Abstract
Understanding the cellular and molecular mechanisms underlying the self-renewal and differentiation of dental epithelial stem cells (DESCs) that support the unlimited growth potential of mouse incisors is critical for developing novel tooth regenerative therapies and unraveling the pathogenesis of odontogenic tumors. However, analysis of DESC properties and regulation has been limited by the lack of an in vitro assay system and well-documented DESC markers. Here, we describe an in vitro sphere culture system to isolate the DESCs from postnatal mouse incisor cervical loops (CLs) where the DESCs are thought to reside. The dissociated cells from CLs were able to expand and form spheres for multiple generations in the culture system. Lineage tracing indicated that DESC within the spheres were epithelial in origin as evident by lineage tracing. Upon stimulation, the sphere cells differentiated into cytokeratin 14- and amelogenin-expressing and mineral material-producing cells. Compared to the CL tissue, sphere cells expressed high levels of expression of Sca-1, CD49f (also designated as integrin α6), and CD44. Fluorescence-activated cell sorting (FACS) analyses of mouse incisor CL cells further showed that the CD49fBright population was enriched in sphere-forming cells. In addition, the CD49fBright population includes both slow-cycling and Lgr5+ DESCs. The in vitro sphere culture system and identification of CD49fBright as a DESC marker provide a novel plateform for enriching DESCs, interrogating how maintenance, cell fate determination, and differentiation of DESCs are regulated, and developing tooth regenerative therapies.
Keywords: tooth epithelial stem cell, sphere culture, CD49f (integrin α6), ameloblast, cervical loop
1. Introduction
Stem cells (SCs) that have the capacity to self-renew and to give rise to differentiated progeny are of broad interest because of their potential in regenerative therapy and their purported role in tumor initiation and relapse. The ability to identify and isolate SCs is essential for understanding molecular mechanisms underlying SC self-renewal and expansion, as well as their roles in tumorigenesis. Several approaches have been used to isolate or define SCs from a variety of organs. These include cell sorting based on SC surface markers [1–3], bromodeoxyuridine (BrdU) or other long-term label retention for slow cycling activities [4, 5], sphere-forming assays for the self-renewal property [6–8], and lineage tracing for their progeny [9]. Emerging evidence shows that two types of SCs exist in various tissues, in separate yet adjoining locations. The slow-cycling SCs can be identified by long-term label retention and active SCs that do not retain labels due to rapid cell divisions can be identified by expression of Lgr5 (Leucine-rich repeat-containing G protein-coupled receptor5) [9–12].
Teeth are highly mineralized organs derived from the dental epithelium and the underlying mesenchymal cells originally from neural crest, which undergo a series of sequential and tightly regulated processes to form a tooth [13–15]. Five different lines of dental stromal cells that possess SC properties have been established from developing or mature human teeth [16–20]. However, progressing in characterizing dental epithelial stem cells (DESCs) has been slow. It has been proposed that adult human teeth do not have DESCs since ameloblasts, the terminally differentiated dental epithelial cells, shed after tooth eruption in humans. Moreover, the lack of culture systems and well-accepted surface markers for DESCs further impede this research. Rodent incisors grow continuously throughout life, which is made possible by the existence of DESCs in the cervical loop (CL) region [21–24]. The presence of DESCs in the CL region is evidenced by the gradual differentiation of ameloblast-lineage cells apical to the incisal direction [25], the directional cell migration demonstrated by vital carbocyanine dye DiI tracking, long-term BrdU retention, cell cycle kinetics studies [23], and in vivo lineage trace of Sox2 expressing cells [26].
It has been proposed that slow cycling DESCs within the CL mainly reside in the stellate reticulum (SR) [23, 24, 27]. Recently, however, by tracing the long-term retention of H2B-GFP fusion protein [5], it has been shown that slow cycling DESCs are mainly located in the outer enamel epithelium (OEE) [28]. Interestingly, similar to rapidly renewing adult tissues, such as intestine and hair follicle, expression of the active SC marker Lgr5 has been found in the SR region of the CL [27]. Several other SC markers, such as Bmi-1, Oct 3/4, and Sox2 have recently been reported to be expressed in the CL [26, 29]. In vivo lineage tracing experiments further shows that the Sox2 positive DESCs give rise to multiple lineages of tooth epithelial cells [26]. However, to date the isolation of DESCs is still problematic because of the lack of DESC surface markers and the paucity of in vitro assay systems for isolating and testing DESC properties.
To address these critical issues, we established an in vitro sphere culture system for DESCs isolated from the CL. Thorough analyses indicated that the DESC sphere cells displayed SC properties and expressed high levels of SC markers, including Sca-1, CD49f (also designated as integrin α6), and CD44. Furthermore, it was demonstrated that CD49fBright was a suitable cell surface marker for identifying and isolating DESCs. Therefore, our study establishes a foundation for enriching and expanding DESCs for dental regenerative treatments and for understanding DESC-related pathogenesis.
2. Materials and Methods
2.1. Animals and isolation of tissues
All animals were housed in the Program of Animal Resources of the Institute of Biosciences and Technology, Texas A&M Health Science Center, and were handled in accordance with the principles and procedure of the Guide for the Care and Use of Laboratory Animals. All experimental procedures were approved by the Institutional Animal Care and Use Committee. Mice carrying the Nkx3.1Cre knock-in alleles [30], ROSA26LacZ [31], ROSA26EYFP [32] reporter alleles, K5rtTA [33], H2B-GFP [5], Lgr5LacZ, Lgr5EGFP-ires-CreERT2 [10], and Lgr4LacZ [34] transgenes were maintained and genotyped as described elsewhere. Inducible K5rtTA-H2BGFP expression was achieved by administration of regular chow containing 0.0625% doxycycline (Harlan Teklad).
2.2. Dissociation of the CL epithelial cells for DESC sphere culture
The CL regions defined as the apical tissue distal to the tooth mineralized portion (Fig. 1A) were dissected from postnatal day (P) 7 mice unless otherwise indicated. The dissected tissue was first incubated in a solution containing 1 mg/ml dispase and 1 mg/ml collagenase I (Life Technologies, Grand Island, NY) for 30 minutes at 37°C. Tissues were further dissociated by incubation in 0.005% trypsin for 25 minutes at 37°C with gentle pipetting. Cells were sieved through a 40 μm cell strainer (Falcon) to obtain a single-cell suspension. The cells were suspended in 50 μl oral epithelial progenitor medium (CnT-24) (Cellntec Advanced cell systems, Switzerland), and mixed with Matrigel (BD Biosciences) at a 1:1 ratio at a density of 50,000 cells/ml in primary cultures and 10,000 cells/ml in subsequent passages. The mixtures were plated around the rims of wells in a 12-well plate and allowed to solidify at 37°C for 30 minutes. After adding 1 ml of CnT-24 medium to each well, the cells were cultured in a CO2 incubator at 37°C. The medium was replenished every 3 days. Ten to fourteen days after plating, spheres with a diameter of over 50 μm were counted. To passage spheres, the medium was aspirated off and Matrigel was digested by incubation in 500 μl of dispase solution (1 mg/ml, dissolved in DPBS) for 30 minutes at 37°C. Digested cultures were collected, pelleted, resuspended, and incubated in 0.005% Trypsin/EDTA (Life Technologies) for 25 minutes at 37°C, and passed through a 40 μm filter. Cells were counted and replated. The differentiation medium was composed of DMEM+10%FBS with 3.0 mM Calcium, 100 nM dexamethasone, 10 mM β-glycerolphosphate, and 50μg/ml L-ascorbic acid. LS8 cells [35] derived from enamel organ and human 293 cells were maintained in 5% FBS-DMEM.
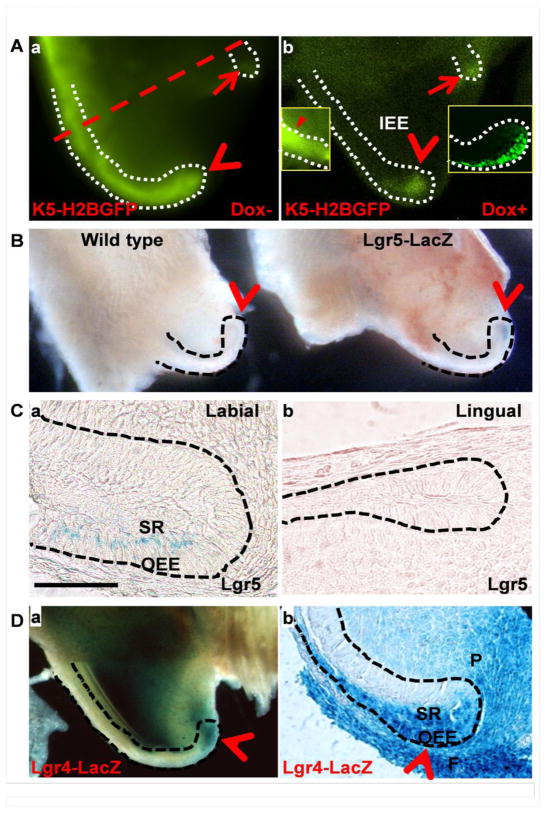
Fig. 1. Identification of label retaining slow-cycling and Lgr5-expressing active dental epithelial stem cells (DESC) in the mouse incisor cervical loop (CL).
A. Representative fluorescent microscopy pictures of K5-H2BGFP mouse maxillary incisor at postnatal 28 day (P28) before (a) or after (b) doxycycline treatment for 7 days. Box 1, the mature ameloblast region of the same tooth; box 2, confocal image of a frozen section of the labial CL shows GFP retaining cells. B. Wholemount X-gal staining of wildtype and Lgr5LacZ maxillary incisor. C. Section of the same tissues in (B) with eosin counterstaining showing that Lgr5-expressing cells were scarce and located in the SR opposite to the OEE. D. Wholemount of X-gal staining of Lgr4LacZ maxillary incisor (a) and section of the same tissue with eosin counterstaining (b) showing Lgr4-expressing cells. Dashed lines outline the CL; arrow heads indicate labial and arrows indicate the lingual CLs; SR, stellate reticulum; OEE, outer enamel epithelium; F, follicular; and P, dental papillary stromal cells.
2.3. Histology and histochemical analyses
Spheres were fixed with 4% paraformaldehyde (PFA) solution for 30 minutes at 4°C. Postnatal mouse heads were fixed with 4% PFA solution at 4°C overnight, and then decalcified by incubation in the decalcifying solution containing 12.5% EDTA and 2.5% PFA for 2 weeks at 4°C. The decalcifying solution was changed twice a week. Fixed tissues were serially dehydrated with ethanol, embedded in paraffin, and completely sectioned according to standard procedures. Immunohistochemical analyses were performed on paraffin sections (5 μm) or frozen sections (10 μm) mounted on Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA). Antigens were retrieved by boiling in citrate buffer (10 mM) for 20 minutes or as suggested by the manufacturers. Frozen incisor CL tissue sections, spheres, and cytospins were fixed in cold acetone for 5 minutes. The sections were incubated with primary antibodies diluted in PBS at 4°C overnight. The sources and concentrations of primary antibodies are: rabbit anti-amelogenin (1:1000 for tissue section, Western blot and 1:2000 for spheres and cells, a generous gift from Dr. Jan C.C.-Hu, University of Michigan, School of Dentistry); mouse anti-CK14 (1:400 for tissue section and cells, 1:3000 for Western blot, Santa Cruz Biotechnology (Santa Cruz, CA); anti-human/mouse CD49f (GoH3; 1:100, BioLegend, San Diego, CA), anti-human/mouse CD44 (1:100, eBioscience, San Diego, CA; clone IM7), and rat anti-Sca-1 (1:100, BD Pharmingen, San Diego, CA; clone D7). Specifically bound antibodies were detected with the ExtraAvidin Peroxidase System from Sigma (Saint Louis, MO) or with FITC- or APC-conjugated secondary antibodies (Life Technologies, Grand Island, NY), and visualized on a Zeiss LSM 510 Confocal Microscope.
To assess alkaline phosphatase activity, the sphere cells were fixed with 4% PFA for 10 minutes and washed with the AP buffer (100 mM Tris-HCl, pH9.5, 100 mM NaCl, 10 mM MgCl) for 10 minutes, followed by color development for 8–10 minutes in the solution containing NBT/BCIP substrate.
Alizarin red S staining was used to assess mineralization in cells and tissue sections. Briefly, cells were fixed with 4% PFA for 10 minutes and washed with distilled water and then stained with 2% alizarin red S, pH 4.2 for 1–2 minutes. Cells or sections were dehydrated in acetone and acetone-xylene (1:1) solution (20 dips each), cleared with xylene, and mounted.
For BrdU labeling assays, BrdU (Sigma, St. Louis, MO) was added to the culture medium at a final concentration of 10 nM after seeding. After incubation for 24 hours, the cells were washed with PBS three times. New culture media were then added to each well. Incorporated BrdU was detected by immunostaining with anti-BrdU antibody (1:500 dilution, Sigma Co, St. Louis, MO). For LacZ staining, the tissues and spheres were first lightly fixed with 0.2% glutaraldehyde for 1 hour or 15 minutes, respectively and then incubated overnight with 1 mg/ml X-Gal at room temperature. The tissues were dehydrated and processed according regular procedures for paraffin embedding after a wash with PBS for 10 minutes, post-fixed with 4% PFA at room temperature overnight, and decalcified as aforementioned.
2.4. In Situ hybridization
Rehydrated paraffin-embedded tissue sections or frozen sections were digested with 20 μg/ml protease K for 7 minutes at room temperature. After prehybridization at 65°C for 2 hours, the hybridization was carried out by overnight incubation at 65°C with 0.5 μg/ml digoxigenin labeled RNA probes for the indicated genes. Non-specifically bound probes were removed by washing four times with 0.1 x DIG washing buffer at 60°C for 30 minutes. Specifically bound probes were detected using alkaline phosphatase-conjugated anti-digoxigenin antibody (Roche, Indianapolis, IN). The Olfm4 probe was constructed as described [10].
2.5. Flow cytometry and cell sorting analysis
Tooth CL or sphere cells were stained with: APC conjugated anti-CD49f (BioLegend, clone GoH3, 20μl/100ul), APC conjugated anti-Sca-1 (BD Pharmingen and Miltenyi Biotec, Auburn, CA; clone D7, 1 mg/ml), or FITC or APC conjugated anti-CD44 (BD Pharmaingen and eBioscience; clone IM7, 1 mg/ml), as well as PE. All antibody incubations, washes, and flow cytometric analyses were performed in cell sorting buffer (1× PBS, 2% FBS). Analyses were conducted on a BD FACSAria I SORP or BD Accuri C6 Flow cytometer, and a minimum of 10,000 counts was acquired for each experimental condition. Fluorescence-activated cell sorting (FACS) was performed on a BD FACSAria I SORP, and cells were sorted into DMEM+20% FBS. Primary antibody labeling for flow cytometry and cell sorting was conducted using a 20-minute ice-cold incubation with antibody dilution according to manufacturer’s suggestions in a volume of 100 μl per 1 million cells in cell sorting buffer. The cells were washed in 1 ml ice-cold cell sorting buffer, resuspended in 0.5 ml cell sorting buffer, and analyzed. Proper isotype controls were used according to manufacturer’s suggestions.
2.6. Gene expression analysis
Total RNA was extracted with the Ribopure RNA isolation reagent (Ambion, TX). Reverse transcription was carried out with SuperScript III (Life Technologies, Grand Island, NY) and random primers. Real-time PCR was performed on Mx3000 (Strategene), using the SYBR Green JumpStart Taq ReadyMix (Sigma, St. Louis, MO) with pairs of primers specific for each transcript and following the manufacturer’s protocol. The ratio between expression levels in the two samples was calculated by relative quantification, using β-actin as a reference transcript for normalization. The primer sequences are listed in Table 1.
Table 1.
Nucleotide sequence of primers
| Genes | Primers |
|---|---|
| Abcg | 5′ GGGTGGCTTTTGTGGCAGGC 3′ 5′ ACTTGCCTCCGCCTGTGGGT 3′ |
| CD44 | 5′ AATTCCGAGGATTCATCCCA 3′ 5′ CGCTGCTGACATCGTCATC 3′ |
| Sca-1 | 5′ ATGGACACTTCTCACACTACAAAG 3′ 5′ TCAGAGCAAGGTCTGCAGGAGGACTG 3′ |
| CD49f | 5′ GTGGCCCAAGGAGATTAGC 3′ 5′ GTTGACGCTGCAGTTGAGA 3′ |
| CD133 | 5′ ACCAACACCAAGAACAAGGC 3′ 5′ GGAGCTGACTTGAATTGAGG 3′ |
| Klf4 | 5′ CACCATGGACCCGGGCGTGGCTGCCAG AAA 3′ 5′ TTAGGCTGTTCTTTTCCGGGGCCACGA 3′ |
| Lgr4 | 5′ TACAGATGCAGCAAATGCCAC 3′ 5′ GGCAGTGATGAACAAGACGCA 3′ |
| Lgr5 | 5′ CCTCTGCTTCCTAGAAGAGTTAC 3′ 5′ CTAGTTCCTTAAGGTTGGAGAGT 3′ |
| CK14 | 5′ GACTTCCGGACCAAGTTTGA 3′ 5′ CTTGAGGCTCTCAATCTGC 3′ |
| Amg | 5′ CCTGCCTCCTGGGAGCAGCTT 3′ 5′ CACGGGCTGTTGAGCTGGCA 3′ |
| Amb | 5′ TGGGGCTCAAGGCATGGCAC 3′ 5′ TGCAGCAGTGGGCTGGAGGA 3′ |
| Jagged1 | 5′ GCACCCGCGACGAGTGTGAT 3′ 5′ TCCCAGGCCTCCACCAGCAA 3′ |
| Notch 1 | 5′ CCCACTGTGAACTGCCCTAT 3′ 5′ CACCCATTGACACACACACA 3′ |
| Notch 2 | 5′ TGCAGCTCAGAGGGCAGCCT 3′ 5′ GCCACGGCACAAGTCCCTCC 3′ |
| ALP | 5′ CATGGGTGGCGGCCGGAAAT 3′ 5′ CCTGGAGGGGTCAAGGGCCA 3′ |
| EMSP-1 | 5′ CCCACCTCTCCATCCAGCAC 3′ 5′ GGCCTTGTAGTCAGTCCATAGC 3′ |
| Tuftelin 1 | 5′ CTGCCTGCAGAAGCTCCGGG 3′ 5′ GGTCTGACTGGTGTCGCCGC 3′ |
| β-actin | 5′ GCACCAAGGTGTGATGGTG 3′ 5′ GGATGCCACAGGATTCCATA 3′ |
2.7. Western blotting analysis
Dissected teeth or cultured cells were homogenized in the cell lysis buffer (0.5% Triton-X-100-PBS), and the lysates were harvested by centrifugation. Western blot analyses were performed as previously described [36]. Quantification of the band intensities was performed using NIH Image J software (http://rsb.info.inh.gov/ij).
2.8. Statistical analyses
Except specified in the result or figure legends, all data were collected from three independent experiments and expressed as means±standard deviation. The statistical significance between means of multiple groups was assessed by ANOVA and between means of two groups was assessed by t-test. Data was analyzed by using SPSS 16.0 software. p≤0.05 was considered significant.
3. Results
3.1. Mouse incisor CLs contain both slow-cycling and Lgr5+ DESCs
To determine whether mouse incisors had slow-cycling SCs, the tetracycline-regulated K5-H2BGFP reporter system was utilized to trace the label retaining cells. Expression of the histone 2B-green fluorescent protein (H2B-GFP) fusion protein was driven by the keratin 5 (K5) promoter under the control of tetracycline-suppressor [5]. All dental epithelial cells were H2B-GFP positive without being treated with doxycycline (Fig. 1A). After doxycycline treatment to turn off H2B-GFP expression for 7 days, the inner enamel epithelium (IEE) where transit amplifying (TA) cells were located was GFP negative. The slow-cycling GFP retaining cells only were present at the labial and lingual CLs and differentiated ameloblast areas (Fig. 1A). Furthermore, the GFP retaining cells in the labial CL were mainly located in the OEE, which was similar to previously reported results [28]. As the Lgr5LacZ reporter alleles had been widely used to identify active SCs [10], we then used the mice carrying Lgr5LacZ alleles to identity active DESCs in the CL. Wholemount LacZ staining revealed that only a few cells in the labial CL were weakly Lgr5+ (Fig. 1B). Tissue section staining showed that the Lgr5LacZ positive cells were mainly located in the SR within the zone adjacent to the OEE (Fig. 1C), which was consistent with in situ hybridization data showing Lgr5 expression in the SR of embryonic incisors [27]. Unlike label retaining/slow-cycling SCs in both labial and lingual CLs, Lgr5 expression was identified only in the labial, but not the lingual, CLs (Fig. 1C). Lgr4 is expressed in slow cycling SCs, active (Lgr5+) SCs, TA cells, and stromal cells surrounding SC niches of dermal papillae and intestine [37, 38]. We then used the Lgr4LacZ reporter to identify the Lgr4-expressing cells in the CL (Fig. 1D). Strong and broad X-gal stained cells were found in both epithelial and stromal cells surrounding the CL. Sections of the wholemount X-gal stained tissues further showed that the Lgr4 expressing cells were located in the OEE and SR regions of the labial CL, as well as the dental follicular and dental papillary stromal cells. Together, these data suggest that similar to other actively renewing organs, the mouse incisor CL contains both slow-cycling and Lgr5+ SCs.
3.2. Establishing a sphere culture system suitable for propagating DESCs
Since the in vitro sphere-forming capacity is a common way to assess the self-renewal property of SCs from various organs [6–8], a sphere culture system optimized for propagating DESCs was established. The CL regions defined as the apical tissue distal to the tooth mineralized portion (Fig. 1A) were dissected from postnatal day (P) 7 mice. The dissociated cells were cultured in Matrigel (Fig. 2Aa). The cells formed spheres within 7–10 days after inoculation with an efficiency of 0.0513%±0.0133%. The average diameter of the spheres was 100 μm or larger. Under the same conditions, immortalized LS8, tooth epithelial cells derived from enamel organ [35], and a differentiated tooth epithelial cell line developed in our laboratory did not form spheres (data not shown). H&E staining of sphere sections revealed that the majority of spheres had an organized, concentric structure (Fig. 2Ab). The cells at the periphery were small, had hyperchromatic nuclei and a high nuclear to cytoplasmic ratio. The cells in the middle were larger with abundant cytoplasm and had a low nuclear/cytoplasmic ratio. The center of the sphere, however, was devoid of cells, but nearly full of non-cellular material possibly secreted by the cells although the composition remained uncharacterized. Cell lineage tracing with Nkx3.1cre-ROSA26lacZ or K5-H2BGFP reporter alleles demonstrated that the sphere cells were epithelial in origin (Fig. 2B). To determine whether the DESC spheres contained label retaining cells that represented slow-cycling DESCs, BrdU labeling was used to trace the slow-cycling cells in the spheres. There were a few BrdU positive cells in the peripheral region even at day 7 after BrdU withdraw, demonstrating the presence of long-term label retaining cells in DESC spheres (Fig. 2Ca). In addition, the tetracycline regulated K5-H2BGFP reporter system was also used to trace the label-retaining cells in DESC spheres. Without the doxycycline treatment, the K5-H2BGFP bearing DESC sphere cells had strong GFP signals (data not shown), but not those bearing the H2BGFP control transgene (Fig. 2Cb, insert). However, only 0.94±0.33% of the sphere cells had retained GFP signals randomly distributed in the peripheral region of the spheres in the presence of doxycycline (Fig. 2Cb). The GFP signals were restricted to the nucleus, which made it distinguishable from dead cells that normally had GFP homogenously distributed throughout the cell. Since the spheres were derived from a single cell, the retained nuclear H2B-GFP signal indicated that the cells were early progeny of the sphere-forming cells. They did not undergo multiple division cycles that would dilute the nuclear H2B-GFP signal.
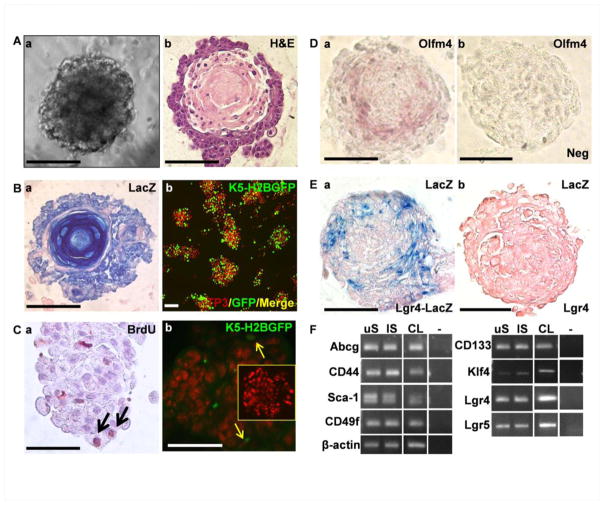
Fig. 2. DESC spheres contain both label retaining and Lgr5-expressing DESCs.
A. Morphology of DESC spheres. a, representative phase contrast images of DESC spheres; b, H&E staining of a DESC sphere section. B. Cell-lineage tracing with Nkx3.1cre-ROSA26LacZ (a) or K5-H2BGFP reporter showing the epithelial origin of DESC spheres (b). C. BrdU (a) or H2BGFP (b) label retaining cells in DESC spheres. Arrows indicate label retaining cells. Insert is a negative control carrying only the H2BGFP transgene. D. In situ hybridization showing Olfm4 (representing Lgr5)-expressing cells in DESCs (a) and sense probe as negative control (b). E. X-gal staining with eosin counterstaining of DESC spheres bearing Lgr4LacZ (a) or wildtype Lgr4 animals (b). F. RT-PCR analyses of total RNAs extracted from 14-day cultured DESC spheres derived from maxillary (uS) or mandibular (lS) incisors or the cervical loop (CL). -, no RT for negative control. To-Pro3 (TP3) was used for nuclear counter staining. Scale bar, 100 μm.
Since expression of Lgr5 is widely used to identify active SCs of various tissue origins [9–12], we then examined Lgr5 expression in DESC spheres. However, the expression of Lgr5 was too weak to be clearly demonstrated. Therefore, we examined the expression of Olfactomedin 4 (Olfm4), an olfactomedin-related glycoprotein coexpressed with Lgr5 and broadly used for tracing Lgr5+ SCs [39]. In situ hybridization revealed that Olfm4 was expressed in the cells at the junction between outer and middle cell layers of the spheres (Fig 2D). Lgr4 is another marker expressed in SCs and TA cells [37, 38]. X-gal staining of Lgr4LacZ positive spheres revealed that Lgr4 was expressed in a similar but slightly broader region than that of Olfm4 (Fig. 2E). RT-PCR analyses further demonstrated that DESC spheres and CLs expressed multiple SC markers, including ATP-binding cassette transporter gene (Abcg), CD44, Sca-1, CD49f, CD133, Klf4, Lgr4, and Lgr5 (Fig. 2F). Together, these results suggest that the DESC spheres contain both slow-cycling and Lgr5+ SCs located at separate, yet adjacent, regions. To date, the DESC spheres have been maintained in culture for more than 10 months and have been subcultured for more than 18 passages. These long-term culture experiments have been repeated three times. In contrast, the lifespan of ameloblasts in primary culture is about 2–3 passages or 8 weeks [40].
3.3. Differentiation of DESC sphere cells into dental epithelium
To determine whether DESC-containing spheres exhibited differentiated dental epithelial cells, immunostaining with anti-cytokeratin and anti-amelogenin was carried out. Although mainly expressed in basal cells in other organs, CK14 is expressed in the whole rodent dental epithelium with the highest expression in fully differentiated cells [41]. The results revealed weak expression of CK14 and scanty or no amelogenin expression in DESC sphere cells (Fig. 3A). To further evaluate the differentiation capability of DESC sphere cells, the spheres were disassociated, plated in regular tissue culture dishes, and cultured in the epithelial medium until confluence was reached. The medium was then changed to the differentiation medium and the cultures were continued for another 2 weeks. The monolayer cultured cells demonstrated different morphology ranging from tightly adherent, small round, to large flattened cells. The majority of the cells expressed CK14 and amelogenin (Fig. 3B). To avoid contamination of pre-existent ameloblasts that would diminish after 2–3 passages, high passage sphere cells were used for the Western blot and semi-quantitative real-time RT-PCR analyses for the expression of CK14 and amelogenin. The results demonstrated that both CK14 and amelogenin were expressed in 2-D cultures of sphere cells, although the expression level of amelogenin was much lower than in tooth tissues (Fig. 3C). Furthermore, the 2-D cultures also expressed ameloblastin, another characteristic protein expressed during amelogenesis (Fig. 3D). Ameloblastin is normally expressed in early stages of amelogenesis. Therefore, it was not surprising to see ameloblastin expression was higher than that of amelogenin.
Fig. 3. Multiple dental epithelial cell lineages from of DESC sphere cells.
A. Sections of DESC spheres were immunostained with anti CK14 or amelogenin (Amg) antibodies as indicated. No primary antibody served as a negative (Neg) control. B. 2D cultures of DESC sphere cells were immunostained with anti CK14 or Amg antibodies as indicated. C. Western blot (a) or real-time RT-PCR (b) analyses of 2D-cultured passage 7 DESC sphere-derived cells for expression levels of CK14 and Amg. Protein extracts of teeth (T) were used a control. The expression levels in (b) were normalized with β-actin loading controls and presented as relative expression ratios using Matrigel cultured spheres as 1. Data are means±sd from 4 replicated samples. D. RT-PCR analyses of Amg and ameloblastin (Amb) in 2D-culture of P8 sphere cells. E. Alizarin Red staining demonstrating calcified cells and products in the 2D-cultured DESC spheres cells or human 293 cells cultured in differentiation medium. F. RT-PCR analyses of total RNAs extracted from 2D cultured sphere-derived cells (A), 14-day cultured DESC sphere cells derived from maxillary (uS), mandibular (lS) incisors, and cervical loop (CL), showing expression of cell lineage-specific markers. -, no RT for negative control. G. Alkaline phosphatase staining of DESC spheres. Arrows indicate ALK positive cells inside the spheres. Jag1, Jagged 1; EMSP1, enamel matrix serine proteinase 1; ALP, alkaline phosphatase. *, p<0.05; scale bar: 100 μm.
Enamel is a highly mineralized tissue, which is primarily composed of crystalized calcium phosphate. To determine whether cells in DESC sphere had the potential to produce mineralized materials, the 2-D cultures of DESC cells were maintained in high calcium differentiation medium for two weeks and then subject to Alizarin Red staining (Fig. 3E). The results showed that the DESC cells were highly enriched in calcium and also were able to produce calcified matrix materials. Under the same conditions, human 293 cells did not exhibit calcium deposits, indicating that the calcium deposition in 2D cultured OESC sphere cells was not due to precipitation of medium calcium, although it had not been examined whether the calcified materials were hydroxyapatite or calcium phosphate. Together, the results demonstrate that DESC sphere cells have potential to differentiate into ameloblasts.
The DESCs give rise not only to the IEE that later differentiate to ameloblasts, but also the OEE, SR, and SI (stratum intermedium). To determine whether DESC sphere cells expressed these indicators of differentiation, RT-PCR analyses were carried out to detect the characteristic differentiation markers for these four lineages (Fig. 3F). Clearly, the DESC spheres expressed specific markers of ameloblasts at different maturation stages, including ameloblastin, enamel matrix serine proteinase 1 (EMSP1), and Tuftelin (early secretory to late maturation, early maturation stage, preameloblast, respectively). In addition, DESC spheres also expressed several characteristic molecules of various dental epithelial cells, including Jagged1 of the IEE, Notch1 of the SR and SI, Notch2 of the OEE and SR, and alkaline phosphatase (ALP) of the SI. Although ALP was expressed in the SI and stromal cells, the spheres were only composed of epithelial lineage cells. Thus, the ALP expressing cells in the spheres were the SI lineage cells (Fig. 3G). Therefore, the results indicated that the DESC spheres contained cells expressing markers of various differentiation stages and lineages. Further experiments are needed to determine whether there are distinct populations of cells in the spheres representing each individual lineage.
3.4. The CD49fBright subpopulation is enriched in sphere-forming cells
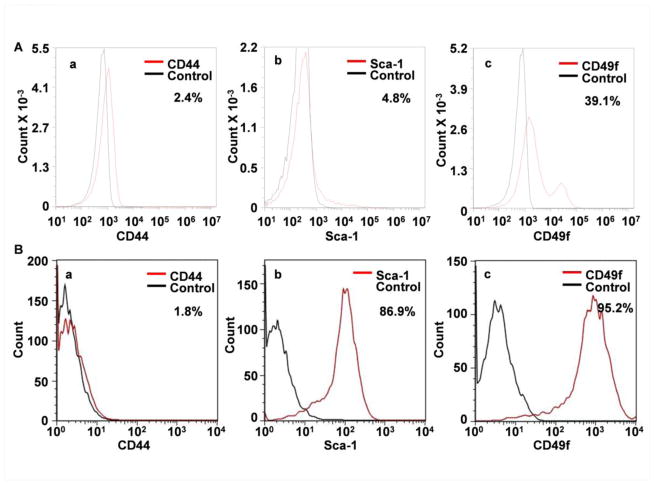
Since the commonly used SC surface markers, CD44, Sca-1, and CD49f were expressed in the CL and DESC spheres (Fig. 2D), we then examined whether these surface markers were suitable for assessing enrichment of DESCs by evaluating the sphere-forming activity of CD44+, Sca-1+, and CD49f+ cells. FACS analyses revealed that CD44, Sca-1, and CD49f were expressed in 2.4±0.3%, 4.8±0.7%, and 39.1±0.4% of maxillary CL cells (Fig. 4A) and 1.8±1.3%, 86.9±3.2%, and 95.2±0.6% of sphere cells (Fig 4B). Surprisingly, CD44+ or Sca-1+ sorting did not enrich cells with high sphere-forming activities; only the CD49f+ cells had high sphere-forming capacity (Fig. 5A). Cell lineage tracing with Nkx3.1Cre and ROSA26YFP reporter alleles revealed that only a fraction of CD49f+ cells in the CL were YFP expressing epithelial cells (Fig. 5B), suggesting that CD49f was also expressed in stromal cells that did not express Nkx3.1Cre. Yet, all DESC spheres were epithelial in origin (Fig. 2A). Therefore, CD49f is a suitable surface marker for enriching sphere-forming DESCs.
Fig. 4. DESC sphere culture enriches Sca-1 and CD49f expressing cells.
FACS analyses of freshly dissociated CL cells (A) or P0 DESC sphere cells (B) labeled with anti-CD44, Sca-1, or CD49f antibodies, demonstrating that the populations of cells expressing Sca-1 or CD49f were significantly increased by sphere culture.
Fig. 5. Cell sorting based on CD49fBright enriches sphere-forming DESCs.
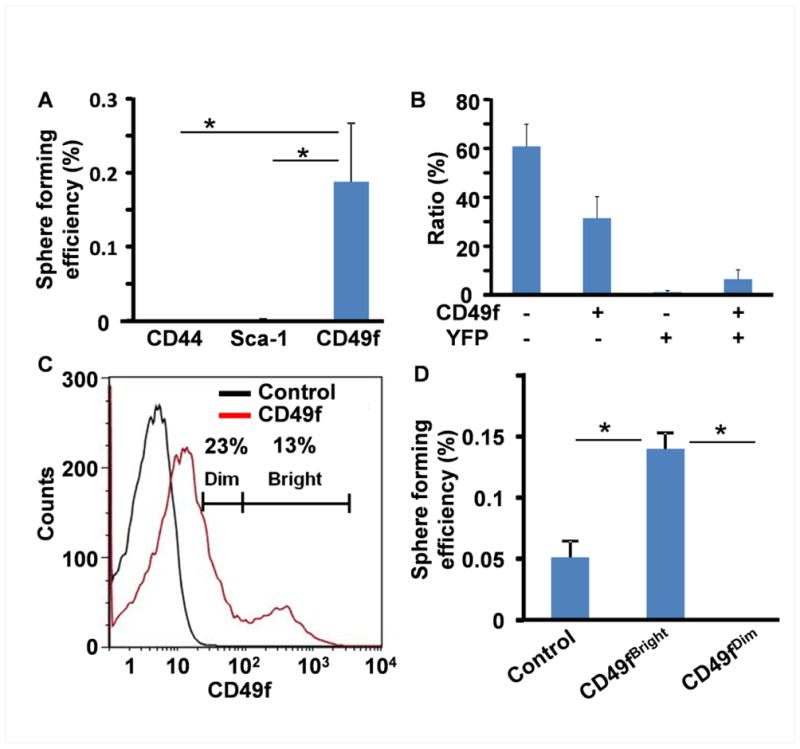
A. Sphere-forming analyses for FACS sorted cells based on CD44, Sca-1, or CD49f, showing that only CD49f+ cells consistently form DESC spheres. B. Two color FACS analyses of freshly dissociated CL cells from Nkx3.1cre-ROSAEYFP mice showing CD49f was expressed in both epithelial (YFP+) and stromal (YFP−) cells. C. CD49f+ cells were arbitrary divided into Dim and Bright groups based on fluorescence intensity. Two third of CD49f+ cells (23% of total population) only exhibit weak CD49f expression. About 13% cells form a separate population exhibiting high CD49f expression. D. Sphere-forming assay showing that only CD49fBright cells have high sphere forming activity. Unsorted freshly dissociated cells were used as the control; *p<0.05.
About 23% of cells in both maxillary and mandibular CLs were weak CD49f+ and 13% of cells were strong CD49f+ (Fig. 5C). Therefore, the CD49f+ population was further separated into CD49fBright and CD49fDim groups. Sphere-forming analyses revealed that CD49fBright cells were 200 folds more efficient in forming spheres than the CD49fDim group (0.14±0.13% versus 0.0007±0.00001%, p<0.001) (Fig. 5D). The CD49fBright cell-derived spheres had been passaged for more than 10 generations, and the sphere-forming efficiency was similar to those derived from unfractionated CL cells. These results suggest that the cells expressing high CD49f also have higher sphere-forming capacity than those non- or low CD49f expressing cells, and that CD49fBright can be used as a surface marker for enriching and identifying DESCs.
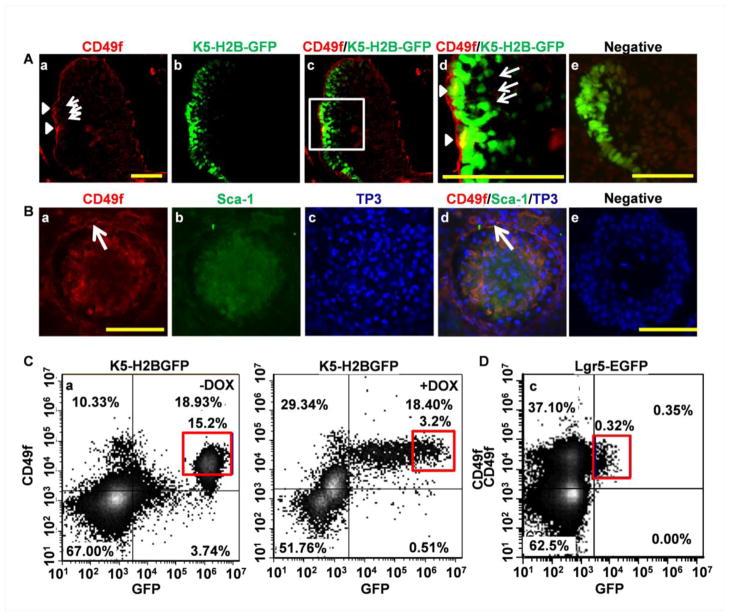
To determine whether CD49f was expressed in slow-cycling DESCs, we then utilized the tetracycline-regulated K5-H2BGFP reporter to trace slow-cycling DESCs. Immunostaining revealed that CD49f was primarily expressed in the basement membrane opposite to the OEE, where the long-term H2B-GFP retaining cells resided (Fig. 6A). In addition, there were some scattered but bright CD49f staining cells in the SR adjoining the OEE, where Lgr5+ cells resided, which was consistent with a previous report [42]. Similarly, bright CD49f stained cells were also found in the middle layer of DESC spheres where the Lgr5+ DESCs were located, as well as at the sphere periphery where the slow cycling cells were found (Fig. 6B). Thus, the results suggest that both slow-cycling and Lgr5+ DESCs were CD49f+.
Fig. 6. Distribution of CD49f+ cells in the labial CL and DESC spheres.
A. Confocal images of CD49f immunostaining of the labial CL of K5-H2BGFP mice 7 days post doxycycline treatment, showing CD49f+ cells at the basement membrane opposite to OEE (arrow head) and scattered CD49f +cells in the stellate reticulum (arrows). B. Confocal images of CD49f and Sca-1 immunostaining of DESC spheres, demonstrating CD49fBright cells in the peripheral region (arrow), scattered CD49f+ cells in the middle of the spheres, and Sca-1+ cells in the middle to center areas of the spheres. TP3 was used for nuclear counterstaining. Scale bar: 100 μm. C. Two-color FACS analyses of freshly prepared CL cells of K5-H2BGFP before and after 7 days doxycycline treatment, showing the majority of GFPhigh cells were also CD49fBright as marked by red box. D. Two-color FACS analyses of freshly prepared CL cells of Lgr5EGFP-IRES-CreERT2, showing the majority of GFP+ cells were also CD49Bright as marked by the box. Scale bar: 100 μm
To further confirm that CD49fBright cells included both slow-cycling and Lgr5+ DESCs, two-color FACS analyses were carried out to determine the expression level of CD49f in K5-H2BGFP retaining and Lgr5+ DESCs (Fig 6C). Before doxycycline treatment, the K5-H2BGFP CL cells had high (105.5–107 units) GFP fluorescence. The CL cells isolated from 7 day doxycycline chased K5-H2BGFP transgenic mice had 18.40±3.02%cells displaying a broad range (104 to 107 units) of GFP expression. Among them, 3.2±0.95% exhibited high (105.5–107 units) GFP (GFPhigh) fluorescence. The majority of the GFPhigh cells were CD49fBright, indicating that the majority of slow-cycling DESCs exhibited high CD49f expression, similar to label retaining SCs in other organs. CL cells isolated from the mice carrying the Lgr5EGFP-ires-CreERT2 reporter allele had 0.35±0.15% of Lgr5+ cells in the maxillary incisor CL. The results were consistent with those derived from Lgr5LacZ reporter mice that Lgr5 was only weakly expressed in a limited number of cells. Interestingly, nearly all GFP positive cells in the Lgr5EGFP-ires-CreERT2 CL were CD49fBright cells (Fig. 6D), indicating that active DESCs exhibited high CD49f expression. Together, the results indicate that the CD49fBright subpopulation includes both label retaining slow-cycling and Lgr5+ DESCs.
4. Discussion
By using the tetracycline-regulated K5-H2BGFP model that marks slow-cycling SCs and the Lgr5LacZ model that labels active SCs, we showed the existence of both slow-cycling and Lgr5+ DESCs in mouse incisor CLs. We also demonstrated that the locations of slow-cycling and Lgr5+ SCs were not overlapping but were adjacent to each other as shown in Fig. 7. Since the sphere-forming ability is a unique property of many tissue SCs, Matrigel sphere cultures have been established for many tissue SCs, including those from the prostate, mammary gland, and neural tissues. Using a similar strategy, we established a sphere culture system for DESCs and demonstrated that DESC spheres contained both slow-cycling and Lgr5+ SCs. Similar to the CL, the two types of DESCs also were located at different areas in the sphere (Fig. 7). We further demonstrated that sphere cells were able to express differentiation markers of different dental epithelial lineages, including the IEE, OEE, SR, and SI. Emerging evidence validates the finding that the slow-cycling SCs can be identified by long-term label retention and active SCs can be identified by expression of Lgr5 [43]. Therefore, this is the first in-depth study that delineates slow-cycling and active DESCs in vivo and the first report of an in vitro DESC culture system that can be used for DESC characterization and related studies.
Fig. 7. Schematic of localization of label retaining and Lgr5+ DESC in the CL and DESC spheres.
Label retaining cells (LRC) and Lgr5+ DESCs coexist in the mouse incisor CL and DESC spheres at separate yet adjoining locations. Am, ameloblasts; D, dentin; E, enamel; IEE, inner enamel epithelium; F, dental follicular stromal cells; O, odontoblasts; OEE, outer enamel epithelium; P, dental papilla stromal cells; SI, stratum intermedium; and SR, stellate reticulum.
RT-PCR analyses revealed that several common SCs surface markers, including Sca-1, CD49f, and CD44 were expressed in both CLs and DESCs.. CD49fBright CL epithelial cells were able to self-renew by forming spheres and to express characteristic genes of all four tooth epithelial lineages. This suggests that the CD49fBright cells represent DESCs and that CD49fBright based FACS can be used to enrich DESCs. Expression of CD49f is remarkably conserved in SCs in different organs, such as prostate [2], mammary gland [1], skin [44], and DESCs as shown in this study. Moreover, the data herein further demonstrated that CD49f expressing cells include the majority of slow-cycling and Lgr5+ DESCs. Therefore, although CD49f is expressed in DESCs and stromal cells, it can be used to enrich DESCs when combined with the sphere-culture system since only DESCs form spheres. CD49f, also called integrin α6, is a receptor for laminin α5 that is a major laminin in the basement membrane of tooth germs. Intense expression of CD49f mRNA and protein has been found localized in the dental epithelium during early stages of tooth morphogenesis [45]. CD49f was first described as cell surface marker for epidermal stem cells by Li et al., in 1998 [46]. In addition to being a surface marker, CD49f also plays an important role in maintaining the stemness property of SCs. Laminin α5 null mice develop small tooth germs with defective epithelial proliferation and disturbed cell polarity. Although the function of CD49f in DESCs is remains to be investigated, treating tooth germ organ cultures with anti-CD49f antibody inhibits tooth germ development [47], suggesting that CD49f may also play a role in maintaining DESCs.
Although Sca-1 is another conserved marker present in both stromal and epithelial SCs of many tissues, including hematopoietic cells [3], mouse incisor pulp progenitor cells [48], lung [49], prostate [2], and pancreas [50] SCs, Sca-1 based sorting did not enrich the sphere-forming DESCs. Moreover, immunostaining demonstrated that Sca-1 was mainly expressed in the middle and center of DESC spheres where TA cells resided (Fig. 6B). This suggests that Sca-1 is expressed more in TA cells instead of DESCs in the dental epithelium, although it can not be excluded that the DESC sphere culture described herein did not provide a suitable environment for propagating Sca-1+ DESCs. The sphere-forming efficiency varied considerably between different populations. CD49fBright cells consistently had a 2.8 fold higher sphere-forming activity than unsorted cells (0.05% unsorted, 0.14% CD49fBright). Although Lgr5-GFP+ cells were scarce (0.4%) in the CL, we were able to isolate Lgr5-GFP+ cells for sphere-forming assays and the sphere-forming efficiency was 0.6%, which was a 12-fold increase compared to unsorted cells. No K5-H2BGFP retaining cells could form spheres in culture, suggesting that the sphere culture system did not contained factors needed to activate slow-cycling DESCs.
FACS analyses showed that only around 2% of cells in the CL region are CD44+. CD44 is intensively expressed in presecretory ameloblasts, the SI, odontoblasts, and the wall of blood vessels, but not in the OEE or at the IEE-OEE junction in developing human tooth germ [51]. The enzyme-based cell dissociation process may remove surface markers and it takes longer than 3 hours to replenish, which may affect the quantity and quality of surface marker based cell sorting. However, this influence is enzyme- and surface marker-specific [52, 53]. It appears that dispase and collagenase dissociation does not affect the cell sorting based on surface marker in SCs of other organs, such as prostate, breast, and skin [1, 54, 55]. Our data here also showed that dispase and collagenase treatments did not affect CD49fBright-based cell sorting for DESCs. However, it can not be ruled out that the negative results for CD44 and Sca-1 based sorting was due to removal of these two surface markers by dispase and collagenase.
In summary, this is the first report on both in vivo and in vitro characterization of slow-cycling and Lgr5+ DESCs in the mouse incisor CL. As the majority of both slow-cycling and Lgr5+ DESCs highly expressed CD49f, CD49fBright can serve as a surface marker for identifying and isolating DESCs. Furthermore, the in vitro DESC sphere culture system provides a new venue to expand and maintain DESCs for exploring the potential of DESCs in tooth regeneration.
Highlights.
Establish a sphere-culture system for mouse dental epithelial stem cells
Dental epithelial stem cells have the capacity to form spheres and to express characteristic genes of multiple dental epithelial lineages.
Identify CD49f (integrin α6) as a surface marker for dental epithelial stem cells
Identify in vivo location of Lgr5+ and slow-cycling dental epithelial stem cells
Acknowledgments
We thank Allan Prejusa and Jonathan Lei for helps in FACS cell sorting and analysis; Drs. Elaine Fuchs, Adam Glick, Hans Clevers, Mingyao Liu, Michael M. Shen, Frank Costantini for providing H2B-GFP, K5rtTA, Lgr5LacZ, Lgr5EGFP-ires-CreERT2, Lgr4LacZ, Nkx3.1Cre, ROSA26EYFP mice; Dr. Jan C.C. Hu for amelogenin antibody; Dr. Hidemitsu Harada for sharing the CL dissection experience; Drs. Nick Barker and Hans Clevers for mLgr5, hLgr5, and mOlfm4 cDNAs; and Dr. Stefan Siwko for critical reading of the manuscript. This work was supported by the National Institutes of Health K08DE020883 (to JYC), T32DE018380 (RD), CA96824 (FW), CA140388 (WLM, FW), CPRIT110555 (FW, WLM), Komen Breast Cancer Foundation (WLM), and the John S. Dunn Research Foundation (WLM).
Abbreviations
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- DESC
dental epithelial stem cell
- CL
cervical loop
- SC
stem cell
- OEE
outer enamel epithelium
- IEE
inner enamel epithelium
- SR
stratum reticulum
- SI
stratum intermedium
- Alp
alkaline phosphatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 2.Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 4.Bickenbach JR, Chism E. Selection and extended growth of murine epidermal stem cells in culture. Exp Cell Res. 1998;244:184–195. doi: 10.1006/excr.1998.4163. [DOI] [PubMed] [Google Scholar]
- 5.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds BA, Rietze RL. Neural stem cells and neurospheres--re-evaluating the relationship. Nat Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 7.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes & development. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xin L, Lukacs RU, Lawson DA, Cheng D, Witte ON. Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells. 2007;25:2760–2769. doi: 10.1634/stemcells.2007-0355. [DOI] [PubMed] [Google Scholar]
- 9.Snippert HJ, Clevers H. Tracking adult stem cells. EMBO Rep. 2011;12:113–122. doi: 10.1038/embor.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 11.Barker N, Rookmaaker MB, Kujala P, Ng A, Leushacke M, Snippert H, van de Wetering M, Tan S, Van Es JH, Huch M, Poulsom R, Verhaar MC, Peters PJ, Clevers H. Lgr5(+ve) stem/progenitor cells contribute to nephron formation during kidney development. Cell Rep. 2012;2:540–552. doi: 10.1016/j.celrep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grobstein C. Mechanisms of organogenetic tissue interaction. Natl Cancer Inst Monogr. 1967;26:279–299. [PubMed] [Google Scholar]
- 14.Thesleff I, Hurmerinta K. Tissue interactions in tooth development. Differentiation. 1981;18:75–88. doi: 10.1111/j.1432-0436.1981.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 15.Thesleff I, Vaahtokari A, Kettunen P, Aberg T. Epithelial-mesenchymal signaling during tooth development. Connect Tissue Res. 1995;32:9–15. doi: 10.3109/03008209509013700. [DOI] [PubMed] [Google Scholar]
- 16.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 17.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 20.Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, Mohl C, Sippel C, Hoffmann KH. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Tummers M, Thesleff I. Root or crown: a developmental choice orchestrated by the differential regulation of the epithelial stem cell niche in the tooth of two rodent species. Development. 2003;130:1049–1057. doi: 10.1242/dev.00332. [DOI] [PubMed] [Google Scholar]
- 22.Yokohama-Tamaki T, Ohshima H, Fujiwara N, Takada Y, Ichimori Y, Wakisaka S, Ohuchi H, Harada H. Cessation of Fgf10 signaling, resulting in a defective dental epithelial stem cell compartment, leads to the transition from crown to root formation. Development. 2006;133:1359–1366. doi: 10.1242/dev.02307. [DOI] [PubMed] [Google Scholar]
- 23.Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA, Thesleff I. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol. 1999;147:105–120. doi: 10.1083/jcb.147.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harada H, Ohshima H. New perspectives on tooth development and the dental stem cell niche. Arch Histol Cytol. 2004;67:1–11. doi: 10.1679/aohc.67.1. [DOI] [PubMed] [Google Scholar]
- 25.Mitsiadis TA, Barrandon O, Rochat A, Barrandon Y, De Bari C. Stem cell niches in mammals. Exp Cell Res. 2007;313:3377–3385. doi: 10.1016/j.yexcr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Juuri E, Saito K, Ahtiainen L, Seidel K, Tummers M, Hochedlinger K, Klein OD, Thesleff I, Michon F. Sox2+ Stem Cells Contribute to All Epithelial Lineages of the Tooth via Sfrp5+ Progenitors. Dev Cell. 2012;23:317–328. doi: 10.1016/j.devcel.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suomalainen M, Thesleff I. Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/beta-catenin signaling in the epithelial stem cells. Developmental dynamics: an official publication of the American Association of Anatomists. 2010;239:364–372. doi: 10.1002/dvdy.22106. [DOI] [PubMed] [Google Scholar]
- 28.Seidel K, Ahn CP, Lyons D, Nee A, Ting K, Brownell I, Cao T, Carano RA, Curran T, Schober M, Fuchs E, Joyner A, Martin GR, de Sauvage FJ, Klein OD. Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development. 2010;137:3753–3761. doi: 10.1242/dev.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Kwon HJ, Harada H, Ohshima H, Cho SW, Jung HS. Expression patterns of ABCG2, Bmi-1, Oct-3/4, and Yap in the developing mouse incisor. Gene expression patterns: GEP. 2011;11:163–170. doi: 10.1016/j.gep.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Lin Y, Liu G, Zhang Y, Hu YP, Yu K, Lin C, McKeehan K, Xuan JW, Ornitz DM, Shen MM, Greenberg N, McKeehan WL, Wang F. Fibroblast growth factor receptor 2 tyrosine kinase is required for prostatic morphogenesis and the acquisition of strict androgen dependency for adult tissue homeostasis. Development. 2007;134:723–734. doi: 10.1242/dev.02765. [DOI] [PubMed] [Google Scholar]
- 31.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature genetics. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 32.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–794. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- 34.Weng J, Luo J, Cheng X, Jin C, Zhou X, Qu J, Tu L, Ai D, Li D, Wang J, Martin JF, Amendt BA, Liu M. Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6081–6086. doi: 10.1073/pnas.0708257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen LS, Couwenhoven RI, Hsu D, Luo W, Snead ML. Maintenance of amelogenin gene expression by transformed epithelial cells of mouse enamel organ. Archives of oral biology. 1992;37:771–778. doi: 10.1016/0003-9969(92)90110-t. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Zhang J, Lin Y, Lan Y, Lin C, Xuan JW, Shen MM, McKeehan WL, Greenberg NM, Wang F. Role of epithelial cell fibroblast growth factor receptor substrate 2alpha in prostate development, regeneration and tumorigenesis. Development. 2008;135:775–784. doi: 10.1242/dev.009910. [DOI] [PubMed] [Google Scholar]
- 37.Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, Stange DE, Toftgard R, Clevers H. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 38.de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 39.van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15–17. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 40.Li W, Machule D, Gao C, DenBesten PK. Growth of ameloblast-lineage cells in a three-dimensional Matrigel environment. Eur J Oral Sci. 2006;114(Suppl 1):159–163. doi: 10.1111/j.1600-0722.2006.00308.x. discussion 164–155, 380–151. [DOI] [PubMed] [Google Scholar]
- 41.Kawano S, Saito M, Handa K, Morotomi T, Toyono T, Seta Y, Nakamura N, Uchida T, Toyoshima K, Ohishi M, Harada H. Characterization of dental epithelial progenitor cells derived from cervical-loop epithelium in a rat lower incisor. Journal of dental research. 2004;83:129–133. doi: 10.1177/154405910408300209. [DOI] [PubMed] [Google Scholar]
- 42.Kieffer-Combeau S, Meyer JM, Lesot H. Cell-matrix interactions and cell-cell junctions during epithelial histo-morphogenesis in the developing mouse incisor. Int J Dev Biol. 2001;45:733–742. [PubMed] [Google Scholar]
- 43.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tani H, Morris RJ, Kaur P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A. 2000;97:10960–10965. doi: 10.1073/pnas.97.20.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salmivirta K, Gullberg D, Hirsch E, Altruda F, Ekblom P. Integrin subunit expression associated with epithelial-mesenchymal interactions during murine tooth development. Developmental dynamics: an official publication of the American Association of Anatomists. 1996;205:104–113. doi: 10.1002/(SICI)1097-0177(199602)205:2<104::AID-AJA2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 46.Li A, Simmons PJ, Kaur P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3902–3907. doi: 10.1073/pnas.95.7.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukumoto S, Miner JH, Ida H, Fukumoto E, Yuasa K, Miyazaki H, Hoffman MP, Yamada Y. Laminin alpha5 is required for dental epithelium growth and polarity and the development of tooth bud and shape. J Biol Chem. 2006;281:5008–5016. doi: 10.1074/jbc.M509295200. [DOI] [PubMed] [Google Scholar]
- 48.Balic A, Aguila HL, Caimano MJ, Francone VP, Mina M. Characterization of stem and progenitor cells in the dental pulp of erupted and unerupted murine molars. Bone. 2010;46:1639–1651. doi: 10.1016/j.bone.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 50.Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci U S A. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Felszeghy S, Modis L, Tammi M, Tammi R. The distribution pattern of the hyaluronan receptor CD44 during human tooth development. Arch Oral Biol. 2001;46:939–945. doi: 10.1016/s0003-9969(01)00053-x. [DOI] [PubMed] [Google Scholar]
- 52.Mulder WM, Koenen H, van de Muysenberg AJ, Bloemena E, Wagstaff J, Scheper RJ. Reduced expression of distinct T-cell CD molecules by collagenase/DNase treatment. Cancer Immunol Immunother. 1994;38:253–258. doi: 10.1007/BF01533516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ford AL, Foulcher E, Goodsall AL, Sedgwick JD. Tissue digestion with dispase substantially reduces lymphocyte and macrophage cell-surface antigen expression. J Immunol Methods. 1996;194:71–75. doi: 10.1016/0022-1759(96)00067-1. [DOI] [PubMed] [Google Scholar]
- 54.Lukacs RU, Goldstein AS, Lawson DA, Cheng D, Witte ON. Isolation, cultivation and characterization of adult murine prostate stem cells. Nat Protoc. 2010;5:702–713. doi: 10.1038/nprot.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szabo AZ, Fong S, Yue L, Zhang K, Strachan LR, Scalapino K, Mancianti ML, Ghadially R. The CD44+ ALDH+ population of human keratinocytes is enriched for epidermal stem cells with long-term repopulating ability. Stem Cells. 2012;31:786–799. doi: 10.1002/stem.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]