Abstract
Objective: The current study was aimed at the investigation of differences in response to photoinactivation between methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive S. aureus (MSSA) isolates. Moreover, we aimed to elucidate if the observed variation resulted from antimicrobial resistance mechanisms and strains' susceptibility to antibiotic therapy. Background data: Because of the emergence of multidrug resistance, the development of alternative antimicrobial strategies seems to be required. The concept of photodynamic inactivation (PDI) involves cell exposure to appropriate wavelength light that leads to the excitation of photosensitizer molecules, resulting in the production of reactive oxygen species responsible for cell inactivation and death. Recently, we have demonstrated a strain-dependent response of S. aureus to photoinactivation, and observed elevated resistance to PDI among MRSA strains. Nevertheless, the mechanism underlying this phenomenon remains unexplained. Methods: S. aureus response to protoporphyrin IX (PPIX)-mediated photoinactivation was studied for 424 MRSA/MSSA isolates. VITEK 2 Advanced Expert System was used to detect antimicrobial resistance mechanisms and strains' susceptibility to antibiotictherapy. Results: Data obtained demonstrated that MRSA are significantly more resistant to photoinactivation than MSSA strains; however, the difference observed did not result from antimicrobial susceptibility or resistance mechanisms. Furthermore, regardless of the strains' origin, a similar effectiveness of PDI could be achieved. Moreover, it was determined that the ability to form biofilms in vitro, and the presence of mec element, does not explain the observed differences between MRSA and MSSA strains. Conclusions: PDI could be highly effective against multidrug resistant pathogens as well as their naïve counterparts. Nevertheless, regardless of the antimicrobial resistance mechanism, the difference in response to PDI between MRSA and MSSA exists.
Introduction
Photodynamic therapy (PDT), which is generally recognized as a cancer treatment, includes photosensitizers (PS) that accumulate in the target cells (i.e., malignant tissues or microorganisms). If the therapy involves microorganisms, then this strategy is termed “photodynamic inactivation” (PDI).1 The appropriate wavelength of visible light is then used to excite the PS molecules to the singlet state, and excited sensitizers undergo triplet state reactions by either type I or type II pathways.2 The type I mechanism involves electron transfer from the triplet state PS to the substrate, producing cytotoxic reactive species such as superoxide or hydroxyl radicals.3 The type II mechanism is based on energy transfer from the triplet state PS to molecular oxygen (ground triplet state) to produce highly cytotoxic singlet oxygen.4 With various photosensitizers and the appropriate wavelength light, Staphylococcus aureus has been observed to be dramatically inactivated in a serial of in vitro studies.5–7 Moreover, methicillin-resistant S. aureus (MRSA) isolated from patients were demonstrated to be completely eradicated because of photodynamic treatment.8 In another study, 16 epidemic MRSA strains were inactivated by PDI, and obtained results indicated that all strains were susceptible to inactivating by PDI. An undoubted benefit of PDI is fact that bacteria are not able to easily develop resistance, and multiresistant strains have shown to be as susceptible to PDI as their naïve counterparts.5;9 However, our research group has recently described the effect of the strain-dependent response of S. aureus to PDI, and indicated that MRSA strains could be characterized by elevated PDI resistance when compared with methicillin-sensitive S. aureus (MSSA) strains.10,11 The mechanism underlying this phenomenon is still poorly understood. Therefore, the current study aimed to provide statistically relevant evidence as to whether the response of MRSA strains to PDI differs significantly from that of MSSA, and whether it results from antimicrobial resistance mechanisms or susceptibility to routinely used antimicrobials.
Materials and Methods
Bacterial isolates
In total, 424 clinical S. aureus strains isolated from 2002 to 2012 at the Provincial Hospital in Gdansk and Hospital in Koszalin, Poland, were used; of these, 265 (62.5%) were MRSA and 159 (37.5%) were MSSA. The isolates were characterized by Gram staining and the ability to produce coagulase and the clumping factor using Slidex Staph Plus (bioMèrieux, Craponne, France). Species were identified using the MALDI-TOF MS (Bruker Daltonics, Bremen, Germany). Of the 424 strains collected, 38.2% (162 isolates) were isolated from surveillance cultures, 70.6% (185 isolates) from patients with local infections, 26.7% (70 isolates) from patients with bacteremia and generalized infections, and 2.7% (7 isolates) from infections connected with endoprostheses. Moreover, the isogenic S. aureus strains Newman (MSSA) and Newman mec-positive (MRSA)12 and isogenic pair of SCCmec-harboring (DK 2574) and SCCmec-lacking (DK 2575) strains were used.13
MALDI-TOF MS
All strains were examined by MALDI-TOF MS using a Microflex LT instrument (Bruker Daltonics, Bremen, Germany), Flexcontrol 3.0 software, and the Biotyper 2.0 database (Bruker Daltonics, Bremen, Germany). The data analysis was performed according to the manufacturer's instructions. The samples were covered with 1 mL matrix solution (a saturated solution of a-cyano-4-hydroxycinnamic acid in 50% acetonitrile, 2.5% trifluoroacetic acid). The analyzed mass range of spectra was 2000–20,000 m/z. Each spectrum was obtained after 240 shots in an automatic acquisition mode. For the identification approach, a mass-to-charge range of 3000–15,000 Da was used. Identification was performed in duplicate and the higher score was retained. The identification score cutoff values were applied on each measurement according to the manufacturer's instructions. According to this score system, a score of 2.0–2.3 is recommended for a probable species identification and a score of>2.3 is recommended for a secure species identification.14
Antibiotic susceptibility testing
Antibiotic susceptibility for penicillin G, cefoxitin, teicoplanin, vancomycin, gentamicin, tetracycline, ciprofloxacin, moxifloxacin, trimethoprim/sulfamethoxazole (co-trimoxazole), fusidic acid, erythromycin, clindamycin, rifampicin, mupirocin, linezolid, and tigecycline was determined by the VITEK 2 system, AST 603 cards (bioMérieux, Craponne, France). S. aureus ATCC 29213 was the control strain and the VITEK 2 minimum inhibitory concentration (MIC) results were interpreted using the Advanced Expert System (AES) of the VITEK 2 according to Clinical and Laboratory Standards Institute (CLSI) recommendations15,16 excluding MIC breakpoints for fusidic acid and tigecycline where European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendations were applied (document V2.0, 2012, http://www.eucast.org). Additionally, macrolide, lincosamide and streptogramin B (MLSB) resistance was tested by “D test” as per CLSI guidelines detecting three phenotypes (inducible and constitutive MLSB as well as MSB phenotype).15
Photosensitizer
Protoporphyrin IX (PPIX) (MP Biomedicals, California, USA) was used as a sensitizer. PPIX was dissolved in dimethyl sulfoxide (DMSO) (Sigma, Munich, Germany) to make a 1 mM stock solution and was stored in the dark at −20°C until use. The concentration of the photosensitizer was determined spectrophotometrically (extinction coefficient 1.6×105 M−1 cm−1, wavelength 408 nm).
Light source
Illumination was performed using a Q.Light® PDT Lamp (b & p® Shweiz AG, Rorschach, Switzerland) (ISO 9001 & EN 46001 - CE 1275) (power of lamp 80 mW; fluence rate 102 mW/cm2; fluence 6.12 J/cm2/min). To obtain the reported fluence, the distance between the device and the samples was 3 cm and the spot area was 2 cm in diameter. The spatial profile of the beam emitted by the Q.Light® PDT Lamp has a Gaussian distribution; therefore, there is ∼10–15% higher energy level in the center of the distributed beam. Therefore, the delivered light energy was determined with the use of light power meter (model LM1, CARL ZEISS, Jena, Germany) in a spot area of 2 cm in diameter. To assure precise illumination of all samples, simultaneous irradiation was performed solely for four wells of microtiter plates fitted perfectly to the spot area. The Q.Light® PDT Lamp emits polarized light (polarization level: 98%) over a wavelength range of 620–780 nm.
PDI studies
All bacterial isolates were subjected to PDI. Bacterial cultures were grown overnight (24 h) at 37°C in nutrient Trypticase Soy Broth (bioMèrieux, Craponne, France) and then diluted with fresh broth to an appropriate density (107/mL bacterial cells) based on densitometry (Densi Meter II, EMO, Hannover, Germany). Diluted S. aureus cultures were incubated with 25 μM PPIX in the dark at 37°C for 30 min. The cells were then transferred to a 96 well microtiter plate (100 μL per well) and illuminated with appropriate light (50 J/cm2) at room temperature for 8 min and 10 sec. Following illumination, 10 μL aliquots were used to determine the colony-forming units (CFU). The contents of the wells were mixed before sampling, and the aliquots were serially diluted 10-fold in phosphate-buffered saline (PBS) (0.13 mM NaCl, 8.1 mM Na2HPO4, 2.68 mM KCl, 1.47 mM KH2PO4) to achieve final dilutions of 10−1 to 10−4, which were then streaked horizontally onto square Trypticase soy agar plates.17 The plates were incubated at 37°C overnight. Controls consisted of untreated bacteria (no photosensitizers or light) kept in 96 well plates for the duration of the illumination. After 18 h of incubation at 37°C in the dark, CFUs were counted and the results were statistically analyzed. Survival fractions were expressed as ratios of CFUs of treated bacteria (with light and photosensitizer) to CFUs of untreated bacteria. The experiment was performed three times.
Spectrophotometric biofilm assay
The spectrophotometric biofilm assay was performed as described by Fowler et al.18 S. aureus strains were grown overnight in trypticase soy broth (bioMèrieux, Craponne, France), containing an additional 1% glucose. Following incubation, the strains were diluted 1:200 in TSB, and 200 μL of the suspension was added to a polystyrene microtiter plate and incubated overnight at 37°C. The wells were washed twice with PBS. For biofilm fixation, 100 μL of 99% methanol was added for 15 min, after which the supernatants were removed and the plates were air dried. Then, 100 μL of a crystal violet (CV) solution was added. After 20 min, the excess CV was removed by washing the plates under running tap water. Finally, bound CV was released by adding 100 μL of 33% acetic acid. The absorbance was measured at 590 nm. All steps were performed at room temperature.19
Statistical analysis
The statistical analyses have been performed using the statistical suite StatSoft. Inc. (2011). STATISTICA (data analysis software system) version 10.0 (www.statsoft.com) and Excel. The quantitive variables were characterized by the arithmetic mean of standard deviation or median or max/min (range) and 95% confidence interval. The qualitative variables were presented with the use of count and percentage. Statistical significance of differences between two groups (unpaired variables model) was processed with the Student's t test. The significance of difference between more than two groups was assessed with F test (ANOVA). In all the calculations, the statistical significance level of p<0.05 was used.
Results
Heterogeneous response of clinical isolates of S. aureus to PDI
PDI was applied to all 424 S. aureus isolates. Heterogeneous response to photoinactivation, ranging from 0.01 to 4.82 log10 unit reductions in viable counts was obtained. Among the MRSA strains, the viable count reduction ranged from 0.01 to 4.51 log10 units, whereas for the MSSA strains, the range was from 0.01 to 4.82 log10 units. According to various responses to PDI, S. aureus isolates were classified as resistant, intermediate-sensitive, and sensitive strains. Furthermore, a different distribution of PDI responders among MRSA and MSSA population was reported (Table 1). The percentage of sensitive strains was greater among the MSSA strains (20.1%) than among the MRSA strains (14.0%) (p<0.05), and a contradictory percentage of PDI resistant S. aureus strains was higher in MRSA population (62.6 vs. 47.8) (p<0.001), demonstrating that, in general, MRSA strains are more resistant to photoinactivation. Comparison of uncategorized values of PDI responses in MRSA and MSSA strains confirmed that MRSA in general are characterized by elevated resistance to photoinactivation (Fig. 1).
Table 1.
Range of Effectiveness of Photosensitization
| No. of strains (%) | ||||
|---|---|---|---|---|
| Log10-unit reduction | Classificationa | MRSA | MSSA | Total |
| 0.01–0.99 | Resistant | 166 (62.4)* | 76 (47.8)* | 242 (57.1) |
| 1–1.99 | Intermediate sensitive | 62 (23.4)* | 51 (32.1)* | 113 (26.6) |
| 2–4.82 | Sensitive | 37 (14.0)** | 32 (20.1)** | 69 (16.3) |
| Total | 265 (62.5) | 159 (37.5) | 424 (100) | |
The cutoffs for the categorization of PDI response was made according to lethal or sublethal damage caused for Staphylococcus aureus strains. Strains reaching lethal or sublethal damage were deemed sensitive or resistant to PDI, respectively. Strains revealing reduction in viable counts that ranged between lethal and sublethal damage were deemed intermediate sensitive.
p<0.001; **p<0.05 (comparison of MRSA vs. MSSA); higher incidence of PDI resistant strains was observed for MRSA group; higher incidence of PDI intermediate sensitive and sensitive strains was reported for MSSA group.
MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus; PDI, photodynamic inactivation.
FIG. 1.
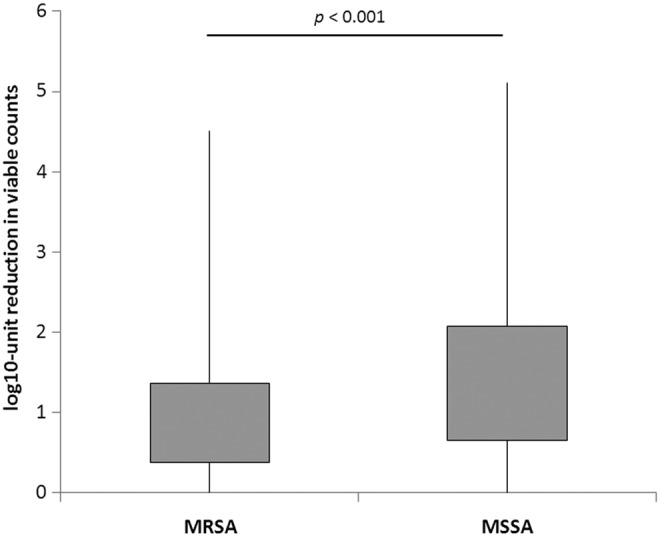
Response of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive S. aureus (MSSA) strains to protoporphyrin IX (PPIX)-mediated photodynamic inactivation (PDI). Each box plot represents the spread of bacterial response across the different clinical isolates. The error bars represent minimum and maximum value of log10 unit reduction in viable counts.
Antimicrobial resistance mechanisms and susceptibility
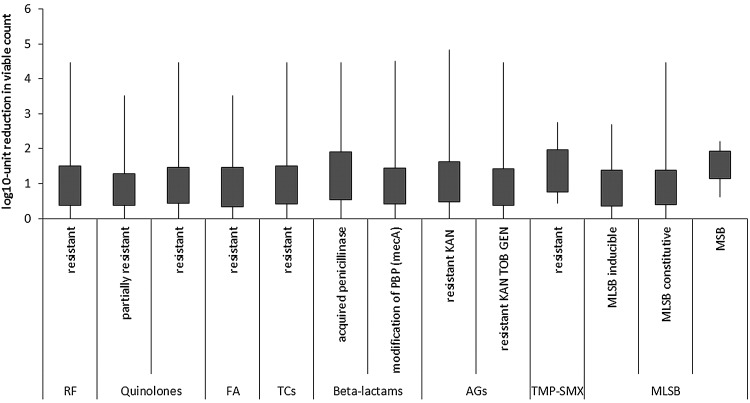
To evaluate if observed differences in response to PDI between MRSA and MSSA strains result from antimicrobial resistance mechanism, the existing resistance mechanisms were identified with the use of VITEK 2 AES. Several resistance mechanisms were identified (Table 2). One should remember that these mechanisms were identified because of interpretation of MIC values. Nevertheless, it is widely accepted that with the use of VITEK 2 AES, the reliable detection of inducible clindamycin resistance and resistance related to β-lactams could be achieved.20 In the case of quinolones and aminoglycosides, multicenter evaluation of the VITEK 2 AES for interpretive reading of antimicrobial resistance tests indicated that agreement between reference genotype data and VITEK 2 AES phenotypic interpretation is high enough to consider these detections reliable, and sufficient for the analysis performed in the current article.21 Moreover, MLSB resistance mechanisms were confirmed with the D test as suggested by CLSI.15 In the case of other resistance, for example, to rifamycins, fusidic acid, tetracyclines, oxazolidinone, and trimethoprim/sulfonamides, the identification of resistance phenotypes rather than underlying mechanisms was described. The similar distribution of PDI-resistant, intermediate-sensitive, and sensitive S. aureus strains across different antimicrobial resistance mechanisms revealed a lack of correlation between existing mechanisms and susceptibility to photoinactivation (Fig. 2). The distribution of PDI responders differs in strains revealing MSB resistance mechanisms; however, the number of representatives of this mechanisms is too low to draw any significant conclusions. Similarly, the lack of correlation between S. aureus antimicrobial resistance mechanisms and susceptibility to PDI was confirmed when compared with uncategorized values of log10 unit reduction (Fig. 3). Next, the susceptibility of S. aureus isolates to recommended antimicrobials was defined, to evaluate its connection with a strain's response to photoinactivation. In accordance to resistance mechanisms, susceptibility testing revealed no impact of S. aureus susceptibility to antimicrobials to PDI response. The distribution of PDI-resistant, intermediate-resistant, and sensitive strains was similar across S. aureus, with different antimicrobial susceptibility (Fig. 4). Moreover, the log10 unit reduction in viable count was also similar across all identified S. aureus groups (Fig. 5).
Table 2.
Resistance Phenotypes Resulting from Interpretation of MIC Values
| No. of isolates | |||
|---|---|---|---|
| Relevant phenotypes and/or genotypes | MRSA | MSSA | Total |
| Resistance to rifamycines | 83 | 11 | 94 |
| Resistance to quinolones | 216 | 21 | 237 |
| Partially resistant | 83 | 10 | 93 |
| Resistant | 133 | 11 | 144 |
| Resistance to fusidic acid | 74 | 5 | 79 |
| Resistance to tetracyclines | 127 | 40 | 167 |
| Resistance to β-lactamsa | 265 | 121 | 386 |
| Modification of PBP (mecA) | 264 | 264 | |
| Acquired penicillinase | 1 | 121 | 122 |
| Resistance to oxazolidinone | 1 | 2 | 3 |
| Resistance to aminoglycosides | 265 | 159 | 424 |
| Resistant to KAN (aph[3’]-III) | 173 | 144 | 317 |
| Resistant to KAN TOB GEN (ant[2]-Ia) | 92 | 15 | 107 |
| Resistance to trimethoprim/sulfonamids | 7 | 7 | |
| Resistance to macrolides/lincosamides | 258 | 156 | 414 |
| MLSB induciblea,b | 11 | 16 | 27 |
| MLSB constitutiveb | 195 | 13 | 208 |
| MSBb | 3 | 3 | 6 |
| Other | 49 | 124 | 173 |
Resistance mechanisms detected by VITEK 2.
Resistance mechanisms detected by D test.
MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive S. aureus; PBP, penicillin-binding protein; GEN, gentamicin; KAN, kanamycin; TOB, tobramycin; MLSB, macrolides, lincosamides, streptogramins B.
FIG. 2.
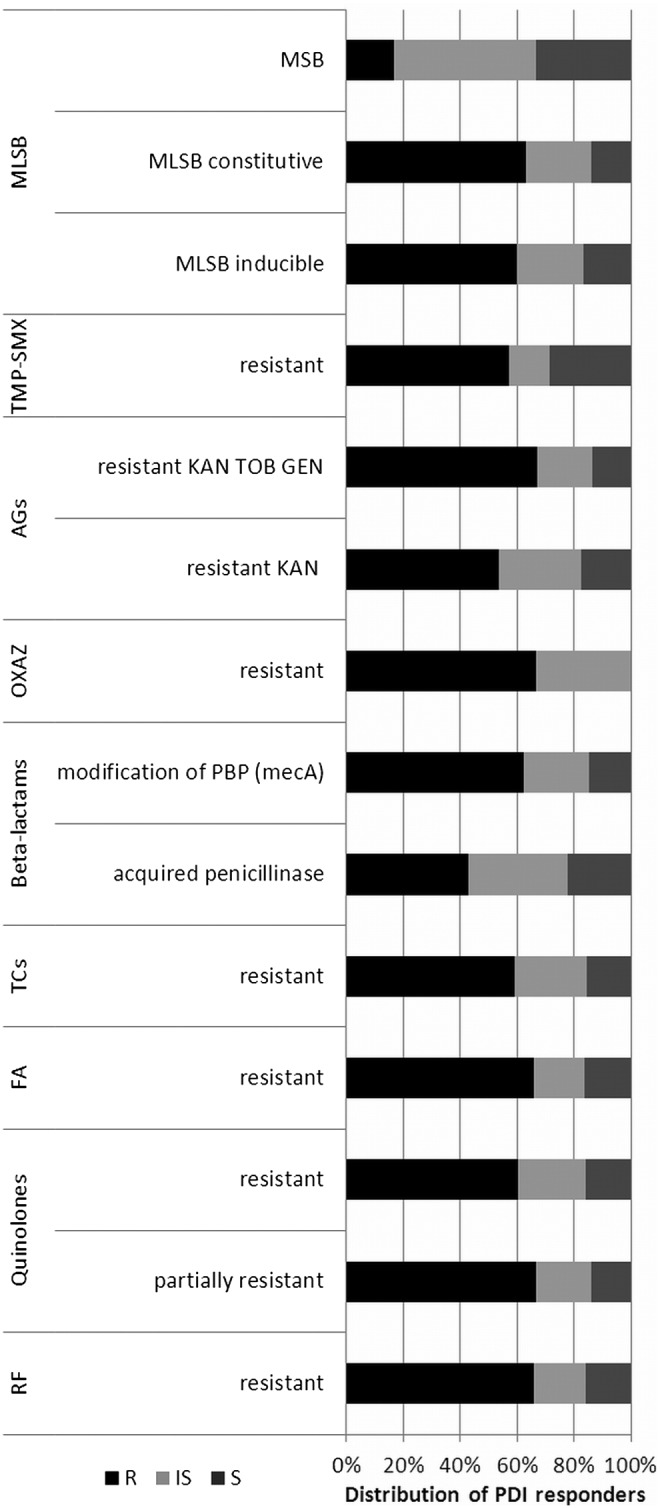
Percentage distribution of photodynamic inactivation (PDI) responders across Staphylococcus aureus groups revealing various antimicrobial resistance mechanisms. R, resistant; IS, intermediate sensitive, S, sensitive (PDI responders). RF, rifamycines, FA, fusidic acid; TCs, tetracyclines; OXAZ, oxazolidinone; AGs, aminoglycosides; TMP-SMX, trimethoprim/sulfamethoxazole; MLSB, macrolides, lincosamides, streptogramins B; KAN, kanamycin; TOB, tobramycin; GEN; gentamycin.
FIG. 3.
Response to protoporphyrin IX (PPIX)-mediated photodynamic inactivation (PDI) of Staphylococcus aureus revealing various resistance mechanisms. Each box plot represents the spread of bacterial response across different clinical isolates. RF, rifamycines; FA, fusidic acid; TCs, tetracyclines; AGs, aminoglycosides; TMP-SMX, trimethoprim/sulfamethoxazole; MLSB, macrolides, lincosamides, streptogramins B; KAN, kanamycin; TOB, tobramycin; GEN; gentamycin. The error bars represent minimum and maximum value of log10 unit reduction in viable counts.
FIG. 4.
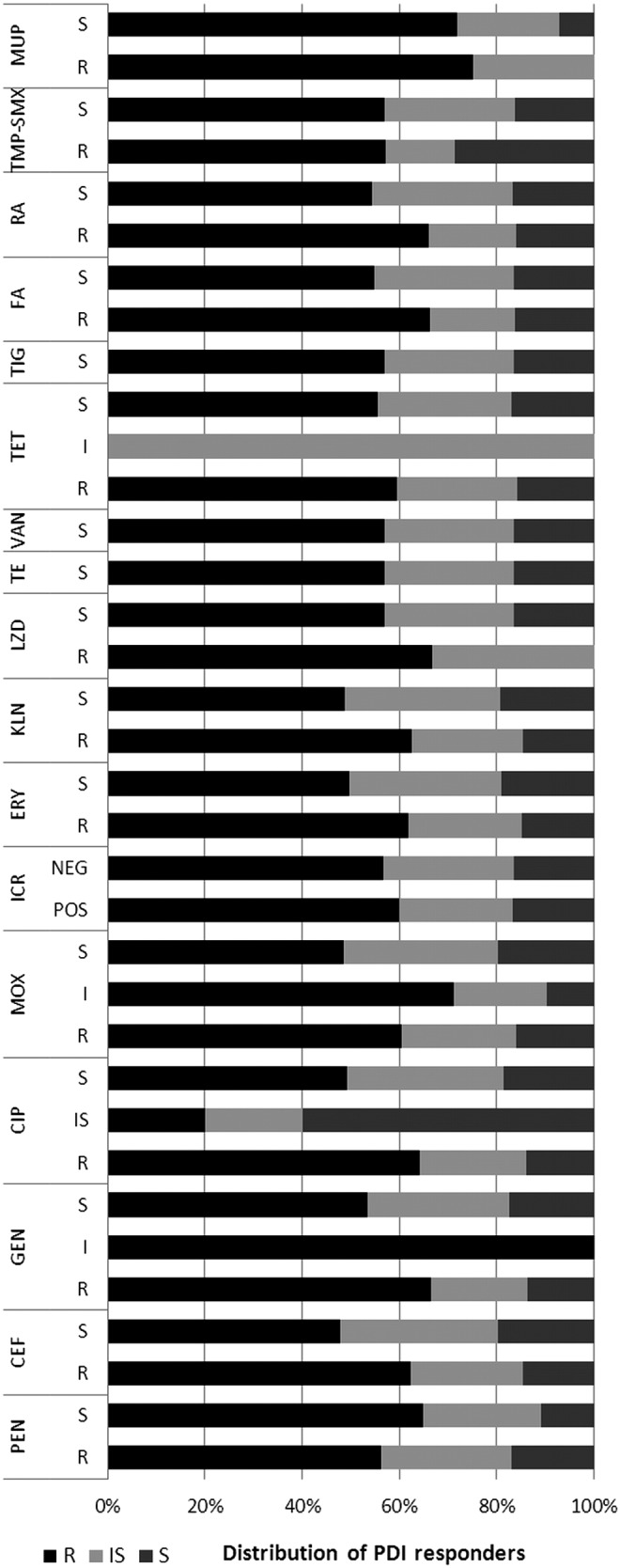
Percentage distribution of photodynamic inactivation (PDI) responders across Staphylococcus aureus groups revealing various antimicrobials susceptibility. PDI responders: R, resistant; IS, intermediate sensitive, S, sensitive. PEN, penicillin; CEF, cefoxitin; GEN, gentamycin; CIP, ciprofloxacin; MOX, moxifloxacin; ICR, inducible clindamycin resistance (POS, positive; NEG, negative); ERY, erythromycin; KLN, clindamycin; LZD, linezolid; TE, teicoplanin; VAN, vancomycin; TET, tetracycline; TIG, tigecycline; FA, fusidic acid; RA, rifampicin; TMP-SMX, trimethoprim/sulfamethoxazole; MUP; mupirocin (category of antimicrobial susceptibility: R, resistant; I, intermediate; S, susceptible).
FIG. 5.
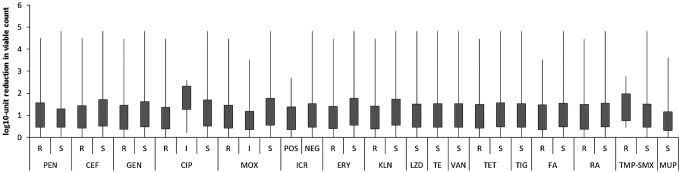
Response to protoporphyrin IX (PPIX)-mediated photodynamic inactivation (PDI) of Staphylococcus aureus revealing various antimicrobial susceptibility. Each box plot represents the spread of bacterial response across different clinical isolates. PEN, penicillin; CEF, cefoxitin; GEN, gentamycin; CIP, ciprofloxacin; MOX, moxifloxacin; ICR, inducible clindamycin resistance (POS, positive; NEG, negative); ERY, erythromycin; KLN, clindamycin; LZD, linezolid; TE, teicoplanin; VAN, vancomycin; TET, tetracycline; TIG, tigecycline; FA, fusidic acid; RA, rifampicin; TMP-SMX, trimethoprim/sulfamethoxazole; MUP; mupirocin (category of antimicrobial susceptibility: R, resistant; I, intermediate; S, susceptible). The error bars represent minimum and maximum value of log10 unit reduction in viable counts.
The same analysis concerning the possible correlation of both resistance mechanisms and susceptibility testing with response to PDI was applied separately for MRSA and MSSA populations. In accordance with results obtained for all S. aureus populations, across MRSA and MSSA groups, no significant correlation was observed, indicating that resistance mechanisms and susceptibility patterns do not influence strain response to PDI (data not shown).
Isolates' origin does not influence PDI response
To evaluate if features connected with strain origin could influence S. aureus response to photoinactivation, the effectiveness of PDI was compared between surveillance isolates and these originated from infected patients. Additionally, the response to PDI was compared accordingly to clinical symptoms, that is, strains isolated from local infections, generalized infections, and endoprosthesis-associated infections. No significant difference was observed, indicating that regardless of isolate origin and whether it is isolated from local, generalized, or endoprosthesis-related infections, similar PDI effectiveness could be reached.
Presence of mec element does not influence response to PDI
Searching for possible differences between MRSA and MSSA strains that could affect S. aureus susceptibility to photoinactivation, we have used S. aureus Newman and its isogenic mec-positive derivative. Introduction of mec element into MSSA Newman strain did not changed strain susceptibility to PDI. Similarly, deletion of SCCmec element in MRSA strain DK 2574 also did not affect S. aureus response to photoinactivation. Obtained results revealed no difference in response to PDI between studied strains, indicating that presence of mec element does not explain the higher resistance of MRSA strains to photodynamic treatment (Fig. 6).
FIG. 6.
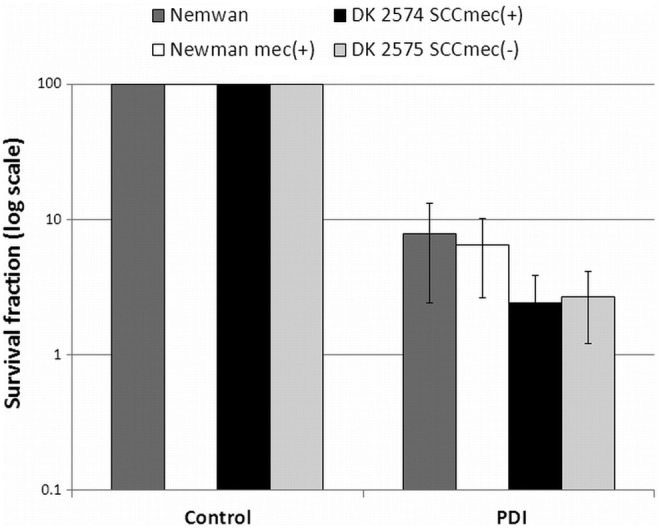
Photoeffect of protoporphyrin IX (PPIX) against isogenic Staphylococcus aureus strains. The cells were illuminated after dark incubation for 30 min at 37°C with 25 μM PPIX with the light dose of 50 J/cm2. The survival rate was calculated from the number of colony-forming units (CFU) in the photodynamic inactivation (PDI)-treated sample divided by the number of CFU in the sample irradiated without photosensitizer. Each experiment was done three times, and error bars show SD.
Ability to produce biofilm in vitro does not explain different PDI response between MRSA and MSSA strains
When searching for differences among the studied S. aureus strains that could determine resistance to PDI, an effort was made to investigate the effect of in vitro biofilm production. The strains were tested in vitro for biofilm production using the spectrophotometric method.19 Each strain was characterized as a weak-, intermediate-, or strong-biofilm producer according to the measured absorbance value (<0.200, between 0.200 and 0.300, and>0.300, respectively). The spectrophotometric assay revealed that distribution of biofilm producing and non-producing strains is similar across MRSA and MSSA populations (no statistically significant difference was observed). The same was true for the distribution of strong-, intermediate-, or weak-biofilm producers (Fig. 7). These results indicate that the ability to produce biofilms does not explain observed differences between MRSA and MSSA populations regarding their response to photodynamic treatment.
FIG. 7.
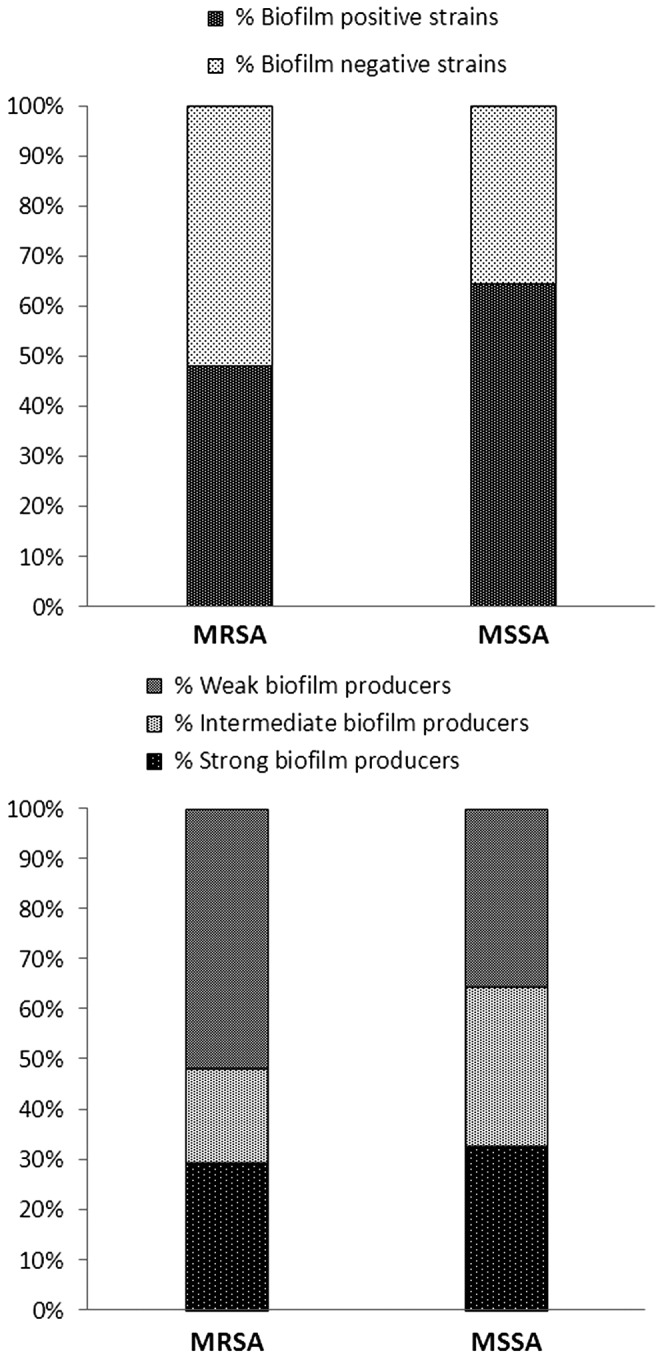
Distribution of biofilm-producing and biofilm-nonproducing strains among methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive S. aureus (MSSA) groups (upper panel). Distribution of weak, intermediate, and strong biofilm producers among MRSA and MSSA groups (lower panel).
Discussion
Applicability of PDI assumes the existence of some foregone conclusions, which are widely cited and presented as undoubted benefits of PDI in comparison with conventional antimicrobial treatments. These certainties include statements of species-dependent response to photoinactivation assuming that, for example, S. aureus is generally susceptible to photodynamic treatment, or statements that multidrug-resistant isolates are as susceptible to PDI as naïve counterparts, assuming that no difference between MRSA and MSSA strains are reported. However, so far no meta-analysis, including of numerous bacterial populations, was performed to evaluate these theses.
Our previously published data concerning 80 clinical S. aureus isolates suggested that response to photoinactivation differs significantly among various strains, indicating that response to PDI is strain rather than species dependent.10 Moreover, on the basis of obtained results, we have stated that MRSA seemed to be more resistant to PDI than were MSSA strains.10 The current study provides hard evidence that response to photodynamic treatment is strain dependent, and that a significant difference between MRSA and MSSA strains according to PDI responses could be observed. It is to be hoped that the difference between MRSA and MSSA strains resulted from factors other than resistance mechanisms, as both the percentage distribution of PDI responders and log10 unit reduction in viable count was similar in all groups of S. aureus isolates that differ in antimicrobial resistance mechanisms and antimicrobial susceptibility. Therefore, in accordance with widely accepted foregone conclusions concerning photodynamic treatment, our studies confirm that PDI could be highly effective against multidrug-resistant pathogens as well as their naïve counterparts. Nevertheless, the truth is that regardless antimicrobial resistance mechanisms, the difference between MRSA and MSSA exists, as MRSA strains are slightly more resistant to photoinactivation.
Observed difference in susceptibility to PDI may result from capsular polysaccharide on the surface of MRSA cells, as indicated by Fournier et al.;22 however, other factors determining the difference between MRSA and MSSA strains must exist, and should be further investigated, excluding differences in drug resistance mechanisms.
Other phenotype features such as biofilm production, efflux pumps and superoxide dismutases have already been investigated by our group, and suggested possible explanations concerning differences in strain response to PDI, however, did not provide the final explanation of observed difference.10,23,24 Some S. aureus are known for being able to produce biofilms in order to enhance their tenacity in biotic surfaces; therefore, biofilm formation could lead to reduced susceptibility to photoinactivation. To investigate whether MRSA and MSSA strains differ in their ability to form biofilms, we have checked if studied strains are biofilm producers. As similar distribution of biofilm producers was observed both in MRSA and MSSA populations, we could conclude that even though biofilm production affects S. aureus susceptibility to PDI, it does not explain observed differences between MRSA and MSSA strains. Additionally, the genetic background of S. aureus strains was analyzed in previously published work concerning Agr and SCCmec elements; however, such an approach did not explain differences in susceptibility to photoinactivation.25 In the current article, the impact of mec element on S. aureus response to PDI was not observed. One reason for various responses to PDI could be an antioxidant defense system. S. aureus strains lacking active superoxide dismutases were significantly more susceptible to PPIX-based photo-killing than their naïve counterparts.24 The same observation was true for a strain deprived of staphyloxanthin, a staphylococcal golden pigment known for its antioxidant properties (Nakonieczna, unpublished results). The effect of extracellular slime on photoinactivation was also evaluated by Gad et al.,26 who indicated that it significantly influenced sensitizer uptake by S. aureus cells, which may result in variable response to PDI. The next possible factor explaining the difference in response to PDI could be the different status of efflux mechanisms. The uptake level of specific sensitizers differs in accordance with efflux pump expression across S. aureus strains, suggesting that pumping the sensitizer out, individual strains could be more resistant to PDI.27
Taking into consideration the abovementioned factors influencing strain susceptibility to photodynamic treatment, we can draw the conclusion that the underlying mechanism is multifactorial. Moreover, some differences between MRSA and MSSA strains must also play a significant role in the effectiveness of PDI, as a significant difference between these groups has been proven in the current article. Moreover, these differences must include factors other than resistance mechanisms, as no difference in antimicrobial resistance has been observed within the current work. There are many different groups of clonal lineages with different genetic backgrounds across S. aureus isolates. Some of them are more associated with epidemic spread, others with livestock. This epidemiological behavior could also lead to differences in tenacity, which might have an impact on the photoinactivation response of the bacterial strain. Therefore, applying PDI against MSSA and/or MRSA strains of such different epidemiological backgrounds, to shed more light on possible differences between MRSA and MSSA regarding their susceptibility to photoinactivation, should be analyzed further.
Conclusions
In accordance with widely accepted foregone conclusions concerning photodynamic treatment, our studies confirm that PDI could be highly effective against multidrug-resistant pathogens, as well as their naïve counterparts. Nevertheless, the truth is that regardless of antimicrobial resistance mechanisms, the difference between MRSA and MSSA exists, as MRSA strains are slightly more resistant to photoinactivation.
Acknowledgments
Dr. Grinholc conceived and designed the experiments. Drs. Grinholc and Rapacka-Zdonczyk performed the experiments. Dr. Grinholc analyzed the. Drs. Grinholc and Bielawski contributed reagents/materials/analysis tools. Grinholc wrote the article. All authors read and approved the final manuscript. The isogenic strains of S. aureus Newman was kindly provided by Brigitte Berger Bachi, Institute of Medical Microbiology, University of Zurich, Switzerland. MALDI-TOF MS analysis and VITEK 2 susceptibility testing were performed at LM Bruss Laboratory in Poland. This work was supported by grant no. 1651/B/P01/2010/39 from the National Science Centre (NCN).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hamblin M.R., and Newman E.L. (1994). On the mechanism of the tumour-localising effect in photodynamic therapy. J. Photochem.Photobiol. B 23, 3–8 [DOI] [PubMed] [Google Scholar]
- 2.Ochsner M. (1997). Photophysical and photobiological processes in the photodynamic therapy of tumours. J. Photochem. Photobiol. B 39,1–18 [DOI] [PubMed] [Google Scholar]
- 3.Athar M., Mukhtar H., and Bickers D.R. (1988). Differential role of reactive oxygen intermediates in photofrin-I- and photofrin-II-mediated photoenhancement of lipid peroxidation in epidermal microsomal membranes. J. Invest Dermatol. 90, 652–657 [DOI] [PubMed] [Google Scholar]
- 4.Redmond R.W., and Gamlin J.N. (1999). A compilation of singlet oxygen yields from biologically relevant molecules. Photochem. Photobiol. 70, 391–475 [PubMed] [Google Scholar]
- 5.Wilson M., and Pratten J. (1995). Lethal photosensitisation of Staphylococcus aureus in vitro: effect of growth phase, serum, and pre-irradiation time. Lasers Surg. Med. 16, 272–276 [DOI] [PubMed] [Google Scholar]
- 6.Embleton M.L., Nair S.P., Cookson B.D., and Wilson M. (2004). Antibody-directed photodynamic therapy of methicillin resistant Staphylococcus aureus. Microb. Drug Resist. 10, 92–97 [DOI] [PubMed] [Google Scholar]
- 7.Zolfaghari P.S., Packer S., Singer M., et al. (2009). In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 9, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajim K.I., Salih D.S., and Rassam Y.Z. (2010). Laser light combined with a photosensitizer may eliminate methicillin-resistant strains of Staphylococcus aureus. Lasers Med. Sci. 25, 743–748 [DOI] [PubMed] [Google Scholar]
- 9.Wainwright M. (1998). Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 42, 13–28 [DOI] [PubMed] [Google Scholar]
- 10.Grinholc M., Szramka B., Kurlenda J., Graczyk A., and Bielawski K.P. (2008). Bactericidal effect of photodynamic inactivation against methicillin-resistant and methicillin-susceptible Staphylococcus aureus is strain-dependent. J. Photochem. Photobiol. B 90, 57–63 [DOI] [PubMed] [Google Scholar]
- 11.Grinholc M., Zawacka–Pankau J., Gwizdek–Wisniewska A., and Bielawski K.P. (2010). Evaluation of the role of the pharmacological inhibition of Staphylococcus aureus multidrug resistance pumps and the variable levels of the uptake of the sensitizer in the strain-dependent response of Staphylococcus aureus to PPArg(2)-based photodynamic inactivation. Photochem. Photobiol. 86, 1118–1126 [DOI] [PubMed] [Google Scholar]
- 12.Vaudaux P.E., Monzillo,V., Francois P., Lew D.P., Foster T.J., and Berger–Bachi B. (1998). Introduction of the mec element (methicillin resistance) into Staphylococcus aureus alters in vitro functional activities of fibrinogen and fibronectin adhesins. Antimicrob. Agents Chemother. 42, 564–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szabados F., Kaase M., Anders A., and Gatermann S.G. (2012). Identical MALDI TOF MS-derived peak profiles in a pair of isogenic SCCmec-harboring and SCCmec-lacking strains of Staphylococcus aureus. J. Infect. 65, 400–405 [DOI] [PubMed] [Google Scholar]
- 14.Szabados F., Woloszyn J., Richter C., Kaase M., and Gatermann S. (2010). Identification of molecularly defined Staphylococcus aureus strains using matrix-assisted laser desorption/ionization time of flight mass spectrometry and the Biotyper 2.0 database. J. Med. Microbiol. 59, 787–790 [DOI] [PubMed] [Google Scholar]
- 15.CLSI (2012). Performance standards for antimicrobial susceptibility testing. CLSI approved standard M100-S22. Wayne, PA: Clinical and Laboratory Standards Institute, [Google Scholar]
- 16.Shittu A., Oyedara O., Abegunrin F., et al. (2012). Characterization of methicillin-susceptible and -resistant staphylococci in the clinical setting: a multicentre study in Nigeria. BMC. Infect. Dis. 12, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jett B.D., Hatter K.L., Huycke M.M., and Gilmore M.S. (1997). Simplified agar plate method for quantifying viable bacteria. Biotechniques 23, 648–650 [DOI] [PubMed] [Google Scholar]
- 18.Fowler V.G., Jr., Fey P.D., Reller L.B., Chamis A.L., Corey G.R., and Rupp M.E. (2001). The intercellular adhesin locus ica is present in clinical isolates of Staphylococcus aureus from bacteremic patients with infected and uninfected prosthetic joints. Med. Microbiol. Immunol. 189, 127–131 [DOI] [PubMed] [Google Scholar]
- 19.Peeters E., Nelis H.J., and Coenye T. (2008). Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 72, 157–165 [DOI] [PubMed] [Google Scholar]
- 20.Winstanley T., and Courvalin P. (2011). Expert systems in clinical microbiology. Clin. Microbiol. Rev. 24, 515–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livermore D.M., Struelens M., Amorim J., et al. (2002). Multicentre evaluation of the VITEK 2 Advanced Expert System for interpretive reading of antimicrobial resistance tests. J. Antimicrob. Chemother. 49, 289–300 [DOI] [PubMed] [Google Scholar]
- 22.Fournier J.M., Boutonnier A., and Bouvet A. (1989). Staphylococcus aureus strains which are not identified by rapid agglutination methods are of capsular serotype 5. J. Clin. Microbiol. 27, 1372–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grinholc M., Zawacka–Pankau J., Gwizdek–Wisniewska A., and Bielawski K.P. (2010). Evaluation of the role of the pharmacological inhibition of Staphylococcus aureus multidrug resistance pumps and the variable levels of the uptake of the sensitizer in the strain-dependent response of Staphylococcus aureus to PPArg(2)-based photodynamic inactivation. Photochem. Photobiol. 86, 1118–1126 [DOI] [PubMed] [Google Scholar]
- 24.Nakonieczna J., Michta E., Rybicka M., Grinholc M., Gwizdek–Wisniewska A., and Bielawski K.P. (2010). Superoxide dismutase is upregulated in Staphylococcus aureus following protoporphyrin-mediated photodynamic inactivation and does not directly influence the response to photodynamic treatment. BMC. Microbiol. 10, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grinholc M., Richter M., Nakonieczna J., Fila G., and Bielawski K.P. (2011). The connection between agr and SCCmec elements of Staphylococcus aureus strains and their response to photodynamic inactivation. Photomed. Laser Surg. 29, 413–419 [DOI] [PubMed] [Google Scholar]
- 26.Gad F., Zahra T., Hasan T., and Hamblin M.R. (2004). Effects of growth phase and extracellular slime on photodynamic inactivation of gram-positive pathogenic bacteria. Antimicrob. Agents Chemother. 48, 2173–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tegos G.P., and Hamblin M.R. (2006). Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob. Agents Chemother. 50, 196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]