Abstract
Purpose
To investigate whether the volumes of rectum exposed to intermediate doses, from 30-50 Gy, contribute to the risk of Grade ≥2 late rectal toxicity among patients with prostate cancer receiving radiotherapy.
Methods and Materials
Data from 1009 patients treated on Radiation Therapy Oncology Group (RTOG) protocol 94-06 were analyzed using three approaches. First, the contribution of intermediate doses to a previously published fit of the Lyman-Kutcher-Burman (LKB) normal-tissue complication probability (NTCP) model was determined. Next, the extent to which intermediate doses provide additional risk information, after taking the LKB model into account, was investigated. Third, the proportion of rectum receiving doses higher than a threshold, VDose, was computed for doses ranging from 5-85 Gy, and a multivariate Cox proportional hazards (PH) model was used to determine which of these parameters were significantly associated with time to Grade ≥2 late rectal toxicity.
Results
Doses <60 Gy have no detectable impact on the fit of the LKB model, as expected based on the small estimate of the volume parameter (n=0.077). Furthermore, there is no detectable difference in late rectal toxicity among cohorts with similar risk estimates from the LKB model but with different volumes of rectum exposed to intermediate doses. The multivariate Cox PH model selected V75 as the only value of VDose significantly associated with late rectal toxicity.
Conclusions
There is no evidence from these data that intermediate doses influence the risk of Grade ≥2 late rectal toxicity. Instead, the critical doses for this endpoint appear to be ≥75 Gy. It is hypothesized that cases of Grade ≥2 late rectal toxicity occurring among patients with V75 less than about 12% may be due to a “background” level of risk, likely due mainly to biological factors.
Keywords: prostate cancer, RTOG, late rectal toxicity, dose-volume histogram
INTRODUCTION
Many dose-volume studies have investigated dosimetric risk factors for late rectal toxicity after radiotherapy. In the recent QUANTEC review (Quantitative Analysis of Normal Tissue Effects in the Clinic), it was noted that the volume of rectum receiving doses ≥60 Gy (V60) is consistently associated with the risk of Grade ≥2 late rectal toxicity or rectal bleeding (1). In contrast, there is much less agreement regarding the role of doses <60 Gy (1).
Several studies have suggested that rectal volumes exposed to intermediate doses, in the 30-50 Gy dose range, contribute significantly to the risk of late rectal toxicity. For example, Skwarchuk et al. found that an increased incidence of rectal bleeding was associated with enclosure of the outer rectal contour by the 50% isodose curve (2). This factor, corresponding to doses in the 35-38 Gy range, retained significance in a multivariate model that included maximum dose to rectum. In a subsequent analysis, Jackson et al. found that V46 for rectal wall was more significantly associated with late rectal bleeding than volumes corresponding to other dose thresholds from 0-80 Gy (3). As a further example, Karlsdottir et al. reported that V40 for rectum was significantly associated with Grade ≥2 late rectal toxicity, but that V20, V60, V65, and V70 were not (4).
Other studies, however, have found no evidence for an impact of intermediate doses on late rectal toxicity (5-8). For example, Koper et al. found that only V60 retained significance in a multivariate model, although both V30 and V60 were significant as univariate factors (6). Similarly, Fiorino et al. found that V50 and V70 were both associated with Grade ≥2 late rectal bleeding in univariate analyses, but only V70 retained significance after multivariate analysis (8).
The difficulty in assessing the relative effects of intermediate versus high doses is due, in large part, to the correlation that typically occurs between volumes of rectum exposed to various doses. This is especially true in single-institution studies, where common conformal treatment techniques are generally used for all patients (1).
The goal of the present study, therefore, was to investigate the contribution of intermediate doses to the risk of late rectal toxicity among patients treated on Radiation Therapy Oncology Group (RTOG) protocol 94-06 (9), a large multi-institutional dose-escalation trial in which a wide variety of conformal techniques was employed by the 42 participating institutions. These included 4-field box, 6-field with opposed laterals and 4 obliques, 7-field with opposed laterals, 4 obliques with a lightly weighted anterior-posterior field, and 4 non-coplanar fields, among others. There were further variations in rectal exposure because some patients were treated to the prostate only, whereas others were treated to prostate plus seminal vesicles for all or part of therapy. These features make the data from RTOG 94-06 ideally suited for clarifying the role of intermediate doses on late rectal toxicity.
METHODS AND MATERIALS
Patient data
The data from RTOG 94-06 are described in detail in our previous study (10). Briefly, the trial included 5 dose levels ranging from 68.4-79.2 Gy delivered in daily fractions of 1.8-2.0 Gy, with no specific normal tissue constraints. Rectal toxicity was scored prospectively using RTOG criteria at prescribed intervals. The dose-volume histogram (DVH) was computed for rectum as a solid structure, as described previously (10). The RTOG Publications Committee and the Institutional Review Boards of The University of Texas MD Anderson Cancer Center, Washington University Medical Center, and the American College of Radiology approved these secondary analyses.
Data analyses
Three approaches were used to investigate the impact of intermediate doses on the incidence of Grade ≥2 late rectal toxicity, as described below.
1. Contribution of intermediate doses to the fit of the LKB model
We previously reported a fit of the LKB model to the Grade ≥2 late rectal toxicity from RTOG 94-06 using a technique in which event times and patient follow-up were taken into account (10). In the LKB model (11,12), the effective dose to an organ at risk is
| (1) |
where n is the volume parameter, Di is the dose to subvolume vi, and the sum extends over all dose bins in the DVH. Complication probability is modeled as a probit function of Deff using two additional parameters, TD50 and m:
| (2) |
where
| (3) |
Here, we first wished to quantify the extent to which intermediate doses contributed to the previous LKB model fit. Specifically, we considered changes in Deff that would result from omission of dose bins from equation (1). “Reduced” Deff values, denoted Deff-D, were computed by omitting the dose bins between D and D+1 Gy, meaning that subvolumes exposed to doses from D to D+1 Gy were hypothetically assumed to have received 0 Gy instead. For example, since the DVH was collected using 0.2-Gy dose bins, Deff-42 was computed by omitting the 5 dose bins from 42-43 Gy. The relative reduction in Deff resulting from this procedure is
| (4) |
which depends on D and on the volume parameter, n.
2. Impact of intermediate doses not incorporated into the LKB model
We next wished to determine whether intermediate doses provide additional risk information, not already incorporated into the LKB model fit. Patient cohorts were identified having similar values of Deff based on the LKB model fit. Each cohort was divided into two equal subgroups depending on the volume of rectum exposed to doses from 30 to 50 Gy, V30 minus V50 (V30-V50). Within each cohort having similar Deff values, the log-rank test was used to compare freedom from Grade ≥2 late rectal toxicity in the subgroups having larger (V30-V50>median) versus smaller (V30-V50<median) rectal volumes exposed to intermediate doses. A difference would indicate that intermediate doses had an impact on toxicity that was not captured by the LKB model.
3. Multivariate analysis of V5 through V85
The third analysis performed was a standard multivariate analysis, unrelated to the fit of the LKB model. The Cox proportional hazards (PH) model was used to investigate the association between V5 through V85, in increments of 5 Gy, and time to Grade ≥2 late rectal toxicity. VDose parameters were added to the model using a forward stepwise procedure, with factors considered to improve the model significantly if the P-value for inclusion was P<0.05.
RESULTS
Patient cohort
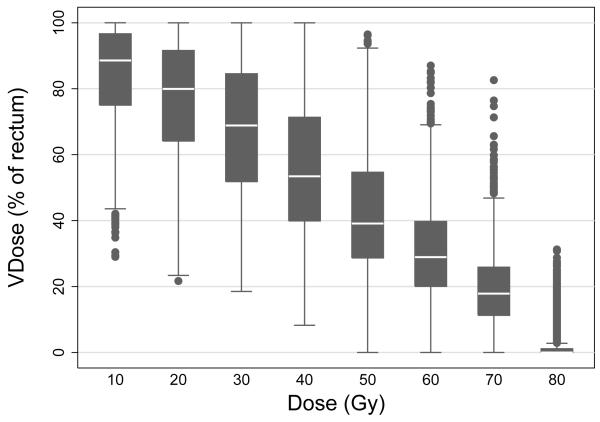
Of the 1084 patients enrolled on RTOG 94-06, data from 1010 patients were available for analyses of late rectal toxicity, as described previously (10). For the present analysis, one patient was excluded who stopped treatment after 15 fractions (without toxicity) and had a maximum dose to rectum of only 28.4 Gy. Figure 1 illustrates the wide variation in rectal DVHs in the present cohort (N=1009). The median follow-up in this cohort was 7.2 (range 0.3-12.4) years.
Figure 1.
Box plots illustrating the wide variation in rectal V10-V80 among patients treated on RTOG 94-06.
Contribution of doses <60 Gy to the fit of the LKB model
In our previous analysis of Grade ≥2 late rectal toxicity among patients treated on RTOG 94-06, we estimated a small value for the LKB volume parameter: n=0.077 (10). It is well known that volume parameters near zero indicate that large doses play the predominant role in determining complication risk. However, we wished to determine whether intermediate doses played any role at all in the LKB model fit.
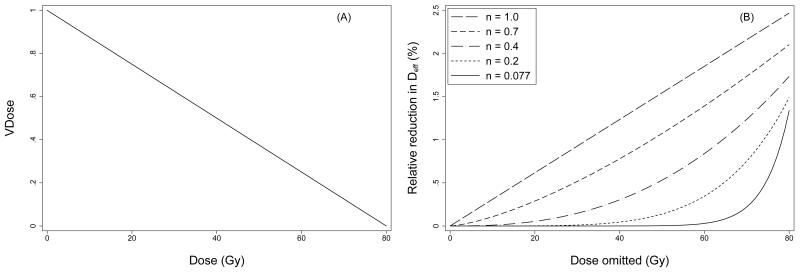
To illustrate our approach, Figure 2A shows a hypothetical DVH in which each dose bin contains an equal sub-volume of the organ at risk. The relative change in Deff resulting from omission of 1-Gy dose bins, computed from this DVH using equation (4), is shown in Figure 2B for several different volume parameter values. For n=0.077, the omission of subvolumes exposed to doses <60 Gy (in 1-Gy increments) results in little or no reduction in Deff. In fact, if all subvolumes exposed to doses <60 Gy are omitted simultaneously, the relative reduction in Deff is <0.1%. By comparison, when n=1, the change in Deff is 1-1.5% with omission of individual 1-Gy dose bins from 30-50 Gy, and the simultaneous omission of all dose bins <60 Gy reduces Deff by nearly 55%.
Figure 2.
Panel A) Hypothetical dose-volume histogram in which each dose bin from 0-80 Gy contains an equal subvolume of the organ at risk. Panel B) Relative reduction in Deff resulting from omission of doses between D and D+1 Gy from the DVH in panel A (equation 4), and plotted as a function of omitted dose D. As shown, the relative reduction in Deff depends on D and on the value of the volume parameter, n.
The calculation illustrated in Figure 2B was applied to the rectal DVH for each patient in the study cohort using the fitted LKB volume parameter, n=0.077. The results were averaged over all patients and plotted as a function of omitted dose D in Figure 3. As expected, omission of 1-Gy dose bins <60 Gy had negligible impact on Deff, and simultaneous exclusion of all doses bins <60 Gy also resulted in almost no change in Deff (median 0.1%, range 0.006-0.8%). This demonstrates that intermediate doses, in the 30-50 Gy dose range, had essentially no impact on our previously reported fit of the LKB model to the RTOG 94-06 late rectal toxicity data, as expected based on the small volume parameter obtained.
Figure 3.
Solid curve: mean relative reduction in Deff, calculated for each patient using equation (4) with volume parameter n=0.077 and averaged over all patients. Dashed curves show ±1 standard deviation.
Impact of intermediate doses after adjusting for the LKB model fit
The next goal was to determine whether rectal volumes exposed to intermediate doses convey additional information about the risk of Grade ≥2 late rectal toxicity, after using the LKB model to take into account the effects of high doses. Three cohorts were identified that each included >150 patients and had Deff values falling in a narrow range: 64-66 Gy, 66-68 Gy, or 68-70 Gy. Although the available sample sizes provide limited power (<80%) to detect statistically significant differences in complication rates unless the differences are relatively large (>15%), each cohort was investigated for evidence of a trend toward increased toxicity among patients with larger volumes of rectum exposed to doses between 30 and 50 Gy, V30-V50. The value of V30-V50 varies widely in each of these cohorts, as illustrated in Figure 4A. Figures 4B-4D show the time to Grade ≥2 late rectal toxicity in each cohort for patients having larger (>median) versus smaller (<median) values of V30 minus V50. These figures illustrate that the volume of rectum exposed to intermediate doses conveys no detectable additional risk of Grade ≥2 late rectal toxicity, once the effect of high doses is taken into account via the LKB model.
Figure 4.
Panel A) Percentage of rectum receiving doses between 30 and 50 Gy (V30 minus V50) plotted against Deff computed using n=0.077. Panels B-D) Kaplan-Meier curves showing freedom from Grade ≥2 late rectal toxicity as a function of time after start of radiotherapy (RT) among patients with Deff ranging from 64-68 Gy, 66-68 Gy, or 68-70 Gy, divided into two subgroups at the median value of V30-V50 for each cohort.
Multivariate analysis of V5 through V85
In the stepwise Cox PH analysis of time to Grade ≥2 late rectal toxicity, the only factor selected for inclusion in the model was V75 (P < 0.001).
DISCUSSION
If there were a clinically significant impact of intermediate doses on Grade ≥2 late rectal toxicity, one would expect this effect to be detectable in the data from RTOG 94-06, which includes >1000 patients from 42 different institutions, employing many different 3D-conformal treatment techniques, and which consequently includes patients with wide variations in the rectal DVH (Figure 1). The results of the current study, however, indicate that intermediate doses do not detectably increase the risk of Grade ≥2 late rectal injury beyond that resulting from high-dose exposure alone.
Instead, the analyses performed here found V75 to be the only dose-volume parameter significantly associated with late rectal toxicity, a finding consistent with several previous studies. In the first published dose-volume study of late rectal toxicity, Benk et al. reported a significantly higher incidence of rectal bleeding among patients with >40% of the anterior rectum exposed to doses ≥75 Gy (14). Later, Boersma et al. concluded that patients receiving ≥75 Gy to >5% of the rectal wall were significantly more likely to experience severe rectal bleeding (15). More recently, in a large multi-institutional study, Fellin et al. investigated threshold doses ranging from 20 to 75 Gy, and found that only V75 was significantly associated with rectal bleeding (16).
The selection of V75 as the only dose-volume parameter significantly associated with late rectal toxicity in RTOG 94-06 was unexpected, given that rectal toxicity was observed among patients with prescription doses <75 Gy. The Kaplan-Meier estimates of Grade ≥2 late rectal toxicity at 5 years among patients treated with 68.4 Gy, 73.8 Gy, and 74 Gy on RTOG 94-06 were 8.9%, 12.5%, and 13.2%, respectively (10). This can be explained in part by the fact that portions of rectum often receive doses higher than the prescription dose, due to the requirement of minimum target dose coverage and the shapes of dose distributions that result from 3D conformal treatment techniques. More than 50% of patients receiving 73.8 Gy had V75 exceeding 10% of rectum, as did nearly 40% of patients treated with 74 Gy. In the group receiving 68.4 Gy, however, none of the patients had V75 >0%, yet Grade ≥2 late rectal toxicity was still observed.
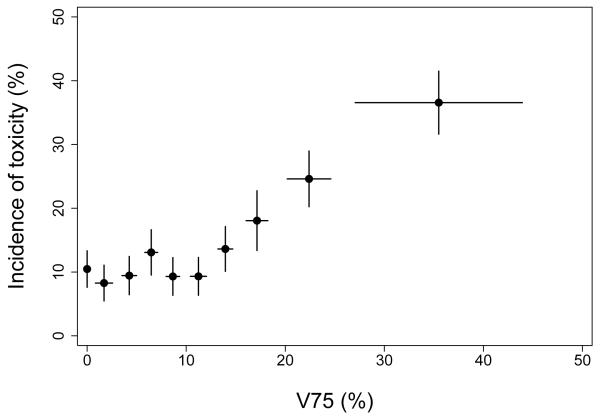
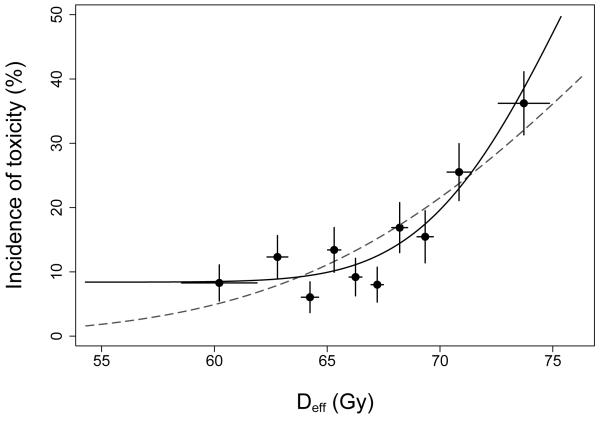
Figure 5 suggests toxicity among patients with V75=0% might be explained by a “background” level of toxicity, perhaps 8-10%, that is independent of the treatment received provided V75 is no larger than about 12%. Evidence for a possible background level of late rectal toxicity is also apparent when the data are analyzed using the LKB model. In Figure 6, the dashed curve shows our previously reported fit of the LKB model (10). The solid curve was obtained by refitting the LKB model after replacing NTCP (equation 2) with b0+(1−b0)·NTCP, where b0 is an additional parameter representing background toxicity. The resulting improvement in model fit was highly significant (P=0.008; likelihood ratio test), with the background estimated to be b0=8.4% (95% CI 0-14.2%). The improvement in fit is visually apparent in Figure 6; the LKB+background model captures the initial “plateau”, where the incidence of toxicity is fairly constant for the first 6 dose groups, and describes the steepness of the rising portion of the curve better than the LKB model.
Figure 5.
Kaplan-Meier incidence of Grade ≥2 late rectal toxicity 8 years after start of radiotherapy in each of 10 patient subgroups, grouped by V75. The cohort with V75=0% includes 117 patients; other cohorts include 99-100 patients each. Points are plotted at the mean value of V75 per subgroup. Horizontal error bars show ± 1 standard deviation; vertical error bars show ± 1 standard error computed using the method of Greenwood (13).
Figure 6.
Kaplan-Meier incidence of Grade ≥2 late rectal toxicity at 8 years after the start of radiotherapy in each of 10 subgroups of 100-101 patients each, grouped by Deff computed using n=0.077. Points are plotted at the mean value of Deff per subgroup. Error bars are as in Figure 5. Curves show fits of the LKB model with (solid) or without (dashed) a background level of toxicity included.
Interestingly, other reports also support the possibility of a similar background incidence of late rectal toxicity. In the publication of Fellin et al., a plot of Grade ≥2 late rectal bleeding against V75 suggests a background rate of ~4-7% (16). In a cohort of patients treated to 79-84 Gy, Chism et al. reported a 9% incidence of Grade 2 gastrointestinal morbidity among patients treated with a lateral rectal shield (17). Söhn et al. plotted the incidence of Grade ≥2 late rectal bleeding as a function of V73.7, and in the group of patients with the lowest exposure (V73.7~8%), the toxicity rate is about 8-10% (18).
If there is a background level of Grade ≥2 late rectal toxicity of approximately 8-10%, what is the explanation for this phenomenon? It seems likely that biological factors play a major role in these “background” events. Some may be due to rectal symptoms such as hemorrhoids that are not a consequence of treatment. A survey of rectal symptoms among nearly 58,000 prostate cancer patients reported a background level of late rectal toxicity among individuals not receiving radiotherapy close to 2% (19), which does not fully account for the background level seen here, although different toxicity scoring criteria may have been used. Other biological factors likely to play a role are comorbidities (10) and genetic differences among patients that affect individual complication risk. In addition, disparities between planned and delivered dose that result in uncertainties in the rectal DVH likely contribute to this phenomenon. It could also be the case that more detailed risk modeling, taking the spatial effects of radiotherapy into account, for example, may eventually clarify how radiation exposure influences the occurrence of these “background” events.
Finally, it should be noted that the conclusions of this study regarding the lack of influence of intermediate doses on late rectal toxicity, the relationship between V75 and toxicity, and the hypothesized background complication level require confirmation before they are used to guide clinical practice. Moreover, these conclusions are specific to the RTOG Grade ≥2 late rectal toxicity endpoint. Evidence has been mounting that different rectal endpoints, including stool frequency, rectal incontinence, abdominal pain, rectal bleeding, etc., are caused by injury to different portions of the gastrointestinal system and may have dose-volume dependencies quite different from one another (8,15,20).
Acknowledgements
Supported in part by grants R01-CA104342, U24-CA81647, U10-CA21661, U10-CA37422 and U10-CA32115 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
There is no evidence that intermediate doses influence the risk of Grade ≥2 late rectal toxicity among patients treated on RTOG 94-06.
REFERENCES
- 1.Michalski JM, Gay H, Jackson A, et al. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;3:S123–S129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skwarchuk M, Jackson A, Zelefsky M, et al. Late rectal toxicity after conformal radiotherapy of prostate cancer (I): Multivariate analysis and dose-response. Int J Radiat Oncol Biol Phys. 2000;47:103–113. doi: 10.1016/s0360-3016(99)00560-x. [DOI] [PubMed] [Google Scholar]
- 3.Jackson A, Skwarchuk MW, Zelefsky MJ, et al. Late rectal bleeding after conformal radiotherapy of prostate cancer (II): Volume effects and dose-volume histograms. Int J Radiat Oncol Biol Phys. 2001;49:685–698. doi: 10.1016/s0360-3016(00)01414-0. [DOI] [PubMed] [Google Scholar]
- 4.Karlsdottir A, Muren LP, Wentzel-Larsen T, et al. Late gastrointestinal morbidity after three-dimensional conformal radiation therapy for prostate cancer fades with time in contrast to genitourinary morbidity. Int J Radiat Oncol Biol Phys. 2008;70:1478–1486. doi: 10.1016/j.ijrobp.2007.08.076. [DOI] [PubMed] [Google Scholar]
- 5.Greco C, Mazzetta C, Cattani F, et al. Finding dose-volume contraints to reduce late rectal toxicity following 3D-conformal radiotherapy (3D-CRT) of prostate cancer. Radiother Oncol. 2003;69:215–222. doi: 10.1016/j.radonc.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Koper PCM, Heemsbergen WD, Hoogeman MS, et al. Impact of volume and location of irradiated rectum wall on rectal blood loss after radiotherapy of prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:1072–1082. doi: 10.1016/j.ijrobp.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Vargas C, Martinez A, Kestin LL, et al. Dose-volume analysis of predictors for chronic rectal toxicity after treatment of prostate cancer with adaptive image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1297–1308. doi: 10.1016/j.ijrobp.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 8.Fiorino C, Fellin G, Rancati T, et al. Clinical and dosimetric predictors of late rectal syndrome after 3D-CRT for localized prostate cancer: Preliminary results of a multicenter prospective study. Int J Radiat Oncol Biol Phys. 2008;70:1130–1137. doi: 10.1016/j.ijrobp.2007.07.2354. [DOI] [PubMed] [Google Scholar]
- 9.Michalski JM, Purdy JA, Winter K, et al. Preliminary report of toxicity following 3D radiation therapy for prostate cancer on 3DOG/RTOG 9406. Int J Radiat Oncol Biol Phys. 2000;46:391–402. doi: 10.1016/s0360-3016(99)00443-5. [DOI] [PubMed] [Google Scholar]
- 10.Tucker SL, Dong L, Bosch WR, et al. Late rectal toxicity on RTOG 94-06: Analysis using a mixture Lyman model. Int J Radiat Oncol Biol Phys. 2010;78:1253–1260. doi: 10.1016/j.ijrobp.2010.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–19. [PubMed] [Google Scholar]
- 12.Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys. 1989;16:1623–1630. doi: 10.1016/0360-3016(89)90972-3. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood M. The natural duration of cancer. Reports on Public Health and Medical Subjects. 1926;33:1–26. [Google Scholar]
- 14.Benk VA, Adams JA, Shipley WU, et al. Late rectal bleeding following combined X-ray and proton high dose irradiation for patients with stages T3-T4 prostate carcinoma. Int J Radiat Oncol Biol Phys. 1993;26:551–557. doi: 10.1016/0360-3016(93)90978-5. [DOI] [PubMed] [Google Scholar]
- 15.Boersma LJ, van den Brink M, Bruce AM, et al. Estimation of the incidence of late bladder and rectum complications after high-dose (70-78 Gy) conformal radiotherapy for prostate cancer, using dose-volume histograms. Int J Radiat Oncol Biol Phys. 1998;41:83–92. doi: 10.1016/s0360-3016(98)00037-6. [DOI] [PubMed] [Google Scholar]
- 16.Fellin G, Fiorino C, Rancati T, et al. Clinical and dosimetric predictors of late rectal toxicity after conformal radiation for localized prostate cancer: Results of a large multicenter observational study. Radiother Oncol. 2009;93:197–202. doi: 10.1016/j.radonc.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Chism DB, Horwitz EM, Hanlon AL, et al. Late morbidity profiles in prostate cancer patients treated to 79-84 Gy by a simple four-field coplanar beam arrangement. Int J Radiat Oncol Biol Phys. 2003;55:71–77. doi: 10.1016/s0360-3016(02)03822-1. [DOI] [PubMed] [Google Scholar]
- 18.Söhn M, Yan D, Liang J, et al. Incidence of late rectal bleeding in high-dose conformal radiotherapy of prostate cancer using equivalent uniform dose-based and dose-volume-based normal tissue complication probability models. Int J Radiat Oncol Biol Phys. 2007;67:1066–1073. doi: 10.1016/j.ijrobp.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giordano SH, Lee A, Kuo YF, et al. Late gastrointestinal toxicity after radiation for prostate cancer. Cancer. 2006;107:423–432. doi: 10.1002/cncr.21999. [DOI] [PubMed] [Google Scholar]
- 20.Peeters STH, Lebesque JV, Heemsbergen WD, et al. Localized volume effects for late rectal and anal toxicity after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2006;64:1151–1161. doi: 10.1016/j.ijrobp.2005.10.002. [DOI] [PubMed] [Google Scholar]