Large-scale manufacture of treatment to cure hepatitis C virus (HCV) is feasible, with target prices of US$100–$250 per 12-week treatment course. These low prices could make widespread access to HCV treatment in low- and middle-income countries a realistic goal.
Keywords: sofosbuvir, daclatasvir, ribavirin, simeprevir, faldaprevir
Abstract
Background. Several combinations of 2 or 3 direct-acting antivirals (DAAs) can cure hepatitis C virus (HCV) in the majority of treatment-naive patients. DAAs for HCV infection have similar mechanisms of action and chemical structures to antiretrovirals for human immunodeficiency virus (HIV) infection. Generic antiretrovirals are currently manufactured at very low prices, to treat 10 million people with HIV/AIDS in developing countries.
Methods. Four HCV DAAs, currently either in phase 3 development or recent approval (daclatasvir, sofosbuvir, simeprevir, faldaprevir), and ribavirin were classified by chemical structure, molecular weight, total daily dose, and complexity of synthesis. The likely range of manufacturing costs per gram of DAA were then projected as formulated product cost, based upon treating a minimum of 1 million patients annually (to arrive at volume demand) combined with an analysis of the complexity of synthesis and a 40% margin for formulation. Projections were then compared with actual costs of antiretrovirals with similar structures.
Results. Minimum manufacturing costs of antiretrovirals were US$0.2–$2.1 per gram. The complexity of chemical synthesis for HCV DAAs was ranked from lowest to highest: ribavirin, daclatasvir, sofosbuvir, faldaprevir, and simeprevir. Predicted manufacturing costs (US dollars) for 12-week courses of HCV DAAs were $21–$63 for ribavirin, $10–$30 for daclatasvir, $68–$136 for sofosbuvir, $100–$210 for faldaprevir, and $130–$270 for simeprevir.
Conclusions. Within the next 15 years, large-scale manufacture of 2 or 3 drug combinations of HCV DAAs is feasible, with minimum target prices of $100–$250 per 12-week treatment course. These low prices could make widespread access to HCV treatment in low- and middle-income countries a realistic goal.
Worldwide, 160–180 million people are antibody positive for Hepatitis C, with up to 500 000 HCV-related deaths per year [1, 2]. The vast majority of these patients are left untreated, with treatment rates ranging from 3.5% in Europe to 21% in the United States [3, 4].
In comparison, approximately 35.3 million are infected with human immunodeficiency virus (HIV), with 1.6 million HIV-related deaths per year [5]. The vast majority of these infections and deaths are in resource-limited settings. Due to remarkable progress in reducing costs of treatment, >10 million patients are now on antiretroviral (ARV) regimens in low- and middle-income countries [5].
People with hepatitis C infection can be cured using modern treatment, but at a very high cost. The US launch prices for 12 weeks of treatment with simeprevir and sofosbuvir, 2 newly approved drugs, are $66 000 and $84 000, respectively [6]. In low- and middle-income countries, access to HCV treatment is extremely limited, mainly because of the complexity of patient management and high costs [7]. Of the 20 countries with the largest HCV epidemics, 12 are classified as low or lower-middle income (Table 1). For widespread treatment of HCV in developing countries to become feasible, we will need short-course antiviral treatment available at very low costs and with minimal diagnostic support.
Table 1.
Hepatitis C Global Prevalence by Country (2010)
| Country | Income Classification | Most Prevalent Genotypes | Anti-HCVa, % | No. Infected |
|---|---|---|---|---|
| China | Upper-middle | 1, 2, 6 | 2.2 | 29 791 212 |
| India | Lower-middle | 1, 3 | 1.5 | 18 216 960 |
| Egypt | Lower-middle | 4 | 14 | 11 826 360 |
| Indonesia | Lower-middle | 1, 2 | 3.9 | 9 436 986 |
| Pakistan | Lower-middle | 3 | 5.9 | 9 422 402 |
| Russia | Upper-middle | 1, 3 | 4.1 | 5 796 498 |
| United States | High | 1, 2, 3 | 1.8 | 5 367 834 |
| Democratic Republic of Congo | Low | 4 | 6.4 | 4 010 240 |
| Nigeria | Lower-middle | 1, 2 | 2.1 | 3 323 439 |
| Japan | High | 1, 2 | 2.4 | 3 058 008 |
| Cameroon | Lower-middle | 1, 2, 4 | 13.8 | 2 754 204 |
| Brazil | Upper-middle | 1, 3 | 1.4 | 2 609 670 |
| Uganda | Low | 1, 4 | 6.6 | 2 230 536 |
| Philippines | Lower-middle | 1 | 2.2 | 1 932 854 |
| Italy | High | 1, 2 | 3.2 | 1 923 136 |
| Ukraine | Lower-middle | 1 | 4.0 | 1 864 840 |
| Uzbekistan | Lower-middle | 1, 3 | 6.5 | 1 774 955 |
| Turkey | Upper-middle | 1 | 2.2 | 1 549 108 |
| Ethiopia | Low | 1, 2, 4 | 1.9 | 1 500 734 |
| Thailand | Upper-middle | 1, 3, 6 | 2.2 | 1 499 058 |
| World's population | 2%–3% | 160–180 million |
Recently, a number of direct-acting antivirals (DAAs) in phase 2 or 3 development have shown sustained virologic response (SVR) rates of up to 100% in noncirrhotic patients and, promisingly, high SVR rates in people with advanced liver disease or previous null response (Table 2). The approval of such drugs will likely see DAA combinations replace interferon-based regimens as the new standard of care [18]. Success of treatment often depends on genotype; although the majority of results are from clinical trials carried out on patients infected with the HCV-1 genotype, encouraging results are emerging for the treatment of both HCV-2 and HCV-3 (Table 2).
Table 2.
Results From Clinical Trials of Interferon-Free Regimens
| Combination | Study Population | Previous Response | Genotype | Treatment Arm(s) | SVR Rate |
|---|---|---|---|---|---|
| Daclatasvir + sofosbuvir ± RBV | AI444–040 [10] Noncirrhotic (n = 170) | Naive | 1 | 12 wk (n = 82) | 95%–98% (SVR-4) |
| 24 wk (n = 44) | 93%–100% (SVR-24) | ||||
| 2/3 | 24 wk (n = 44) | 88%–100% (SVR-24) | |||
| Daclatasvir + asunaprevir + BMS-791325 | AI443-014 [11] Noncirrhotic (n = 32) | Naive | 1 | 24 wk (n = 16) | 94% (SVR-4) |
| 12 wk (n = 16) | 94% (SVR-12) | ||||
| Daclatasvir + asunaprevir | AI447-011 [12] (n = 38) | Null response | 1b | Once daily (n = 20) | 65% (SVR-12) |
| Twice daily (n = 18) | 78% (SVR-12) | ||||
| Sofosbuvir + RBV ± GS-5885 | ELECTRON [13] Noncirrhotic (n = 120) | Naive | 1 | 12 wk, 3-drug (n = 25) | 100% (SVR-12) |
| 12 wk, 2-drug (n = 25) | 84% (SVR-12) | ||||
| Null response | 12 wk, 3-drug (n = 9) | 100% (SVR-12) | |||
| Naive | 2/3 | 12 wk, 2-drug (n = 11) | 100% (SVR-24) | ||
| 8 wk, 2-drug (n = 25) | 64% (SVR-12) | ||||
| Experienced | 12 wk, 2-drug (n = 25) | 68% (SVR-12) | |||
| Sofosbuvir + weight-based RBV or low-dose RBV | SPARE [14] Noncirrhotic (n = 10); all stages fibrosis (n = 50) | Naive | 1 | 24 wk, noncirrhotic, WB (n = 10) | 90% (SVR-12) |
| 24 wk, WB (n = 25) | 72% (SVR-4) | ||||
| 24 wk, LD (n = 25) | 56% (SVR-4) | ||||
| Sofosbuvir + RBV | POSITRON [15] (n = 207 + 71 placebo) | IFN-ineligible | 2 | 12 wk (n = 109) | 93% (SVR-12) |
| 3 | 12 wk (n = 98) | 61% (SVR-12) | |||
| FUSION [15] (n = 201) | Experienced | 2 | 12 wk (n = 36) | 86% (SVR-12) | |
| 16 wk (n = 32) | 94% (SVR-12) | ||||
| 3 | 12 wk (n = 64) | 30% (SVR-12) | |||
| 16 wk (n = 63) | 62% (SVR-12) | ||||
| Sofosbuvir + simeprevir ± RBV | COSMOS [16]; noncirrhotic (n = 80) | Null response | 1 | 24 wk, 3-drug (n = 24) | 67% (SVR-8) |
| 24 wk, 2-drug (n = 15) | 100% (SVR-8) | ||||
| 12 wk, 3-drug (n = 27) | 96% (SVR-8) | ||||
| 12 wk, 2-drug (n = 14) | 93% (SVR-8) | ||||
| Faldaprevir + BI207127 ± RBV | SOUND-C2 [17] (n = 329 [33 = F4]) | Naive | 1 | 28 wk, 3-drug (n = 316) | Up to 69% (SVR-12) |
| 28 wk, 2-drug (n = 46) | 39% (SVR-12) |
Abbreviations: IFN, interferon; LD, low-dose; RBV, ribavirin; SVR-4, undetectable hepatitis C virus RNA 4 weeks after finished treatment; SVR-12, undetectable hepatitis C virus RNA 12 weeks after finished treatment; SVR-24, undetectable hepatitis C virus RNA 24 weeks after finished treatment; WB, weight-based.
DAAs for HCV infection have similar mechanisms of action and chemical structures to antiretrovirals currently in use for the treatment of HIV. These drugs are also similarly intended for oral delivery using relatively uncomplicated formulation technologies. Over the last 2 decades, generic competition, increased purchase volumes, and improvements in manufacturing processes (both active pharmaceutical ingredients [APIs] and formulation) have driven the cost of HIV ARV treatment down by >99%, with standard triple therapy now costing as little as US$60 per patient per year [19].
These prices could not have been imagined when triple ARV drug combinations were introduced at more than US$10 000 per patient per year in the late 1990s [7]. Following the important precedent set by access to treatment for HIV infection, the aim of this analysis was to estimate the minimum cost of HCV treatment, assuming the same strategic market dynamics as used to supply ARVs to people with HIV/AIDS in developing countries.
METHODS
In this analysis, the molecular structures of HCV DAAs were compared with their closest analogues in the treatment of HIV. We evaluated the likely routes of manufacturing as published by the originator companies and assumed a volume demand based on 1–5 million patients per year to arrive at approximate costs of DAA APIs. We then added on a 40% margin for finished production manufacturing (formulation) to arrive at a projected cost of therapy.
The purpose of this analysis is to logically speculate whether DAAs can be provided for millions of people at a reasonable cost. The analysis is not meant to be exact or to arrive at a “most likely optimized cost” for any individual or combination DAA therapy. Very little information is presently available to estimate actual commercial formulation costs for DAAs. These DAAs are all delivered orally using conventional technologies. Projected API costs for these DAAs range from US$1400–to US$21 000 per kilogram; as such, the very high relative costs of API would justify a 40% increment as a reasonable add-on for estimating cost of the finished dosage form.
The minimum treatment costs of comparator HIV ARVs were calculated using the lowest prices reported by manufacturers to Médecins Sans Frontières in 2012 [19]. Minimum costs per gram of ARVs ranged from US$0.20 to US$0.90 per gram for nucleoside analogues, US$0.50 per gram for nucleotide analogues, and US$0.70–$2.10 per gram for protease inhibitors (Table 3).
Table 3.
Minimum Costs of HIV Antiretrovirals, by Increasing Cost per Gram
| Agent | Chemical Formula | Molecular Weight, g/mol | Daily Dose, mg | Overall Dose Per Year, g | Cost Per Gram, US$ | Cost Per Year, US$ |
|---|---|---|---|---|---|---|
| Lamivudine | C8H11N3O3S | 229 | 300 | 110 | 0.19 | 21 |
| Zidovudine | C10H13N504 | 267 | 600 | 219 | 0.34 | 75 |
| Tenofovir disoproxil fumarate | C23H34N5O14P | 636 | 300 | 110 | 0.52 | 57 |
| Indinavira | C36H47N5O4 | 614 | 1600 | 584 | 0.67 | 394 |
| Abacavir | C14H18N6O | 286 | 600 | 219 | 0.77 | 169 |
| Emtricitabine | C8H10FN3O3S | 247 | 200 | 73 | 0.79 | 58 |
| Stavudine | C10H12N2O4 | 224 | 60 | 22 | 0.86 | 19 |
| Lopinavir/ritonavirb | C37H48N4O5 | 629 | 800/200 = 1000 | 365 | 1.01 | 368 |
| Darunavira | C27H37N3O7S | 548 | 1200 | 438 | 1.83 | 803 |
| Saquinavira | C38H50N6O5 | 671 | 2000 | 730 | 1.87 | 1366 |
| Atazanavira | C38H52N6O7 | 705 | 300 | 110 | 2.11 | 231 |
a Prices of protease inhibitors do not include the cost of the ritonavir booster drug, except for lopinavir/ritonavir.
b Chemical formula and molecular weight for lopinavir only.
Source: MSF Drug Access Team [19].
Four HCV DAAs were included in this study: daclatasvir (phase 3), sofosbuvir (approved December 2013), faldaprevir (phase 3), and simeprevir (approved November 2013). Ribavirin, already available in generic form, was also included in this analysis due to its likely inclusion in future drug regimens. Using the most likely daily dosage identified from clinical trials, the total drug requirement for each DAA was calculated for a 12-week course of each HCV DAA. To estimate the manufacturing cost of each HCV DAA, current routes of synthesis and critical cost-limiting raw materials were taken from the patent literature. In an alternative comparison, each compound was matched to the closest equivalent HIV ARV based on structural similarity.
Production costs per gram of HCV DAA were assumed to be 1–10 times higher than the equivalent HIV ARV, dependent on the complexity of chemical synthesis. Complexity was assessed by identifying the most likely cost-limiting intermediates for the synthesis of each DAA. Additional considerations included ease and number of steps of manufacture and availability and cost of starting materials. Using this estimate for the production cost per gram of drug together with the total drug requirement, an estimate for the minimum cost of a 12-week course of treatment with each HCV DAA was calculated. These costs were used to provide estimates for the production costs of 2- or 3-drug combination therapy based on the combinations currently being investigated in clinical trials (Table 2).
RESULTS
Based on molecular weight, chemical structure, class, and dose, the HIV ARV most comparable to each HCV DAA were as follows: zidovudine to ribavirin, atazanavir to daclatasvir, tenofovir and stavudine to sofosbuvir, darunavir to faldaprevir, and lopinavir to simeprevir. Table 4 shows the HCV DAA and most comparable HIV ARV as well as the likely cost limiting raw materials in production. A summary of the estimated costs per person for a 12-week course of each HCV DAA is shown in Table 5.
Table 4.
Hepatitis C Virus Direct-Acting Antiviral Structure and Likely Cost Limiting Raw Material in Production
| HCV DAA Agent | HCV DAA Structure and Retrosynthesis | Comparator HIV Agent |
|---|---|---|
| Ribavirin C8H12N4O5 MW: 244 g/mol | 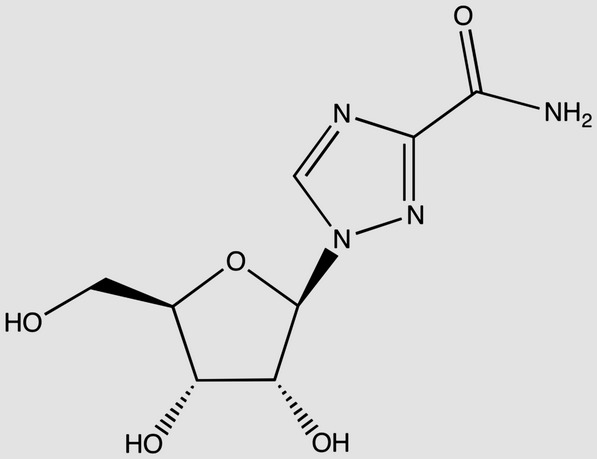 |
Zidovudine C10H13N5O4 MW: 267 g/mol |
| Daclatasvir C40H50N8O6 MW: 739 g/mol |
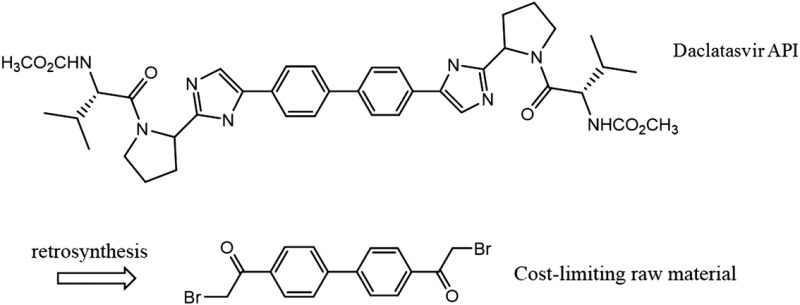 |
Atazanavir C38H52N6O7 MW: 705 g/mol |
| Sofosbuvira C22H29FN3O9P MW: 529 g/mol |
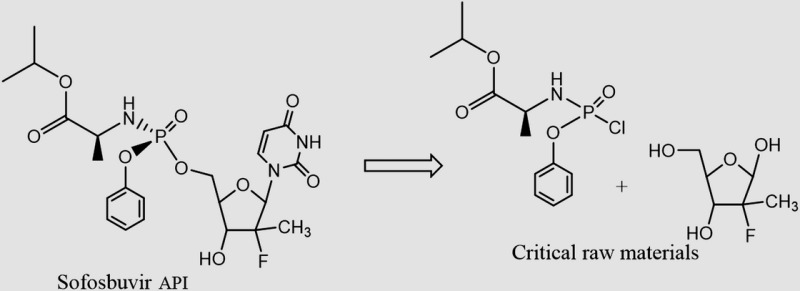 |
TDF C23H34N5O14P MW: 636 g/mol |
| Faldaprevir C40H49BrN6O9S MW: 870 g/mol |
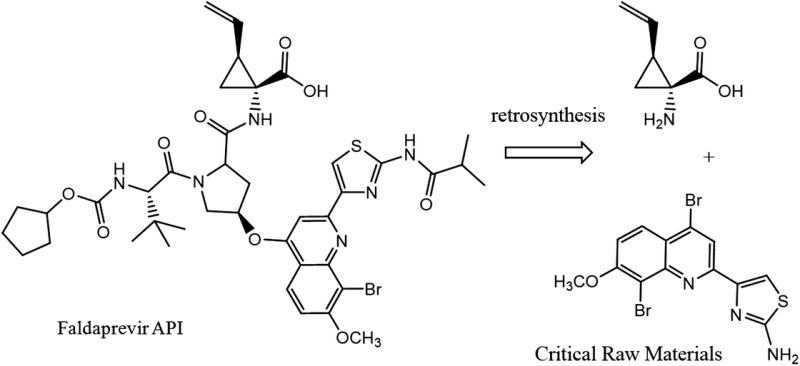 |
Darunavir C27H37N3O7S MW: 548 g/mol |
| Simeprevir C38H47N5O7S2 MW: 750 g/mol |
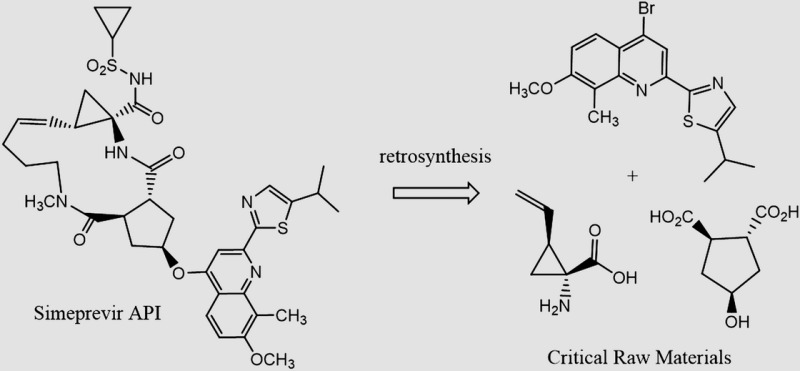 |
Lopinavir/r C37H48N4O5 MW: 629 g/mol |
Abbreviations: API, active pharmaceutical ingredients; DAA, direct-acting antiviral; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MW, molecular weight; TDF, tenofovir.
a Sofosbuvir is presumably synthesized as a mixture of diastereomers on phosphorous. Only one of these 2 diastereomers is desired in the active pharmaceutical ingredients. Separation of diastereomers can be a substantial contributor to the cost of API production, particularly when an undesired diastereomer cannot be efficiently recycled. See US patent 7 390 791 and US application 20130065856 for the tenofovir alafenamide fumarate (TAF) prodrug version of tenofovir, which contains a “ProTide” moiety identical to that present in sofosbuvir. Efficient recycling of diastereomers has been demonstrated for TAF, but we do not presume this is the case for sofosbuvir.
Table 5.
Predicted Minimum Costs of Hepatitis C Virus Direct-Acting Antivirals
| Agent | Daily Dose, mg | Overall Dose Per 12 wk, g | Estimated Cost per Gram, US$ | Predicted Cost, US$ |
|---|---|---|---|---|
| Ribavirin | 1000–1200 | 84–101 | 0.29–0.41a | $34–$48b |
| Daclatasvir | 60 | 5 | 2–6 | $10–$30 |
| Sofosbuvir | 400 | 34 | 2–4 | $68–$136 |
| Faldaprevir | 120 | 10 | 10–21 | $100–$210 |
| Simeprevir | 150 | 13 | 10–21 | $130–$270 |
a Current range of active pharmaceutical ingredients cost per gram from 3 Chinese suppliers.
b Shows cost for 1000 mg daily dose; $41–$58 for 1200-mg daily dose of ribavirin; adjusted with a 40% markup for formulation.
Ribavirin
With a daily dose of 1000–1200 mg dependent on patient weight, a 12-week course of treatment with ribavirin will require between 84.0 and 100.8 grams of API. This results in a range of demand between 84 and 504 metric tons of API to treat 1–5 million patients. Ribavirin is a nucleoside analogue with a molecular weight of 244 g/mol. Based on this and the chemical formula (Table 3), zidovudine was considered to be the closest equivalent HIV relative of ribavirin (also a nucleoside analogue with molecular weight 267 g/mol). Analysis revealed that ribavirin has a relatively simple chemical synthesis [20]. Using zidovudine alongside knowledge of the current costs of ribavirin, production costs were estimated to lie between US$0.29 and US$0.41 per gram, giving potential costs for a 12-week course of ribavirin of US$34–$48 for the dose of 1000 mg per day, and US$41–$58 for the dose of 1200 mg/day.
Daclatasvir
At the dose of 60 mg/day, a 12-week course of treatment would require 5.0 grams of daclatasvir API. Daclatasvir is a NS5A inhibitor with a molecular weight of 739 g/mol. Treating 1–5 million patients with daclatasvir would require 5–25 metric tons of API. Daclatasvir was deemed most structurally comparable to atazanavir, a protease inhibitor with a molecular weight of 705 g/mol (Table 3). Daclatasvir has a straightforward synthesis with the cost-limiting intermediate being the substituted biphenyl compound shown (Table 5) [21, 22]. There is wide availability of cheap starting materials to synthesize the side-chains of daclatasvir. The estimated production costs of daclatasvir finished product are between US$2 and US$6 per gram. At a daily dose of 60 mg, the estimated production costs for a 12-week course of treatment were US$10–$30.
Sofosbuvir
Delivered at a 400 mg daily dose, a 12-week course of treatment with sofosbuvir will require 33.6 grams of API. This results in a range of API demand between 33.6 and 168 metric tons to treat 1–5 million patients. With a molecular weight of 529 g/mol and chemical formula of C22H29FN3O9P, sofosbuvir was considered most structurally comparable to tenofovir, with a molecular weight of 636 g/mol and chemical formula C23H34N5O14P (Table 3). The cost-limiting raw material/intermediate for the synthesis of sofosbuvir API is the 2′-fluoro-2′-methylfuranose intermediate (Table 5) [23–25]. Although a number of approved drugs have similar structures, the presence of both a methyl and a fluoro substituent at the 2′ position makes this intermediate cost-limiting. We have estimated API production costs for sofosbuvir between US$2 and US$4 per gram; sofosbuvir is considered most closely analogous in cost to the HIV drug stavudine, which is relatively expensive in terms of manufacturing costs per gram. An estimated cost for 12 weeks of treatment with sofosbuvir API is US$68–$136.
Faldaprevir
At a daily dose of 120 mg, 10.1 grams of faldaprevir API will provide a 12-week course of treatment. Treating 1–5 million patients with faldepravir would require 10.1–50.5 metric tons of API. Faldaprevir is a protease inhibitor with a molecular weight of 870 g and chemical formula of C40H49BrN6O9S (Table 3). As a result of this high molecular weight and complicated chemical structure darunavir was deemed the most cost-comparable with faldaprevir. Faldaprevir manufacture requires a tetra-substituted quinoline and a vinyl-cyclopropane amino acid as raw materials for the synthesis of the API (Table 5) [25, 26]. Due to the difficult synthesis, estimated production costs of US$10–$21 per gram were applied. Accordingly, a 12-week course of treatment with faldaprevir could cost between US$100 and US$210.
Simeprevir
At a daily dose of 150 mg, a course of treatment with simeprevir would require 13 grams of simeprevir API. Treating 1–5 million patients with simeprevir would require 13–65 tons of API. In terms of class, molecular weight and chemical structure, lopinavir/ritonavir was considered the equivalent HIV ARV of simeprevir, although atazanavir was included in comparing cost estimates (Table 3). Simeprevir is a medium-ring macrocycle that utilizes a ring-closing metathesis reaction in the late stages of API manufacturing, which is challenging (Table 5). Novel raw materials entered into the synthesis include a tetra-substituted quinoline and the same vinyl-cyclopropane amino acid as used in the synthesis of faldaprevir [27]. Production costs were estimated at US$10 to US$21 per gram, giving an estimated cost of treatment of US$130–$270 for 12 weeks.
Combination Therapies
Table 6 shows the estimated prices of combination therapy based on the DAA drug prices calculated in this analysis. Using the minimum costs, a 12-week course of treatment with daclatasvir and sofosbuvir could cost a minimum of US$78 per person. A treatment with sofosbuvir and simeprevir could cost US$198 for 12 weeks with the addition of ribavirin increasing this cost to US$232 per person. For some patients or some regimens, a 24-week treatment might be necessary, doubling treatment costs.
Table 6.
Potential Regimen Costs
| Regimen | Duration, wk | Predicted Cost, US$ |
|---|---|---|
| Daclatasvir + sofosbuvir | 12 | $78–$166 |
| Daclatasvir + sofosbuvir + ribavirin | 12 | $112–$214 |
| Sofosbuvir + ribavirin | 12 | $102–$184 |
| Sofosbuvir + simeprevir | 12 | $198–$406 |
| Sofosbuvir + simeprevir + ribavirin | 12 | $232–$454 |
DISCUSSION
Fifteen years ago, universal access to antiretroviral therapy for HIV/AIDS in developing countries was considered too complex and expensive to be feasible. With the invention of effective, simple therapy, a market was created for generic competition that in turn made treatment affordable, and along with the associated international funding, treatment scale-up became possible [28]. The situation of HCV treatment today is reminiscent of treatment for HIV/AIDS in the year 2000.
Our analysis suggests that 2- or 3-drug combinations of interferon-free HCV treatments could cost US$100–$250 for a 12-week course of treatment. These low prices coupled with the high SVR rates established in several trials shows the potential for large-scale, low-cost HCV treatment in developing countries, with the potential to repeat the model of low-cost HIV treatment that has benefited millions of people. This model of treatment is based on simplified, standardized treatment approaches using tolerable, easy-to-administer regimens that are supportive of task shifting (care delivery by lesser trained health staff) and good patient adherence, and could be facilitated by widespread access to oral, short-course DAA therapy in low-income countries [29].
The cost of production of HIV ARVs has fallen progressively over the past decade through increased market competition, increased volumes, and efficiencies in manufacturing processes. The Clinton Health Access Initiative pioneered purchasing from quality-assured generic pharmaceutical manufacturers in India and raw material manufacturers in China to ensure the lowest sustainable costs for some ARVs, lowering prices dramatically [30]. Our estimated unit costs of HCV DAAs per gram are still far higher than the current costs of HIV ARVs, and it could be assumed that the cost of HCV DAAs may further decrease over time through process optimization and the cheaper sourcing of raw materials as volume demand drives competition and process efficiencies.
We have been conservative in our estimations vs the history of costs for HIV drug production. API syntheses begin with raw materials of rather simple structure that are combined in a specific and modular fashion to build the more complex structures of drugs. When a commercial market already exists for these raw materials, their contribution to cost is rather modest. When raw materials with no previous commercial demand are used, these can contribute very substantially to cost. Efavirenz, an HIV-1 reverse transcriptase inhibitor, provides an illustration of this. Cyclopropylacetylene (CPA) is a raw material for the synthesis of efavirenz. During human clinical trials, when the demand for CPA was only a few metric tons, CPA cost was US$800–$1350 per kilogram. When the drug was approved (1998) and demand for CPA was about 50 tons per year, the price of CPA had fallen to US$300–$350 per kilogram. Today, with a global demand for efavirenz of >800 tons per year, CPA can be purchased for US$50–$60 per kilogram. Efavirenz was launched in 1998 at an API cost of $1800 per kilogram. The current best cost for the API is about $120 per kilogram from Indian generic suppliers.
The DAA molecules in this analysis each contain at least 1 novel raw material that will be expensive in the early phases of commercial introduction. Similar to the reductions in the cost of efavirenz, the estimated costs in this analysis can only be justified if we can guarantee the eventual procurement of large orders. With the DAAs investigated in this study, 12 weeks of treatment for 1 million patients would require between 5 and 34 metric tons of API, with ribavirin requiring between 84 and 101 tons of active ingredient. Treatment for only a fraction of the 185 million people infected with HCV could ensure order sizes in a similar region to HIV ARVs.
With the introduction of these new HCV DAAs, the methods used to diagnose and monitor HCV are likely to be greatly simplified [31]. To ensure widespread treatment, the costs of diagnostic and monitoring tests will also need to fall. In the same way that the cost of HIV treatment has decreased, the cost of HIV diagnostics and monitoring has rapidly declined over the last few decades, with commercial HIV RNA tests now costing less than US$2 per test [32].
There are several limitations with this analysis. First, to calculate more precise costs, more detailed analysis of the API and formulation processes for production will be necessary. Very little specific information is currently available about formulations of the DAAs. However, all of the DAAs are for oral delivery and—to the best available knowledge—use relatively common drug release technologies. This is in parallel with the formulation of ARVs. Given this, we applied a 40% conversion cost of API to finished product, noting that this figure is not exceeded for any of the large-scale ARV combinations being marketed. Secondly, access to the HCV DAAs at minimum prices in developing countries will strongly depend on the level of enforcement of patent restrictions. These price estimates are based on the previous experience with production of ARVs for HIV treatment, which assumes market competition through generic manufacture. Legal mechanisms such as voluntary or compulsory licenses may be needed to overcome patent barriers and stimulate such competition. Patents for daclatasvir [33], sofosbuvir [34], faldaprevir [35, 36], and simeprevir will remain in force until at least 2025 [37], after which time it should be possible to produce generic versions at much lower cost, provided no additional patents are granted for modifications such as route of synthesis, crystalline structures, or methods of use. Such “evergreen” patenting has happened repeatedly in HIV drug development and could further delay the introduction of generic DAAs [19, 38]. In the near future, there need to be negotiations with the patent holders on voluntary licensing, access prices for low- and middle-income countries, and mass production of low-cost DAAs. Unless these DAAs are widely introduced, current death rates from HCV of 500 000 people per year will continue for many years to come.
Finally, our analysis is limited to HCV DAAs that have been mainly evaluated in genotype 1 HCV. Although sofosbuvir has recently been approved in combination with ribavirin for the treatment of genotypes 2 and 3, further research needs to be conducted to ensure pan-genotypic coverage of HCV.
The high cost of drugs is often justified by the need to recover costs of research and development. In the case of ARVs for HIV, many of these costs were assumed by the public sector, where parts of the drug discovery and development occurred [39]. Several mechanisms have been used by originator companies to allow access to ARVs in low-income settings. These include differential pricing (charging more in high-income countries) and voluntary licensing (allowing third-party generic manufacture). Similar mechanisms could be employed for HCV DAAs to ensure these drugs are affordable, while also providing a financial return on the costs of research and development [40]. Commitments by national governments to scale up ARV therapy, with support from international donors, were also critical to leveraging prices by increasing the size and predictability of the HIV market. This will be an essential factor in lowering drug prices and increasing access to HCV DAAs [41].
Expanding access through greater affordability will also confer indirect benefits. Currently, a large proportion of untreated patients continue to spread the HCV pandemic worldwide. Lessons learned from HIV suggests that with the introduction of strong community programs for testing, and high rates of treatment coverage and retention, expanded access to treatment is also likely to have a pronounced effect on HCV transmission, a benefit already suggested in modeling studies [42].
Widespread access to HCV DAAs will also require fast regulatory approvals of new drugs, political will, establishment of national viral hepatitis programs, early access programs to start treating people who are most at risk, and accepted international HCV treatment guidelines.
In conclusion, widespread access to combinations of HCV DAAs is feasible, with potential target prices of US$100–$250 per person for a 12-week treatment course. Progressive reductions in these costs are likely through optimization of chemical synthesis and cheaper sourcing of raw materials. These low prices could make widespread access to HCV treatment in low- and middle-income countries a realistic goal, with substantial individual and public health benefits.
Notes
Author contributions. A. H. wrote the original plan for analysis and coordinated the project. B. S. conducted the literature search and compiled the data for analysis. J. F. worked on the routes of chemical synthesis and raw materials. N. F. worked on the patent access issues. S. K. worked on the clinical issues in treatment of hepatitis C. All authors critically reviewed the manuscript and approved the final version.
Potential conflicts of interest. A. H. has received consultancy payments from Janssen, not connected with this project. S. K. has received consultancy payments and travel grants from pharmaceutical companies, not connected with this project. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–42. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 cause of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Razavi H, Estes C, Pasini K, Gower E, Hindman S. HCV treatment rate in select European countries in 2004–2010. J Hepatol. 2013;58(suppl 1):S22–3. [Google Scholar]
- 4.Volk ML. Antiviral therapy for hepatitis C: why are so few patients being treated? J Antimicrob Chemother. 2010;65:1327–9. doi: 10.1093/jac/dkq157. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. HIV/AIDS fact sheet. Updated October 2013. Available at: http://www.who.int/hiv/en/ Accessed 8 November 2013.
- 6.Fair Pricing Coalition. Activists condemn Gilead for exorbitant price of its new hepatitis C drug. 9 December 2013. Available at: http://www.thebody.com/content/73462/activists-condemn-gilead-for-exorbitant-price-of-t.html. Accessed 11 December 2013.
- 7.Ford N, Singh K, Cooke GS, et al. Expanding access to treatment for hepatitis C in resource-limited settings: lessons from HIV/AIDS. Clin Infect Dis. 2012;54:145–72. doi: 10.1093/cid/cis227. [DOI] [PubMed] [Google Scholar]
- 8.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–15. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 9.Negro F, Alberti A. The global health burden of hepatitis C virus infection. Liver Int. 2011;31(suppl 2):1–3. doi: 10.1111/j.1478-3231.2011.02537.x. [DOI] [PubMed] [Google Scholar]
- 10.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. AI444040 Study Group. High rate of sustained virologic response with the all-oral combination of daclatasvir (NS5a inhibitor) plus sofosbuvir (nucleotide NS5b inhibitor) with or without ribavirin, in treatment-naive patients chronically infected with HCV GT 1, 2, or 3. 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), Boston, MA,; 9–12 November 2012. [Google Scholar]
- 11.Everson GT, Sims KD, Rodriguez-Torres M, et al. An interferon-free, ribavirin-free 12-week regimen of daclatasvir (DCV), asunaprevir (ASV), and BMS-791325 yielded SVR4 of 94% in treatment-nave patients with genotype (GT) 1 chronic hepatitis C virus (HCV) infection. 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 9–12 November 2012; Boston, MA. [Google Scholar]
- 12.Lok DF, Gardiner DF, Hezode C, et al. Sustained virologic response in chronic HCV genotype (GT) 1-infected null responders with combination of daclatasvir (DCV; NS5A inhibitor) and asunaprevir (ASV; NS3 inhibitor) with or without peginterferon alfa-2a/ribavirin (PEG/RBV) Hepatology. 2012;56(S1):230A–1A. [Google Scholar]
- 13.Gane EJ, Stedman CA, Hyland RH, et al. Once daily sofosbuvir (GS-7977) regimens in HCV genotype 1–3: the ELECTRON trial. Hepatology. 2012;56(S1):306A–7A. [Google Scholar]
- 14.Osiunusi A, Heytens L, Lee YJ, et al. High efficacy of GS-7977 in combination with low or full dose ribavirin for 24 weeks in difficult to treat HCV infected genotype 1 patients. 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), Boston, MA,; 9–12 November 2012. [Google Scholar]
- 15.Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–77. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 16.Lawitz E, Ghalib R, Rodriguez-Torres M, et al. COSMOS Study: SVR4 results of a once daily regimen of simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in HCV genotype 1 null responders. 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, GA,; 3–6 March 2013. [Google Scholar]
- 17.Zeuzem S, Soriano V, Asselah T, et al. Interferon (IFN)-free combination treatment with the HCV NS3/4A protease inhibitor faldaprevir (BI 201335) and the non-nucleoside NS5B inhibitor BI 207127±ribavirin: final results of SOUND-C2 and predictors of response. Hepatology. 2012;56(suppl 1):308A–9A. [Google Scholar]
- 18.No authors listed. A brighter future in the fight against hepatitis. Nat Med. 2013;19:791. doi: 10.1038/nm.3269. [DOI] [PubMed] [Google Scholar]
- 19.MSF Drug Access Team Untangling the web of antiretroviral price reductions. 2012. 15th edition, Available at: http://www.msfaccess.org/reports. Accessed June 2013.
- 20.Witkowski JT, Robins RK, Sidwell RW, Simon LN. Design, synthesis, and broad spectrum antiviral activity of 1-D-ribofuranosyl-1,2,4-triazole-3-carboxamide and related nucleosides. J Med Chem. 1972;15:1150–4. doi: 10.1021/jm00281a014. [DOI] [PubMed] [Google Scholar]
- 21.Gao M, Nettles RE, Belema M, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465:96–100. doi: 10.1038/nature08960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng G, Hamm J, Pack SK, et al. Bristol-Myers Squibb Company. Process for synthesising compounds useful for treating hepatitis C. Patent application WO2009/020825 A1. 12 February 2009.
- 23.Chun BK, Wang P. Pharmasset, Inc. Preparation of 2′-fluoro-2′-alkyl-substituted or other optionally substituted ribofuranosyl pyrimidines and purines and their derivatives. Patent application WO2006/031725 A3. 16 April 2009.
- 24.Chun BK, Pamulapati GR, Rachakonda S, et al. Pharmasset, Inc. Nucleoside phosphoramidates. Patent application US2011/0251152 A1. 13 October 2011.
- 25.White PW, Llinàs-Brunet M, Amad M, et al. Preclinical characterization of BI 201335, a C-terminal carboxylic acid inhibitor of the hepatitis C virus NS3-NS4A protease. Antimicrob Agents Chemother. 2010;54:4611–8. doi: 10.1128/AAC.00787-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Llinàs-Brunet M, Bailey MD, Goudreau N, et al. Discovery of a potent and selective noncovalent linear inhibitor of the hepatitis C virus NS3 protease (BI 201335) J Med Chem. 2010;53:6466–76. doi: 10.1021/jm100690x. [DOI] [PubMed] [Google Scholar]
- 27.Horvath A, Wuyts S, Depré D, et al. Janssen Pharmaceuticals, Inc: improved process for preparing an intermediate of the macrocyclic protease inhibitor TMC435. Patent application WO2013/061285 A1. 2 May 2013.
- 28.Schwartländer B, Grubb I, Perriëns J. The 10-year struggle to provide antiretroviral treatment to people with HIV in the developing world. Lancet. 2006;368:541–6. doi: 10.1016/S0140-6736(06)69164-2. [DOI] [PubMed] [Google Scholar]
- 29.Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–10. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 30.The Clinton Health Access Initiative. Antiretroviral (ARV) ceiling price list. May 2013. Available at: http://www.clintonhealthaccess.org/news-and-information/key-CHAI-documents. Accessed 20 June 2013.
- 31.MSF Access Campaign. The diagnosis and treatment of hepatitis C: a technical landscape. Available at: http://www.msfaccess.org/our-work/hiv-aids/article/2024. Accessed 20 June 2013.
- 32.Wu G, Zaman MH. Low-cost tools for diagnosing and monitoring HIV infection in low-resource settings. Bull World Health Organ. 2012;90:914–20. doi: 10.2471/BLT.12.102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bristol-Myers Squibb Company. Form 10-K: annual report pursuant to section 13 or 15(d) of the Securities Exchange Act of 1934. Fiscal year ending 31 December 2011. Page 9.
- 34.Pharmasset, Inc. Form 10-K: annual report pursuant to section 13 or 15(d) of the Securities Exchange Act of 1934. Fiscal year ending 30 September 2011. Page 26.
- 35.Llinàs-Brunet M, Bailey MD, Bhardwaj P, et al. Boehringer Ingelheim International. Hepatitis C inhibitor compounds. Patent application US7585845 B2. 8 September 2009.
- 36.Busacca CA, Frutos RP, Haddad N, et al. Boehringer Ingelheim International. Process for preparing acyclic HCV protease inhibitors. Patent application US7514557 B2. 7 April 2009.
- 37.Medivir. Stockholm: Boeringher-Ingelheim; 2012. 2012 annual report. 26. [Google Scholar]
- 38.Kapczynski A. Engineered in India—patent law 2.0. N Engl J Med. 2013;369:497–9. doi: 10.1056/NEJMp1304400. [DOI] [PubMed] [Google Scholar]
- 39.Chirac P, von Schoen-Angerer T, Kasper T, Ford N. AIDS: patent rights versus patient's rights. Lancet. 2000;356:502. doi: 10.1016/S0140-6736(00)02566-6. [DOI] [PubMed] [Google Scholar]
- 40.Moon S, Jambert E, Childs M, von Schoen-Angerer T. A win-win solution? A critical analysis of tiered pricing to improve access to medicines in developing countries. Global Health. 2011;7:39. doi: 10.1186/1744-8603-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes C, Coggin W, Jamieson D, et al. Use of generic antiretroviral agents and cost savings in PEPFAR treatment programs. JAMA. 2010;304:313–20. doi: 10.1001/jama.2010.993. [DOI] [PubMed] [Google Scholar]
- 42.Durier N, Nguyen C, White LJ. Treatment of hepatitis C as prevention: a modelling case studying in Vietnam. PLoS One. 2012;7:e34548. doi: 10.1371/journal.pone.0034548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. The World Bank. How we classify countries by income. Available at: http://data.worldbank.org/about/country-classifications/country-and-lending-groups . Accessed 20 January 2014.


