Abstract
Background. Pneumococcus, meningococcus, and Haemophilus influenzae cause a similar spectrum of infections in the ear, lung, blood, and brain. They share cross-reactive antigens that bind to the laminin receptor of the blood-brain barrier as a molecular basis for neurotropism, and this step in pathogenesis was addressed in vaccine design.
Methods. Biologically active peptides derived from choline-binding protein A (CbpA) of pneumococcus were identified and then genetically fused to L460D pneumolysoid. The fusion construct was tested for vaccine efficacy in mouse models of nasopharyngeal carriage, otitis media, pneumonia, sepsis, and meningitis.
Results. The CbpA peptide–L460D pneumolysoid fusion protein was more broadly immunogenic than pneumolysoid alone, and antibodies were active in vitro against Streptococcus pneumoniae, Neisseria meningitidis, and H. influenzae. Passive and active immunization protected mice from pneumococcal carriage, otitis media, pneumonia, bacteremia, meningitis, and meningococcal sepsis.
Conclusions. The CbpA peptide–L460D pneumolysoid fusion protein was broadly protective against pneumococcal infection, with the potential for additional protection against other meningeal pathogens.
Keywords: Streptococcus pneumoniae, meningitis, pneumonia, vaccine, meningococcus
Children younger than 5 years are at high risk for invasive disease caused by Streptococcus pneumoniae (pneumococcus), Neisseria meningitidis (meningococcus), and Haemophilus influenzae. While effective vaccines have been developed against a subset of each of these pathogens, most of the >90 serotypes of pneumococcus, all non–type b H. influenzae, and the highly prevalent serogroup B meningococcus are not covered by these vaccines. The use of capsule-based vaccine strategies has resulted in serotype replacement by nonvaccine capsular serotypes or complete loss of capsule [1, 2]. Unencapsulated strains, previously relegated to mild mucosal infections, have emerged as causes of otitis media, bacteremia, and meningitis [2]. Thus, development of conserved protein–based vaccines has become increasingly important. Recently, efforts to add proteins to vaccines or substitute the protein components of conjugate vaccines with virulence determinants from these pathogens have gained support. However, a limitation of this approach is that the proteins under consideration are species specific. We sought to test shared determinants of infection as potential protein-based vaccines.
Pneumococcus, meningococcus, and Haemophilus cause a similar spectrum of disease ranging from otitis media and pneumonia to sepsis and meningitis. The 3 pathogens share the ability to bind to the laminin receptor of the blood-brain barrier as a molecular basis for neurotropism during meningitis [3]. This approach has not been applied to vaccine design and raises the possibility that a broadly protective vaccine against meningitis might be developed on the basis of the commonality between bacterial ligands that target the cerebrovascular endothelium.
Pneumococcal pneumolysin (Ply) and choline-binding protein A (CbpA) are 2 well-characterized major virulence factors that contribute to the development of invasive disease [4]. Pneumolysin is a cholesterol-dependent cytolysin that induces pore formation in the membrane of eukaryotic cells [5]. Vaccinating with various attenuated toxoid versions of Ply (pneumolysoids) demonstrated efficacy against multiple stages of infection in animal models, particularly bacteremia [6–8]. Two noncytolytic toxoids used in this study are L460D and Δ6D385N. L460D is unable to bind cholesterol [5], and Δ6D385N is unable to form pores in cell membranes and has a reduced ability to activate complement [8].
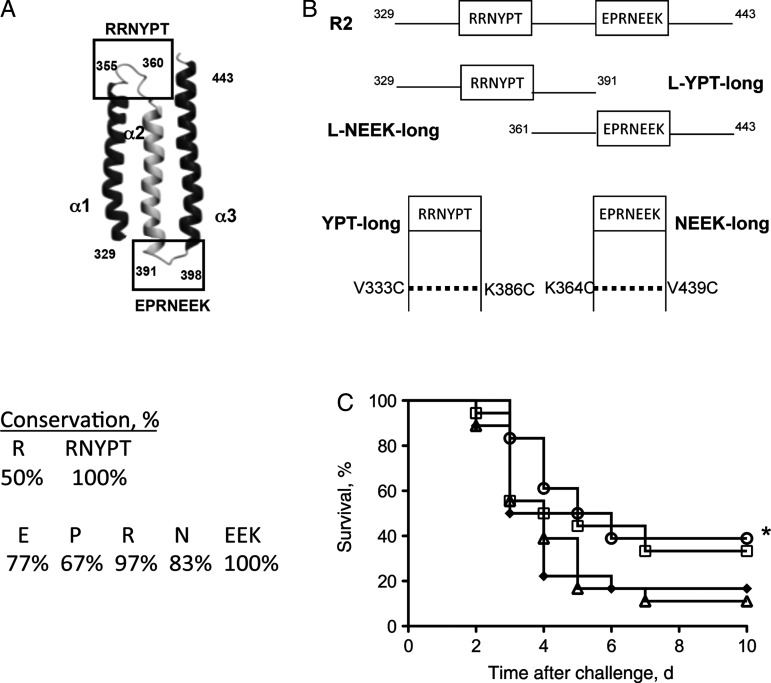
CbpA [9] is a highly protective vaccine antigen in animal models of pneumococcal infection [6]. In humans challenged with pneumococci, immunoglobulin G (IgG) titers against CbpA were highest among all antigens tested [10]. The N-terminus contains 2 nearly identical repeat domains (R domains) that each fold into antiparallel helices, and turns connecting the helices show extremely high sequence conservation (Figure 1A) [11, 12]. The RRNYPT sequence binds to the epithelial polymeric immunoglobulin receptor (pIgR) [12], and the sequence EPRNEEK binds to the laminin receptor of the blood-brain barrier [3]. Importantly, H. influenzae and N. meningitidis also bind to the same domain of the laminin receptor. Although their ligands (PilQ, PorA, and OmpP2 [13]) are not homologous to CbpA by sequence, they are cross-reactive with antibodies against CbpA [3].
Figure 1.
Construction of CbpA peptides. A, Schematic representation of the R2 domain of CbpA. Two nonhelical loop regions (boxes) link the 3 antiparallel α-helices. The RRNYPT motif binds to the pIgR receptor on epithelial cells, and the EPRNEEK motif binds to laminin receptor of endothelial cells. Amino acid numbers are indicated. The percentage conservation of sequence from 30 clinical isolates is listed. B, Regions of R2 were expressed as wild-type (referred to as linear: L-YPT-long, L-NEEK-long: 62 and 82 amino acids, respectively) or dual Cys-containing (YPT-long or NEEK-long) polypeptides made by substituting cysteine residues as indicated (referred to as “looped”). C, Survival of mice (n = 18 per group) immunized with 10 μg of linear (diamonds) or looped (circles) NEEK-long, full-length R2 (squares), or BSA (triangles) in 3 doses 2 weeks apart followed by challenge with TIGR4X intranasally (1 × 107 colony-forming units/mouse) 2 weeks after immunization. Data are pooled from 3 independent experiments. *Significantly different from BSA (P = .03).
Protein-based vaccines for pneumococcal disease are likely to require multiple antigens in the same formulation. A combination of pneumolysoid and CbpA is an attractive vaccine concept as it addresses pathological processes in the nasopharynx, ear, lung, blood, and brain. Creating single constructs that combine protective epitopes of antigens decreases the number of proteins in a multicomponent vaccine while maintaining broad antigen coverage. We designed and optimized a protein-based pneumococcal vaccine with the potential to ameliorate 3 common forms of bacterial meningitis by fusing the receptor binding domains of CbpA to a toxoid form of pneumolysin.
MATERIALS AND METHODS
Bacterial Strains and Growth Conditions
S. pneumoniae strains used included serotype 4 TIGR4 (T4) and its cbpA-, unencapsulated (T4R) or bioluminescent (TIGR4X) derivatives, serotype 2 D39, and clinical isolates 6B and 19F. Liquid cultures were grown without aeration at 37°C in a 5% CO2 incubator until an OD620 of 0.4–0.5 was reached [18]. N. meningitidis serogroup A strain 13 077 and H. influenzae serotype b strain 10 211 were obtained from ATCC and grown overnight on chocolate agar plates (VWR) at 37°C in a 5% CO2 incubator. Bacteria were transferred to brain-heart infusion medium supplemented with hemin (10 µg/mL) and NAD (10 µg/mL) and grown shaking at 37°C to an OD620 of 0.4–0.5.
Production of Protein Antigens and Synthetic CbpA Peptides
The CbpA R2 domain-derived, dual-helix constructs and related polypeptide variants were amplified by polymerase chain reaction (PCR) from TIGR4 genomic DNA, using primers listed in Supplementary Table 1. PCR products were subcloned into the NdeI/EcoRI sites of vector pET28a (Novagen). The Cys-containing YPT and NEEK constructs were made using the pET28a plasmid for the wild-type constructs as templates for the Quikchange site-directed mutagenesis kit (Stratagene). Briefly, to create the Cys-containing YPT construct, amino acids V333 and K386 of L-YPT were mutated to cysteines, using primers V333C and K386C, respectively. To create the Cys-containing NEEK construct, amino acids K364 and V439 of L-NEEK were mutated to cysteines, using primers K364C and V439C, respectively. Clones incorporating the desired mutations were amplified and verified using DNA sequencing. Peptides containing the YPT and NEEK sequences were synthesized by the SJCRH Hartwell Center. Polypeptides joined to measles T-cell epitopes (TCEs) were also synthesized and included TCE-YPT and TCE-NEEK (Table 1). Polypeptides were purified by high-performance liquid chromatography, lyophilized, and dissolved in H2O. Polypeptides with cysteine substitutions were incubated overnight at room temperature to allow for spontaneous disulfide bond formation to create helical hairpin structures similar to those observed in the wild-type CbpA R2 domain [11].
Table 1.
Sequences of Peptides and Fusion Constructs
| Peptide | CbpA Amino Acid | Sequence |
|---|---|---|
| L-YPTlong | 329–391 | MPEKKVAEAEKKVEEAKKKAEDQKEEDRRNYPTNTYKTLELEIAESDVEVKKAELELVKEEAKE |
| L-NEEKlong | 361–443 | MNTYKTLELEIAESDVEVKKAELELVKEEAKEPRNEEKVKQAKAEVESKKAEATRLEKIKTDRKKA EEEAKRKAAEEDKVKEKP |
| YPTlong | 329–391 | MPEKKCAEAEKKVEEAKKKAEDQKEEDRRNYPTNTYKTLELEIAESDVEVKKAELELVCEEAKE |
| NEEKlong | 361–443 | MNTYCTLELEIAESDVEVKKAELELVKEEAKEPRNEEKVKQAKAEVESKKAEATRLEKIKTDRKKAE EEAKRKAAEEDKCKEKP |
| TCE-YPT | 344–372 | qyikanskfigitggACKKAEDQKEEDRRNYPTNTYKTLELECA |
| TCE-NEEK | 386–402 | qyikanskfigitqyikanskfigitggKECAKEPRNEEKVKQCK |
| YPT-L460D-NEEK (YLN) | 344–373, 386–402 | MACKKAEDQKEEDRRNYPTNTYKTLELECAEGG-L460D-KECAKEPRNEEKVKQCK |
| YPT-Δ6D385N-NEEK | 344–373, 386–402 | MACKKAEDQKEEDRRNYPTNTYKTLELECAEGG-Δ6D385N-KECAKEPRNEEKVKQCK |
Cysteine mutations are underlined. The YPT and NEEK sequences are in bold. The TCE epitopes are in lower case letters. Amino acid numbers correspond to TIGR4 CbpA.
To create CbpA-pneumolysoid fusion constructs, primers YPTNde and NEEKSac that contained the respective CbpA sequences were used to amplify pneumolysin toxoid Δ6D385N (provided by Tim Mitchell, University of Glasgow) or L460D from its original construct. PCR products were digested and cloned into the NdeI/SacI sites of pET28a. Clones with correct inserts were determined by DNA sequencing and expressed in BL21(DE3) cells. Liquid cultures were induced overnight at 22°C with 0.07 mM IPTG, lysed with Bugbuster HT (Novagen), and subsequently purified over a His-Select Ni++ column (Sigma). Purified proteins were dialyzed into phosphate-buffered saline (PBS) and stored at −80°C with 10% glycerol. L460D and YLN were stored in 10 mM His, pH 6.0, with 15% trehalose [19]. All proteins were purified by the SJCRH Protein Production Facility.
Anti-CbpA Peptide Antibody Production and Functional Analysis
Polyclonal rabbit antiserum to CbpA, YLN, L460D, or alum and monoclonal antibodies were developed at Rockland Immunochemicals. Clone 14A3 was selected from mice immunized with YPT, while clones 3G12 and 3H11 were selected from mice immunized with NEEK. Adhesion and invasion of Detroit nasopharyngeal epithelial cells and rBCEC6 rat brain endothelial cells were assessed as described elsewhere [18]. T4R cells were incubated with 25 µg/mL of monoclonal antibody or a 1:100 dilution of polyclonal antibody for 30 minutes before infection with 107 colony-forming units (CFU)/well. Assays were repeated 3–4 times with 4 wells per sample. For all passive-protection studies, 300 µg of monoclonal antibody (n = 10/group, 2 experiments) or 100 µL of rabbit polyclonal antibody antibody (n = 10/group, 3 experiments) were given intraperitoneally 1 hour before challenge and 18 hours after challenge.
Immunization Studies
All animal experiments were done in accordance with the St Jude Institutional Animal Care and Use Committee, using 6–7-week-old female BALB/c mice (Jackson Labs). For initial immunizations, mice were primed with 10 µg of protein or 200 µg of peptide in a 1:1 dilution of CFA (Sigma). Boosts were given in a 1:1 dilution of IFA (Sigma) at weeks 2 and 4. For pneumolysoid and CbpA-pneumolysoid fusion protein immunizations, mice were primed with 10 µg of protein containing 130 µg of alum (Brenntag), and boosts were given at weeks 2 and 4. Serum was collected from mice on week 5. Mice were challenged with 1 × 107 CFU TIGR4X intratracheally on week 7. Meningitis was confirmed through Xenogen imaging and plating cerebrospinal fluid. For meningitis and survival, the data represent 8 independent studies of >7 mice per group. In the pneumonia study, lungs were collected at 72 hours after challenge for histopathologic analysis (5 mice/group). For nasopharyngeal carriage, mice were challenged intranasally with a nonlethal dose of strain 19F at 5 × 105 CFU or 107 CFU of TIGR4X. Nasal washes were collected and plated weekly for 3 weeks (3 independent studies of >5–6 mice per group). For otitis media, mice were challenged intranasally with bioluminescent otitis strain 19FX at 1 × 105 CFU/100 µL. Xenogen imaging of the head was performed 6 hours after challenge and then every 12 hours for 3 days [20]. Otitis was defined by bioluminescent signal in one or both ears as previously described.
Functional Antibody Measurements
Serum samples from immunized mice were taken one week after the second boost. IgG titers were determined by enzyme-linked immunosorbent assay (ELISA). Plates were coated with either single protein antigens (0.1 µg/well) or intact bacteria (106 CFU/well). IgG titer was determined as the reciprocal of the highest dilution to give positive recognition of the epitope. Enzyme-linked immunosorbent spot (ELISPOT) analysis of spleen cells 10 days after the final boost was performed as described to determine activation of peptide-specific T cells [21]. To determine the ability of serum to neutralize wild-type pneumolysin, inhibition of hemolysis was assayed as previously described [19].
Colony blotting was used to establish cross-reactivity of Neisseria and Haemophilus strains with sera from rabbits immunized with CbpA or vaccine constructs. Mutants deleted for ligands for laminin receptor (3) were tested in parallel as controls. All strains were grown in liquid culture and spotted onto a polyvinylidene fluoride membrane, using a dot blot apparatus. After brief drying, the membrane was blocked and probed with rabbit polyclonal antibody (1:5000) to CbpA, YLN, or L460D.
Solid-Phase Peptide ELISAs
Sera from immunized mice (10–12 per condition) were pooled and used in a peptide-based ELISA to determine immunogenic epitopes. Peptides of the PLY protein (accession number NP_359331.1), consisting of 10 amino acids with 8 overlapping, were synthesized onto polyethylene pins in a 96-well format (The Serum Analyte and Biomarker Core, The Oklahoma Medical Research Foundation). Pins were blocked with 3% milk in PBS at room temperature for 1 hour. Peptides were then incubated in mouse sera (1:100 in 3% milk in PBS, plus 0.05% Tween) in 96-well plates, with pins inserted into individual wells for 4 hours at room temperature. Pins were then washed with PBS-Tween and incubated with antimouse secondary antibody conjugated to alkaline phosphatase (Sigma) diluted 1:5000 overnight at 4°C. Pins were then washed and incubated with p-nitrophenyl phosphate (PNPP; Sigma) in substrate buffer (167 mM sodium bicarbonate, 12 mM sodium carbonate, 100 mM glycine, 984 mM magnesium chloride hexahydrate, and 1 mM zinc chloride; pH 10.4) at 37°C for 30 minutes, at which time the positive control sera had reached an OD of 1. Absorbance was then read at 410 nm (reference absorbance at 490 nm). An epitope was defined as positive if ≥2 consecutive epitopes showed an absorbance of ≥4 SDs above the average OD for the pin set.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism V5.0. Differences in survival curves were determined by the Mantel-Cox log-rank test; percentages with meningitis was compared using the Fisher exact test. All in vitro cell assays were analyzed by the Student t test. The Mann-Whitney U test was used to compare antibody titers, nasal washes, and blood titers. A P value of <.05 was determined to be statistically significant.
RESULTS
Activities of Looped Peptide Domains of CbpA
The 2 highly conserved nonhelical turns between α-helices α1-α2 and α2-α3 of the R2 domain of CbpA that enable binding to host cells in the nasopharynx (RRNYPT) and at the blood-brain barrier (EPRNEEK; Figure 1A) were targeted for vaccine development. Since in the native CbpA structure these regions form hairpin-like (nonlinear) loops [11], we prepared recombinant constructs 60–80 amino acids long containing each loop with either wild-type sequences (L-YPT-long and L-NEEK-long; Figure 1B) or with 2 mutations each to introduce Cys residues that we postulated could promote the native-like α-helical conformations through cross-linking (YPT-long and NEEK-long; Figure 1B and Table 1). The Cys mutant constructs were shown to form native-like α-helical secondary structure, using circular dichroism spectropolarimetry (Supplementary Figure 1). When the Cys mutant and linear EPRNEEK constructs were compared as vaccines against pneumococcal bacteremia, only the Cys mutant polypeptide significantly improved survival (Figure 1C).
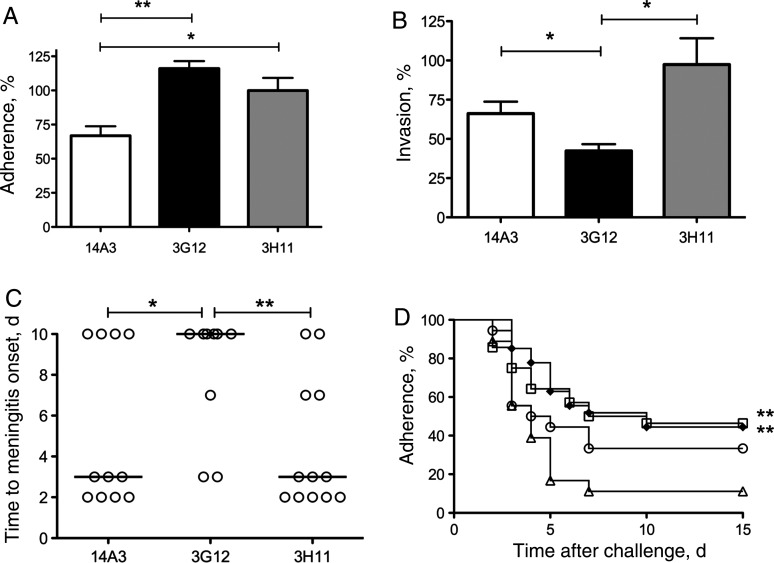
Anti-CbpA monoclonal antibodies 14A3 and 3G12 that bound to the Cys mutant RRNYPT or EPRNEEK peptides by ELISA (Supplementary Figure 2) were bioactive. Antibodies 14A3 and 3G12 decreased pneumococcal adherence to Detroit epithelial cells in vitro (Figure 2A) and blocked pneumococcal invasion of cerebral endothelial cells (Figure 2B), significantly prevented the development of meningitis (Figure 2C), and conferred 71% survival as compared to 14% for the 3H11 control (P = .0019). These data suggest that maintenance of tertiary structure of the Cys mutant peptides may be a factor important for optimal vaccine responses.
Figure 2.
Bioactivities of antipeptide monoclonal antibodies. Three anti-CbpA monoclonal antibodies were mapped by enzyme-linked immunosorbent assay to peptide fragments spanning CbpA (Supplementary Figure 1). 14A3 binds RRNYPT motif, 3G12 binds the ERPNEEK motif, and 3H11 is a negative control binding the N terminus of the R2 domain. Inhibition of pneumococcal adhesion to Detroit nasopharyngeal epithelial cells (A) or invasion of rBCEC6 cerebral endothelial cells (B) by preincubating strain T4R (107 colony-forming units [CFU]) with 25 µg α-CbpA monoclonal antibody 30 minutes before addition to eukaryotic cells. Adhesion was quantified as total viable bacteria per well, and invasion was calculated as bacteria surviving antibiotic treatment to kill extracellular bacteria. Data are represented as a percentage of value with no antibody added (100% = mean ± SD of 3 wells; 172 ± 8 CFU for Detroit cells and 146 ± 14 CFU for rBCEC6 cells). The results are an average of ≥3 independent assays. *P = .047, **P = .0052 (A); *P < .04 (B). C, Day after TIGR4X intratracheal challenge that mice developed meningitis after intraperitoneal passive antibody treatment (n = 5–6/group, repeated twice). The median is represented by a solid line. Each circle represents an individual mouse. Ten surviving mice did not develop meningitis. *P = .019; **P = .0018. D, Survival of mice immunized with TCE-peptide vaccines as above and challenged with TIGR4X pneumococcus (intratracheal 107 CFU): TCE-YPT (squares); TCE-NEEK (diamonds); recombinant R2 positive control (circles); alum negative control (triangles). Data were pooled from 3 independent experiments (n = 26 per group). **P < .001.
Protective Activity of CbpA Peptides Linked to TCEs
Recent evidence has suggested that T-cell activity may contribute to protection against nasopharyngeal colonization [14]. This suggested that linking CbpA peptides to larger proteins with TCE might be advantageous. To first examine whether CbpA peptides would be recognized in such a context, Cys-containing 30 amino acid peptides spanning the conserved YPT and NEEK loops were synthesized and linked to a known promiscuous measles TCE (Table 1) [15]. The generation of TCE-specific T cells was demonstrated by ELISPOT in vaccinated mice (Supplementary Figure 2). Immunization of mice with the TCE peptides also elicited protection in a model of pneumococcal bacteremia, indicating that the CbpA components were recognized in the fusion construct (Figure 2D).
Protective Activity of CbpA-Pneumolysoid Fusions
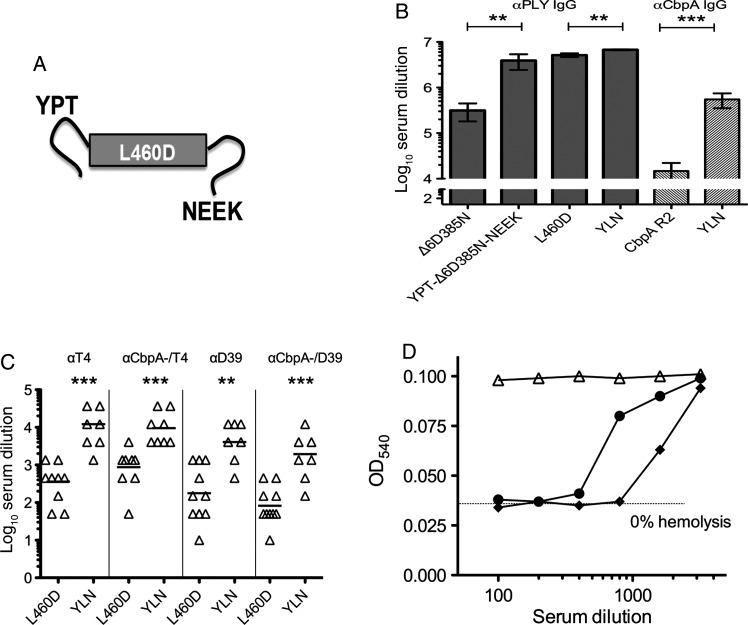
To further broaden protection of a fusion construct and provide pneumococcal-specific T-cell stimulation, the DNA encoding the 30 amino acid Cys-containing CbpA peptides (YPT and NEEK) was fused to the construct for Δ6D385N or L460D pneumolysoids (termed YLN- Δ6D385N-NEEK and YLN, respectively; Figure 3A and Table 1). Peptide-toxoid fusions engendered IgG titers against pneumolysin that not only exceeded toxoid alone in magnitude (Figure 3B) but also enhanced responses to a broader range of pneumolysin epitopes (Figure 4). Thus, immunogenicity of the toxoid was improved by addition of the N- and C-terminal CbpA peptide fusions.
Figure 3.
Immunization with pneumolysoid-CbpA fusion protein elicits bioactive antibodies against both CbpA and PLY. A, Schematic diagram of fusion of YPT and NEEK peptides to L460D. B, Titer of α-PLY or α-CbpA immunoglobulin G (IgG) antibodies in immunized mouse serum. C, Titer of IgG antibodies to whole bacteria in immunized mouse serum (wild-type serotype 4 TIGR4 [T4] or wild-type serotype 2 D39 and their respective isogenic cbpA deletion mutants. D, Neutralization of pneumolysin-induced hemolysis by serum from mice immunized with either YLN (diamonds), L460D (circles), or alum (triangles). Dotted line represents complete inhibition of lysis. Anti-hemolytic titer, 1:800 for anti-YLN vs 1:200 for anti-L460D.
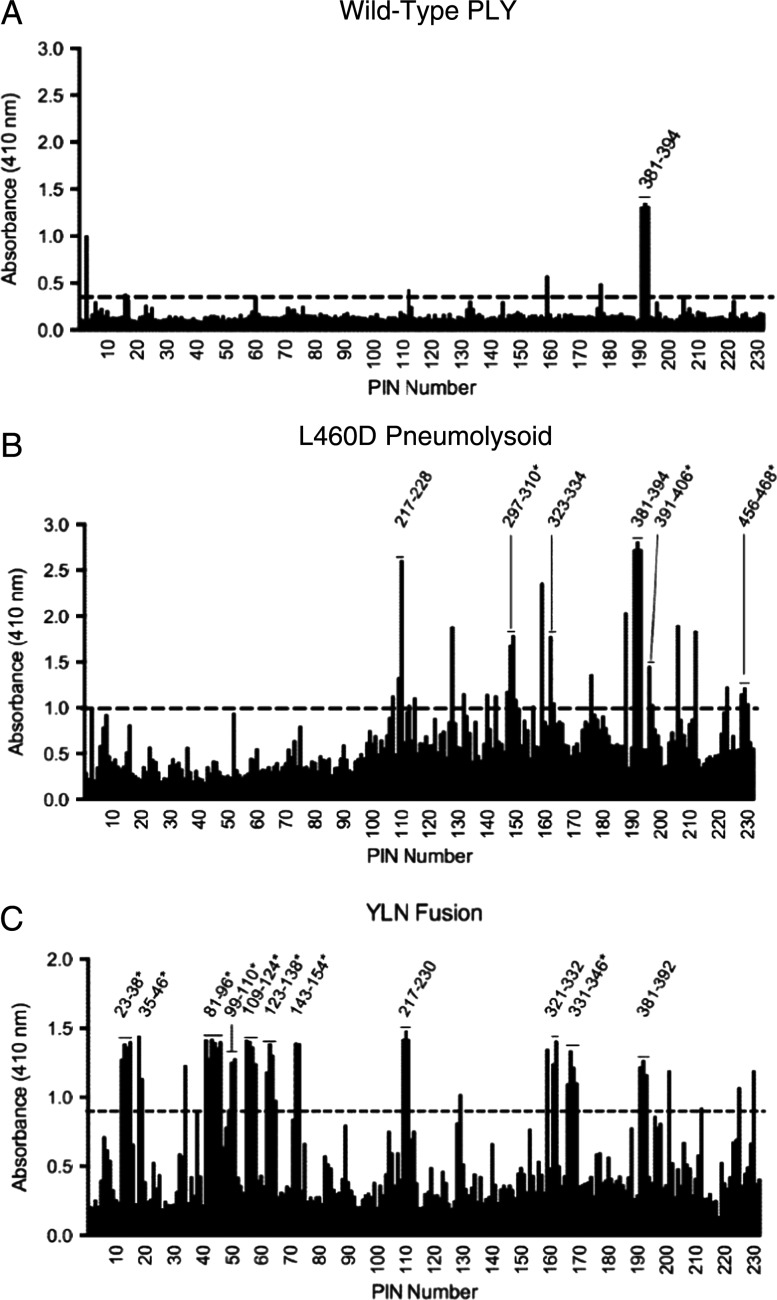
Figure 4.
Breadth of pneumolysin epitopes recognized by mouse serum. PLY solid-phase enzyme-linked immunosorbent assay was used to identify immunogenic PLY epitopes in pooled sera from mice immunized with wild-type PLY (A), L460D (B), or YLN (C). Numbers shown above each peak correspond to amino acid residue numbers in PLY. The dashed black line indicates the cutoff of 4 SDs. Reactivity of the antibody with ≥2 contiguous peptides was required to assign a positive epitope. The results in A and B are representative of ≥2 mapping results. The results in C are representative of a single experiment because of limited sera. The horizontal bars provide the width of the antigenic region. In some cases where there are ≥4 contiguous peptides recognized by the antibody, it is likely that >1 epitope exists.
CbpA peptide fusion to Δ6D385N toxoid significantly increased survival (50% for YPT-Δ6D385N-NEEK as compared to 10% for toxoid alone; P = .0098; n = 14) and lowered the rate of meningitis (20% for YPT-Δ6D385N-NEEK as compared to 50% for toxoid alone). However, protection appeared to be stronger for the L460D fusion construct YLN, and therefore this construct was further characterized.
Immunization with the YLN elicited high-titer antibodies directed at CbpA and non-CbpA epitopes expressed by intact bacteria of serotypes 2 and 4 (Figure 3C), and these antibodies neutralized toxin-induced cytolysis more effectively than antibodies to toxoid alone (Figure 3D). Serum from YLN-immunized mice decreased bacterial binding to epithelial cells (anti-YLN, 70% ± 8.7% of alum control; P = .016) and invasion of endothelial cells (anti-YLN, 71% ± 4.8% of alum control; P = .003), while serum from L460D immunized mice did not.
Antisera developed in rabbits immunized with YLN fusion or L460D toxoid were used in a passive protection model of sepsis in mice. Mice given anti-YLN rabbit serum 1 hour before and 18 hours after challenge showed protection from death following challenge with pneumococcal serotypes 4 (TIGR4X) and 2 (D39). Survival 1 week after TIGR4X challenge was 67% for YLN versus 50% for L460D and 20% for preimmune serum (P = .019 YLN vs preimmune serum). Survival 1 week after D39 challenge was 70% for YLN versus 40% for mice given either antiserum to L460D or preimmune serum (P = .24; data not shown).
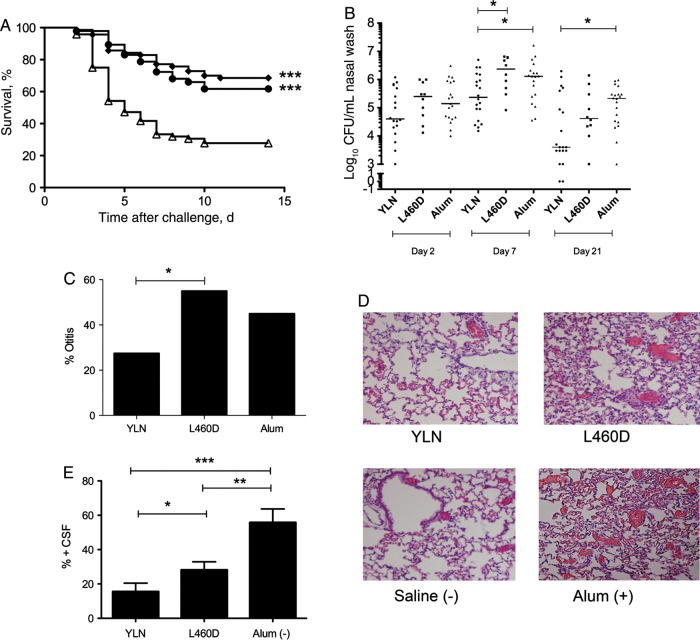
To test the active protection activity of the YLN fusion vaccine in different models of pneumococcal infection, mice were immunized with either YLN fusion or L460D, and nasopharyngeal carriage, otitis media, pneumonia, meningitis, and overall survival were monitored. Survival in a sepsis model, a site targeted by L460D, was equivalent between immunization with YLN or L460D, indicative of no adverse effect of the fusion on the protective activity of the toxoid (Figure 5A). Colonization, a site targeted by the YPT peptide component of YLN, was tested by intranasal inoculation of pneumococcal strain 19F. Mice immunized with YLN showed a significantly greater decrease in nasopharyngeal wash bacterial titers at days 7 and 21 as compared to L460D or alum (Figure 5B). In an otitis media model using bioluminescent strain 19FX, YLN significantly decreased the incidence of infection, as detected by Xenogen imaging over 4 days (Figure 5C). For pneumonia, a site targeted by both CbpA and pneumolysin antigens, lungs were harvested 72 hours after intratracheal challenge. Histopathologic analyses in the alum group showed marked cellular infiltrate in alveoli consistent with advanced pneumonia. L460D-immunized mice showed widespread alveolar hemorrhage with some areas of consolidation indicative of modest protection. The YLN-immunized lungs showed normal architecture with scant cellular infiltrate, a pattern equivalent to the noninfected controls and clearly better protection than seen with L460D alone (Figure 5D). In a meningitis model, a site targeted by the NEEK peptide component, there was marked protection in the YLN immunized mice as compared to L460D alone or alum control (Figure 5E). These data supported the individual contributions of each component of the YLN fusion to broad-based protection in multiple pneumococcal infection models.
Figure 5.
In vivo protective activities of immunization with YLN-fusion. A, Survival time in days of mice immunized with YLN (diamonds), L460D (circles), or alum (triangles) and challenged intratracheally with T4X (n > 60 per group; ***P < .0001). B, Mice (5–10/group) were immunized as indicated and challenged intranasally with strain 19F at 2 × 105 colony-forming units (CFU). Log bacterial CFU per milliliter over 21 days in nasal wash is shown for each mouse. Results are pooled from 2 independent experiments. *P < .04. C, Incidence of otitis media following intranasal challenge with 19F (5 × 106 CFU) and sequential Xenogen imaging (n = 20; P = .049). D, Lung histopathologic findings (by hematoxylin-eosin stain) 72 hours after intratracheal challenge with T4X (107 CFU) in mice immunized with YLN, L460D, or alum. Data for a nonimmunized mouse given only saline intratracheally is shown as a negative control. E, Development of meningitis in mice immunized and challenged as in panel A. Data represent an average of 8 independent experiments (n = 8–10 for each study). *P = .049; **P = .0092; ***P = .0004.
Potential for Cross-protection Against Meningococcal and Haemophilus Infection
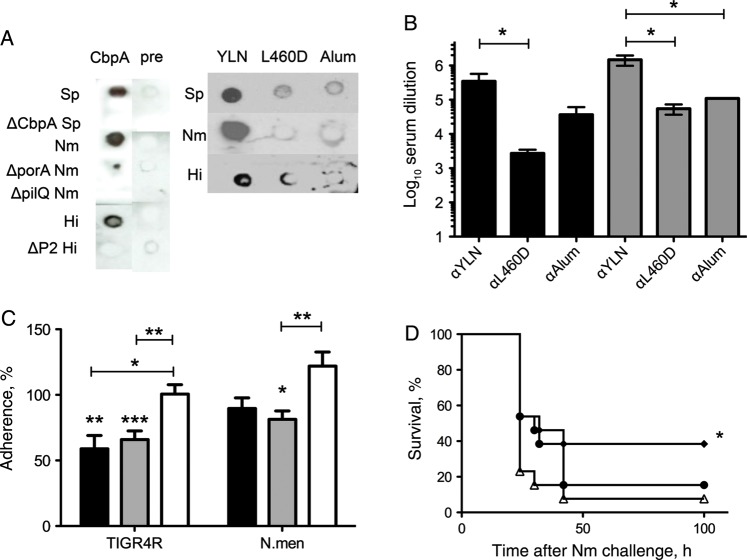
S. pneumoniae, H. influenzae, and N. meningitidis are known to bind to the same domain of the laminin receptor at the blood-brain barrier [3]. The shared mechanism of binding could possibly translate to cross-protection from disease in vivo. Polyclonal antibodies against the R2 domain of CbpA or YLN cross-reacted with both H. influenzae and N. meningitidis in colony blots (Figure 6A) and by ELISA (Figure 6B). Additionally, antibodies against CbpA or YLN cross-inhibited bacterial adhesion to endothelial cells in vitro, compared with cells treated with antibodies against toxoid or alum alone (Figure 6C). Mice immunized with YLN showed significant survival benefit against meningococcal infection as compared to alum, while L460D alone did not (Figure 6D). This suggests an epitope provided by CbpA elicits cross-protection against other meningeal pathogens.
Figure 6.
Protective potential of YLN-fusion against Haemophilus influenzae and Neisseria meningitidis. A, Colony blots of pneumococcus (Sp), H. influenzae (Hi), and meningococcus (Nm; wild type and indicated deletion mutants) treated with polyclonal rabbit serum to CbpA, preimmune serum, YLN, L460D, or alum. *P < .02; **P < .003; ***P < .0004. B, The enzyme-linked immunosorbent assay value represents the geometric mean of the highest dilution of serum that bound Nm (black bars) or Hi (gray bars) bacteria (5 wells each). *P = .02. C, Inhibition of adherence to rBCEC6 cells by α-CbpA antibody (black bars), α-YLN (gray bars), or α-L460D (white bars). Inhibition with α-alum represents 100%: Sp, 1.3 × 105; Hi, 7.6 × 106; and Nm, 2.7 × 104 colony-forming units (CFU)/mL. Antisera were used at a 1:100 dilution. Data represent 4 independent experiments of 4 wells each. *P < .02; **P < .003; ***P = .0004. D, Survival time in hours of mice immunized with YLN (diamonds), L460D (circles), or alum (triangles) and challenged intraperitoneally with N. meningitidis (106 CFU intraperitoneally; n = 15). *P < .05 vs alum; P> 0.5 for L460D vs alum.
DISCUSSION
Capsular vaccines have proven highly effective in preventing invasive disease caused by pneumococcus, meningococcus, and Haemophilus. However, serotype replacement and emergence of unencapsulated invasive strains have forced reevaluation of this strategy [1, 2]. Novel protein vaccines that are highly immunogenic, T-cell–dependent, antigenically conserved, and protective across serotypes are being tested to various degrees of success [16, 17]. Multivalent pneumococcal capsule vaccines conjugated to either Haemophilus protein D (PHiD-CV) or meningococcal C (9VPnC-MnCC) exemplify the effort to combine protection across species [7].
It is generally accepted that optimal efficacy of a protein-based vaccine will require a combination of antigens to broadly protect against all forms of disease, even within a bacterial species. However, a multicomponent vaccine must also be cost-effective, limiting the number of antigens that can be assembled together. This study explored pneumococcal CbpA/pneumolysoid fusions as the basis of a cross-species vaccine against pneumococcus, meningococcus, and H. influenzae. The concept of a fusion protein was used to encompass the protection of multiple antigens in a single construct.
CbpA domains represented as peptides with Cys mutations to enable native-like tertiary structure were effective protective antigens. The YPT-long construct required the Cys mutations and disulfide bond formation to adopt native α-helical structure, while both the wild-type and Cys mutant NEEK-long constructs displayed α-helical structure. Peptides containing Cys mutations generated antibodies reactive with native CbpA, inhibited bacterial adherence and invasion, and were protective against in vivo challenge. Monoclonal antibodies reactive with the Cys-containing CbpA peptides decreased pneumococcal adherence to epithelial and endothelial cells in vitro and protected mice against pneumococcal carriage, sepsis, and meningitis. Thus, Cys-containing CbpA peptides known to target bacterial interactions with receptors on nasopharyngeal epithelium and blood-brain barrier endothelium individually acted as effective vaccine antigens.
To expand protective efficacy against pneumonia and sepsis, the CbpA peptides were genetically fused to full-length pneumolysoid proteins. The fusion enhanced the magnitude and breadth of antibodies against the pneumolysoid, an unexpected benefit of the construct. This suggests that the addition of the peptides altered the structure of the pneumolysoid so as to increase availability of epitopes normally less available in the unsubstituted protein. These unmasked epitopes added to protection in vivo, suggesting that they may be at least transiently available in the wild-type pneumolysin. The YLN fusion also resulted in higher-titer antibodies to CbpA than the R2 domain alone, perhaps by increasing the presentation of the small CbpA peptide to the immune system as compared to its availability in native CbpA. These antibodies blocked nasopharyngeal carriage and interactions with the blood-brain barrier in vitro and protected against pneumococcal infection in the nasopharynx, ear, lung, blood, and brain. Finally, immunization with YLN elicited antibodies cross-reactive with Haemophilus and meningococcus, based on the shared NEEK epitope activity as a laminin receptor ligand. Animal models of meningococcal meningitis are limited, and this is even more so for Haemophilus. However, there was a measurable benefit against meningococcal sepsis in animals immunized with the pneumococcal construct. Testing of the vaccine in more-complex models adapted to these pathogens seems warranted.
With the emergence of pneumococcal infections caused by nonvaccine serotypes and multidrug-resistant strains, a broadly effective vaccine may be the best strategy for decreasing morbidity and mortality. The CbpA peptide–pneumolysoid fusion protein is a viable candidate for such a vaccine, a construct that appears to demonstrate synergy between components rather than simple additivity. Further, protective activity might extend to meningitis caused by Haemophilus and meningococcus.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author
Notes
Acknowledgments. We thank Amy Iverson, for work with the otitis media model; Dr Julia Hurwitz, for aid in completing the ELISPOT analysis shown in Supplementary Figure 2; Dr Tim Mitchell, for generously providing Δ6D385N pneumolysoid; and Dr James Paton, for providing some of the sera from immunized mice for Figure 4.
Financial support. This work was supported by the National Institutes of Health (grant R01 AI27913 to E. I. T.), the American Lebanese Syrian Associated Charities (to E. I. T.), and PATH.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Croucher NJ, Harris SR, Fraser C, et al. Rapid pneumococcal evolution in response to clinical interventions. Science. 2011;331:430–4. doi: 10.1126/science.1198545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gkentzi D, Slack MP, Ladhani SN. The burden of nonencapsulated Haemophilus influenzae in children and potential for prevention. Cur Opin Infect Dis. 2012;25:266–72. doi: 10.1097/QCO.0b013e32835310a4. [DOI] [PubMed] [Google Scholar]
- 3.Orihuela CJ, Mahdavi J, Thornton J, et al. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J Clin Invest. 2009;119:1638–46. doi: 10.1172/JCI36759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orihuela CJ, Gao G, Francis KP, Yu J, Tuomanen EI. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J Infect Dis. 2004;190:1661–69. doi: 10.1086/424596. [DOI] [PubMed] [Google Scholar]
- 5.Farrand AJ, LaChapelle S, Hotze EM, Johnson AE, Tweten RK. Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proc Natl Acad Sci U S A. 2010;107:4341–6. doi: 10.1073/pnas.0911581107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogunniyi AD, Woodrow MC, Poolman JT, Paton JC. Protection against Streptococcus pneumoniae elicited by immunization with pneumolysin and CbpA. Infect Immun. 2001;69:5997–6003. doi: 10.1128/IAI.69.10.5997-6003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salha D, Szeto J, Myers L, et al. Neutralizing antibodies elicited by a novel detoxified pneumolysin derivative, PlyD1, provide protection against both pneumococcal infection and lung injury. Infect Immun. 2012;80:2212–20. doi: 10.1128/IAI.06348-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell TJ, Andrew PW, Saunders FK, Smith AN, Boulnois GJ. Complement activation and antibody binding by pneumolysin via a region of the toxin homologous to a human acute-phase protein. Mol Microbiol. 1991;5:1883–8. doi: 10.1111/j.1365-2958.1991.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 9.Rosenow C, Ryan P, Weiser JN, et al. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol. 1997;25:819–29. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 10.McCool TL, Cate TR, Moy G, Weiser JN. The immune response to pneumococcal proteins during experimental human carriage. J Exp Med. 2002;195:359–65. doi: 10.1084/jem.20011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo R, Mann B, Lewis WS, et al. Solution structure of choline binding protein A, the major adhesin of Streptococcus pneumoniae. EMBO J. 2005;24:34–43. doi: 10.1038/sj.emboj.7600490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammerschmidt S, Tillig MP, Wolff S, Vaerman JP, Chhatwal GS. Species-specific binding of human secretory component to SpsA protein of Streptococcus pneumoniae via a hexapeptide motif. Mol Microbiol. 2000;36:726–36. doi: 10.1046/j.1365-2958.2000.01897.x. [DOI] [PubMed] [Google Scholar]
- 13.Abouseada NM, Assafi MS, Mahdavi J, et al. Mapping the laminin receptor binding domains of Neisseria meningitidis PorA and Haemophilus influenzae OmpP2. PloS One. 2012;7:e46233. doi: 10.1371/journal.pone.0046233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trzcinski K, Thompson CM, Srivastava A, et al. Protection against nasopharyngeal colonization by Streptococcus pneumoniae is mediated by antigen-specific CD4+ T cells. Infect Immun. 2008;76:2678–84. doi: 10.1128/IAI.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demotz S, Matricardi P, Lanzavecchia A, Corradin G. A novel and simple procedure for determining T cell epitopes in protein antigens. J Immunol Methods. 1989;122:67–72. doi: 10.1016/0022-1759(89)90335-9. [DOI] [PubMed] [Google Scholar]
- 16.Grabenstein JD, Klugman KP. A century of pneumococcal vaccination research in humans. Clin Microbiol Infect. 2012;5:15–24. doi: 10.1111/j.1469-0691.2012.03943.x. [DOI] [PubMed] [Google Scholar]
- 17.Tan TQ. Pediatric invasive pneumococcal disease in the United States in the era of pneumococcal conjugate vaccines. Clin Microbiol Rev. 2012;25:409–18. doi: 10.1128/CMR.00018-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radin JN, Orihuela CJ, Murti G, et al. ß arrestin 1 participates in platelet activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect Immun. 2005;73:7827–35. doi: 10.1128/IAI.73.12.7827-7835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu L, Joshi SB, Liyanage MR, et al. Physical characterization and formulation development of a recombinanta pneumolysoid protein-based pneumococcal vaccine. J Pharm Sci. 2103;102:387–400. doi: 10.1002/jps.23375. [DOI] [PubMed] [Google Scholar]
- 20.McCullers JA, Karlstrom A, Iverson AR, Loeffler JM, Fischetti VA. Novel strategy to prevent otitis media caused by colonizing Streptococcus pneumoniae. PLoS Pathog. 2007;3:e28. doi: 10.1371/journal.ppat.0030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown SA, Stambas J, Zhan X, et al. Clustering of Th cell epitopes on exposed regions of HIV envelope despite defects in antibody activity. J Immunol. 2003;171:4140–8. doi: 10.4049/jimmunol.171.8.4140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.