Abstract
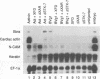
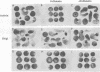
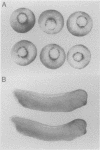
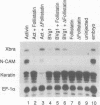
Activin and Vg1, two members of the TGF-beta family, are believed to play roles in mesoderm induction and axis formation in the amphibian embryo. Both molecules are provided maternally, either as protein (activin) or as RNA and protein (Vg1), and experiments with a truncated form of a type IIB activin receptor have led to the conclusion that activin is required for induction of mesoderm in vivo. In this paper we first show that truncated versions of two different Xenopus activin receptors also have severe effects on the activity of the mature region of Vg1, suggesting that such receptors may block the function of several members of the TGF-beta family. We go on to demonstrate that follistatin, a secreted protein which binds activin and blocks its activity, does not interfere with Vg1 signalling. Furthermore, overexpression of follistatin mRNA in Xenopus embryos does not perturb mesoderm formation. Taken together, our data show that the effects of truncated activin receptors on Xenopus development can be explained by the inhibition of Vg1 activity, while the lack of effect of follistatin argues against a function for activin in mesoderm induction.
Full text
PDF
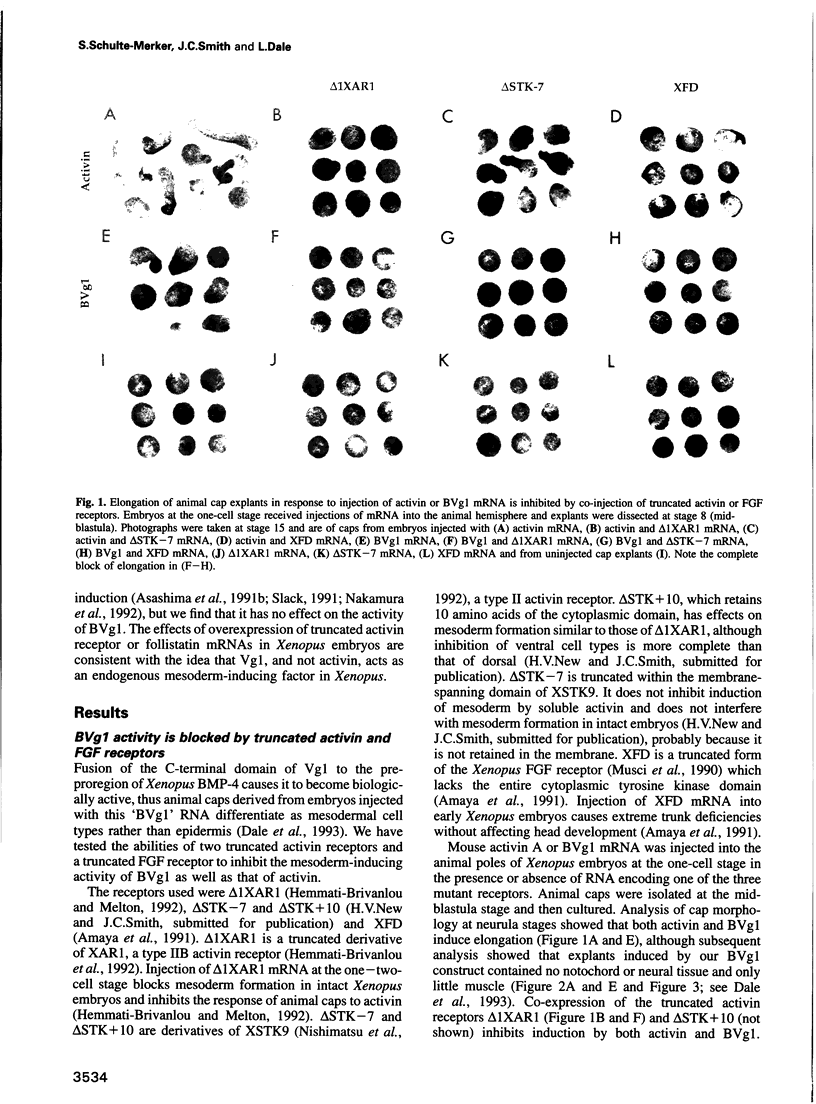
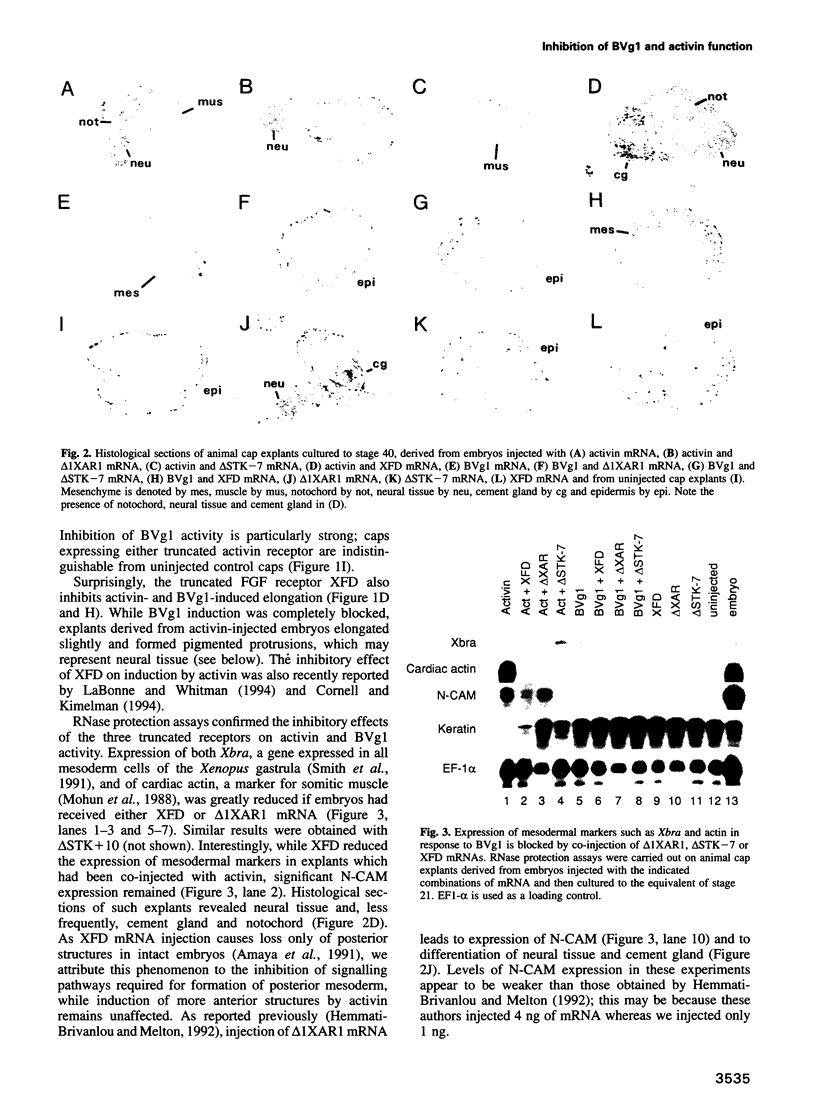
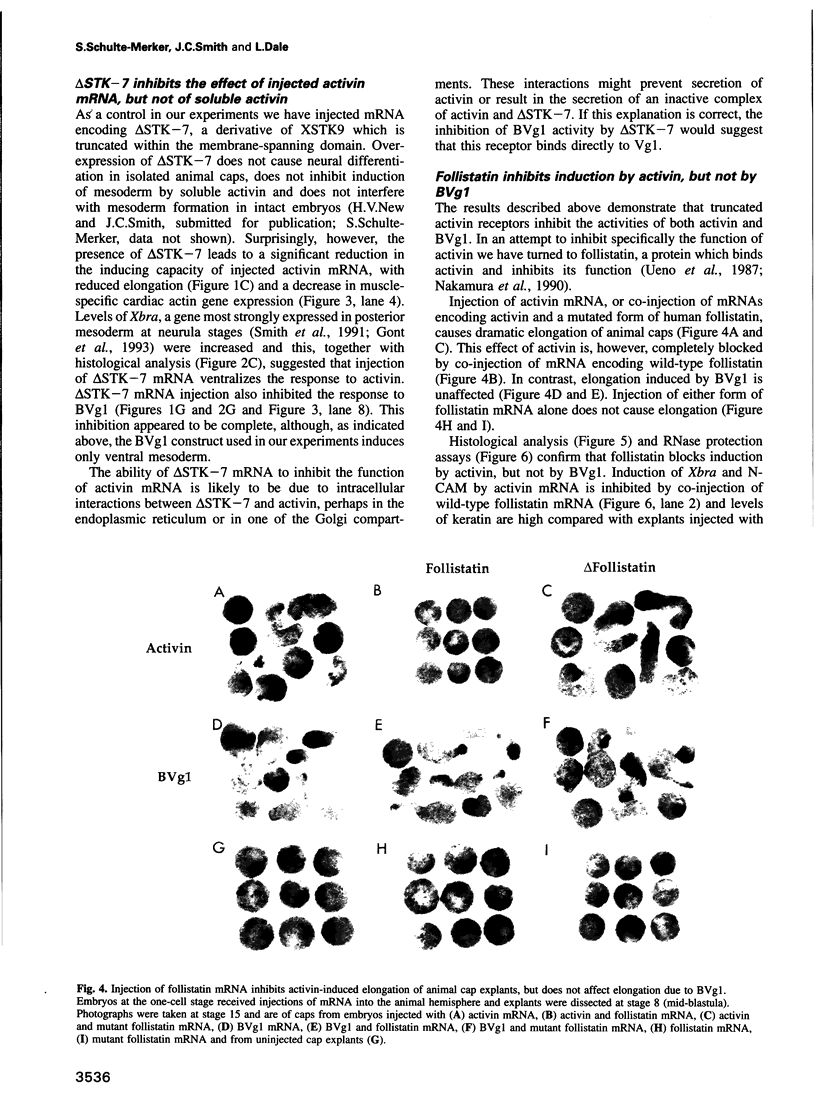

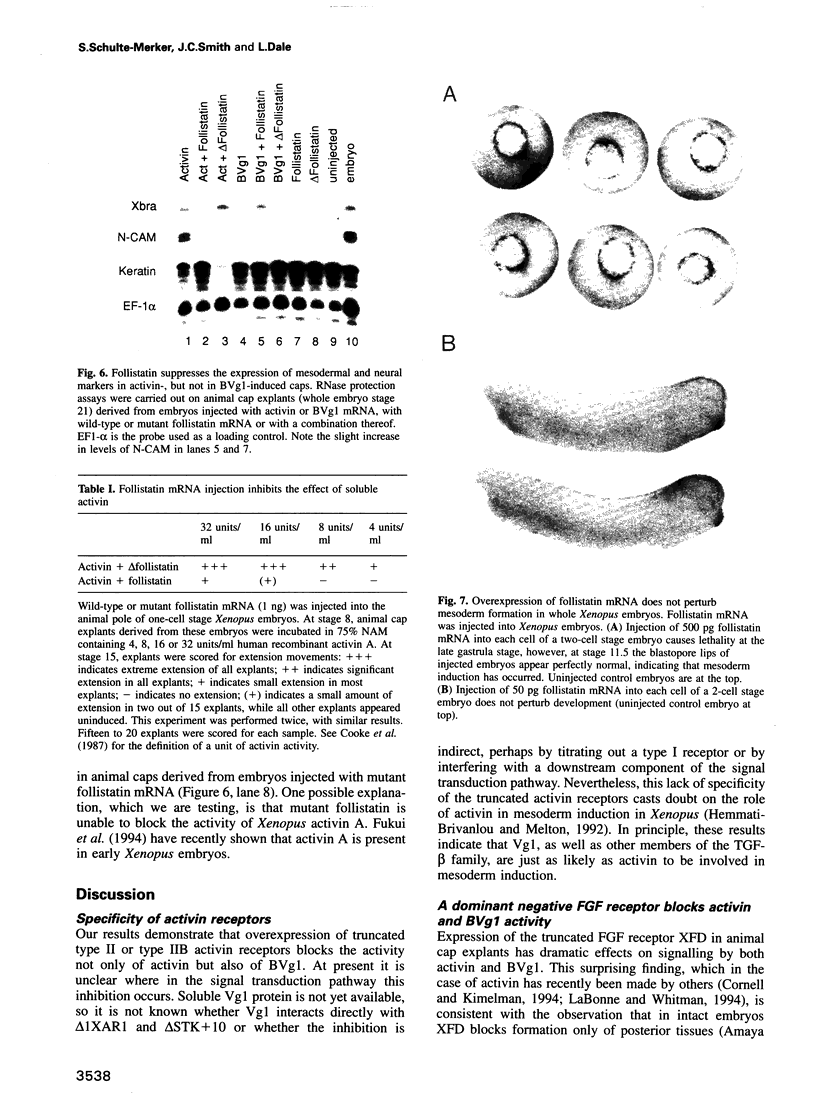



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano R. M., Arkell R., Beddington R. S., Smith J. C. Expression of inhibin subunits and follistatin during postimplantation mouse development: decidual expression of activin and expression of follistatin in primitive streak, somites and hindbrain. Development. 1994 Apr;120(4):803–813. doi: 10.1242/dev.120.4.803. [DOI] [PubMed] [Google Scholar]
- Albano R. M., Groome N., Smith J. C. Activins are expressed in preimplantation mouse embryos and in ES and EC cells and are regulated on their differentiation. Development. 1993 Feb;117(2):711–723. doi: 10.1242/dev.117.2.711. [DOI] [PubMed] [Google Scholar]
- Amaya E., Musci T. J., Kirschner M. W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991 Jul 26;66(2):257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Asashima M., Nakano H., Uchiyama H., Sugino H., Nakamura T., Eto Y., Ejima D., Nishimatsu S., Ueno N., Kinoshita K. Presence of activin (erythroid differentiation factor) in unfertilized eggs and blastulae of Xenopus laevis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6511–6514. doi: 10.1073/pnas.88.15.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attisano L., Cárcamo J., Ventura F., Weis F. M., Massagué J., Wrana J. L. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell. 1993 Nov 19;75(4):671–680. doi: 10.1016/0092-8674(93)90488-c. [DOI] [PubMed] [Google Scholar]
- Attisano L., Wrana J. L., Cheifetz S., Massagué J. Novel activin receptors: distinct genes and alternative mRNA splicing generate a repertoire of serine/threonine kinase receptors. Cell. 1992 Jan 10;68(1):97–108. doi: 10.1016/0092-8674(92)90209-u. [DOI] [PubMed] [Google Scholar]
- Beddington R. S. Induction of a second neural axis by the mouse node. Development. 1994 Mar;120(3):613–620. doi: 10.1242/dev.120.3.613. [DOI] [PubMed] [Google Scholar]
- Cho K. W., De Robertis E. M. Differential activation of Xenopus homeo box genes by mesoderm-inducing growth factors and retinoic acid. Genes Dev. 1990 Nov;4(11):1910–1916. doi: 10.1101/gad.4.11.1910. [DOI] [PubMed] [Google Scholar]
- Cooke J., Smith J. C., Smith E. J., Yaqoob M. The organization of mesodermal pattern in Xenopus laevis: experiments using a Xenopus mesoderm-inducing factor. Development. 1987 Dec;101(4):893–908. doi: 10.1242/dev.101.4.893. [DOI] [PubMed] [Google Scholar]
- Cornell R. A., Kimelman D. Activin-mediated mesoderm induction requires FGF. Development. 1994 Feb;120(2):453–462. doi: 10.1242/dev.120.2.453. [DOI] [PubMed] [Google Scholar]
- Cunliffe V., Smith J. C. Ectopic mesoderm formation in Xenopus embryos caused by widespread expression of a Brachyury homologue. Nature. 1992 Jul 30;358(6385):427–430. doi: 10.1038/358427a0. [DOI] [PubMed] [Google Scholar]
- Cunliffe V., Smith J. C. Specification of mesodermal pattern in Xenopus laevis by interactions between Brachyury, noggin and Xwnt-8. EMBO J. 1994 Jan 15;13(2):349–359. doi: 10.1002/j.1460-2075.1994.tb06268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale L., Howes G., Price B. M., Smith J. C. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development. 1992 Jun;115(2):573–585. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- Dale L., Matthews G., Colman A. Secretion and mesoderm-inducing activity of the TGF-beta-related domain of Xenopus Vg1. EMBO J. 1993 Dec;12(12):4471–4480. doi: 10.1002/j.1460-2075.1993.tb06136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale L., Matthews G., Tabe L., Colman A. Developmental expression of the protein product of Vg1, a localized maternal mRNA in the frog Xenopus laevis. EMBO J. 1989 Apr;8(4):1057–1065. doi: 10.1002/j.1460-2075.1989.tb03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui A., Nakamura T., Uchiyama H., Sugino K., Sugino H., Asashima M. Identification of activins A, AB, and B and follistatin proteins in Xenopus embryos. Dev Biol. 1994 May;163(1):279–281. doi: 10.1006/dbio.1994.1143. [DOI] [PubMed] [Google Scholar]
- Gont L. K., Steinbeisser H., Blumberg B., de Robertis E. M. Tail formation as a continuation of gastrulation: the multiple cell populations of the Xenopus tailbud derive from the late blastopore lip. Development. 1993 Dec;119(4):991–1004. doi: 10.1242/dev.119.4.991. [DOI] [PubMed] [Google Scholar]
- Green J. B., Howes G., Symes K., Cooke J., Smith J. C. The biological effects of XTC-MIF: quantitative comparison with Xenopus bFGF. Development. 1990 Jan;108(1):173–183. doi: 10.1242/dev.108.1.173. [DOI] [PubMed] [Google Scholar]
- Green J. B., New H. V., Smith J. C. Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell. 1992 Nov 27;71(5):731–739. doi: 10.1016/0092-8674(92)90550-v. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Kelly O. G., Melton D. A. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994 Apr 22;77(2):283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Melton D. A. A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature. 1992 Oct 15;359(6396):609–614. doi: 10.1038/359609a0. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Wright D. A., Melton D. A. Embryonic expression and functional analysis of a Xenopus activin receptor. Dev Dyn. 1992 May;194(1):1–11. doi: 10.1002/aja.1001940102. [DOI] [PubMed] [Google Scholar]
- Inouye S., Guo Y., Ling N., Shimasaki S. Site-specific mutagenesis of human follistatin. Biochem Biophys Res Commun. 1991 Aug 30;179(1):352–358. doi: 10.1016/0006-291x(91)91377-o. [DOI] [PubMed] [Google Scholar]
- Isaacs H. V., Tannahill D., Slack J. M. Expression of a novel FGF in the Xenopus embryo. A new candidate inducing factor for mesoderm formation and anteroposterior specification. Development. 1992 Mar;114(3):711–720. doi: 10.1242/dev.114.3.711. [DOI] [PubMed] [Google Scholar]
- Jones C. M., Lyons K. M., Lapan P. M., Wright C. V., Hogan B. L. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992 Jun;115(2):639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster M., Plessow S., Clement J. H., Lorenz A., Tiedemann H., Knöchel W. Bone morphogenetic protein 4 (BMP-4), a member of the TGF-beta family, in early embryos of Xenopus laevis: analysis of mesoderm inducing activity. Mech Dev. 1991 Mar;33(3):191–199. doi: 10.1016/0925-4773(91)90027-4. [DOI] [PubMed] [Google Scholar]
- LaBonne C., Whitman M. Mesoderm induction by activin requires FGF-mediated intracellular signals. Development. 1994 Feb;120(2):463–472. doi: 10.1242/dev.120.2.463. [DOI] [PubMed] [Google Scholar]
- Lamb T. M., Knecht A. K., Smith W. C., Stachel S. E., Economides A. N., Stahl N., Yancopolous G. D., Harland R. M. Neural induction by the secreted polypeptide noggin. Science. 1993 Oct 29;262(5134):713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Massagué J. Receptors for the TGF-beta family. Cell. 1992 Jun 26;69(7):1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Vale W. W. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991 Jun 14;65(6):973–982. doi: 10.1016/0092-8674(91)90549-e. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Vale W. W., Kintner C. R. Cloning of a second type of activin receptor and functional characterization in Xenopus embryos. Science. 1992 Mar 27;255(5052):1702–1705. doi: 10.1126/science.1313188. [DOI] [PubMed] [Google Scholar]
- Mohun T., Garrett N., Stutz F., Sophr G. A third striated muscle actin gene is expressed during early development in the amphibian Xenopus laevis. J Mol Biol. 1988 Jul 5;202(1):67–76. doi: 10.1016/0022-2836(88)90519-0. [DOI] [PubMed] [Google Scholar]
- Musci T. J., Amaya E., Kirschner M. W. Regulation of the fibroblast growth factor receptor in early Xenopus embryos. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8365–8369. doi: 10.1073/pnas.87.21.8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Hasegawa Y., Sugino K., Kogawa K., Titani K., Sugino H. Follistatin inhibits activin-induced differentiation of rat follicular granulosa cells in vitro. Biochim Biophys Acta. 1992 Apr 30;1135(1):103–109. doi: 10.1016/0167-4889(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Takio K., Eto Y., Shibai H., Titani K., Sugino H. Activin-binding protein from rat ovary is follistatin. Science. 1990 Feb 16;247(4944):836–838. doi: 10.1126/science.2106159. [DOI] [PubMed] [Google Scholar]
- Nishimatsu S., Oda S., Murakami K., Ueno N. Multiple genes for Xenopus activin receptor expressed during early embryogenesis. FEBS Lett. 1992 May 25;303(1):81–84. doi: 10.1016/0014-5793(92)80482-v. [DOI] [PubMed] [Google Scholar]
- Rebagliati M. R., Weeks D. L., Harvey R. P., Melton D. A. Identification and cloning of localized maternal RNAs from Xenopus eggs. Cell. 1985 Oct;42(3):769–777. doi: 10.1016/0092-8674(85)90273-9. [DOI] [PubMed] [Google Scholar]
- Sargent M. G., Bennett M. F. Identification in Xenopus of a structural homologue of the Drosophila gene snail. Development. 1990 Aug;109(4):967–973. doi: 10.1242/dev.109.4.967. [DOI] [PubMed] [Google Scholar]
- Sive H. L. The frog prince-ss: a molecular formula for dorsoventral patterning in Xenopus. Genes Dev. 1993 Jan;7(1):1–12. doi: 10.1101/gad.7.1.1. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Darlington B. G., Heath J. K., Godsave S. F. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987 Mar 12;326(6109):197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Slack J. M. Inducing factors in Xenopus early embryos. Curr Biol. 1994 Feb 1;4(2):116–126. doi: 10.1016/s0960-9822(94)00027-8. [DOI] [PubMed] [Google Scholar]
- Slack J. M. Regional biosynthetic markers in the early amphibian embryo. J Embryol Exp Morphol. 1984 Apr;80:289–319. [PubMed] [Google Scholar]
- Slack J. M. The nature of the mesoderm-inducing signal in Xenopus: a transfilter induction study. Development. 1991 Oct;113(2):661–669. doi: 10.1242/dev.113.2.661. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Green J. B., Weigel D., Herrmann B. G. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991 Oct 4;67(1):79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Van Nimmen K., Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990 Jun 21;345(6277):729–731. doi: 10.1038/345729a0. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Slack J. M. Dorsalization and neural induction: properties of the organizer in Xenopus laevis. J Embryol Exp Morphol. 1983 Dec;78:299–317. [PubMed] [Google Scholar]
- Tannahill D., Melton D. A. Localized synthesis of the Vg1 protein during early Xenopus development. Development. 1989 Aug;106(4):775–785. doi: 10.1242/dev.106.4.775. [DOI] [PubMed] [Google Scholar]
- Thomsen G. H., Melton D. A. Processed Vg1 protein is an axial mesoderm inducer in Xenopus. Cell. 1993 Aug 13;74(3):433–441. doi: 10.1016/0092-8674(93)80045-g. [DOI] [PubMed] [Google Scholar]
- Thomsen G., Woolf T., Whitman M., Sokol S., Vaughan J., Vale W., Melton D. A. Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell. 1990 Nov 2;63(3):485–493. doi: 10.1016/0092-8674(90)90445-k. [DOI] [PubMed] [Google Scholar]
- Ueno N., Ling N., Ying S. Y., Esch F., Shimasaki S., Guillemin R. Isolation and partial characterization of follistatin: a single-chain Mr 35,000 monomeric protein that inhibits the release of follicle-stimulating hormone. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8282–8286. doi: 10.1073/pnas.84.23.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli A., Matzuk M. M., Gardner H. A., Lee K. F., Jaenisch R. Activin/inhibin beta B subunit gene disruption leads to defects in eyelid development and female reproduction. Genes Dev. 1994 Feb 15;8(4):414–427. doi: 10.1101/gad.8.4.414. [DOI] [PubMed] [Google Scholar]
- Weeks D. L., Melton D. A. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell. 1987 Dec 4;51(5):861–867. doi: 10.1016/0092-8674(87)90109-7. [DOI] [PubMed] [Google Scholar]