Abstract
The gravity-dependent behavior of Paramecium biaurelia and Euglena gracilis have previously been studied on ground and in real microgravity. To validate whether high magnetic field exposure indeed provides a ground-based facility to mimic functional weightlessness, as has been suggested earlier, both cell types were observed during exposure in a strong homogeneous magnetic field (up to 30 T) and a strong magnetic field gradient. While swimming, Paramecium cells were aligned along the magnetic field lines; orientation of Euglena was perpendicular, demonstrating that the magnetic field determines the orientation and thus prevents the organisms from the random swimming known to occur in real microgravity. Exposing Astasia longa, a flagellate that is closely related to Euglena but lacks chloroplasts and the photoreceptor, as well as the chloroplast-free mutant E. gracilis 1F, to a high magnetic field revealed no reorientation to the perpendicular direction as in the case of wild-type E. gracilis, indicating the existence of an anisotropic structure (chloroplasts) that determines the direction of passive orientation. Immobilized Euglena and Paramecium cells could not be levitated even in the highest available magnetic field gradient as sedimentation persisted with little impact of the field on the sedimentation velocities. We conclude that magnetic fields are not suited as a microgravity simulation for gravitactic unicellular organisms due to the strong effect of the magnetic field itself, which masks the effects known from experiments in real microgravity. Key Words: Levitation—Microgravity—Gravitaxis—Gravikinesis—Gravity. Astrobiology 14, 205–215.
1. Introduction
As Earth's gravity has not significantly changed since the origin of life billions of years ago, experiments under altered gravity conditions allow researchers to address the impact of the unique gravitational stimulus on living organisms with regard to development, physiology, and behavior. As experiments under real microgravity can only infrequently be accomplished for obvious logistical constraints and limitations, as well as exorbitant costs, researchers try to simulate these conditions in ground-based facilities (Herranz et al., 2013). Strong static magnetic field gradients are one approach that has been used with the intent to compensate for Earth's gravitational field for the exposed systems (Berry and Geim, 1997). Due to the magnetic susceptibilities of material that is exposed to a magnetic field gradient within an inhomogeneous field, that material experiences a force against or toward high field areas (Brooks et al., 2000). Diamagnetic materials will be pushed away from the field center. If a vertical magnetic field is applied and the diamagnetic material is exposed in the gradient above the field center, it will experience a force acting against the gravitational force. Magnetic levitation thus is achieved when the magnetic force exactly counterbalances the gravitational force; the resulting situation is predicted to be comparable with “0g” (Valles et al., 1997; Meisel and Brooks, 2012).
1.1. Magnetic levitation versus real microgravity—the biological test systems
Various biological systems have been exposed to magnetic levitation ranging from cell cultures (e.g., Babbick et al., 2007; Hammer et al., 2009), bacteria (Dijkstra et al., 2011; Liu et al., 2011), and yeast (Coleman et al., 2007) to more complex systems, such as plants (Arabidopsis) and insects (Drosophila melanogaster) (Herranz et al., 2012), frog eggs (Valles et al., 1997), frogs (Berry and Geim, 1997), and mice (Liu et al., 2010).
Behavioral issues based on magnetic levitation have not been satisfactorily compared and interpreted on the basis of effects seen under actual spaceflight and thus real microgravity conditions. Consequently, it remains difficult to make a distinction between the effects of a magnetic field (for review, see Galland and Pazur, 2005) and the effects of real microgravity on behavioral phenomena. Can microgravity be achieved by exposing samples to a high magnetic field gradient? To answer this question, a suitable test system is needed.
In the present study, we exposed protists to vertical strong magnetic fields and field gradients with online microscopic observation. These organisms were chosen due to their clearly visible graviresponses and the detailed knowledge of their behavior in real microgravity conditions (Machemer and Bräucker, 1992; Hemmersbach and Häder, 1999; Häder et al., 2005, 2006).
Paramecium and the chloroplast-bearing Euglena swim by the beat of their movement organelles (cilia, flagellum). Both organisms use stimuli from the environment for their orientation, which results, for example, in phototaxis, chemotaxis, galvantotaxis, magnetotaxis, and gravitaxis (Häder et al., 2005). Under normal gravity conditions (1g), Paramecium and Euglena show a negative gravitaxis, which means that their preferred swimming orientation is directed upward. Gravity also can induce changes in the speed of locomotion, a phenomenon called gravikinesis (Machemer et al., 1991). Due to their actively speeding up during upward swimming and deceleration during downward swimming, protists (mainly ciliates) manage to at least partially counterbalance their passive sedimentation, which enables them to stay in optimal habitats. Häder et al. (1997) and Machemer-Röhnisch et al. (1999) discussed the controversial subject of gravikinesis with regard to Euglena.
According to a recent working model, gravitaxis and gravikinesis are the result of a physiological mechanism supported by physical properties of the cell (for review see Häder et al., 2005; Machemer and Bräucker, 1992). Besides the passive torque of the cells, which is based on an unequal mass distribution (buoyancy effect), the cells perceive gravity via a modified statolith mechanism: as the cytoplasm of the protists exceeds the density of the surrounding medium by about 4%, it sediments. As a consequence, a force acts on different parts of the cell membrane depending on the orientation of the cell and thereby stimulates mechanosensitive ion channels. It has been suggested that triggering a distinct orientational response is based on a polar distribution of the ion channels, which indeed has been shown in the case of Paramecium by electrophysiological studies (Krause et al., 2010) and has been postulated in the case of Euglena (Lebert and Häder, 1996). Due to the stimulation of the sedimenting mass of the cytoplasm, the resulting change in calcium/potassium conductance of the cell membrane leads to a change of the membrane potential, which triggers reorientational movements of the cilia/flagella and finally the orientation of the whole cell.
Exposing Euglena and Paramecium to real microgravity conditions (drop tower, parabolic flights of aircrafts, or rockets) results within seconds in a loss of gravitaxis and gravikinesis (e.g., Hemmersbach-Krause et al., 1993a, 1993b; Häder et al., 1995, 1996).
In circumstances where magnetic levitation provides an experimental approach to achieve “weightlessness” (microgravity) conditions, a similar behavior as that of swimming cells in real microgravity is to be expected, that is, the transition from gravitaxis to random swimming and equal swimming velocities in all directions. If levitation can be achieved, immobilized cells should no longer sediment, as is the case in 1g, but decrease their sedimentation velocity and finally float.
Several reports on the swimming behavior of protists under the influence of a magnetic field have already been presented and state that the behavior of Paramecium is affected such that changes in swimming velocities and increases in directional turns in swimming paths occur (Rosen and Rosen, 1990). Parallel swimming of P. caudatum with respect to the direction of the applied strong magnetic field (∼3 T) (Fujiwara et al., 2006; Guevorkian and Valles, 2006a, 2006b), as well as perpendicular swimming of P. aurelia to a static magnetic field of moderate intensity (0.68 T) (Nakaoka et al., 2002), has been observed. Tanimoto et al. (2002) described magnetotaxis of E. gracilis in a horizontal magnetic field gradient. The authors made a short statement that no magnetotaxis was observed in a uniform horizontal magnetic field, which in turn induces a perpendicular movement of living and dead Euglena with respect to the field direction.
In the present study, we addressed this aspect, that is, the impact of structural properties of the cells on the direction of orientation in a magnetic field, by direct observation and comparison of the swimming behavior of P. biaurelia, E. gracilis, and the chloroplast-free species Astasia longa and E. gracilis 1F in magnetic field strengths up to 30 T. The comprehensive understanding of these protists' behavior, that is, gravitaxis at 1g, random distribution in real microgravity, as well as sedimentation of immobilized cells in 1g, qualifies them as test systems to answer the following questions: Does magnetic levitation provide microgravity conditions, and can levitation of the protists be achieved?
2. Material and Methods
2.1. Cell cultures
Paramecium biaurelia Sonneborn(CCAP, Culture Collection of Algae and Protozoa, Ambleside, UK) was cultured at 20°C in straw medium (pH 7.2) in darkness. A total of 15 g of straw was cooked in 1 l A. bidest. for 15 min. This medium was disposed. After further cooking of the straw in 1 l A. bidest., the medium was ready to use after cooling. The culture was started by inoculation with 200 mL of an already existing cell culture. This medium was selected according to previously performed experiments in microgravity (Hemmersbach-Krause et al., 1993a). Cells were enriched without centrifugation to avoid mechanical stress before the start of the experiments. To do so, the cells were transferred to falcon flasks, where they assembled due to their negative gravitaxis and positive aerotaxis in the neck of the bottle, from where they were finally transferred to the observation chambers. Euglena gracilis, Astasia longa, and E. gracilis 1F were obtained from the Culture Collection of Algae at Goettingen University (SAG; Goettingen, Germany). Euglena gracilis was cultured in mineral medium (Starr, 1964) at 20°C and 24 h continuous light of about 18 W/m2 from mixed cool white and warm-tone fluorescent lamps. Astasia longa and E. gracilis 1F were cultivated in complex medium (Checcucci et al., 1976) in 100 mL Erlenmeyer flasks at 20°C in continuous darkness. The age of the cultures for experimentation was at least two weeks. If a culture was too dense for video analysis (quantified by image analysis), it was diluted with filtered medium.
2.2. Cell immobilization
For sedimentation experiments, P. biaurelia was immobilized by means of NiCl2 (0.04 g/100 mL). According to Kuznicki (1963), NiCl2 was dropwise added (but did not exceed a final concentration of 0.125 mM) under microscopic control to achieve the arrest of the ciliary beat and thus stop swimming but allow contractile vacuole activity to persist. Euglena gracilis, Astasia longa, and E. gracilis 1F were temporarily immobilized by means of liquid nitrogen. An amount of 500 μL of cell culture was placed into a safe lock flask and dropped for 60 s in liquid nitrogen. The frozen cell culture was thawed on ice. This procedure does not kill the cells but immobilizes them for 2–3 h. Microscopic observation revealed no impact on the cell form by the applied immobilization procedures.
2.3. Experiments in the magnet
The experiments were performed in a 50 mm bore Florida-Bitter magnet up to a field strength of 30 T at the High Field Magnet Laboratory at Nijmegen, the Netherlands. The exposition bore was tempered at 20°C by water inside a double-walled metal tube, which leaves a 40 mm diameter exposition area for the experiment. Observation of the cells in the magnet was possible by a special mirror imaging system and a CCD camera for recording (Fig. 1). The focal distance of the lens for observation of Paramecium was 250 mm, for observation of Euglena 80 mm. The cells were observed in two positions within the magnet. At position 1, in the center of the magnet, the field strength (B0) is maximum, and the field gradient is zero. This position is used to study the effect of the magnetic field under normal gravity conditions. At position 2, 82 mm above the field center, the field strength is lower (B=0.76 B0), but the gradient of the magnetic field is strongest. This results in a magnetic force upward (opposed to the gravitational force) that is proportional to the product of the field strength and the field gradient (BB′-value) of BB′=4.95 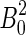 m−1, leading to a maximum BB′ of 4455 T2/m (at B0=30 T) (Beaugnon and Tournier, 1991). Due to the configuration and the restricted space within the magnet, we could not use circular chambers. Such circular chambers are, due to their chamber geometry, better suited for orientation studies and were also used in our microgravity experiments (Hemmersbach-Krause et al.,
1993a). For exposition in the magnet, the cells had to be observed in rather small cuvettes (Präzisionsküvetten, Hellma, Müllheim, Germany) as follows: Paramecium in cuvettes of 52×12.5×3.5 mm (350 μL cell culture), Euglena, Astasia, and E. gracilis 1F in 45×12.5×0.2 mm (50 μL). Control experiments under 1g conditions revealed no impact on vitality and alignment and thus confirmed suitability of the hardware for the experiments. To avoid phototactic behavior of the cells, the observation was performed with a red LED lamp (wavelength 630 nm), as the photoreceptor of Euglena gracilis is not sensitive in the red spectral region. In consequence, transition to red light is equal to transition into darkness from the perspective of the cells. Furthermore, the construction of the cuvette avoids any gradient of visible light, and the duration of the experiment by far exceeds the initial time of photophobic response. We can therefore certify that the change of irradiation did not interfere with the response in the magnetic field.
m−1, leading to a maximum BB′ of 4455 T2/m (at B0=30 T) (Beaugnon and Tournier, 1991). Due to the configuration and the restricted space within the magnet, we could not use circular chambers. Such circular chambers are, due to their chamber geometry, better suited for orientation studies and were also used in our microgravity experiments (Hemmersbach-Krause et al.,
1993a). For exposition in the magnet, the cells had to be observed in rather small cuvettes (Präzisionsküvetten, Hellma, Müllheim, Germany) as follows: Paramecium in cuvettes of 52×12.5×3.5 mm (350 μL cell culture), Euglena, Astasia, and E. gracilis 1F in 45×12.5×0.2 mm (50 μL). Control experiments under 1g conditions revealed no impact on vitality and alignment and thus confirmed suitability of the hardware for the experiments. To avoid phototactic behavior of the cells, the observation was performed with a red LED lamp (wavelength 630 nm), as the photoreceptor of Euglena gracilis is not sensitive in the red spectral region. In consequence, transition to red light is equal to transition into darkness from the perspective of the cells. Furthermore, the construction of the cuvette avoids any gradient of visible light, and the duration of the experiment by far exceeds the initial time of photophobic response. We can therefore certify that the change of irradiation did not interfere with the response in the magnetic field.
FIG. 1.
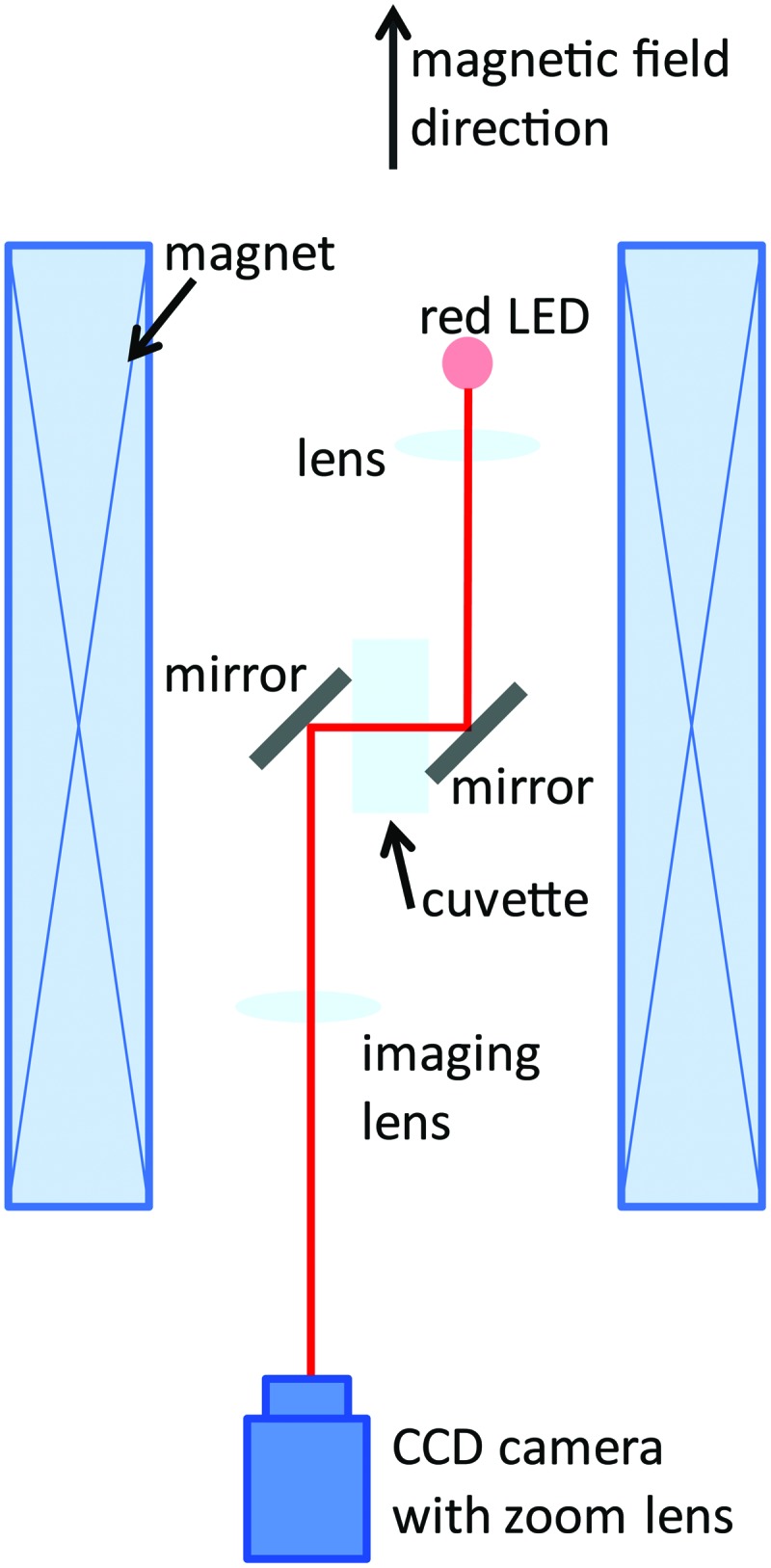
Optical imaging setup within a water-cooled Florida-Bitter electromagnet. The magnetic field strength (0–30 T) and the magnetic field gradient depend on the value of the electrical current through the magnet coil and the vertical position within the magnet. The light beam of a light-emitting diode (LED, wavelength 630 nm) is collimated using a lens, guided through the sample cuvette by aluminum mirrors, and focused on a charge-coupled device (CCD) camera with an imaging lens [focal length 250 mm (Paramecium) or 80 mm (Euglena)] and a zoom lens. (Color graphics available online at www.liebertonline.com/ast)
The measurements of sedimentation and swimming at each of the different field intensities were performed for 2 min. Control measurements for the total exposure time (60 min) were performed in the home lab at Cologne and within the magnet at 0 T (local field), which means that the magnet was switched off and just the local, geomagnetic field of Nijmegen was acting.
2.4. Analysis of the swimming tracks
Video tracks from the CCD camera were directly stored on a computer as wmv files. The video sequences were analyzed with a cell tracking system (Wintrack 2000, Lebert and Häder, 1999). The Wintrack version used in this project did not have the capacity to read digital signals, so it was necessary to analogize the wmv signal first. To capture the images, the movie was displayed by a computer with the screen resolution set to 800×600 pixels. The digital signal of the VGA output was again transferred into an analog video signal by way of a VGA-to-S-video converter (Universal PC-TV Konverter, Conrad, Hirschau, Germany). The resulting analog signal was connected to a second computer equipped with a USB-frame grabber (G3 USB 2.0, Terratec, Nettetal, Germany), which digitized the video stream again. Despite the 3-fold conversion of the signal, the video quality was not noticeably impaired. Objects were detected by brightness differences between cells and the background. For each single video frame (40 ms), the system analyzed the spatial position of every cell. To perform motion analysis, the software measures the shift of each object after five consecutive frames (200 ms). Resulting single movement vectors were statistically pooled to calculate mean population measurements such as mean velocity, motility, or precision of movement of a cell. In the present study, we focused primarily on the alignment parameter, which indicates whether a cell moves more vertically (along the y axis) or horizontally (along the x axis).
The alignment parameter is defined by the equation
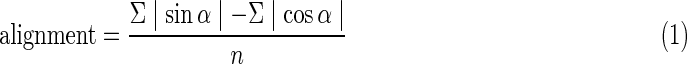 |
Alignment of +1 is an absolute parallel orientation of the cells to the y axis, whereas 0 is a random swimming distribution of the cells. The value −1 represents a parallel adjustment to the x axis.
Another parameter employed is the r value, which is a measure for precision of orientation. The r value ranges from 0 (random orientation) to 1 (precise orientation) and is calculated as follows (Batschelet, 1985):
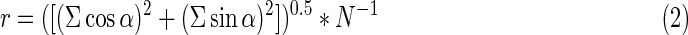 |
with α: deviation from stimulus direction (here, acceleration), N: number of recorded cell tracks.
To yield more detailed information from the tracks, all single movement vectors were retrieved and classified according to the angle of movement; cells aligned to sectors 157.5–202.5° and 337.5–22.5° are classified as vertically oriented, cells aligned to sectors 67.5–112.5° and 247.5–292.5° as horizontally/perpendicular oriented, and cells aligned to the remaining sectors as diagonal oriented. It is important to note that the angle representation is not Cartesian.
Gravikinesis was calculated according to Machemer et al. (1991):
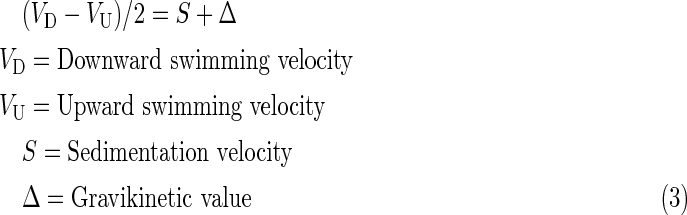 |
2.5. Result analyses
Data are given as mean±standard deviation. Gaussian distributed data were compared by using the Student t test. In the case of data that were not normally distributed, the Mann-Witney U test was applied. Significance level: α=0.05. Test for normality was Kolmogorov–Smirnov with Lilliefors correction. All tests were calculated with SPSS (version 19).
3. Results
Successful adaptation of a microscope device within the magnetic facility enabled online observation of the cells during their exposure in the magnetic field under different field intensities.
3.1. Levitation of immobilized cells was not achieved
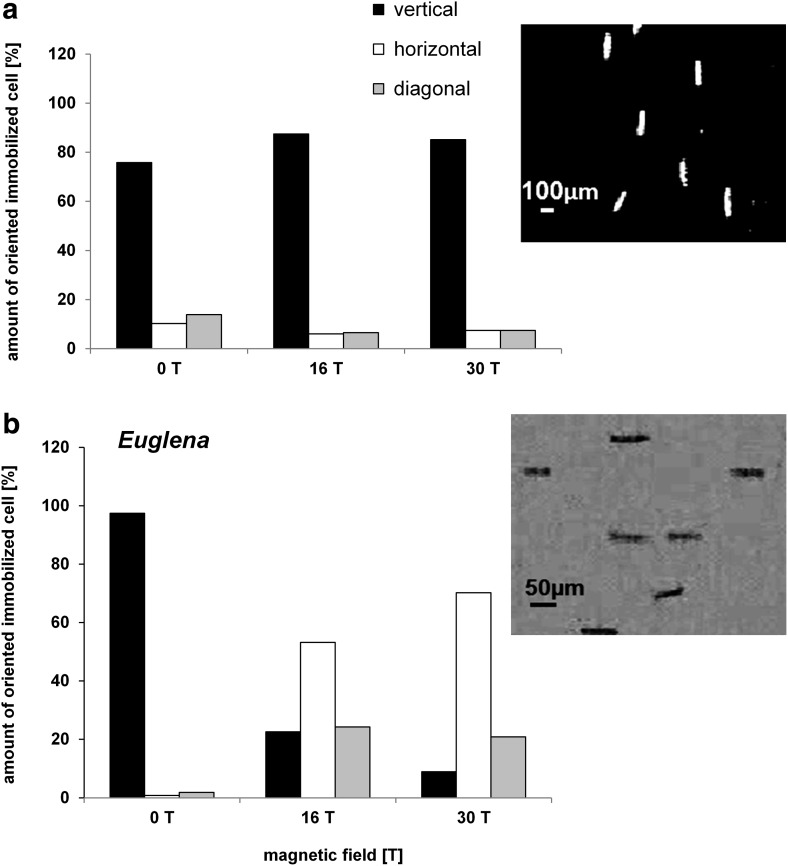
To determine the levitation point of immobilized Euglena and Paramecium cells, the cells were transferred into the magnet (at position 2, 82 mm above the field center) and observed under a stepwise increased magnetic field strength. Sedimentation of immobilized Paramecium and Euglena could not be compensated for even at the highest field strength used, which was B0=30 T (BB′=4455 T2/m). (Note that the actual maximum field strength is then 22.8 T at this position 2, 82 mm above the field center). Both cell types still sedimented; thus their levitation could not be achieved. The sedimentation speed of Paramecium biaurelia was even slightly (but significantly) higher in the magnetic field gradient [74.2±6.7 μm/s at BB′=0; 78.8±8.0 μm/s at BB′=4455 T2/m (n>90)]. The sedimentation speed of Euglena gracilis was lower at increased strength of the magnetic field [BB′=0: 13.8±2.5 μm/s to BB′=4455 T2/m: 10.9 μm/s±6.5 (n>92)]. Immobilized cells showed different angular alignments of their longitudinal axis with respect to the direction of the magnetic field. Paramecium cells were aligned in parallel (vertical orientation of its long axis); in contrast, Euglena cells were aligned perpendicular (horizontal position) with respect to the direction of the field (Fig. 2). At 0 T (control, magnet off), immobilized Paramecium cells sedimented predominantly with a vertical orientation (75%). The percentage of vertically aligned cells rose to 87% under the influence of the magnetic field. A quite different sedimentation behavior was revealed in the case of Euglena. At 0 T, Euglena cells also sedimented predominately with a vertical orientation (97%); however, under the influence of the magnetic field, 70% of the cells were forced in a horizontal position, while only 8% remained vertically oriented (Fig. 2b).
FIG. 2.
Orientation of immobilized cells (a) Paramecium biaurelia, (b) Euglena gracilis in an inhomogeneous magnetic field gradient, demonstrated by the percentage of cells in vertical (0°/180°±22.5°, black), horizontal (90°/270°±22.5°, white), and diagonal (45°/135°/225°/315°±22.5°, gray) direction. Each column represents n>1500 cells. Corresponding microscope pictures demonstrate the species-specific orientation with respect to the vertically applied magnetic field of 30 T (for imaging setup see Fig. 1).
To disentangle the effect of the magnetic field itself and the magnetic levitation forces, the orientation of immobilized cells was not only observed in the inhomogeneous field (position 2, 82 mm above the field center) but also compared with the behavior in the field center (position 1, field center). By this approach, it could be tested whether the passive orientation is a direct effect of the magnetic field itself, which was in fact the case for our systems due to the following results. In the field center, immobilized Paramecium also aligned in the same manner with respect to the field lines, as revealed by measurements at five different field intensities in 3 T steps. At 3 T, 90% of the Paramecium cells were vertically aligned, reaching a maximum alignment of 94% at 12 T. The same was true for immobilized Euglena, which reoriented perpendicular to the magnetic field.
3.2. Orientation and alignment of swimming cells in the magnetic field
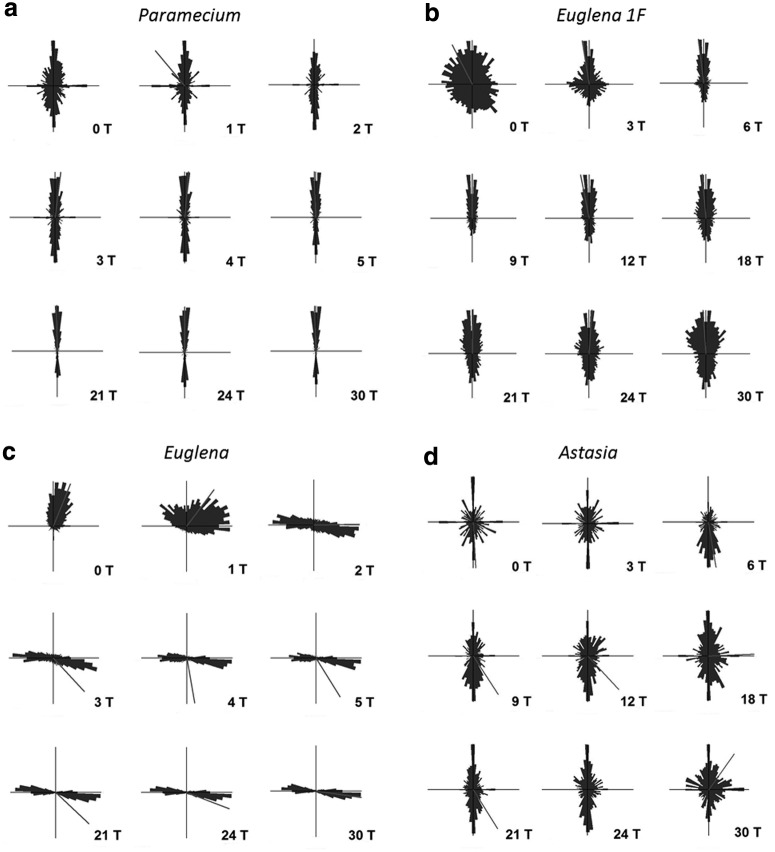
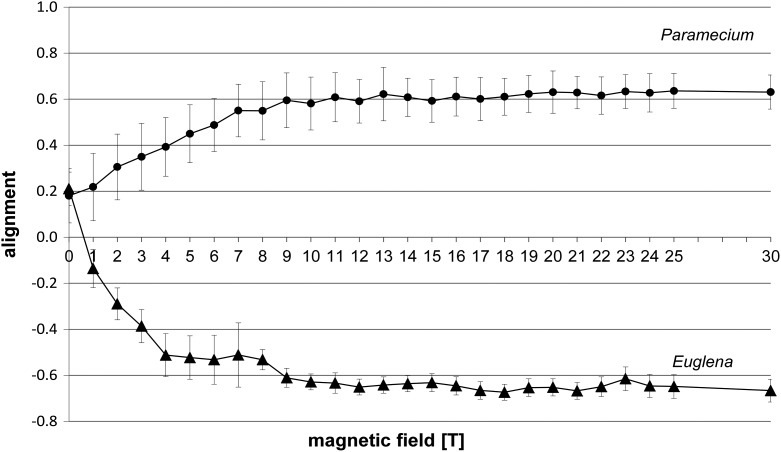
Without the magnetic field, observation of swimming Paramecium cells showed a bipolar distribution with a slight preference of upward swimming (a weak negative gravitaxis). Representative circular histograms are shown in Fig. 3 (0 T). The value of the alignment was 0.18 (±0.12) at 0 T (Fig. 4). Application of a magnetic field up to 30 T induced a precision of the swimming direction parallel to the applied magnetic field (Fig. 3).
FIG. 3.
Circular histograms demonstrating the degree of orientation of mobile (a) Paramecium biaurelia, (b) chloroplast-free E. gracilis 1F, (c) Euglena gracilis, and (d) Astasia longa at different magnetic field intensities. Each histogram represents n>2000 cells.
FIG. 4.
Alignment of mobile cells Paramecium biaurelia and Euglena gracilis at different magnetic field intensities measured in the field center (position 1, field center). Each point of measurement represents n>150 cells.
The increase of alignment correlated with the increase of the magnetic field intensity and remained from 11 T on in the range of ≥0.6 up to the maximum of 30 T (Fig. 4). Analysis of the swimming velocities revealed that gravikinesis still exists at 30 T. With the values of downward, upward, and sedimentation velocities, a gravikinetic value of −43 μm/s was calculated (downward swimming velocity 378 μm/s; upward swimming velocity 306 μm/s), which is similar to the situation at 1g, without the influence of a magnetic field.
Without a magnetic field, Euglena also demonstrated a negative gravitaxis with a degree of alignment of 0.21±0.07 (Fig. 4). The corresponding circular histogram shows a significant upward orientation (r value 0.5, theta 22°) (Fig. 3; 0 T). Application of the magnetic field forced the swimming cells to a perpendicular alignment and, thus, a horizontal swimming direction with respect to the applied magnetic field (Fig. 4). While gravitaxis of Euglena is characterized by a pronounced orientation normally in one direction, the orientation of the cells under the influence of the magnetic field becomes bimodal (biphasic), losing the preference to one direction (Fig. 3). The degree of the alignment increased very quickly up to a magnetic field strength of 10 T (0.68±0.05) and remained in this range up to the maximum of 30 T tested (Fig. 4).
3.3. Structural cellular characteristics influence orientation within the magnetic field center
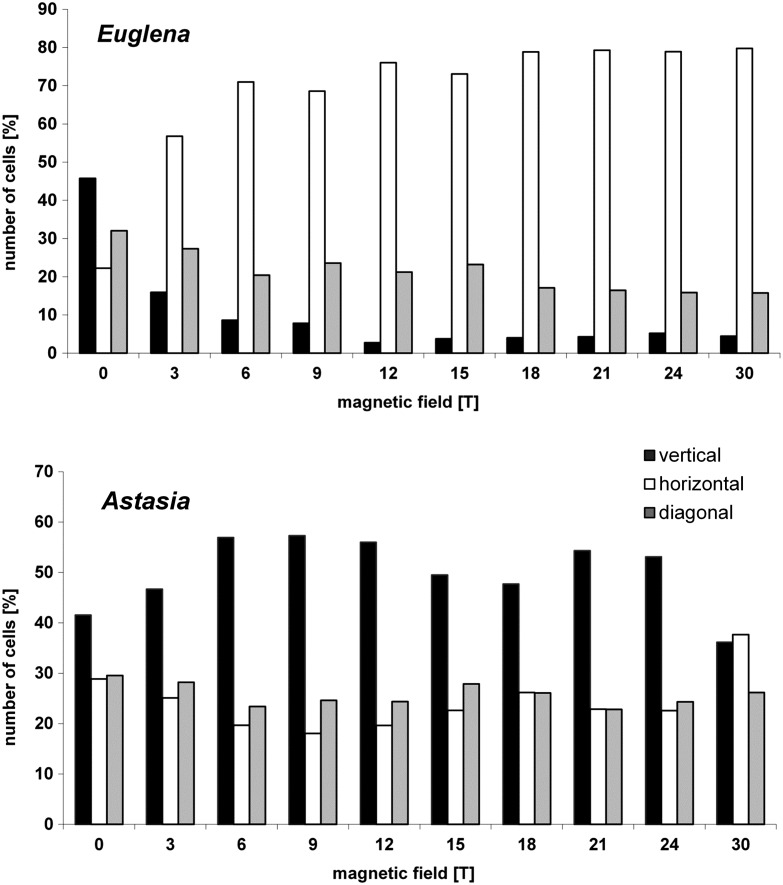
To contribute to the understanding of the different swimming and orientation directions of the exposed protists, the chloroplast-free mutant of Euglena—E. gracilis 1F—as well as Astasia longa, a flagellate similar to Euglena but lacking chloroplasts and the photoreceptor—were exposed to high magnetic fields (field center, position 1). Both entities showed a completely different behavior compared to the wild-type E. gracilis. Immobilized E. gracilis 1F sedimented at 0 T with a preference of vertical orientation (68%) (n=4642). Sedimentation persisted with rising magnetic field strength, though orientation of the immobilized cells shifted to a preference of a horizontal (42% at 10 T) and diagonal (43% at 10 T) direction.
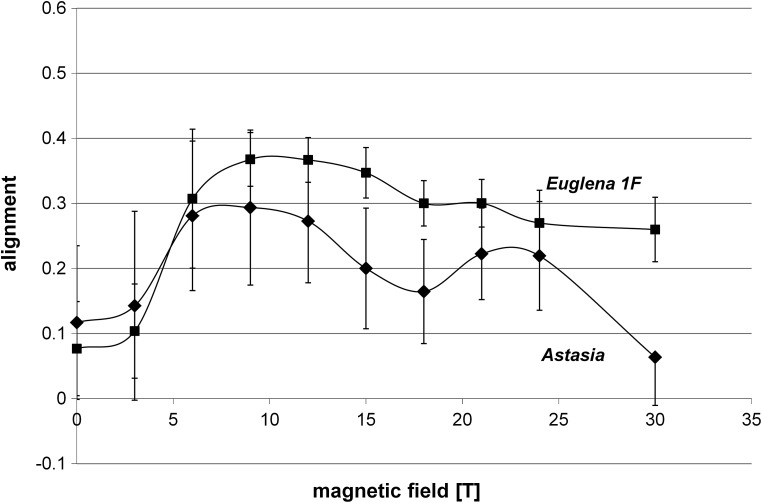
Swimming E. gracilis 1F showed a random distribution with a slight preference of upward swimming and thus a weak negative gravitaxis at 0 T (Fig. 3b). With rising magnetic field strength, the precision of orientation and alignment of E. gracilis 1F increased with respect to the direction of the applied field. The maximum of vertical alignment was reached at 12 T (0.37±1.13); then it slightly declined at 30 T, alignment 0.26±0.09 (Fig. 5). To summarize, swimming E. gracilis 1F remained vertically aligned with increasing magnetic field strength, which was similar to Paramecium behavior, though the alignment for E. gracilis 1F was less pronounced than was that of Paramecium. Wild-type E. gracilis, however, shifted from the vertical to a horizontal orientation with respect to the field direction.
FIG. 5.
Alignment of mobile cells E. gracilis 1F and Astasia at different magnetic field intensities measured in the field center (position 1, field center). Each point of measurement represents n>150.
Immobile Astasia longa sedimented at 0 T preferentially vertically oriented. Increasing the magnetic field induced a decrease of vertically aligned immobilized cells, while the amount of diagonally and horizontally oriented cells increased, which was in contrast to Euglena in that they shifted from a vertical to a horizontal orientation. Mobile Astasia longa showed a bipolar distribution with respect to the vertical at 0 T (Figs. 3d, 5, 6). Increasing magnetic field strength had no severe impact on the orientation and the degree of alignment. Thus, in contrast to E. gracilis and immobilized Astasia longa cells, the vertical orientation of swimming Astasia longa persisted (Fig. 3, Fig. 6).
FIG. 6.
Orientation of swimming Euglena and Astasia in the field center, differentiated in vertical (0°/180°±22.5°, black), horizontal (90°/270°±22.5°, white), and diagonal (45°/135°/225°/315°±22.5°, gray) direction. Each column in represents>2300 (Euglena) and >50 (Astasia) cells. Notice the corresponding shift from vertically aligned mobile wild-type Euglena in contrast to the chloroplast-free Astasia.
4. Discussion
In the literature, magnetic levitation frequently is recommended as a ground-based experimental approach to achieve a reduced gravity environment and in best cases “weightlessness” conditions due to the levitation of diamagnetic substances/objects. The biological model systems selected in the present study offer a significant advantage in that they can be easily observed and their gravity-dependent behavior (gravitaxis, gravikinesis) is well studied and established under normal gravity (1g) and under real microgravity (free fall) conditions. Because of this, we chose the degree of orientation, the swimming and sedimentation velocities, and the linearity of swimming paths as experimental parameters for our comparative approach (real microgravity vs. magnetic levitation).
4.1. Reason for alignment
Our study contributes to the search for structures that determine the orientation of the model systems subjected to the magnetic field. Our finding of the parallel alignment of Paramecium biaurelia in a high magnetic field confirms the data of Guevorkian and Valles (2006a) and Fujiwara et al. (2006), who exposed P. caudatum to a vertical or horizontal high magnetic field with a maximum of 9 T. By comparing the orientation of swimming P. caudatum cells with immobilized ones, Guevorkian and Valles (2006a, 2006b) concluded that the observed orientation and increased alignment in the magnetic field >3 T is the result of a passive response to a torque that the magnetic field exerts on diamagnetic anisotropic structures in the cell cortex. This means that cells align according to their anisotropic magnetic susceptibility (Rosen, 2003). The alignment has to be defined by a structure with a high magnetic susceptibility, which has a determined location in the cell or a repetitive structure. In this context, Guevorkian and Valles (2006a) suggested “cortical units” as repetitive structures in the pellicle of Paramecium. Except for the region of the oral groove, the cortex consists of identical units including the cell membrane, cilia, trichocysts, fibrils, and microtubular structures (Hausmann et al., 2003). Modeling of the components and the assumption of the net anisotropy of the units support the hypothesis that these structures determine the alignment of the cells along the magnetic field. This was shown for different Paramecium species as follows: P. multimicronucleatum, P. tetraaurelia, P. caudatum (Guevorkian and Valles, 2006b), and now for P. biaurelia in the course of the present study. Nakaoka et al. (2011) assumed that membrane lipid fluidity is an important factor that determines the magnetic orientation. They found differences, parallel as well as perpendicular swimming of different species (syngens) from the P. aurelia complex. Also, syngen-dependent variations in magnetic sensitivity were correlated with membrane fluidity, obviously due to variations in lipid synthesis. In a previous study, this group also investigated isolated cell organelles with respect to their diamagnetic anisotropy and thus orientation within a static magnetic field of 0.78 T; cilia as well as trichocyts oriented in parallel, while isolated macronuclei showed no tendency to align. Thus, orientation is determined by the diamagnetic anisotropy of the cell organelles (Nakaoka et al., 2002).
In contrast, swimming as well as immobilized Euglena gracilis demonstrated a perpendicular orientation with respect to the applied field, which thus confirms the observation of Tanimoto et al. (2002) with E. gracilis at 8 T. Our data obtained with the chloroplast-free mutants Astasia longa and E. gracilis 1F demonstrate a completely different behavior in the magnetic field compared to wild-type Euglena. Furthermore, the orientation of swimming cells differed from that of immobilized cells. We conclude that the existence of chloroplasts and their characteristic distribution within the cell determines the perpendicular reorientation of Euglena wild type, as it has been shown that isolated chloroplasts orientate perpendicular to a magnetic field (Geacintov et al., 1971, 1974; Papp and Meszena, 1982). Due to an association with the actin cytoskeleton, the 10–15 chloroplasts in Euglena are kept in parallel position to the long axis of the cell (Schöpfer, 1989), which guarantees an optimal absorption of the light. This arrangement of the chloroplasts is obviously responsible for the perpendicular orientation of Euglena and the lack of this response of the chloroplast-free species. In contrast to E. gracilis 1F, Astasia longa has no photoreceptor and as a consequence lost the capacity of phototactic orientation (Ntefidou and Häder, 2005). Our data show no significant difference in the behavior of these two species compared to Euglena, excluding the photoreceptor as a determining factor for the passive orientation in the magnetic field. We will now consider the different alignment of mobile and immobilized cells in the case of the chloroplast-free Euglena species. The cells contain different structures of different anisotropic values. It can be assumed that the passive orientation is determined by the structure with the highest anisotropy. As swimming cells rotate, there is no constant situation, and the magnetic force might increase the torque of different structures over time. The increase of diagonally aligned immobile cells might be caused by the active swimming of the cells and/or by the helical arrangement (Storch and Welsch, 2002) of the microtubules in the pellicle of Euglena. Isolated microtubules orient in parallel with respect to a magnetic field (Vassilev et al., 1982); thus, their helical arrangement in Euglena makes the diagonal orientation of the chloroplast-free cells likely. Due to our observations, we can assume that in the case of chloroplast-bearing cells the anisotropy of the chloroplast rather than that of the microtubules determines the orientation.
4.2. Levitation
Even at BB′=4455 T2/m (B0=30 T), the maximum that can be achieved in the magnet used, immobilized cells of P. biaurelia and E. gracilis still sedimented, which indicates that their point of levitation is not achieved under the applied experimental conditions.
Under increasing magnetic field, the sedimentation velocity of Euglena slightly decreased, and that of Paramecium was even slightly enhanced. The reason for this is due to the different orientations and thus the induced drag and friction. A sedimenting body being aligned in parallel to the direction of the g-vector induces less turbulences in the surrounding medium and consequently an increased sedimentation velocity (Paramecium) than a horizontal or diagonal aligned one (Euglena).
The applied magnetic field gradient of BB′=4455 T2/m is not sufficient to levitate the cells. By using the cell parameters for Paramecium given by Guevorkian and Valles (2006b), a BB′ value of=7044 T2/m is needed to exert a sufficient force on the cells to levitate them, which is technically challenging but feasible with the use of either a 45 T hybrid magnet (National High Magnetic Field Laboratory, Tallahassee) or the new 38 T Florida-Bitter magnet at the High Field Magnet Laboratory at Nijmegen, which is currently under construction.
Guevorkian and Valles (2006a, 2006b) tried to overcome this problem by manipulating the medium in which the cells were to be exposed. They showed that, by application of Gd-DTPA (gadolinium diethylene triamine pentaacetate), the magnetic susceptibility of the medium is changed due to the so-called magneto Archimedes effect, a method successfully used in material sciences (Hirota et al., 2002). By this approach, it is principally possible to levitate Paramecium. The parameter of judgment was the disappearance of the g-dependent swimming velocities (gravikinesis). They demonstrated that, in a magnetically simulated gravity environment, the gravikinetic behavior of Paramecium caudatum is similar to those observed in centrifugation experiments. A linearity of the gravikinetic response was shown between −5g and 5g, meaning that the cells swim faster downward compared to upward at 5g and vice versa at −5g. Consequently, a “0g” value exists that is characterized by similar swimming velocities in both directions and thus a loss of gravikinesis in Paramecium, as it is observed in microgravity. However, untreated cells in their original medium, as was used for our experiments, still showed a gravikinesis at 30 T, meaning that upward and downward swimming velocities differed, which is not the case in real microgravity, where sedimentation has no impact. Even at 30 T, Paramecium is still able to partially compensate their sedimentation velocity due to an increased upward swimming and a decreased downward swimming compared to cells without gravikinesis, in which sedimentation simply adds to downward swimming velocities and subtracts from upward swimming. The gravikinetic value of Paramecium of −43 μm/s at 30 T is in the range of published values for Paramecium species at 1g. We decided against the technical approach used by Guevorkian and Valles (2006a, 2006b), as gadolinium ions are known to act as blockers of mechanosensitive ion channels (Millet and Pickard, 1988) and have severe impacts on the behavior of protists. Already low concentrations (4–10 μM) of the trivalent cation gadolinium alter the membrane potential of Paramecium tetraurelia, which suggests an interference with membrane channels and reduction in the gravity-induced modulation of the swimming speed (gravikinesis) (Nagel and Machemer, 2000). Gadolinium ions (100 μM) even specifically inhibit gravitaxis of Euglena, without impact on motility and phototaxis (Lebert et al., 1997).
Because all living matter is diamagnetic, it is in principle possible to levitate biological specimens, obviously without any detrimental effects. However, due to the fact that living objects contain diamagnetic and paramagnetic substances, the inhomogeneous magnetic field induces opposite effects that might induce strange sensations during levitation of the object (Berry and Geim, 1997).
Even if a system can be levitated, it is not possible to separate the experience of a reduced gravity stimulation from the strong influences of the magnetic field itself, which has also been shown in the case of mammalian cell cultures as revealed by alterations of the actin systems by the magnetic field itself (Moes et al., 2011). We cannot exclude that the transfer of an object and thus its previous experience from a small local field (near geomagnetic) to a huge field in the tesla range induces new effects such as the induction of an electric current that might change, for example, the electrical potential of cell membranes. In our experimental approach, the application of the field does not occur instantly but in a slow sweep up that takes several minutes, which is assumed to have no appreciable induced electrical current in the exposed cell. Due to the low conductance value of Paramecium, a potential electrical effect would be low and is difficult to estimate and only measurable by electrophysiological studies of a cell in the magnet. If electrical signals are induced, this would in addition mask expected effects of functional weightlessness.
With respect to gravitactic protists, we conclude that exposure to a strong magnetic field does not provide functional weightlessness conditions for them as indicated by persisting gravitaxis, gravikinesis, and sedimentation, which all disappear in real microgravity. Whether this conclusion holds for systems other than those tested will have to be investigated. The restricted area of exposure within a magnet, and thus limited material that can be used, results in further restrictions as to how life science experiments can be carried out within a magnet, and these restrictions should be carefully considered in future studies.
Control experiments and an understanding of what occurs in real microgravity are an absolutely necessity if researchers are to avoid misinterpretation of results obtained in a levitron or other type of ground-based facility with the intent to achieve functional weightlessness.
Acknowledgments
Magnetic levitation at the High Field Magnet Laboratory was granted by EuroMagNET II to R.H. and grant 20WB0828 of the German Space Administration on behalf of Bundesministerium für Wirtschaft und Technologie to M.L.
References
- Babbick M., Dijkstra C., Larkin O.J., Anthony P., Davey M.R., Power J.B., Lowe K.C., Cogoli-Greuter M., and Hampp R. (2007) Expression of transcription factors after short-term exposure of Arabidopsis thaliana cell cultures to hypergravity and simulated microgravity (2-D/3-D clinorotation, magnetic levitation). Adv Space Res 39:1182–1189 [Google Scholar]
- Batschelet E. (1985) Circular Statistics in Biology, Academic Press, London [Google Scholar]
- Beaugnon E. and Tournier R. (1991) Levitation of organic materials. Nature 349:470 [Google Scholar]
- Berry M.V. and Geim A.K. (1997) Of flying frogs and levitrons. European Journal of Physics 18:307–313 [Google Scholar]
- Brooks J.S., Reavis J.A., Medwood R.A., Stalcup T.F., Meisel M.W., Steinberg E., Arnowitz L., Stover C.C., and Perenboom J.A.A.J. (2000) New opportunities in science, materials, and biological systems in the low-gravity (magnetic levitation) environment. J Appl Phys 87:6194–6199 [Google Scholar]
- Checcucci A., Golombetti G., Fererra R., and Lenci F. (1976) Action spectra for photoaccumulation of green and colourless Euglena: evidence for identification of receptor pigments. Photochem Photobiol 23:51–54 [DOI] [PubMed] [Google Scholar]
- Coleman C.B., Gonzalez-Villalobos R.A., Allen P.L., Johanson K., Guevorkian K., Valles J.M., and Hammond T.G. (2007) Diamagnetic levitation changes growth, cell cycle, and gene expression of Saccharomyces cerevisiae. Biotechnol Bioeng 98:854–863 [DOI] [PubMed] [Google Scholar]
- Dijkstra C.E., Larkin O.J., Anthony P., Davey M.R., Eaves L., Rees C.E., and Hill R.J. (2011) Diamagnetic levitation enhances growth of liquid bacterial cultures by increasing oxygen availability. J R Soc Interface 8:334–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y., Tomishige M., Yasuhiro I., Fujiwara M., Shibata N., Kosaka T., Hosoya H., and Tanimoto Y. (2006) Effect of horizontal strong static magnetic field on swimming behaviour of Paramecium caudatum. Mol Phys 104:1659–1666 [Google Scholar]
- Galland P. and Pazur A. (2005) Magnetoreception in plants. J Plant Res 118:371–389 [DOI] [PubMed] [Google Scholar]
- Geacintov N.E., Van Nostrand F., Pope M., and Tinkel J.B. (1971) Magnetic field effect on the chlorophyll fluorescence in Chlorella. Biochim Biophys Acta 226:486–491 [DOI] [PubMed] [Google Scholar]
- Geacintov N.E., Van Nostrand F., and Becker J.F. (1974) Polarized light spectroscopy of photosynthetic membranes in magneto-oriented whole cells and chloroplasts. Fluorescence and dichroism. Biochim Biophys Acta 347:443–463 [DOI] [PubMed] [Google Scholar]
- Guevorkian K. and Valles J.M. (2006a) Aligning Paramecium caudatum with static magnetic fields. Biophys J 90:3004–3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevorkian K. and Valles J.M. (2006b) Swimming Paramecium in magnetically simulated enhanced, reduced, and inverted gravity environments. Proc Natl Acad Sci USA 35:13051–13056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häder D.-P., Rosum A., Schäfer J., and Hemmersbach R. (1995) Gravitaxis in the flagellate Euglena gracilis is controlled by an active gravireceptor. J Plant Physiol 146:474–480 [PubMed] [Google Scholar]
- Häder D.-P., Rosum A., Schäfer J., and Hemmersbach R. (1996) Graviperception in the flagellate Euglena gracilis during a shuttle space flight. J Biotechnol 47:261–269 [DOI] [PubMed] [Google Scholar]
- Häder D.-P., Porst M., Tahedl H., Richter P., and Lebert M. (1997) Gravitactic orientation in the flagellate Euglena gracilis. Microgravity Sci Technol 10:53–57 [PubMed] [Google Scholar]
- Häder D.-P., Hemmersbach R., and Lebert M. (2005) Gravity and the Behavior of Unicellular Organisms, Cambridge University Press, New York [Google Scholar]
- Häder D.-P., Richter P., and Lebert M. (2006) Signal transduction in gravisensing of flagellates. Signal Transduct 6:422–431 [Google Scholar]
- Hammer B.E., Kidder L.S., Williams P.C., and Xu W.W. (2009) Magnetic levitation of MC3T3 osteoblast cells as a ground-based simulation of microgravity. Microgravity Sci Technol 21:311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann K., Hülsmann N., and Radeck R. (2003) Protistology, Schweizerbart'sche Verlagsbuchhandlung, Stuttgart [Google Scholar]
- Hemmersbach R. and Häder D.-P. (1999) Graviresponses of unicellular organisms. FASEB J 13:69–75 [DOI] [PubMed] [Google Scholar]
- Hemmersbach-Krause R., Briegleb W., Häder D.-P., Vogel K., Grothe D., and Meyer I. (1993a) Orientation of Paramecium under the conditions of weightlessness. J Eukaryot Microbiol 40:439–446 [DOI] [PubMed] [Google Scholar]
- Hemmersbach-Krause R., Briegleb W., Vogel K., and Häder D.-P. (1993b) Swimming velocity of Paramecium under the conditions of weightlessness. Acta Protozool 32:229–236 [PubMed] [Google Scholar]
- Herranz R., Larkin O.J., Dijkstra C.E., Hill R.J.A., Anthony P., Davey M.R., Eaves L., van Loon J.J.W.A., Medina F.J., and Marco R. (2012) Microgravity simulation by diamagnetic levitation: effects of a strong gradient magnetic field on the transcriptional profile of Drosophila melanogaster. BMC Genomics 13, 10.1186/1471-2164-13-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz R., Anken R., Boonstra J., Braun M., Christianen P.C.M., de Geest M., Hauslage J., Hilbig R., Hill R.J.A., Lebert M., Medina F.J., Vagt N., Ullrich O., van Loon J.J.W.A., and Hemmersbach R. (2013) Ground-based facilities for simulation of microgravity: organism-specific recommendations for their use, and recommended terminology. Astrobiology 13:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota N., Ikezoe Y., Uetake H., Kaihatsu T., Takayama T., and Kitazawa K. (2002) Magneto-Archimedes levitation and its application. Riken Review 44:159–161 [Google Scholar]
- Krause M., Bräucker R., and Hemmersbach R. (2010) Gravikinesis in Stylonychia mytilus is based on membrane potential changes. J Exp Biol 213:161–171 [DOI] [PubMed] [Google Scholar]
- Kuznicki L. (1963) Reversible immobilization of Paramecium caudatum evoked by nickel ions. Acta Protozool 13:301–312 [Google Scholar]
- Lebert M. and Häder D.-P. (1996) How Euglena tells up from down. Nature 379:590. [DOI] [PubMed] [Google Scholar]
- Lebert M. and Häder D.-P. (1999) Image analysis: a versatile tool for numerous applications. G.I.T. Imaging Microscopy 1:5–6 [Google Scholar]
- Lebert M., Richter P., and Häder D.-P. (1997) Signal perception and transduction of gravitaxis in Euglena gracilis. J Plant Physiol 150:685–690 [Google Scholar]
- Liu Y., Zhu D.-M., Strayer D.M., and Israelsson U.E. (2010) Magnetic levitation of large water droplets and mice. Adv Space Res 45:208–213 [Google Scholar]
- Liu M., Gao H., Shang P., Zhou X., Ashforth E., Zhuo Y., Chen D., Ren B., Liu Z., and Zhang L. (2011) Magnetic field is the dominant factor to induce the response of Streptomyces avermitilis in altered gravity simulated by diamagnetic levitation. PLoS One 6:e24697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machemer H. and Bräucker R. (1992) Gravireception and graviresponses in ciliates. Acta Protozool 31:185–214 [PubMed] [Google Scholar]
- Machemer H., Machemer-Röhnisch S., Bräucker R., and Takahashi K. (1991). Gravikinesis in Paramecium: theory and isolation of a physiological response to the natural gravity vector. J Comp Physiol A 168:1–12 [Google Scholar]
- Machemer-Röhnisch S., Nagel U., and Machemer H. (1999) A gravity-induced regulation of swimming speed in Euglena gracilis. J Comp Physiol A 185:517–527 [Google Scholar]
- Meisel M.W. and Brooks J.S. (2012) A perspective of magnetic levitation as an Earth-based low gravity analogue: what it is and what it ain't. Gravitational and Space Research 26:55–59 [Google Scholar]
- Millet B. and Pickard B.G. (1988) Gadolinium ion is an inhibitor suitable for testing the putative role of stretch-activated ion channels in geotropism and thigmotropism. Biophys J 53:A155 [Google Scholar]
- Moes M.J.A., Gielen J.C., Bleichrodt R.-J., van Loon J.J.W.A., Christianen P.C., and Boonstra J. (2011) Simulation of microgravity by magnetic levitation and random positioning: effect on human A431 cell morphology. Microgravity Sci Technol 23:249–261 [Google Scholar]
- Nagel U. and Machemer H. (2000) Effects of gadolinium on electrical membrane properties and behaviour in Paramecium tetraurelia. Eur J Protistol 36:161–168 [Google Scholar]
- Nakaoka Y., Takeda R., and Shimizu K. (2002) Orientation of Paramecium in a DC magnetic field. Bioelectromagnetics 23:607–613 [DOI] [PubMed] [Google Scholar]
- Nakaoka Y., Itoh J., and Shimizu K. (2011) Orientation of Paramecium swimming in a static magnetic field: dependence on membrane lipid fluidity. Bioelectromagnetics 32:66–72 [DOI] [PubMed] [Google Scholar]
- Ntefidou M. and Häder D.-P. (2005) Photoactivated adenylyl cyclase (PAC) genes in the flagellate Euglena gracilis mutant strains. Photochem Photobiol Sci 4:732–739 [DOI] [PubMed] [Google Scholar]
- Papp E. and Meszena G. (1982) Field concentration and temperature dependence of fluorescence polarization of magnetically oriented chloroplasts. Biophys J 39:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A.D. (2003) Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell Biochem Biophys 39:163–173 [DOI] [PubMed] [Google Scholar]
- Rosen A.D. and Rosen M.S. (1990) Magnetic field influence on Paramecium motility. Life Sci 46:1509–1515 [DOI] [PubMed] [Google Scholar]
- Schöpfer P. (1989) Experimentelle Pflanzenphysiologie, Springer, Berlin, Heidelberg, pp 402–404 [Google Scholar]
- Starr R.C. (1964) The culture of algae at Indiana University. Am J Bot 51:1013–1014 [Google Scholar]
- Storch V. and Welsch U. (2002) Kükenthals Leitfaden für das Zoologische Praktikum, Spektrum Akademischer Verlag, Heidelberg, Berlin [Google Scholar]
- Tanimoto Y., Izumi S., Furuta K., Suzuki T., Fujiwara Y., Fujiwara M., Hirata T., and Yamada S. (2002) Effects of high magnetic field on Euglena gracilis. International Journal of Applied Electromagnetics and Mechanics 14:311–316 [Google Scholar]
- Valles J.M., Jr., Lin K., Denegre J.M., and Mowry K.L. (1997) Stable magnetic field gradient levitation of Xenopus laevis: toward low-gravity simulation. Biophys J 73:1130–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev P.M., Dronzine R.T., Vassileva M.P., and Georgiev G.A. (1982) Parallel arrays of microtubules formed in electric and magnetic fields. Biosci Rep 2:1025–1029 [DOI] [PubMed] [Google Scholar]