Abstract
The U.S. dietary supplement market increased by 7.5% in 2012 compared with 2011, reaching $32.5 billion in sales. Therefore, federally supported research on dietary supplements is important to determine their health effects, safety, and efficacy. A portfolio analysis was performed across the NIH and the Office of Dietary Supplements (ODS) for fiscal years (FYs) 2009–2011 by using the databases Human Nutrition Research Information Management (HNRIM) and Computer Access to Research on Dietary Supplements (CARDS). The results indicated that total NIH dietary supplement–related funding for FYs 2009–2011 was $855 million ($295 million in 2009, $311 million in 2010, and $249 million in 2011). The institutes and centers with the highest investment in dietary supplement research were as follows: the National Heart, Lung, and Blood Institute ($135 million); the National Cancer Institute ($188 million); the National Center for Complementary and Alternative Medicine ($99 million); the National Institute of Diabetes and Digestive and Kidney Diseases ($68 million); the National Institute of Environmental Health Sciences ($58 million); and the ODS ($32 million). The dietary supplement ingredients receiving the most funding were botanicals (22%), vitamins (20%), lipids (14%), and minerals and trace elements (10%). The top 3 outcome research areas were cancer (61% of total dietary supplement investment), cardiovascular disease (47%), and women’s reproductive health (38%). In FYs 2009, 2010, and 2011, the ODS provided 3.5%, 3.6%, and 4.1%, respectively, of the NIH investment in dietary supplement research. ODS funding focused on cellular, enzymatic, or molecular mechanisms (64% of total ODS funding). This portfolio analysis demonstrates that the NIH has committed substantial funding to dietary supplement research in an effort to expand the scientific knowledge base on the efficacy and safety of dietary supplements.
Americans spent $32.5 billion on dietary supplements in 2012, a 7.5% increase compared with 2011. This converts to >$100 spent monthly on dietary supplements by every man, woman, and child in the United States. Despite their widespread use, there is a lot of confusion about the health benefits, efficacy, and safety of dietary supplements. Currently, approximately half of adults report using ≥1 dietary supplements to “improve” and “maintain” overall health. By definition, dietary supplements are products intended to supplement the diet; they are not drugs and therefore are not intended to prevent, diagnose, treat, mitigate, or cure diseases. Less than one-quarter of supplements used by adults are recommended by a health care professional, and their effectiveness is often questionable (1–3). The NIH supports the funding of dietary supplement research to investigate their potential roles in promoting health and reducing the risk of chronic disease. To aid in this effort, the Dietary Supplement Health and Education Act authorized the establishment of the Office of Dietary Supplements (ODS)5 at the NIH in 1995. The mission of ODS is to strengthen the knowledge and understanding of dietary supplements by evaluating scientific information, stimulating and supporting research, disseminating research results, and educating the public to foster and enhance quality of life and health for the U.S. population (4). As a result, the ODS places high priority on working with NIH institutes and centers (ICs) to identify opportunities to cofund outstanding research grants related to dietary supplements. Currently, the ODS spends ∼50% of its budget in cofunding of research projects with other NIH ICs.
The NIH uses the Research, Condition, and Disease Categorization system to define the 200 categories, including nutrition, which the NIH reports to Congress annually. Dietary supplements are categorized under nutrition. This system uses text data mining in conjunction with NIH-wide definitions used to match projects to research spending categories. The definitions (fingerprints) are a list of terms and concepts selected by NIH scientific experts to define a research category. The NIH nutrition fingerprint was created by nutrition science experts representing many NIH ICs. The fingerprint is compared with each NIH-funded research project by searching titles, abstracts, and specific aims to generate a list of research projects that are nutrition-related. Once the nutrition fingerprint has identified the nutrition-related projects, they are downloaded into the Human Nutrition Research Information Management (HNRIM) database (5, 6). Nutrition experts further categorized research projects downloaded into HNRIM by nutrition-related topic (vitamin, mineral, lipids, dietary supplements, etc.). Research projects coded as dietary supplement–related are then also downloaded into the NIH ODS’ Computer Access to Research on Dietary Supplements (CARDS) database, where they are categorized by specific supplement ingredient, research area, and study type (7, 8). A research project might be categorized for multiple supplement ingredients; therefore, it could be included in a portfolio more than once.
In this report, we present an analysis of the dietary supplement research portfolio across the NIH and ODS. We selected projects funded between fiscal years (FYs) 2009 and 2011 and coded as “Dietary Supplements: Nutrient Ingredients” and “Dietary Supplements: Botanical and Other Nonnutrient Ingredients” in the HNRIM database (5). The category “Dietary Supplements: Nutrient Ingredients” was subcategorized as carbohydrates, lipids, alcohols, proteins and amino acids, vitamins, minerals and essential trace elements, water and electrolytes, fiber, and other nutrients in food. “Dietary Supplements: Botanical and Other Nonnutrient Ingredients” was subcategorized as either botanical or other nonnutrient ingredients.
Briefly, a “nutrient ingredient” was defined as any essential or nonessential nutrient or other food constituent that is typically described in standard nutrition reference texts or that falls within the review parameters of the Food and Nutrition Board, National Academy of Sciences, in consideration of DRIs. Thus, this category would include substances recognized as essential nutrients such as iron, vitamin C, essential amino acids, and substances not generally recognized as being essential but that have or may have a dietary or nutrient role in humans, such as fish oil, conjugated linoleic acid, glutamine, and carnitine. A “botanical” includes any plant-derived material, whether fresh, preserved, or dried full plants, plant parts, plant species mixtures, plant extracts, “herbs” or “herbal products,” regardless of whether it meets the dictionary definition of herb, or that is composed of parts, extracts, or preparations of wood plants. Other “nonnutrient ingredients” comprise a broad and diverse group of substances that are neither of plant origin nor alone could be viewed as “nutrients” within the commonsense meaning of the term. For example, such substances could include microorganisms and some of their constituents, such as prebiotics, probiotics, coenzyme Q10, melatonin, shark cartilage, etc. (5). All projects were downloaded from HNRIM and Excel files were created for each year. Data were analyzed and graphs were generated by using Excel (Microsoft). Variables of interests were number of awarded projects and dollar amount invested by each of the NIH ICs as well as projects coded as nutrient ingredients and botanical and other nonnutrient ingredients. The search results were reviewed by ODS staff for validation. Projects cofunded by different ICs were counted for each funding IC and the appropriate dollar amount was assigned. In addition, grants were assigned to all appropriate categories. For example, a grant was counted twice if it was categorized for both cancer and cardiovascular disease. The average percentage spent on dietary supplements by an IC was calculated by using the total IC obligation as reported by the NIH Office of Policy, Planning, and Evaluation. Dietary supplement categories with an expenditure of 1% were grouped under “other nutrients.” The CARDS database was searched to identify the specific research areas reported for FYs 2009 and 2010 in NIH-funded dietary supplement research. At the time of the analysis, research areas were not available in CARDS for FY 2011.
The NIH spent $855.7 million on dietary supplement research during FYs 2009–2011 ($295 million in 2009, $311 million in 2010, and $249 million in 2011). The decrease in funding between 2010 and 2011 is likely a result of increased expenditures in 2009 and 2010 because of the American Recovery and Reinvestment Act. On the other hand, the ODS spent $31.7 million on dietary supplement research. ODS investment increased from 3.5% of total NIH investment in dietary supplement research in 2009 to 4.1% in 2011 (Table 1). Twenty-six ICs and the ODS were analyzed for expenditures on dietary supplement–related research. The 6 main institutes at the NIH that fund dietary supplement–related research are as follows: the National Heart, Lung, and Blood Institute; the National Cancer Institute; the National Center for Complementary and Alternative Medicine (NCCAM); the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute of Environmental Health Sciences; and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Table 2).
TABLE 1.
Comparison of dietary supplement research across the whole NIH and the ODS in fiscal years 2009, 2010, and 20111
| Year | NIH total | ODS total | NIH |
| $, millions | $, millions | % of total | |
| 2009 | 294.5 | 10.4 | 3.5 |
| 2010 | 311.0 | 11.1 | 3.6 |
| 2011 | 249.2 | 10.2 | 4.1 |
ODS, Office of Dietary Supplements.
TABLE 2.
Funding of dietary supplements research at the NIH institutes, offices, and centers1
| NIH institutes, offices, and centers | 2009 | 2010 | 2011 |
| $, millions | $, millions | $, millions | |
| NHLBI | 57.9 | 37.4 | 39.6 |
| NCI | 48.5 | 68.0 | 71.8 |
| NCCAM | 38.3 | 39.0 | 22.3 |
| NIDDK | 25.6 | 21.9 | 20.2 |
| NIEHS | 23.8 | 22.3 | 12.8 |
| NICHD | 21.0 | 41.2 | 17.2 |
| NIA | 15.4 | 12.7 | 9.0 |
| NCRR | 11.6 | 8.4 | 4.9 |
| ODS | 10.4 | 11.1 | 10.2 |
| NINDS | 8.9 | 9.9 | 3.3 |
| NEI | 6.0 | 13.4 | 4.9 |
| NIAAA | 5.3 | 4.8 | 5.9 |
| NIDCR | 2.8 | 2.4 | 2.5 |
| OD2 | 4.7 | 1.1 | 7.6 |
| NIGMS | 2.5 | 2.2 | 1.2 |
| NIAID | 2.2 | 3.5 | 4.5 |
| NIMH | 2.2 | 2.8 | 1.9 |
| NIAMS | 1.4 | 2.4 | 3.1 |
| RMOD | 1.2 | 1.2 | 1.2 |
| NINR | 1.1 | 1.3 | 1.1 |
| NIMHD | 1.0 | 0.9 | 0.6 |
| NIDA | 0.9 | 0.9 | 0.6 |
| NHGRI | 0.8 | 0.9 | 1.0 |
| FIC | 0.4 | 0.4 | 0.0 |
| NIDCD | 0.3 | 0.3 | 1.3 |
| NLM | 0.06 | 0.05 | 0.0 |
FIC, Fogarty International Center; NCCAM, National Center for Complementary and Alternative Medicine; NCI, National Cancer Institute; NCRR, National Center for Research Resources; NEI, National Eye Institute; NHGRI, National Human Genome Research Institute; NHLBI, National, Heart, Lung, and Blood Institute; NIA, National Institute on Aging; NIAAA, National Institute on Alcohol Abuse and Alcoholism; NIAID, National Institute of Allergy and Infectious Diseases; NIAMS, National Institute of Arthritis and Musculoskeletal and Skin Diseases; NICHD, Eunice Kennedy Shriver National Institute of Child Health and Human Development; NIDA, National Institute on Drug Abuse; NIDCD, National Institute on Deafness and Other Communication Disorders; NIDCR, National Institute of Dental and Craniofacial Research; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; NIEHS, National Institute of Environmental Health Sciences; NIGMS, National Institute of General Medical Sciences; NIMH, National Institute of Mental Health; NIMHD, National Institute on Minority Health and Health Disparities; NINDS, National Institute of Neurological Disorders and Stroke; NINR, National Institute of Nursing Research; NLM, National Library of Medicine; OD, Office of the Director; ODS, Office of Dietary Supplements; RMOD, Road Map/Common Fund.
For this analysis, the OD includes the Office of Behavioral and Social Sciences Research, the Office of Research on Women’s Health, and the Office of Extramural Research. Although the ODS is part of the OD, its data are being reported separately.
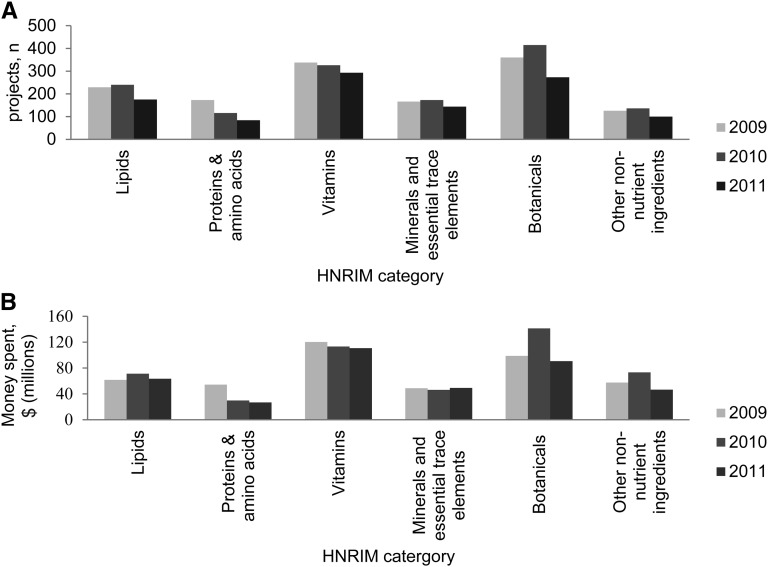
We also investigated the top HNRIM nutrient categories funded across the NIH and ODS. We found that lipids, protein and amino acids, vitamins, minerals and trace elements, and botanical and other nonnutrient ingredients were the main categories funded at the NIH in FYs 2009–2011. The total number of projects and the dollar amount invested showed the same trend during the 3 y analyzed (Fig. 1). The area of botanicals received the highest number of projects and dollar amounts across NIH ICs. For all future analyses, the average of the total funding was taken for FYs 2009–2011. Once the data were averaged, the percentage of total obligations per NIH institute spent on dietary supplement research was calculated and reported in Table 3. The results indicate that although the National Cancer Institute was the top funder of dietary supplement research, it spent only 1.25% of its total obligation on this type of research. In contrast, the NCCAM spent the most (26% of its total obligation) on dietary supplement research.
FIGURE 1.
Number of projects (A) and money spent (B) for the 6 main HNRIM categories funded at the NIH related to dietary supplement research in fiscal years 2009, 2010, and 2011. If applicable, grants were assigned to multiple categories; therefore, a grant could have been counted multiple times. HNRIM, Human Nutrition Research and Information Management database.
TABLE 3.
Average percentages of total institute obligations spent on dietary supplement research by the primary NIH institutes funding this type of research and the ODS in fiscal years 2009–20111
| NIH ICs | Dietary supplement research funding | Total IC obligations | Dietary supplements |
| $, millions | $, millions | % of total obligations | |
| NCI | 62.8 | 5024.0 | 1.25 |
| NHLBI | 45.0 | 3059.0 | 1.47 |
| NCCAM | 33.2 | 127.0 | 26.12 |
| NIDDK | 22.6 | 1937.0 | 1.17 |
| NICHD | 26.5 | 1312.0 | 2.02 |
| NIA | 12.4 | 1095.0 | 1.13 |
| ODS | 10.6 | — | — |
IC, institute and center; NCCAM, National Center for Complementary and Alternative Medicine; NCI, National Cancer Institute; NHLBI, National, Heart, Lung, and Blood Institute; NIA, National Institute on Aging; NICHD, Eunice Kennedy Shriver National Institute of Child Health and Human Development; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; ODS, Office of Dietary Supplements.
When analyzing number of projects for a specific nutrient category, we found that the most frequently funded dietary supplement categories across the NIH during 2009–2011 were as follows: botanicals (22%), vitamins (20%), lipids (14%), minerals and trace elements (10%), nonnutrient ingredients (8%), and proteins and amino acids (8%) (Table 4). Interestingly, the ODS had a very similar trend when compared with the NIH [botanicals (24%), lipids (17%), and vitamins (16%)], but spending by the institutes varied. Taken together, the data indicate that differences in the percentage of projects related to the dietary supplement categories among the ICs reflect variations in their research interests, priorities, and overall missions.
TABLE 4.
Type of dietary supplement research funded by all of NIH, ODS, NCCAM, NCI, NHLBI, NIA, NICHD, and NIDDK for fiscal years 2009, 2010, and 20111
| Supplement category |
||||||||
| NIH ICs | Carbohydrates | Lipids | Proteins and amino acids | Vitamins | Minerals and essential trace elements | Botanicals | Other nonnutrient ingredients | Other nutrients2 |
| % | ||||||||
| NIH | 5 | 14 | 8 | 20 | 10 | 223 | 8 | 13 |
| ODS | 6 | 17 | 8 | 16 | 11 | 243 | 7 | 11 |
| NCCAM | 0 | 13 | 4 | 5 | 2 | 523 | 18 | 6 |
| NCI | 2 | 8 | 2 | 20 | 8 | 363 | 5 | 18 |
| NHLBI | 7 | 17 | 15 | 253 | 5 | 10 | 5 | 17 |
| NIA | 2 | 19 | 8 | 253 | 9 | 17 | 8 | 12 |
| NICHD | 1 | 7 | 13 | 24 | 253 | 5 | 13 | 12 |
| NIDDK | 6 | 13 | 7 | 293 | 15 | 11 | 5 | 14 |
IC, institute and center; NCCAM, National Center for Complementary and Alternative Medicine; NCI, National Cancer Institute; NHLBI, National, Heart, Lung, and Blood Institute; NIA, National Institute on Aging; NICHD, Eunice Kennedy Shriver National Institute of Child Health and Human Development; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; ODS, Office of Dietary Supplements.
Dietary supplement categories such as fiber, alcohols, and water and electrolytes were combined under “other nutrients” when <1%.
Values represent the dietary supplement category with the highest percentage within that IC.
In addition to investigating the different dietary supplement categories, we were also interested in ascertaining the top research areas of investment. The top research areas funded across the NIH were cancer, cardiovascular, women’s reproductive health, immune function, and the central nervous system (Table 5). In contrast, the top research-funded areas at the ODS were cellular, enzymatic or molecular mechanisms, cardiovascular disease, diabetes, and cognitive function (Table 6).
TABLE 5.
Funding of dietary supplement research for selected research areas by the NIH in fiscal years 2009 and 20101
| Research area | NIH funding | NIH dietary supplement funding2 |
| $, millions | % | |
| Cancer | 184.8 | 61.0 |
| Cardiovascular disease | 143.7 | 47.4 |
| Women’s reproductive health | 117.3 | 38.7 |
| Immune function | 95.7 | 31.6 |
| Central nervous system | 94.0 | 31.0 |
| Cellular, enzymatic, or molecular mechanisms | 70.7 | 23.3 |
| Pediatric topics | 47.6 | 15.7 |
| GI function | 45.8 | 15.1 |
| Musculoskeletal system | 44.9 | 14.8 |
| Obesity | 40.2 | 13.2 |
| Diabetes | 37.3 | 12.3 |
| Nutrient requirements/metabolism | 36.7 | 12.1 |
| Respiratory system | 33.6 | 11.0 |
| Cognitive function | 30.4 | 10.0 |
| Aging | 26.2 | 8.6 |
| Addictions | 9.6 | 3.1 |
| Antioxidant function | 7.6 | 2.5 |
Source: Computer Access to Research on Dietary Supplements (CARDS) database (6). GI, gastrointestinal.
If applicable, grants were assigned to multiple categories. Therefore, a grant could have been counted multiple times.
TABLE 6.
Dietary supplement research for selected research areas by the ODS in fiscal years 2009 and 20101
| ODS funding |
|||
| Research area | $, millions | % | NIH funding on dietary supplements2 |
| % | |||
| Cellular, enzymatic, or molecular mechanisms | 6.9 | 64.7 | 9.9 |
| Cardiovascular disease | 5.3 | 49.9 | 3.7 |
| Diabetes | 3.4 | 32.0 | 9.2 |
| Cancer | 3.1 | 29.7 | 1.7 |
| Cognitive function | 2.0 | 18.6 | 6.6 |
| Respiratory system | 1.7 | 16.1 | 5.2 |
| Aging | 1.7 | 16.0 | 6.6 |
| GI function | 1.4 | 13.9 | 3.3 |
| Antioxidant function | 0.8 | 7.9 | 11.1 |
| Addictions | 0.2 | 2.5 | 2.8 |
Source: Computer Access to Research on Dietary Supplements (CARDS) database (6). GI, gastrointestinal; ODS, Office of Dietary Supplements.
If applicable, grants were assigned to multiple categories. Therefore, a grant could have been counted multiple times.
Our analysis indicated that the NIH has a commitment to invest in dietary supplement research to expand the scientific knowledge base on supplement efficacy and safety by continuing to fund new research on these areas. Also, most NIH ICs support some type of research related to dietary supplements in line with their mission statements. For instance, the NCCAM, whose mission is to support science to investigate the usefulness and safety of complementary and alternative medicine interventions and their roles in improving health and health care, spent a large portion (26%) of its total obligation on dietary supplements, mainly botanicals, which are widely used as alternative medicine (9).
A Nutrition Business Journal report indicates that the top dietary supplements sold in 2013 were vitamins (32.2%), botanicals and herbs (17.1%), and sports nutrition products (12.7%); vitamin sales alone surpassed $5 billion/y (10). Dietary supplement research at the NIH is mainly focused on botanicals and vitamins, correlating with the top products consumed by the general public.
The ultimate goal of the NIH is to improve the overall health of Americans by providing funding to investigate the causes, diagnosis, prevention, and cure of human diseases. Our portfolio analysis shows that in the area of dietary supplements, the NIH is largely focusing on research related to cancer and cardiovascular disease. The ODS fills a gap by cofunding research related to understanding the mechanisms of action of dietary supplement ingredients and their potential roles in health and disease. The ODS also funds the database CARDS, which provides information about dietary supplement research to researchers, health professionals, and the general public (8).
In conclusion, results from this portfolio analysis show that dietary supplement research is well represented across the NIH ICs and the ODS. Furthermore, there is a solid commitment to strengthen the knowledge of dietary supplements through research and dissemination of results. The ultimate goal is to provide the public with the necessary scientific evidence, risks, and benefits to make informed deci incorporating dietary supplements into their diets and lifestyles.
Acknowledgments
The authors thank Paul Coates, director of the ODS, for his support in completing this analysis. M.L.G.-C. wrote the manuscript, and M.L.G.-C., C.D.D., K.S.R., and E.A.W. were involved in the study design, data analysis, data interpretation, and manuscript content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CARDS, Computer Access to Research on Dietary Supplements; FY, fiscal year; HNRIM, Human Nutrition Research Information Management; IC, institute and center; NCCAM, National Center for Complementary and Alternative Medicine; ODS, Office of Dietary Supplements.
Literature Cited
- 1.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013;173:355–61 [DOI] [PubMed] [Google Scholar]
- 2.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel PR, Betz JM, Sempos CT, Picciano MF. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141:261–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160:339–49 [DOI] [PubMed] [Google Scholar]
- 4. Office of Dietary Supplements; NIH. Dietary supplement fact sheets [cited 2013 Aug]. Available from: http://dietary-supplements.info.nih.gov/
- 5. Human Nutrition Research Information Management (HNRIM) [homepage on the Internet] [cited 2012 Nov 21]. Available from: http://hnrim.nih.gov.
- 6. NIH. Computer Access to Research on Dietary Supplements (CARDS) database [cited 2013 Feb–Sep]. Available from: http://cards.nih.gov.
- 7.Haggans CJ, Regan KS, Brown LM, Wang C, Krebs-Smith J, Coates PM, Swanson CA. Computer Access to Research on Dietary Supplements: a database of federally funded dietary supplement research. J Nutr. 2005;135:1796–915987867 [Google Scholar]
- 8. National Center for Complementary and Alternative Medicine (NCCAM). Facts at-a-glance and mission [cited 2013 Nov 13]. Available from: http://nccam.nih.gov/about/ataglance.
- 9.Regan KS, Wambogo EA, Haggans CJ. NIH and USDA funding on dietary supplement research, 1999–2007. J Nutr. 2011;141:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. U.S. Dietary supplements: dietary supplements vs. U.S. nutrition industry. Nutrition Business Journal. 2013 Penton Media, Inc. p. 40.