Abstract
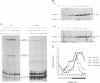
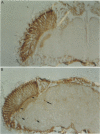
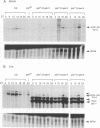
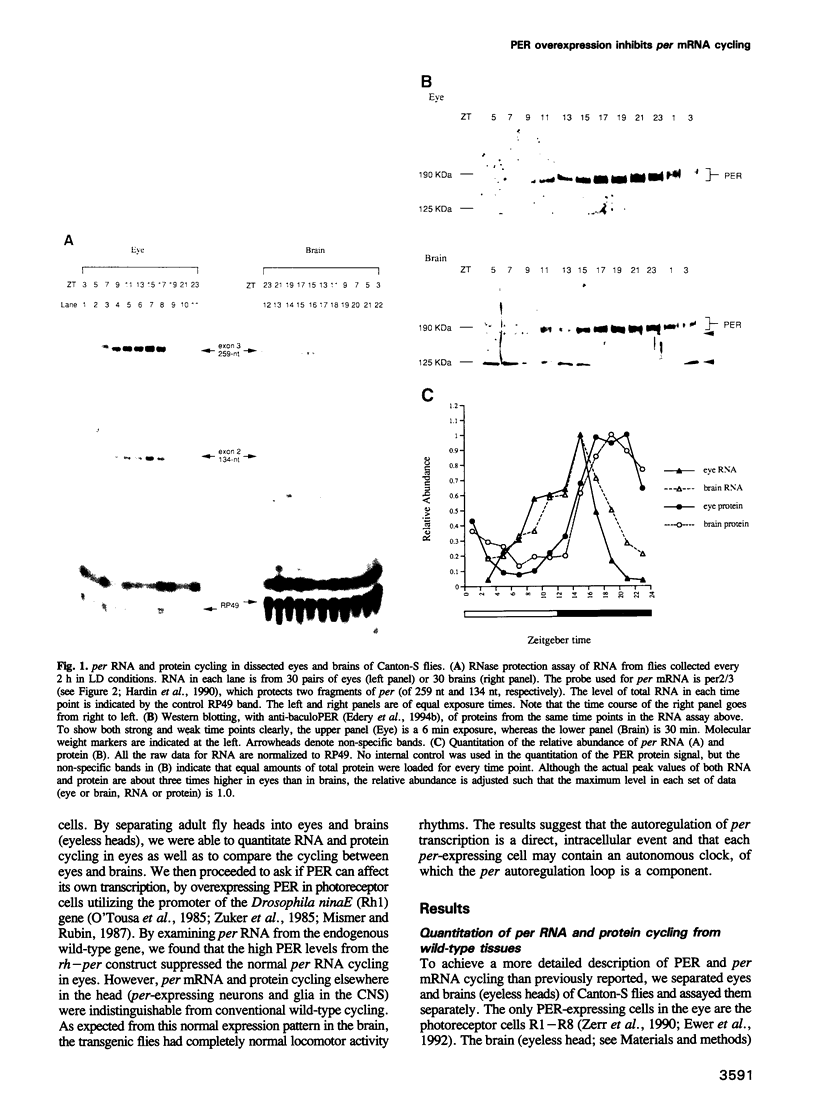
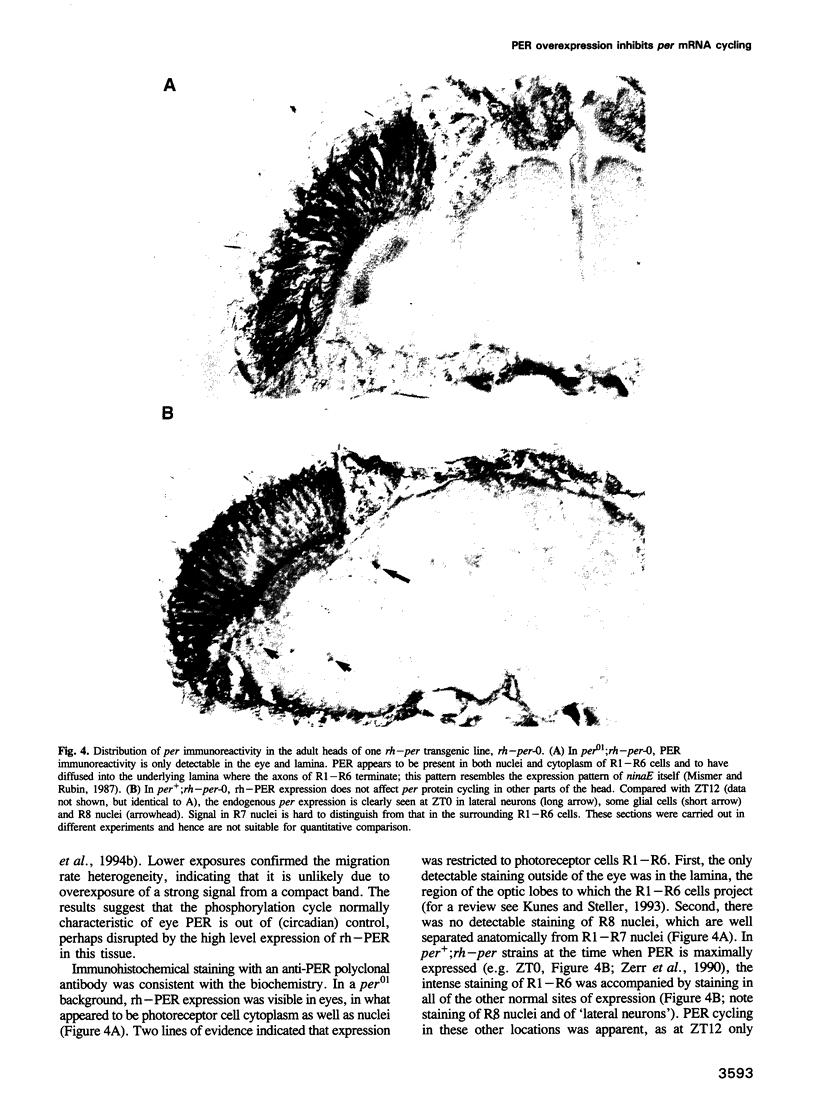
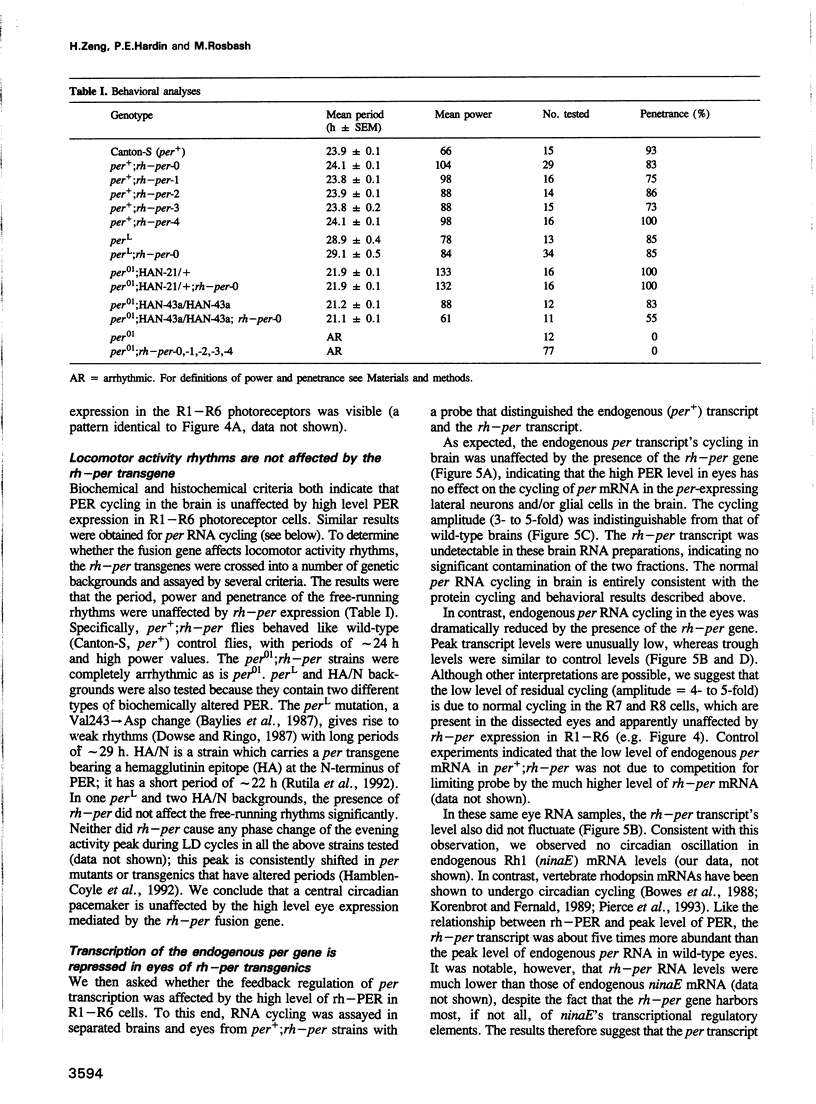
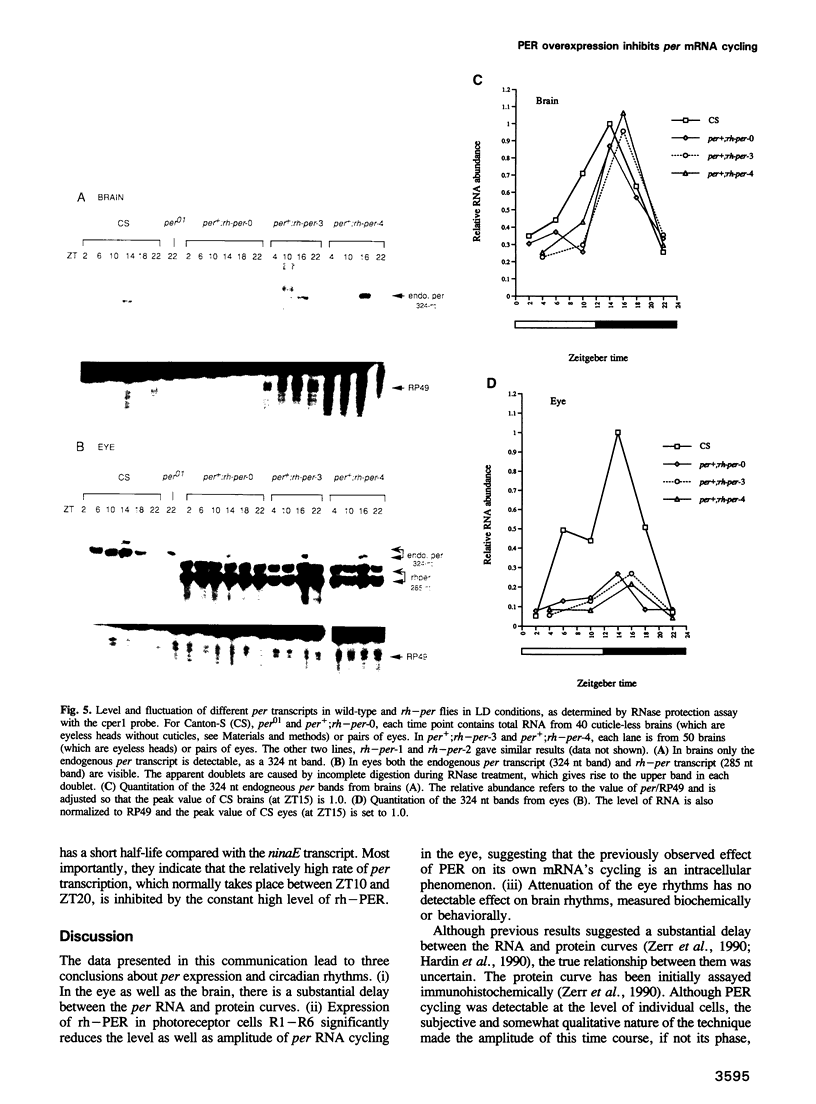
The Drosophila period gene (per) is a likely component of a circadian pacemaker. per protein (PER) participates in the regulation of its own expression, at least in part at the transcriptional level. There is at present no direct evidence that the effect of PER on its own transcription is intracellular. Results presented in this paper show that (i) the circadian oscillations of both per mRNA and PER protein are quantitatively similar in eye photoreceptor cells and in brain; (ii) constitutive overexpression of PER only in photoreceptors R1-R6 represses endogenous per RNA cycling in these cells but not in other per-expressing cells; (iii) the overexpression construct has no effect on locomotor activity rhythms. These results indicate that the autoregulation of per expression is a direct, intracellular event and suggest that each per-expressing cell contains an autonomous oscillator of which the per feedback loop is a component.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson B. D., Johnson K. A., Loros J. J., Dunlap J. C. Negative feedback defining a circadian clock: autoregulation of the clock gene frequency. Science. 1994 Mar 18;263(5153):1578–1584. doi: 10.1126/science.8128244. [DOI] [PubMed] [Google Scholar]
- Bargiello T. A., Jackson F. R., Young M. W. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature. 1984 Dec 20;312(5996):752–754. doi: 10.1038/312752a0. [DOI] [PubMed] [Google Scholar]
- Baylies M. K., Bargiello T. A., Jackson F. R., Young M. W. Changes in abundance or structure of the per gene product can alter periodicity of the Drosophila clock. 1987 Mar 26-Apr 1Nature. 326(6111):390–392. doi: 10.1038/326390a0. [DOI] [PubMed] [Google Scholar]
- Bowes C., van Veen T., Farber D. B. Opsin, G-protein and 48-kDa protein in normal and rd mouse retinas: developmental expression of mRNAs and proteins and light/dark cycling of mRNAs. Exp Eye Res. 1988 Sep;47(3):369–390. doi: 10.1016/0014-4835(88)90049-8. [DOI] [PubMed] [Google Scholar]
- Chen D. M., Christianson J. S., Sapp R. J., Stark W. S. Visual receptor cycle in normal and period mutant Drosophila: microspectrophotometry, electrophysiology, and ultrastructural morphometry. Vis Neurosci. 1992 Aug;9(2):125–135. doi: 10.1017/s0952523800009585. [DOI] [PubMed] [Google Scholar]
- Citri Y., Colot H. V., Jacquier A. C., Yu Q., Hall J. C., Baltimore D., Rosbash M. A family of unusually spliced biologically active transcripts encoded by a Drosophila clock gene. Nature. 1987 Mar 5;326(6108):42–47. doi: 10.1038/326042a0. [DOI] [PubMed] [Google Scholar]
- Dowse H. B., Ringo J. M. Further evidence that the circadian clock in Drosophila is a population of coupled ultradian oscillators. J Biol Rhythms. 1987 Spring;2(1):65–76. doi: 10.1177/074873048700200106. [DOI] [PubMed] [Google Scholar]
- Edery I., Rutila J. E., Rosbash M. Phase shifting of the circadian clock by induction of the Drosophila period protein. Science. 1994 Jan 14;263(5144):237–240. doi: 10.1126/science.8284676. [DOI] [PubMed] [Google Scholar]
- Edery I., Zwiebel L. J., Dembinska M. E., Rosbash M. Temporal phosphorylation of the Drosophila period protein. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2260–2264. doi: 10.1073/pnas.91.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewer J., Frisch B., Hamblen-Coyle M. J., Rosbash M., Hall J. C. Expression of the period clock gene within different cell types in the brain of Drosophila adults and mosaic analysis of these cells' influence on circadian behavioral rhythms. J Neurosci. 1992 Sep;12(9):3321–3349. doi: 10.1523/JNEUROSCI.12-09-03321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch B., Hardin P. E., Hamblen-Coyle M. J., Rosbash M., Hall J. C. A promoterless period gene mediates behavioral rhythmicity and cyclical per expression in a restricted subset of the Drosophila nervous system. Neuron. 1994 Mar;12(3):555–570. doi: 10.1016/0896-6273(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Hall J. C., Rosbash M. Mutations and molecules influencing biological rhythms. Annu Rev Neurosci. 1988;11:373–393. doi: 10.1146/annurev.ne.11.030188.002105. [DOI] [PubMed] [Google Scholar]
- Hamblen M., Zehring W. A., Kyriacou C. P., Reddy P., Yu Q., Wheeler D. A., Zwiebel L. J., Konopka R. J., Rosbash M., Hall J. C. Germ-line transformation involving DNA from the period locus in Drosophila melanogaster: overlapping genomic fragments that restore circadian and ultradian rhythmicity to per0 and per- mutants. J Neurogenet. 1986 Sep;3(5):249–291. doi: 10.3109/01677068609106855. [DOI] [PubMed] [Google Scholar]
- Hardin P. E., Hall J. C., Rosbash M. Circadian oscillations in period gene mRNA levels are transcriptionally regulated. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11711–11715. doi: 10.1073/pnas.89.24.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin P. E., Hall J. C., Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990 Feb 8;343(6258):536–540. doi: 10.1038/343536a0. [DOI] [PubMed] [Google Scholar]
- Huang Z. J., Edery I., Rosbash M. PAS is a dimerization domain common to Drosophila period and several transcription factors. Nature. 1993 Jul 15;364(6434):259–262. doi: 10.1038/364259a0. [DOI] [PubMed] [Google Scholar]
- Jackson F. R., Bargiello T. A., Yun S. H., Young M. W. Product of per locus of Drosophila shares homology with proteoglycans. Nature. 1986 Mar 13;320(6058):185–188. doi: 10.1038/320185a0. [DOI] [PubMed] [Google Scholar]
- Konopka R. J., Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot J. I., Fernald R. D. Circadian rhythm and light regulate opsin mRNA in rod photoreceptors. Nature. 1989 Feb 2;337(6206):454–457. doi: 10.1038/337454a0. [DOI] [PubMed] [Google Scholar]
- Kunes S., Steller H. Topography in the Drosophila visual system. Curr Opin Neurobiol. 1993 Feb;3(1):53–59. doi: 10.1016/0959-4388(93)90035-w. [DOI] [PubMed] [Google Scholar]
- Liu X., Lorenz L., Yu Q. N., Hall J. C., Rosbash M. Spatial and temporal expression of the period gene in Drosophila melanogaster. Genes Dev. 1988 Feb;2(2):228–238. doi: 10.1101/gad.2.2.228. [DOI] [PubMed] [Google Scholar]
- Liu X., Zwiebel L. J., Hinton D., Benzer S., Hall J. C., Rosbash M. The period gene encodes a predominantly nuclear protein in adult Drosophila. J Neurosci. 1992 Jul;12(7):2735–2744. doi: 10.1523/JNEUROSCI.12-07-02735.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel S., Geusz M. E., Zaritsky J. J., Block G. D. Circadian rhythm in membrane conductance expressed in isolated neurons. Science. 1993 Jan 8;259(5092):239–241. doi: 10.1126/science.8421785. [DOI] [PubMed] [Google Scholar]
- Mismer D., Rubin G. M. Analysis of the promoter of the ninaE opsin gene in Drosophila melanogaster. Genetics. 1987 Aug;116(4):565–578. doi: 10.1093/genetics/116.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Tousa J. E., Baehr W., Martin R. L., Hirsh J., Pak W. L., Applebury M. L. The Drosophila ninaE gene encodes an opsin. Cell. 1985 Apr;40(4):839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- Pierce M. E., Sheshberadaran H., Zhang Z., Fox L. E., Applebury M. L., Takahashi J. S. Circadian regulation of iodopsin gene expression in embryonic photoreceptors in retinal cell culture. Neuron. 1993 Apr;10(4):579–584. doi: 10.1016/0896-6273(93)90161-j. [DOI] [PubMed] [Google Scholar]
- Robertson L. M., Takahashi J. S. Circadian clock in cell culture: II. In vitro photic entrainment of melatonin oscillation from dissociated chick pineal cells. J Neurosci. 1988 Jan;8(1):22–30. doi: 10.1523/JNEUROSCI.08-01-00022.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbash M., Hall J. C. The molecular biology of circadian rhythms. Neuron. 1989 Oct;3(4):387–398. doi: 10.1016/0896-6273(89)90199-2. [DOI] [PubMed] [Google Scholar]
- Rutila J. E., Edery I., Hall J. C., Rosbash M. The analysis of new short-period circadian rhythm mutants suggests features of D. melanogaster period gene function. J Neurogenet. 1992 May;8(2):101–113. doi: 10.3109/01677069209084155. [DOI] [PubMed] [Google Scholar]
- Saez L., Young M. W. In situ localization of the per clock protein during development of Drosophila melanogaster. Mol Cell Biol. 1988 Dec;8(12):5378–5385. doi: 10.1128/mcb.8.12.5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A., Price J. L., Man B., Young M. W. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science. 1994 Mar 18;263(5153):1603–1606. doi: 10.1126/science.8128246. [DOI] [PubMed] [Google Scholar]
- Siwicki K. K., Eastman C., Petersen G., Rosbash M., Hall J. C. Antibodies to the period gene product of Drosophila reveal diverse tissue distribution and rhythmic changes in the visual system. Neuron. 1988 Apr;1(2):141–150. doi: 10.1016/0896-6273(88)90198-5. [DOI] [PubMed] [Google Scholar]
- Vosshall L. B., Price J. L., Sehgal A., Saez L., Young M. W. Block in nuclear localization of period protein by a second clock mutation, timeless. Science. 1994 Mar 18;263(5153):1606–1609. doi: 10.1126/science.8128247. [DOI] [PubMed] [Google Scholar]
- Wheeler D. A., Hamblen-Coyle M. J., Dushay M. S., Hall J. C. Behavior in light-dark cycles of Drosophila mutants that are arrhythmic, blind, or both. J Biol Rhythms. 1993 Spring;8(1):67–94. doi: 10.1177/074873049300800106. [DOI] [PubMed] [Google Scholar]
- Wuarin J., Falvey E., Lavery D., Talbot D., Schmidt E., Ossipow V., Fonjallaz P., Schibler U. The role of the transcriptional activator protein DBP in circadian liver gene expression. J Cell Sci Suppl. 1992;16:123–127. doi: 10.1242/jcs.1992.supplement_16.15. [DOI] [PubMed] [Google Scholar]
- Yu Q., Jacquier A. C., Citri Y., Hamblen M., Hall J. C., Rosbash M. Molecular mapping of point mutations in the period gene that stop or speed up biological clocks in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1987 Feb;84(3):784–788. doi: 10.1073/pnas.84.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehring W. A., Wheeler D. A., Reddy P., Konopka R. J., Kyriacou C. P., Rosbash M., Hall J. C. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell. 1984 Dec;39(2 Pt 1):369–376. doi: 10.1016/0092-8674(84)90015-1. [DOI] [PubMed] [Google Scholar]
- Zerr D. M., Hall J. C., Rosbash M., Siwicki K. K. Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J Neurosci. 1990 Aug;10(8):2749–2762. doi: 10.1523/JNEUROSCI.10-08-02749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker C. S., Cowman A. F., Rubin G. M. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 1985 Apr;40(4):851–858. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]
- Zwiebel L. J., Hardin P. E., Liu X., Hall J. C., Rosbash M. A post-transcriptional mechanism contributes to circadian cycling of a per-beta-galactosidase fusion protein. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3882–3886. doi: 10.1073/pnas.88.9.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]