial function are present.
Effects of exercise training on endothelial function
In mostly Caucasian populations, aerobic exercise training has been shown to improve endothelial function in individuals with significant cardiovascular disease risk (e.g., diabetes, coronary artery disease (CAD), metabolic syndrome),11,26,27 but responses to exercise training have been less consistent in healthy individuals.6,28 The results of the present study indicate that aerobic exercise training did not improve endothelial function in apparently healthy African American or Caucasian postmenopausal women. Our results are consistent with exercise training studies in apparently healthy postmenopausal populations without cardiovascular disease.29–31 Casey et al.30 found no significant change in FMD after 18 weeks of treadmill training in healthy subjects (n=10). Similarly, Black et al.32 reported no significant change in FMD after 12 weeks of aerobic training in sedentary postmenopausal women (n=6), but an improvement in FMD approached significance (p=0.07) after 24 weeks of training. Pierce and colleagues31 reported that 8 weeks of aerobic exercise training was associated with a significant improvement in FMD in older men (from 4.6 to 7.1%) but had no effect on FMD in estrogen-deficient postmenopausal women, despite similar improvements in maximal exercise treadmill time after training. Although no specific mechanism was identified explaining these gender differences, the authors indicated that differences in peak shear rate or area under the shear rate curve did not explain the lack of an exercise training effect on FMD in postmenopausal women.31
It is unlikely that the lack of a significant improvement in FMD observed in the present study is explained by the fact that walking/jogging exercise is predominately a lower-body exercise. Other studies have observed increased FMD with walking/jogging exercise and brachial artery FMD measures.11,33 In addition, increased blood flow/antegrade shear stress (the stimulus for improvements in endothelial function after exercise training)34 are present in the non-working musculature/vasculature (i.e., brachial artery) during predominately lower-body aerobic exercise.35,36 Although improvements in endothelial function only occurred in subjects with impaired endothelial function in the present study, we observed improvements in fitness levels in both African American and Caucasian women, which is associated with reduced risk for CVD and type 2 diabetes.37
Exercise training in women with impaired endothelial function at baseline
The impact of aerobic exercise training on endothelial function may be dependent on whether endothelial dysfunction is present at baseline. Based on consistent observations of improved endothelial function in subjects with cardiovascular risk, and inconsistent results in apparently healthy participants, Green et al.6 suggested that baseline endothelial function may be an important factor determining whether FMD can be increased with aerobic training. Pierce et al.31 also speculated that exercise training may be more effective for postmenopausal women with more severely impaired endothelial function. The only report addressing this issue directly was performed by Swift et al.38 who found that in postmenopausal women with elevated blood pressure or hypertension, the greatest improvement in FMD was found in women with endothelial dysfunction at baseline. In the present study, we observed a similar relationship in a sample of healthier postmenopausal women. In order to reduce bias when classifying participants with endothelial dysfunction, we performed two separate analyses based on (1) previous data in the literature (above and below 4.5%),8 and (2) the median FMD value of our study sample (upper and lower 50th percentile).
No significant differences were observed in the demographic characteristics (age, waist circumference, fitness, body fat, weight) between women with impaired or normal FMD at baseline. A limitation of the analysis is the possibility of a “regression to the mean” phenomenon. However, in support of our conclusions, we did not observe a significant decrease in endothelial function in women without impaired FMD at baseline. Therefore, aerobic exercise appears to be an effective intervention to improve endothelial function in apparently healthy postmenopausal women with endothelial dysfunction who have the greatest risk of future hypertension7 and CVD events8 based on epidemiological data. For sedentary individuals who begin an aerobic exercise program with normal FMD, exercise may help to preserve healthy endothelial function.
Several limitations exist in the present study. Due to difficulty in participant recruitment, we were unable to have a non-exercise training control group. Additionally, there was a small sample of postmenopausal African American participants. However, we feel that the sample size is appropriate considering the pilot nature of this trial. In addition, we did not measure endothelial-independent dilation. Pierce et al.31 reported that exercise training did not affect endothelial-independent dilation in their postmenopausal subjects. Lastly, FMD values were not corrected for shear AUC. However, evidence exists that shear stress normalization may only be appropriate for young adults, as Thijssen et al.39 observed no significant correlation between FMD and various methods of shear stress correction in older adults. Additionally, previous studies have observed significant relationships between FMD uncorrected for shear and cardiovascular disease risk.8,40
In conclusion, the present study suggests that in sedentary African American and Caucasian postmenopausal women, improvement in endothelial function in the response to aerobic exercise training is related to the severity of brachial artery FMD impairment prior to initiating the training program, but not by race. The results of this pilot study suggest that future studies should evaluate responses to exercise training on endothelial function in higher risk populations (e.g., adults with cardiovascular disease, diabetics, etc.) in which endothelial dysfunction may be more prevalent.
Acknowledgments
We would like to thank all of the student volunteers who assisted in the exercise training portion of this research study. In addition, we would like to thank the General Clinical Research Center (GCRC) nursing, exercise laboratories, and spin/core laboratories for their work and professionalism during this project. Supported by National Institutes of Health grant RR 00847 to the University of Virginia GCRC.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update. Circulation 2010;121:e46–e215 [DOI] [PubMed] [Google Scholar]
- 2.Campia U, Cardillo C, Panza JA. Ethnic differences in the vasoconstrictor activity of endogenous endothelin-1 in hypertensive patients. Circulation 2004;109:3191–3195 [DOI] [PubMed] [Google Scholar]
- 3.Perregaux D, Chaudhuri A, Rao S, et al. Brachial vascular reactivity in blacks. Hypertension 2000;36:866–871 [DOI] [PubMed] [Google Scholar]
- 4.Loehr LR, Espeland MA, Sutton-Tyrrell K, et al. Racial differences in endothelial function in postmenopausal women. Am Heart J 2004;148:606–611 [DOI] [PubMed] [Google Scholar]
- 5.Campia U, Choucair WK, Bryant MB, et al. Reduced endothelium-dependent and -independent dilation of conductance arteries in african americans. J Am Coll Cardiol 2002;40:754–760 [DOI] [PubMed] [Google Scholar]
- 6.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 2004;561:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi R, Chiurlia E, Nuzzo A, et al. Flow-mediated vasodilation and the risk of developing hypertension in healthy postmenopausal women. J Am Coll Cardiol 2004;44:1636–1640 [DOI] [PubMed] [Google Scholar]
- 8.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol 2008;51:997–1002 [DOI] [PubMed] [Google Scholar]
- 9.Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: Predisposition of african americans to vascular diseases. Circulation 2004;109:2511–2517 [DOI] [PubMed] [Google Scholar]
- 10.Melikian N, Wheatcroft SB, Ogah OS, et al. Asymmetric dimethylarginine and reduced nitric oxide bioavailability in young black african men. Hypertension 2007;49:873–877 [DOI] [PubMed] [Google Scholar]
- 11.Maiorana A, O'Driscoll G, Cheetham C, et al. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol 2001;38:860–866 [DOI] [PubMed] [Google Scholar]
- 12.Vona M, Rossi A, Capodaglio P, et al. Impact of physical training and detraining on endothelium-dependent vasodilation in patients with recent acute myocardial infarction. Am Heart J 2004;147:1039–1046 [DOI] [PubMed] [Google Scholar]
- 13.Thomas AJ, Eberly LE, Davey Smith G, Neaton JD, Stamler J. Race/ethnicity, income, major risk factors, and cardiovascular disease mortality. Am J Public Health 2005;95:1417–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease. Ann Intern Med 1976;85:447–452 [DOI] [PubMed] [Google Scholar]
- 15.Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc 1995;27:1692–1697 [PubMed] [Google Scholar]
- 16.Siri WE. Body-composition from fluid spaces and density—analysis of methods. Nutrition. 1993;9:481–491 [PubMed] [Google Scholar]
- 17.Ortiz O, Russell M, Daley TL, et al. Differences in skeletal-muscle and bone-mineral mass between black-and-white females and their relevance to estimates of body-composition. Am J Clin Nutr 1992;55:8–13 [DOI] [PubMed] [Google Scholar]
- 18.Woodman RJ, Playford DA, Watts GF, et al. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 2001;91:929–937 [DOI] [PubMed] [Google Scholar]
- 19.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the international brachial artery reactivity task force. J Am Coll Cardiol 2002;39:257–265 [DOI] [PubMed] [Google Scholar]
- 20.Gokce N, Holbrook M, Duffy SJ, et al. Effects of race and hypertension on flow-mediated and nitroglycerin-mediated dilation of the brachial artery. Hypertension 2001;38:1349–1354 [DOI] [PubMed] [Google Scholar]
- 21.Palmieri EA, Palmieri V, Innelli P, et al. Aerobic exercise performance correlates with post-ischemic flow-mediated dilation of the brachial artery in young healthy men. Eur J Appl Physiol 2005;94:113–117 [DOI] [PubMed] [Google Scholar]
- 22.Rinder MR, Spina RJ, Ehsani AA. Enhanced endothelium-dependent vasodilation in older endurance-trained men. J Appl Physiol 2000;88:761–766 [DOI] [PubMed] [Google Scholar]
- 23.Rywik TM, Blackman MR, Yataco AR, et al. Enhanced endothelial vasoreactivity in endurance-trained older men. J Appl Physiol 1999;87:2136–2142 [DOI] [PubMed] [Google Scholar]
- 24.Hunter GR, Weinsier RL, Darnell BE, Zuckerman PA, Goran MI. Racial differences in energy expenditure and aerobic fitness in premenopausal women. Am J Clin Nutr 2000;71:500–506 [DOI] [PubMed] [Google Scholar]
- 25.LaMonte MJ, Durstine JL, Yanowitz FG, et al. Cardiorespiratory fitness and c-reactive protein among a tri-ethnic sample of women. Circulation 2002;106:403–406 [DOI] [PubMed] [Google Scholar]
- 26.Lavrencic A, Salobir BG, Keber I. Physical training improves flow-mediated dilation in patients with the polymetabolic syndrome. Arterioscler Thromb Vasc Biol 2000;20:551–555 [DOI] [PubMed] [Google Scholar]
- 27.Walsh JH, Bilsborough W, Maiorana A, et al. Exercise training improves conduit vessel function in patients with coronary artery disease. J Appl Physiol 2003;95:20–25 [DOI] [PubMed] [Google Scholar]
- 28.Moyna NM, Thompson PD. The effect of physical activity on endothelial function in man. Acta Physiol. Scand. 2004;180:113–123 [DOI] [PubMed] [Google Scholar]
- 29.Atkinson G, Batterham AM, Black MA, et al. Is the ratio of flow-mediated dilation and shear rate a statistically sound approach to normalization in cross-sectional studies on endothelial function? J Appl Physiol 2009;107:1893–1899 [DOI] [PubMed] [Google Scholar]
- 30.Casey DP, Pierce GL, Howe KS, Mering MC, Braith RW. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur J Appl Physiol 2007;100:403–408 [DOI] [PubMed] [Google Scholar]
- 31.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci 2010;120:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black MA, Cable NT, Thijssen DHJ, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1109–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westhoff TH, Franke N, Schmidt S, et al. Beta-blockers do not impair the cardiovascular benefits of endurance training in hypertensives. J Hum Hypertens 2007;21:486–493 [DOI] [PubMed] [Google Scholar]
- 34.Tinken TM, Thijssen DHJ, Hopkins N, et al. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 2008;55:312–318 [DOI] [PubMed] [Google Scholar]
- 35.Simmons GH, Padilla J, Young CN, et al. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: Role of thermoregulatory vasodilation. J Appl Physiol 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka H, Shimizu S, Ohmori F, et al. Increases in blood flow and shear stress to nonworking limbs during incremental exercise. Med Sci Sports Exerc 2006;38:81–85 [DOI] [PubMed] [Google Scholar]
- 37.Swift DL, Lavie CJ, Johannsen NM, et al. Physical activity, cardiorespiratory fitness, and exercise training in primary and secondary coronary prevention. Circ J 2013;77:281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swift DL, Earnest CP, Blair SN, Church TS. The effect of different doses of aerobic exercise training on endothelial function in postmenopausal women with elevated blood pressure: Results from the DREW study. Br J Sports Med 2012;46:753–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thijssen DHJ, Bullens LM, van Bemmel MM, et al. Does arterial shear explain the magnitude of flow-mediated dilation?: A comparison between young and older humans. Am J Physiol Heart Circ Physiol 2009;296:H57–H64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeboah J, Crouse JR, Hsu F-C, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The cardiovascular health study. Circulation 2007;115:2390–2397 [DOI] [PubMed] [Google Scholar]