Abstract
Once released into the environment, engineered nanoparticles (eNPs) are subjected to processes that may alter their physical or chemical properties, potentially altering their toxicity vis-à-vis the as-synthesized materials. We examined the toxicity to zebrafish embryos of CdSecore/ZnSshell quantum dots (QDs) before and after exposure to an in vitro chemical model designed to simulate oxidative weathering in soil environments based on a reductant-driven Fenton’s reaction. Exposure to these oxidative conditions resulted in severe degradation of the QDs: the Zn shell eroded, Cd2+ and selenium were released, and amorphous Se-containing aggregates were formed. Weathered QDs exhibited higher potency than did as-synthesized QDs. Morphological endpoints of toxicity included pericardial, ocular and yolk sac edema, non-depleted yolk, spinal curvature, tail malformations, and craniofacial malformations. To better understand the selenium-like toxicity observed in QD exposures, we examined the toxicity of selenite, selenate and amorphous selenium nanoparticles (SeNPs). Selenite exposures resulted in high mortality to embryos/larvae while selenate and SeNPs were non-toxic. Co-exposures to SeNPs + CdCl2 resulted in dramatic increase in mortality and recapitulated the morphological endpoints of toxicity observed with weathered QD exposures. Cadmium body burden was increased in larvae exposed to weathered QDs or SeNP + CdCl2 suggesting the increased potency of weathered QDs was due to selenium modulation of cadmium toxicity. Our findings highlight the need to examine the toxicity of eNPs after they have undergone environmental weathering processes.
INTRODUCTION
Increased production and use of engineered nanoparticles (eNPs) makes their release into the environment inevitable. Once released into the environment, eNPs may be subjected to processes that alter their physical and chemical properties in ways that affect their toxicity.1-4 These processes may be collectively termed “weathering”. Aggregation/agglomeration of eNPs is recognized as an important process affecting eNP fate and has consequences on their toxicity.5-7 Depending on eNP composition and that of intentional coatings, eNPs may also be altered by simple dissolution, redox transformations, photolysis, and the loss or acquisition of organic or inorganic coatings.6, 8-15 These transformations may impact eNP suspension stability.6,12,14,16 Environmentally induced alterations to eNPs have the potential to influence their bioavailability, uptake, and toxicity.17
Most current knowledge about eNP toxicity stems from research focused on intact, as-synthesized NPs.2,18 However, organisms are unlikely to be exposed to NPs in their as-synthesized forms.1 Environmental alterations of eNPs is expected to alter uptake and toxicity. For example, exposure of CdSe quantum dots (QDs) to acidic or alkaline conditions increases toxicity to bacteria due to release of Cd2+ and SeO32−.8 Dissolution of ZnO NPs (i.e., release of Zn2+) has been shown to explain NP toxicity to bacteria,6 but release of Zn2+ alone could not explain toxicity to Daphnia magna.19 As zero-valent iron NPs oxidize to magnetite (Fe3O4) and maghemite (Fe2O3), the antimicrobial capacity of the particles diminishes as the oxidation state of Fe increases.20 The same trend has been observed in rodent microglial cells, where more oxidized forms of iron induce lower levels of oxidative stress.21 As a further example, exposure to an aged TiO2 nanocomposite (T-Lite™) used in sunscreens has a small effect on developing zebrafish (Danio rerio).22 To our knowledge, aside from this latter example, effects of weathered eNPs on aquatic vertebrates have not been reported.
We previously developed an in vitro chemical model based on the extracellular chemistry of lignolytic fungi23,24 to allow investigation of the oxidative weathering of eNPs under conditions representative of oxic soil environments.9 The model consists of a methoxyhydroquinone (MHQ)-promoted Fenton’s reaction leading to the production of hydroxyl radicals (H2O2 + Fe2+ → •OH + OH− + Fe3+) and other reactive oxygen species (ROS). Exposure of PEGylated CdSecore/ZnSshell quantum dots (QDs) to this model system caused dissolution of the QDs, releasing Cd2+ from the nanoparticle core and producing amorphous Se-containing aggregates.9 The toxicity of the as-synthesized CdSecore/ZnSshell QDs has been investigated,25 but that of the products of oxidative weathering has not yet been reported.
The objectives of this study were to (1) evaluate changes in toxicity induced by oxidative weathering vis-à-vis as-synthesized QDs; and (2) provide insight into the causes of observed changes in toxicity. To accomplish these objectives we employed the zebrafish embryo as a model organism. The zebrafish embryo is a well-established and widely used vertebrate model in developmental toxicity,26,27 and has recently been shown to have utility in assessing the toxicity of eNPs.25,28-33 Oxidative weathering of QDs results in a complex mixture of products, complicating the determination of mechanisms of toxicity. We therefore adopted a reductionist approach and examined the toxicity to zebrafish embryos of Se nanoparticles, dissolved cadmium, dissolved selenium species, and their mixtures.
MATERIALS AND METHODS
Chemicals
The following chemicals were purchased from Sigma-Aldrich Chemicals (Milwaukee, WI): CdO (99.5%), trioctylphosphine oxide (TOPO, technical grade, 90%), trioctylphosphine (TOP, technical grade, 90%), zinc stearate (technical grade), sulfur powder (99.98%), selenium powder (100 mesh, >99.5%), tetramethylammonium hydroxide solution (TMAH, ~2.2 M) in methanol, ZnCl2 (>98%), and CdCl2 (99%). Ferrous sulfate (ACS certified), hydrazine (99%), selenous acid (98%), sodium selenate (99%), sodium selenite (99%), and selenium tetrachloride (>98%) were obtained from Fisher Scientific (Waltham, MA). We procured 2,5-dimethoxyhydroquinone (97%) from Alfa Aesar (Ward Hill, MA) and PEG5000-OCH3 (>96%) from RAPP polymer (Germany).
Nanoparticle Syntheses
Synthesis of the CdSecore/ZnSshell QDs was described previously.9,25 The CdSe core diameter was 2.6 ± 0.1 nm; the ZnS shell thickness did not exceed 1 nm.9 Coordinating ligands from the synthesis were exchanged with 5000-Da PEG-thiol (PEG5000) in chloroform. After functionalization, the solvent was evaporated, and QDs were suspended in ultrapure water (18 MΩ-cm resistivity, Barnstead NANOpure Ultrapure Water System, Dubuque, IA, USA). In the exposure medium (vide infra) the hydrodynamic diameter and ζ-potential of the PEG5000-QDs were 21 ± 1 nm and −2.0 ± 0.6 mV.25 After 24 h in exposure medium dissolved Cd concentrations were ≤ 28 μM.25 The characterization of the QDs used in this study are detailed elsewhere.9,25
Amorphous SeNPs were synthesized via hydrazine (N2H4) reduction of selenous acid (H2SeO3) in ultrapure water similar to published methods.34 To keep the SeNP solutions similar to the QD solutions, we used PEG-thiol as the coordinating ligand rather than bovine serum albumin. Briefly, a 0.35 M N2H4 solution (5 mL) was reacted in 20 mL ultrapure water at room temperature for 10 min. To this solution, 10 mL 0.07 M H2SeO3 and excess PEG5000-thiol was added. The solution was stirred for 2 h then stored at 4 °C.
Oxidative Weathering of CdSecore/ZnSshell QDs
PEGylated QDs were subjected to the MHQ-driven Fenton’s reaction.9 Solutions containing 2 μM QDs (7 × 1015 QD·L-1), 200 μM H2O2, 20 μM Fe2+ and 20 μM MHQ in 10 mM acetate buffer (pH 4.1) were allowed to react overnight (16-18 h) in the dark. The reaction was quenched by adjusting pH to 6 with NaOH. Oxidative weathering of the PEG5000-CdSecore/ZnSshell QDs by the MHQ-promoted Fenton’s reaction resulted in the erosion of the ZnS shell, release of Cd from the core, and formation of amorphous selenium aggregates ~10s to 100s of nm in diameter (as determined by transmission electron microscopy, selected area electron diffraction, and energy dispersive X-ray spectroscopy).9 These findings are reported in detail elsewhere.9 Amounts of dissolved and (nano)particulate Cd, Zn, and Se present after weathering are provided in Table S1 in the Supporting Information. We operationally defined dissolved and (nano)particulate species as those passing and retained by a 10 kDa MWCO filter (pore size ~2.8 nm).9,25 Solutions were diluted to the desired QD concentrations for zebrafish experiments in embryo medium immediately before dosing (see below for composition).
Scanning Electron Microscopy (SEM)
SeNPs were spin-cast onto doped silicon wafers for imaging by a Leo Supra55 VP SEM. Data were obtained using 2 kV incident electron energy using the standard in-lens detector.
Ultraviolet-visible (UV-Vis) Spectroscopy
UV-Vis spectra of NP suspensions were obtained using a Shimadzu PC-2401 UV-Visible spectrophotometer. The core diameter and number concentration of as-synthesized QDs was estimated from the position and absorbance of the first exciton peak centered between 525 and 530 nm.9,35 While the accuracy of UV-visible absorbance measurements have received criticism,36 such estimates are considered acceptable for comparative analyses. Concentrations of SeNPs were determined by matching spectra from 450-600 nm to UV-Vis spectra obtained from MHQ-driven Fenton’s reaction exposed QDs.9
Raman Spectroscopy
Raman spectra were recorded on a Thermo Scientific DXR Raman microscope using a 780 nm excitation from a diode-pumped, solid-state laser. Scans (32) were accumulated at 3 cm-1 resolution using 0.1-1 mW laser power. Care was taken to not alter chemical compounds due to laser power. Samples were first run at low power to assess any changes from interaction with the laser prior to increasing power to produce spectra with higher S/N ratio.
Inductively Coupled Plasma-Mass Spectrometry (ICP-MS)
Digests of zebrafish larvae (vide infra) were analyzed by magnetic-sector ICP-MS (Thermo Finnigan Element 2) at the Wisconsin State Lab of Hygiene to determine Cd and Se body burden. Unexposed control larvae had Cd and Se concentrations of 0.0055 ± 0.0004 μg·g-1 and 0.14 ± 0.01 μg·g-1, respectively.
Zebrafish Exposures
Beginning 4-6 hours post fertilization (hpf) zebrafish embryos (AB strain) were exposed to graded concentrations (corresponding to 0.2 to 200 μM Cd) of as-synthesized or weathered QDs suspended in embryo medium (58 mM CaCl2, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 0.5 mM HEPES, pH 7; ionic strength (I) = 0.18 M). For comparison, we also exposed fish to CdCl2, Na2SeO3, Na2SeO4, SeNPs and PEG5000-thiol. Exposures were conducted in a 96-well plate format with a single embryo per well. Dosing solutions (100 μL) were renewed daily, and zebrafish embryos/larvae were monitored for mortality and morphological signs of toxicity until 120 hpf. Experiments were conducted in triplicate, and data are presented as the mean ± SE.
Morphological endpoints of toxicity were evaluated at 120 hpf following exposure to QD concentrations equivalent to 20 μM Cd, a concentration that produced consistent morphological endpoints for both weathered and as-synthesized QDs. Larvae (n = 12) were immobilized in 3% methylcellulose and imaged live (2.5×) in lateral orientation. Images were analyzed for total body length, yolk sac edema and pericardial edema.
To determine cadmium and selenium body burdens, larvae (15-20 per replicate) were collected at 120 hpf after exposure to 20 μM Cd or Se equivalents as-synthesized QDs, weathered QDs, or controls (viz. embryo media, CdCl2, Na2SeO3, SeNP or SeNP + CdCl2), euthanized with 3-aminobenzoic acid ethylester, rinsed 3× and transferred to pre-weighed microcentrifuge tubes. Wet weights were measured following removal of excess water, and larvae were digested overnight in 5 M HNO3 at 60 °C. Cadmium and selenium concentrations were determined by ICP-MS (vide supra).
Statistical Analyses
Statistical significance of differences between treatments and control was determined using Student’s t-test. Mortality data at 120 hpf were used to calculate median lethal concentrations (LC50 values) and 95% confidence intervals (CI95%) by probit analysis (USEPA Probit Analysis Program, Ver 1.5). Median effects concentrations (EC50 values) were determined for selected morphological endpoints of toxicity from incidence data using the trimmed Spearman-Karber method (USEPA Trimmed Spearman-Karber Analysis Program, Ver 1.5). LC50 and EC50 values were compared among treatments for statistical significance by two-way analysis of variance (ANOVA). The level of significance for all analyses was p ≤ 0.05.
RESULTS AND DISCUSSION
Toxicity of Oxidatively Weathered CdSecore/ZnSshell QDs
We obtained dose-response relationships for mortality induced by exposure to as-synthesized and oxidatively weathered PEG5000-CdSecore/ZnSshell QDs (Figure 1a). The LC50 for the as-synthesized PEG5000 QDs was 42 (CI95%: 24-75) μM Cd equivalents as we previously reported.25 Weathering produced a leftward shift in dose-response curves (Figure 1a) and a decline in LC50 value to 14 (CI95%: 9-21) μM Cd equivalents. The mortality induced by both the as-synthesized and oxidatively weathered QDs was higher than that produced by an equivalent concentration of CdCl2 (Figure 1a). The higher toxicity of intact QDs relative to CdCl2 is consonant with our previous findings.25
Figure 1. Toxicity to zebrafish embryos of intact and oxidatively weathered PEG5000-CdSecore/ZnSshell quantum dots (QDs).
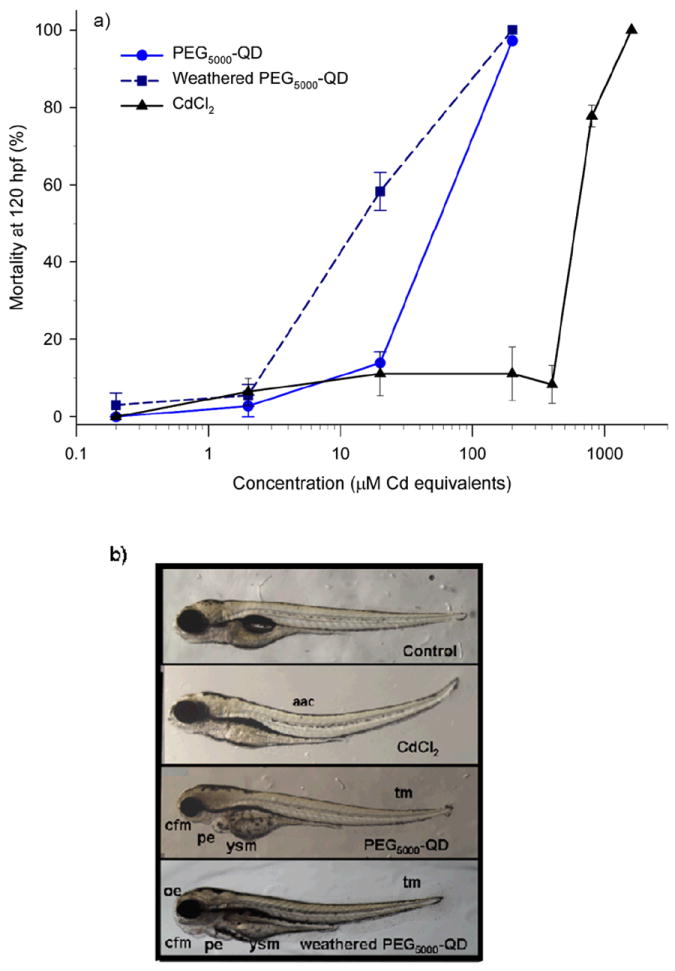
(a) Dose-response relationships for mortality at 120 hours post fertilization (hpf) induced by intact and weathered QDs. Data for CdCl2 shown as a reference. (b) Morphological malformations at 120 hpf resulting from exposure to 20 μM Cd equivalents of as-synthesized QDs, weathered QDs or CdCl2. Morphological endpoints of toxicity included altered axial curvatures (aac), yolk sac malformations (ysm), pericardial edema (pe), tail malformations (tm), ocular edema (oe), opaque tissues and craniofacial malformations (cfm). In (a), calculated LC50 values (95% confidence intervals, CI95%) for PEG5000-QD, weathered PEG5000-QDs, and CdCl2 were 42 (CI95%: 24-75), 14 (CI95%: 9-21), and 409 (CI95%: 270-563) μM Cd equivalents, respectively.
The types of morphological endpoints of toxicity observed did not differ between as-synthesized and weathered QDs (Figure 1b). Morphological endpoints included pericardial, ocular and yolk sac edema (i.e., swelling due to fluid retention), low absorption of the yolk, spinal curvature, tail malformation, opaque tissues (particularly in the head region), craniofacial malformation and failure to inflate the swim bladder. Some of these endpoints fit the profile of Cd toxicity (i.e., spinal curvature, pericardial edema, ocular edema),37,38 while others correspond to those associated with Se toxicity (i.e., pericardial edema, yolk sac edema, low absorption of the yolk).39
Weathered PEGylated QDs displayed increased potency for inducing morphological endpoints of toxicity relative to as-synthesized QDs. That is, weathered QDs produced morphological malformations both at lower concentrations and in more larvae than did as-synthesized QDs. Exposure to weathered PEG5000-QDs at a concentration of 20 μM Cd equivalents (i.e., 6.7 × 1016 QD·L-1) significantly increased the incidences of pericardial edema, yolk sac malformations and craniofacial malformations relative to the as-synthesized QDs (Figure 2). Increases in incidence of pericardial edema and craniofacial malformations were also observed upon exposure to 2 μM Cd equivalents weathered vs. as-synthesized QDs (data not shown). We calculated EC50 values for pericardial edema, yolk sac malformations, and craniofacial malformations for as-synthesized and weathered QDs. For as-synthesized PEG5000-QDs, EC50 values for pericardial edema, yolk sac malformations and craniofacial malformations were 18 (CI95%: 12-28), 9 (CI95%: 6-12) and 9 (CI95%: 5-14) μM Cd equivalents; for weathered PEG5000-QDs, the EC50 values for these responses decreased to 8 (CI95%: 6-11), 4 (CI95%: 3-5) and 3 (CI95%: 2-4) μM Cd equivalents, respectively.
Figure 2. Incidence of selected morphological endpoints of toxicity.
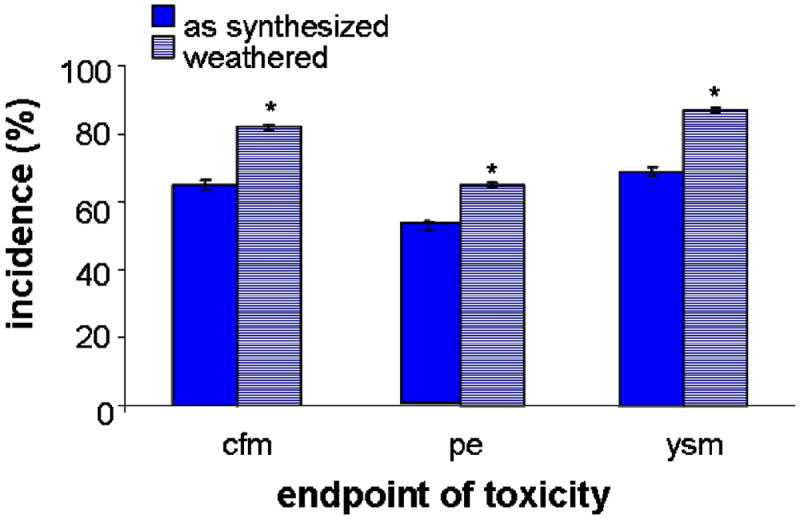
Bars show percent of larvae showing craniofacial malformations (cfm), pericardial edema (pe), and yolk sac malformations (ysm) after 120 h exposure to 20 μM Cd equivalents as-synthesized or oxidatively weathered PEG5000-QDs. *denotes incidence differs significantly (p < 0.05).
We quantified three morphological endpoints of toxicity following exposure to 20 μM Cd equivalents as-synthesized or weathered QDs: total body length, pericardial edema and yolk sac edema (Figure 3). Total body length and pericardial edema differed between as-synthesized and weathered PEG5000-QDs (p ≤ 0.05; Figure 3 a,c), while yolk sac malformations (i.e., edema, lack of adsorption) did not (p > 0.05; Figure 3b). These data indicate that exposure to the weathered QDs produced more severe effects for some morphological endpoints further demonstrating that weathered QDs were more toxic than their as-synthesized counterparts.
Figure 3. Severity of selected morphological endpoints of toxicity.
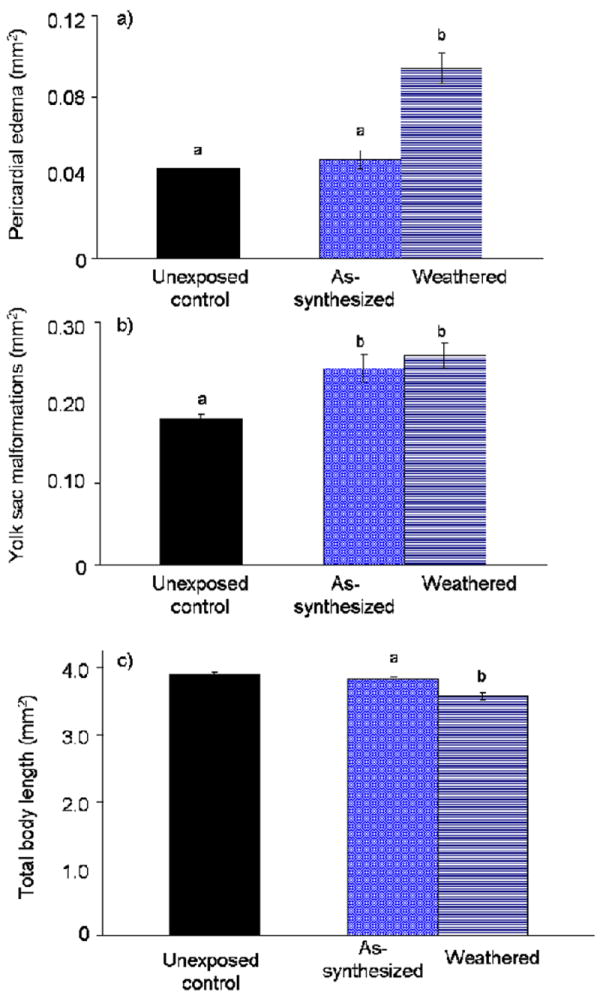
Unexposed larvae and those exposed to 20 μM Cd equivalents as-synthesized or oxidatively weathered PEG5000-QDs were assessed 120 hpf for (a) total body length; (b) yolk sac area, including edema; and (c) pericardial area. Letters denote significant differences (p ≤ 0.05) among treatments.
Toxicity of Dissolved Inorganic Selenium Species
Few prior studies have reported the toxicity of inorganic selenium compounds to zebrafish embryos/larvae.40,41 Furthermore, the morphological endpoints of toxicity caused by selenium compounds have not been described. Selenium was expected to contribute to the toxicity of oxidatively weathered CdSecore/ZnSshell QDs due to its oxidation to soluble species during the weathering process. We therefore investigated the toxicity to zebrafish embryos/larvae of three dissolved inorganic selenium compounds (i.e., sodium selenate, sodium selenite and selenium tetrachloride).
Dose-response relationships were obtained for Na2SeO4, Na2SeO3 and SeCl4 at 120 hpf (Figure 4a). Sodium salts of selenium anions are water-soluble. Selenium tetrachloride decomposes in water to yield selenous acids (pH was adjusted to 7 in exposure solutions with NaOH). The most potent selenium compound tested was SeO32− (LC50 = 5.1 (CI95%: 3.7-7.1) μM Se) followed by SeCl4 (LC50 = 240 (CI95%: 193-280) μM Se). No mortality above baseline was observed following exposure to Na2SeO4 over the concentration range tested (0-200 μM Se). Prevalent morphological effects observed after exposure of zebrafish embryos/larvae included yolk sac edema, failure to adsorb the yolk, yolk discoloration, and pericardial edema. These endpoints of toxicity were produced at much lower concentrations of SeO32− than SeCl4 (Figure 4b). Morphological endpoints of toxicity produced by exposure to SeO32− were similar to those described for other fishes exposed to this selenium oxyanion.39 These data suggest that dissolved Se species may play a role in the toxicity. However, dissolved Se cannot account for the total toxicity of weathered QDs because, at a given dose, only a fraction (~76%) of Se was dissolved (Table S1). Additionally, larvae exposed to inorganic selenium species do not show all endpoints of toxicity of those exposed to weathered QDs.
Figure 4. Toxicity of dissolved inorganic selenium species to zebrafish larvae.
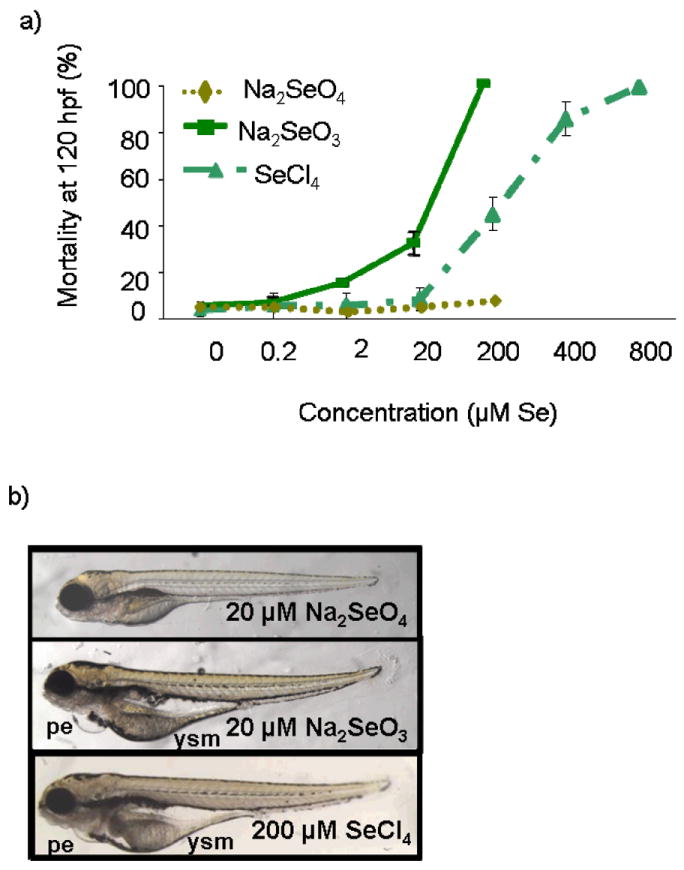
(a) Dose-response relationships for Na2SeO3, Na2SeO4, and SeCl4. Data are means ± SE of 3 replicates. In some cases, error bars are smaller than the size of the symbols. (b) Morphological endpoints of toxicity at 120 hours post fertilization (hpf) resulting from exposure to 20 μM Na2SeO3, 20 μM Na2SeO4 or 200 μM SeCl4. Morphological endpoints of toxicity include pericardial edema (pe) and yolk sac malformations (ysm).
Cadmium and Selenium Body Burdens
We measured Cd and Se body burden by ICP-MS in larvae exposed to 20 μM Cd equivalents of as-synthesized or weathered QDs. For comparison, we measured body burden in larvae exposed to CdCl2, Na2SeO3, SeNPs and SeNP + CdCl2. Cadmium body burden in exposed larvae increased in the following order: as-synthesized QDs < weathered QDs < CdCl2 < SeNPs + CdCl2 treatment groups (Figure 5a). The difference in Cd body burden between larvae exposed to weathered and as-synthesized QDs was small but significant (p = 0.05). Cadmium body burden was higher in larvae co-exposed to SeNPs + CdCl2 than in those exposed to CdCl2 alone (Figure 5a). Selenium body burden in exposed larvae increased in the following order: weathered QDs < as-synthesized QDs < SeNPs + CdCl2 < SeNPs < Na2SeO3 treatments (Figure 5b). The Se body burden was lower in larvae co-exposed to SeNPs + CdCl2 than in those exposed to SeNPs alone. Similarly, larvae exposed to weathered QDs contained significantly less Se (p < 0.001) than those exposed to as-synthesized QDs. The highest Se body burden resulted from exposure to Na2SeO3.
Figure 5. (a) Cd and (b) Se body burdens.
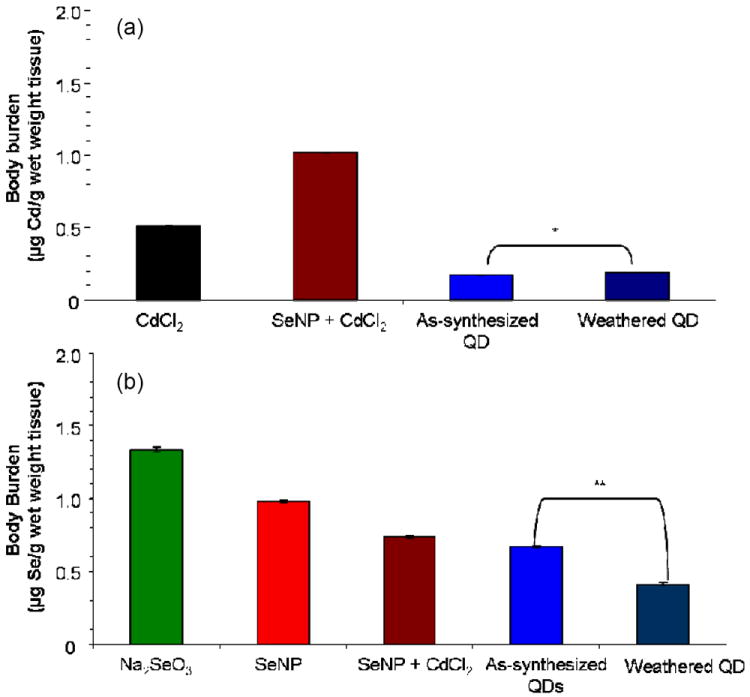
Body burdens for Cd or Se were determined for larvae at 120 hpf exposed to 20 μM Cd or Se equivalents of as-synthesized or weathered PEG5000-QDs, CdCl2, Na2SeO3, SeNPs, or SeNP + CdCl2. Values represent mean ± SE, n = 3 pools of 15-20 larvae each. In some cases, error bars were too small to be visible. Difference between as-synthesized and weathered QDs: *, p = 0.05; **, p < 0.001.
Differences between As-synthesized and Oxidatively Weathered QDs
In principle, several differences between as-synthesized and weathered QD dosing suspensions may have contributed to the higher potency of the latter. In the case of the weathered QDs dosing suspensions contained particulate and dissolved QD products and assay components. Exposures to relevant concentrations of individual assay components did not produce noticeable signs of toxicity. Assay components in combination, produced no mortality, but did cause craniofacial malformations at the highest dose examined (Figure S1). No other endpoints were observed. Degradation of PEG under oxidative conditions is known to produce potentially toxic byproducts.42,43 We examined the potential toxicity of oxidized PEG ligands in two ways. One product of PEG cleavage is ethylene glycol (EG). We therefore exposed zebrafish embryos to EG concentrations equivalent to the amount of PEG present (PEG5000 = 113 EG units) in QD exposures but failed to observe morphological malformations or mortality (Figure S1). Degradation products other than EG can be produced from PEG oxidation. We therefore exposed PEG-thiol to the MHQ-driven Fenton reaction and then exposed zebrafish embryos to the oxidatively degraded PEG. Exposures to oxidatively degraded PEG at concentrations relevant for the QD exposures failed to elicit noticeable toxic responses (Figure S1).
To determine the contribution to toxicity of the dissolved and (nano)particulate fractions, we filtered weathered QD solutions through centrifugal concentrators (10 kDa nominal molecular weight cutoff; pore size ~2.8 nm).9 Zebrafish embryos/larvae were exposed to each fraction at doses consistent with the dosing range used for QD solutions. Morphological malformations were observed when larvae were exposed to either fraction; however, exposure to the (nano)particulate fraction produced more pronounced toxicity (Figure S2). These results led to the hypothesis that the amorphous Se-containing aggregates, produced following oxidative weathering, contributed to the toxicity of weathered QDs.
Toxicity of Se0 Nanoparticles
Oxidative weathering of CdSecore/ZnSshell QDs by the MHQ-promoted Fenton’s reaction produced amorphous selenium aggregates ~10s to 100s nm in diameter. Amounts of dissolved and particulate Se present after weathering are provided in Table S1. We operationally defined particulate selenium as Se not passing a 10 kDa MWCO filter (pore size ~2.8 nm).
To examine toxicity due to the selenium aggregates, we synthesized ~150- to 300-nm amorphous Se0 nanoparticles (SeNPs) (Figure 6a). Raman spectra of SeNPs exhibited peaks at 234 and 251 cm-1 (Figure 6b) consistent with elemental selenium in a (predominately) six-membered ring network structure.44 Selenium NP concentrations comparable to those of Se-aggregates in weathered QD solutions did not cause appreciable mortality (<10%; Figure 6c). Furthermore, few morphological endpoints of toxicity were apparent (Figure 6d). At the highest SeNP dose tested, minimal yolk absorption was evident. These data suggest that the increased potency of weathered QDs cannot be explained solely by exposure to the aggregates of selenium particles formed during simulated oxidative weathering.
Figure 6.
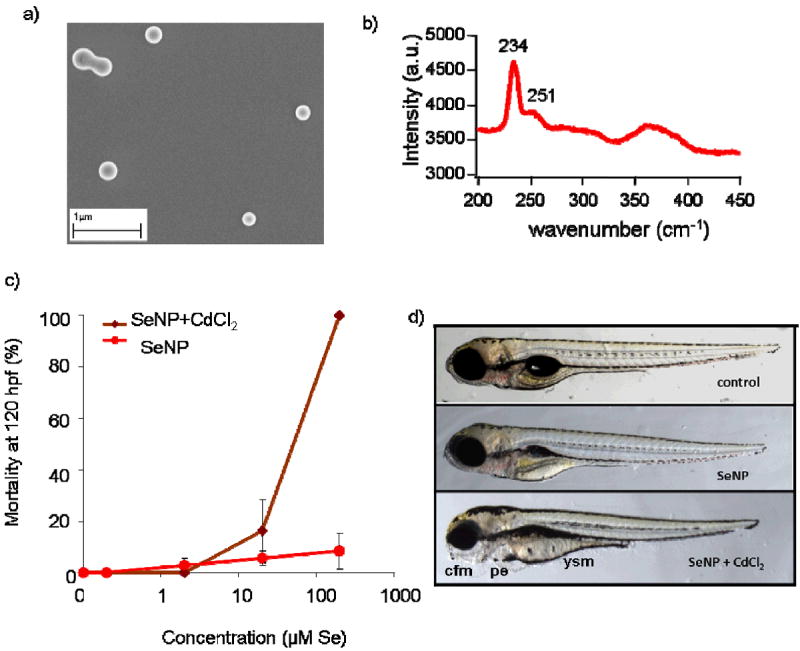
Characterization and toxicity of selenium nanoparticles (SeNPs). (a) Scanning electron micrograph of SeNPs. (b) Raman spectrum of SeNPs. Peaks at 234 and 251 cm-1 are consistent with elemental selenium in a six-membered ring structure.49 (c) Dose-response relationships for SeNPs, SeNPs + CdCl2. The LC50 value for SeNP + CdCl2 was 27 (CI95%: 17-40) μM Cd equivalents. In some cases, error bars are smaller than the size of the symbols. (d) Images of zebrafish larvae at 120 hpf exposed to SeNPs in the presence or absence of CdCl2.
Bovine serum albumin-coated elemental SeNPs (36 nm) have previously been shown to be more lethal to adult medaka (Oryzias latipes) than sodium selenite due to hyperaccumulation of selenium in nanoparticle form.45 In the present study, however, we found the synthesized SeNPs to be less toxic than sodium selenite (cf. Figures 4a and 6c). The discrepancy in the toxicities of SeNPs most likely resulted from the differing SeNP sizes, ligands, and fish species tested.
Toxicity of Se Nanoparticles and Cadmium
Weathered QD solutions contain not only SeNPs, which we have shown to have minimal toxicity (vide supra), but free Cd2+ ions as well. We therefore examined the toxicity of co-administered CdCl2 and SeNPs as they likely both contributed to the toxicity of weathered QDs. At completion of in vitro weathering, the dissolved fraction contained ~70% and ~76% of the total Cd and Se; the particulate fraction contained ~19% of the total Cd and ~25% of the total Se (Table S1). Based on these data, we used a 1:1 Cd:Se ratio in experiments examining the joint toxicity of Cd and Se. Interestingly, co-exposure of zebrafish embryos to SeNPs and CdCl2 produced higher mortality than did either CdCl2 or SeNPs alone (Figure 6c). The calculated LC50 value for SeNP and CdCl2 co-exposures was 27 (CI95%: 17-40) μM Se (or Cd) equivalents (the units are equivalent because co-exposures were conducted using a 1:1 Se-to-Cd ratio). These values are much lower than LC50 values for CdCl2 alone (409 μM Cd).25 Importantly, co-exposure to SeNPs and CdCl2 recapitulated the profile of morphological endpoints produced by exposure to weathered QDs (cf. Figures 1b and 6d). A similar left-shift in dose-response curve was found in co-exposures of Cd2+ and SeO32− compared to SeO32- alone (Figure S3). No shift in mortality was observed in larvae co-exposed to Cd2+ and SeO42- (data not shown). These data may partially explain the toxicity noted in larvae exposed to weathered QD filtrate (Figure S1). Based on solution conditions, one would expect the dissolved Se to be primarily SeO32-. The mortality and morphological endpoints of toxicity observed with zebrafish exposure to the dissolved fraction of weathered QD solutions are consistent with this expectation (Figure S2). We would not expect to note toxicity if the major dissolved species was SeO42-. Additionally, these results indicates that weathered QD toxicity most likely results from both nanoparticulate and dissolved species.
Interactions between Cd and Se in vivo are complex and not fully understood. Low levels of Se can at least partially ameliorate symptoms of Cd toxicity.47 Co-exposure to selenite leads the redistribution of Cd to higher molecular weight proteins within tissues relative to exposures to Cd2+ alone.47 The mechanism behind Se-induced redistribution of Cd is not currently understood. However, rescue of Cd toxicity by Se depends on the concentration and speciation of Se. We previously examined metallothionein (MT) expression as a marker for Cd exposure and found that MT expression correlated weakly with Cd body burden.25 In that study, the QD exposures resulted in co-exposure to Se and Cd, potentially leading to a redistribution of Cd that lowered the amount of MT expressed relative to the Cd body burden.
Our results show an increase in potency and lethality of PEGylated CdSecore/ZnSshell QDs to zebrafish embryos/larvae following simulated oxidative weathering. The increase in toxicity appears attributable in large part to Cd toxicity but is modulated by Se in the QD suspensions. The increased Cd body burden in larvae exposed to weathered QDs may be due to the adsorption of Cd to the SeNP surface enhancing uptake of Cd or reducing Cd elimination. However, since both as-synthesized and weathered QDs are more toxic than equivalent amounts of Cd, selenium appears to play a role in QD toxicity.
Environmental Implications
We have shown that weathering processes that may occur in the environment have the potential to alter eNP toxicity. In the system investigated here, oxidative weathering led to increased toxicity of PEGylated CdSecore/ZnSshell QDs. We expect the impact of environmental weathering on toxicity to depend on NP composition, the nature of the ligands on the NPs, and the particular weathering process. Assessment of the environmental impacts of NPs should consider the effects of other environmental processes (e.g., reductive environments, photodegradation, microbial alterations) on NPs of a variety of compositions. In addition to potential changes in toxicity, environmental alterations to NPs are expected to influence their fate and transport and directly affect on the potential for environmental exposure.
Our data suggest that adsorption of metals to NPs, including NPs formed under environmental conditions, may alter uptake and subsequent toxicity of the adsorbed metal. Enhanced uptake of metals, such as Cd and As, has been shown in fish when co-exposed with TiO2 NPs.48-50 Once released to the environment, NPs may serve as adsorbents for metal and non-metal contaminants.51-53 Such “contaminant-loaded” NPs may exhibit toxicity differing from that of the NP alone. This is expected to be especially important for NPs employed in wastewater treatment and environmental remediation. Research directed at understanding metal adsorption to NPs is therefore warranted, particularly in relation to uptake, distribution and retention of adsorbed metals.
Our experiments with intact and weathered QDs as well as inorganic selenium species, highlights the importance of selenium speciation in toxicity to fish. While this is not surprising given previous research in birds and mammals,54,55 the literature contains scant information on selenium toxicity to fishes. Furthermore, Se present in solution impacted Cd toxicity. The literature on co-exposure of heavy metals (e.g., Cd, Hg) and Se focuses primarily on the amelioration toxicity by Se. Our findings suggest the role of Se in heavy metal toxicity is more complex than this and may be a fruitful area of research.
Supplementary Material
Acknowledgments
This research was funded by the National Science Foundation (initiated under DMR-0425880 and completed under DMR-082760). TKH was supported by grant number T32ES007015 from the National Institute of Environmental Health Sciences. Article contents are solely the responsibility of the authors and do not represent official views of the sponsors.
Footnotes
Supporting Information. Text and figures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Wiesner MR, Lowry GV, Alvarez P, Dionysiou D, Biswas P. Assessing the risks of manufactured nanomaterials. Environ Sci Technol. 2006;40:4336–4345. doi: 10.1021/es062726m. [DOI] [PubMed] [Google Scholar]
- 2.Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR. Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem. 2008;27:1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- 3.Levard C, Hotze EM, Lowry GV, Brown GE., Jr Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ Sci Technol. 2012;46:6900–6914. doi: 10.1021/es2037405. [DOI] [PubMed] [Google Scholar]
- 4.Lowry GV, Holtze EM, Bernhardt ES, Dionysiou DD, Pedersen JA, Wiesner MR, Xing B. Environmental occurrence, behavior and ecological effects of engineered nanomaterials. J Environ Qual. 2010;39:1867–1874. doi: 10.2134/jeq2010.0297. [DOI] [PubMed] [Google Scholar]
- 5.Petosa AR, Jaisi DP, Quevedo IR, Elimelech M, Tufenkji N. Aggregation and deposition of engineered nanomaterials in aquatic environments: Role of physicochemical interactions. Environ Sci Technol. 2010;44:6532–6549. doi: 10.1021/es100598h. [DOI] [PubMed] [Google Scholar]
- 6.Li M, Pokhrel S, Jin X, Mädler L, Damoiseaux R, Hoek EMV. Stability, bioavailability, and bacterial toxicity of ZnO and iron-doped ZnO nanoparticles in aquatic media. Environ Sci Technol. 2011;45:755–761. doi: 10.1021/es102266g. [DOI] [PubMed] [Google Scholar]
- 7.Zhu X, Wang J, Zhang X, Chang Y, Chen Y. The impact of ZnO nanoparticle aggregates on the embryonic development of zebrafish (Danio rerio) Nanotechnol. 2009;20:1–9. doi: 10.1088/0957-4484/20/19/195103. [DOI] [PubMed] [Google Scholar]
- 8.Mahendra S, Zhu H, Colvin VL, Alvarez PJ. Quantum dot weathering results in microbial toxicity. Environ Sci Technol. 2008;42:9424–9430. doi: 10.1021/es8023385. [DOI] [PubMed] [Google Scholar]
- 9.Metz KM, Mangham AN, Bierman MJ, Jin S, Hamers RJ, Pedersen JA. Engineered nanomaterial transformation under oxidative environmental conditions: Development of an in vitro biomimetic assay. Environ Sci Technol. 2009;43:1598–1604. doi: 10.1021/es802217y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Hurt RH. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ Sci Technol. 2010;44:2169–2175. doi: 10.1021/es9035557. [DOI] [PubMed] [Google Scholar]
- 11.Reinsch BC, Forsberg B, Penn RL, Kim CS, Lowry GV. Chemical transformations during aging of zerovalent iron nanoparticles in the presence of common groundwater dissolved constituents. Environ Sci Technol. 2010;44:3455–3461. doi: 10.1021/es902924h. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Cho M, Fortner JD, Hughes JB, Kim J-H. Transformations of aggregated C60 in the aqueous phase by UV irradiation. Environ Sci Technol. 2009;43:4878–4883. doi: 10.1021/es8035972. [DOI] [PubMed] [Google Scholar]
- 13.Auffan M, Pedeutour M, Rose J, Masion A, Ziarelli F, Borschneck D, Chaneac C, et al. Structural degradation at the surface of a TiO2-based nanomaterial used in cosmetics. Environ Sci Technol. 2010;44:2689–2694. doi: 10.1021/es903757q. [DOI] [PubMed] [Google Scholar]
- 14.Auffan M, Rose J, Wiesner MR, Bottero J. Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro. Environ Pollut. 2009;157:1127–1133. doi: 10.1016/j.envpol.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Yan W, Herzing AA, Li X, Kiely CJ, Zhang W. Structural evolution of Pd-doped nanoscale zero-valent iron (nZVI) in aqueous media and implications for particle aging and reactivity. Environ Sci Technol. 2010;44:4288–4294. doi: 10.1021/es100051q. [DOI] [PubMed] [Google Scholar]
- 16.Hou W-C, Jafvert CT. Photochemical transformation of aqueous C60 clusters in sunlight. Environ Sci Technol. 2009;43:362–367. doi: 10.1021/es802465z. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, Louis K, Heideman W, Hamers RJ, Peterson RE, Pedersen JA. Effect of DOM on TiO2 nanoparticle toxicity to zebrafish embryos. Environ Sci Technol. 2013;47:4718–4725. doi: 10.1021/es3047334. [DOI] [PubMed] [Google Scholar]
- 18.Oberdörster G, Stone V, Donaldson K. Toxicology of nanoparticles: A historical perspective. Nanotoxicol. 2007;1:2–25. [Google Scholar]
- 19.Poynton HC, Lazorchak JM, Impellitteri CA, Smith ME, Rogers K, Patra M, Hammer KA, Allen HJ, Vulpe CD. Differential gene expression in Daphnia magna suggests distinct modes of action and bioavailability for ZnO nanoparticls and Zn ions. Environ Sci Technol. 2011;45:762–768. doi: 10.1021/es102501z. [DOI] [PubMed] [Google Scholar]
- 20.Auffan M, Achouak W, Rose J, Chanéac C, Waite DT, Maison A, Woicik J, Wiesner MR, Bottero JY. Relation between redox state of iron-based nanoparticles and their cytotoxicity towards Escherichia coli. Environ Sci Technol. 2008;42:6730–6735. doi: 10.1021/es800086f. [DOI] [PubMed] [Google Scholar]
- 21.Phenrat T, Long TC, Lowry GV, Veronesi B. Partial oxidation (“aging”) and surface modification decrease the toxicity of nanosized zerovalent iron. Environ Sci Technol. 2009;43:195–200. doi: 10.1021/es801955n. [DOI] [PubMed] [Google Scholar]
- 22.Fouqueray M, Noury P, Dherret L, Chaurand P, Abbaci K, Labille J, Rose J, Garric J. Exposure of juvenile Danio rerio to aged TiO2 nanomaterial from sunscreen. Environ Sci Pollut Res. 2013;20:3340–3350. doi: 10.1007/s11356-012-1256-7. [DOI] [PubMed] [Google Scholar]
- 23.Kerem Z, Jensen KA, Hammel KE. Biodegradative mechanism of the brown rot basidiomycete Gloeophyllum trabeum: evidence for an extracellular hydroquinone-driven Fenton reaction. FEBS Lett. 1999;446:49–54. doi: 10.1016/s0014-5793(99)00180-5. [DOI] [PubMed] [Google Scholar]
- 24.Jensen KA, Houtman CJ, Ryan ZC, Hammel KE. Pathways for extracellular Fenton chemistry in the brown rot basidiomycete Gloeophyllum trabeum. Appl Environ Microb. 2001;67:2705–2711. doi: 10.1128/AEM.67.6.2705-2711.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.KingHeiden TC, Wiecinski PN, Magham AN, Metz KM, Nesbit D, Pedersen JA, Hamers RJ, Heideman W, Peterson RE. Quantum dot nanotoxicity assessment using the zebrafish embryo. Environ Sci Technol. 2009;43:1605–1611. doi: 10.1021/es801925c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- 27.Spitsbergen JM, Kent ML. The state of the art of the zebrafish model for toxicology and toxicologic pathology research Advantages and current limitations. Toxicol Pathol. 2003;31(Suppl):62–87. doi: 10.1080/01926230390174959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sijie Lin S, Zhao Y, Nel AE, Lin S. Zebrafish: An in vivo model for nano EHS studies. Small. 2013;9:1608–1618. doi: 10.1002/smll.201202115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Usenko CY, Harper SL, Tanguay RL. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon. 2007;45:1891–1898. doi: 10.1016/j.carbon.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Usenko CY, Harper SL, Tanguay RL. Fullerene C-60 exposure elicits an oxidative stress response in embryonic zebrafish. Toxicol Appl Pharmacol. 2008;229:44–55. doi: 10.1016/j.taap.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bar-Ilan O, Albrecht RM, Fako VE, Furgeson DY. Toxicity assessment of multisized gold and silver nanoparticles in zebrafish embryos. Small. 2009;5:1897–1910. doi: 10.1002/smll.200801716. [DOI] [PubMed] [Google Scholar]
- 32.King Heiden TC, Dengler E, Kao WJ, Heideman W, Peterson RE. Developmental toxicity of low generation PAMAM dendrimers in zebrafish. Toxicol Appl Pharmacol. 2007;225:70–79. doi: 10.1016/j.taap.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bar-Ilan O, Chuang CC, Schwahn DJ, Yang S, Joshi S, Pedersen JA, Hamers RJ, Peterson RE, Heideman W. TiO2 nanoparticle exposure spanning zebrafish development: photo-dependent toxicity at parts per billion concentrations. Environ Sci Technol. 2013;47:4726–4733. doi: 10.1021/es304514r. [DOI] [PubMed] [Google Scholar]
- 34.Jeong UY, Xia YN. Synthesis and crystallization of monodisperse spherical colloids of amorphous selenium. Adv Mater. 2005;17:102. [Google Scholar]
- 35.Yu WW, Qu LH, Guo WZ, Peng XG. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater. 2003;15:2854–2860. [Google Scholar]
- 36.Yu WW, Chang E, Falkner JC, Zhang JY, Al-Somali AM, Sayes CM, Johns J, Drezek R, Colvin VL. Forming biocompatible and nonaggregated nanocrystals in water using amphiphilic polymers. J Am Chem Soc. 2007;129:2871–2879. doi: 10.1021/ja067184n. [DOI] [PubMed] [Google Scholar]
- 37.Blechinger SR, Warren JT, Jr, Kuwada JY, Krone PH. Developmental toxicology of cadmium in living embryos of a stable transgenic zebrafish line. Environ Health Perspect. 2002;95:300–312. doi: 10.1289/ehp.021101041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hen Chow ES, Cheng SH. Cadmium affects muscle type development and axon growth in zebrafish embryonic somitogenesis. Toxicol Sci. 2003;73:149–159. doi: 10.1093/toxsci/kfg046. [DOI] [PubMed] [Google Scholar]
- 39.Lemly AD. Symptoms and implications of selenium toxicity in the fish: the Belews lake case example. Aquatic Toxicol. 2002;57:39–49. doi: 10.1016/s0166-445x(01)00264-8. [DOI] [PubMed] [Google Scholar]
- 40.Niimi AJ, LaHam QN. Selenium toxicity on the early life stages of zebrafish (Brachydanio rerio) J Fish Res Board Can. 1975;6:803–806. [Google Scholar]
- 41.Niimi AJ, LaHam QN. Relative toxicity of organic and inorganic compounds of selenium to newly hatched zebrafish (Brachydanio rerio) Can J Zool. 1976;54:501–509. doi: 10.1139/z76-056. [DOI] [PubMed] [Google Scholar]
- 42.Chen BQ, Evans JRG, Holding S. Decomposition of poly(ethylene glycol) in nanocomposites. J Appl Polym Sci. 2004;94:548–552. [Google Scholar]
- 43.Han S, Kim C, Kwon D. Thermal/oxidative degradation and stabilization of polyethylene glycol. Polymer. 1997;38:317–323. [Google Scholar]
- 44.Oremland RS, Herbel MJ, Blum JS, Langley S, Beveridge TJ, Ajayan PM, Sutto T, Ellis AV, Curran S. Structural and spectral features of selenium nanospheres produced by se-respiring bacteria. Appl Environ Microbiol. 2004;70:52. doi: 10.1128/AEM.70.1.52-60.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li HC, Zhang JS, Wang T, Luo WR, Zhou QF, Jiang GB. Elemental selenium particles at nano-size (Nano-Se) are more toxic to medaka (Oryzias latipes) as a consequence of hyper-accumulation of selenium: A comparison with sodium selenite. Aquatic Toxicol. 2008;89:251–256. doi: 10.1016/j.aquatox.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Zwolak I, Zaporowska H. Selenium interactions and toxicity: A review. Cell Biol Toxicol. 2012;28:31–46. doi: 10.1007/s10565-011-9203-9. [DOI] [PubMed] [Google Scholar]
- 47.Chen RW, Whanger PD, Weswig PH. Selenium-induced redistribution of cadmium binding to tissue proteins: a possible mechanism of protection against cadmium toxicity. Bioinorg Chem. 1975;4:125–133. doi: 10.1016/s0006-3061(00)81021-2. [DOI] [PubMed] [Google Scholar]
- 48.Sun H, Zhang X, Niu Q, Chen Y, Crittenden JC. Enhanced accumulation of arsenate in carp in the presence of titanium dioxide nanoparticles. Water Air Soil Pollut. 2007;178:245–254. [Google Scholar]
- 49.Zhang X, Sun H, Zhang Z, Niu Q, Chen Y, Crittenden JC. Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles. Chemosphere. 2007;67:160–166. doi: 10.1016/j.chemosphere.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Hu X, Chen Q, Jiang L, Yu Z, Jiang D, Yin D. Combined effects of titanium dioxide and humic acid on the bioaccumulation of cadmium in zebrafish. Environ Pollut. 2011;159:1151–1158. doi: 10.1016/j.envpol.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Yang K, Xing B. Sorption of phenanthrene by humic acid-coated nanosized TiO2 and ZnO. Environ Sci Technol. 2009;43:1845–1851. doi: 10.1021/es802880m. [DOI] [PubMed] [Google Scholar]
- 52.Wang XL, Liu Y, Tao S, Xing B. Relative importance of multiple mechanisms in sorption of organic compounds by multiwalled carbon nanotubes. Carbon. 2010;48:3721–3728. [Google Scholar]
- 53.Gai K, Shi B, Yan X, Wang D. Effect of dispersion on adsorption of atrazine by aqueous suspensions of fullerenes. Environ Sci Technol. 2011;45:5959–5965. doi: 10.1021/es103595g. [DOI] [PubMed] [Google Scholar]
- 54.Domingo JL. Metal induced developmental toxicity in mammals-a review. J Toxicol Environ Health. 1994;42:123–141. doi: 10.1080/15287399409531868. [DOI] [PubMed] [Google Scholar]
- 55.Spallholtz JE, Hoffman DJ. Selenium toxicity: Causes and effects in aquatic birds. Aquatic Toxicol. 2002;57:27–37. doi: 10.1016/s0166-445x(01)00268-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


