Abstract
In malaria holoendemic settings, decreased parasitemia and clinical disease is associated with age and cumulative exposure. The relative contribution of acquired immunity against various stages of the parasite life cycle is not well understood. In particular, it is not known whether changes in infection dynamics can be best explained by decreasing rates of infection, or by decreased growth rates of parasites in blood. Here, we analyze the dynamics of Plasmodium falciparum infection after treatment in a cohort of 197 healthy study participants of different ages. We use both polymerase chain reaction (PCR) and microscopy detection of parasitemia in order to understand parasite growth rates and infection rates over time. The more sensitive PCR assay detects parasites earlier than microscopy, and demonstrates a higher overall prevalence of infection than microscopy alone. The delay between PCR and microscopy detection is significantly longer in adults compared with children, consistent with slower parasite growth with age. We estimated the parasite multiplication rate from delay to PCR and microscopy detections of parasitemia. We find that both the delay between PCR and microscopy infection as well as the differing reinfection dynamics in different age groups are best explained by a slowing of parasite growth with age.
Keywords: Malaria, immunity, blood stage, mathematical modeling, Plasmodium falciparum
Plasmodium falciparum infection is estimated to cause over 1 million deaths annually [1]. The majority of symptomatic malaria occurs in children aged less than 5 years, and increasing age and exposure to infection is associated with a reduced risk of pathology and reduced level of parasites observed in the blood. The mechanisms of this acquired resistance to P. falciparum are unclear. Cell-mediated and humoral immune responses targeting pre-erythrocytic stages may reduce the number of sporozoites that invade hepatocytes and/or impair progression through hepatic schizogony, thereby preventing the initiation of new Figure 2A blood-stage infections. Alternatively, immune responses targeting the erythrocytic stage of infection may inhibit parasite replication and slow or prevent parasite growth in the blood. A number of studies have attempted to correlate the levels of different immune responses and protection from malaria [2–8]. However, it has often proved difficult to find an association between a particular immune response and protection. Moreover, because exposure to repeated infection is thought to be necessary to induce age-associated resistance, this would likely also induce a variety of immune responses (be they protective or not), confounding the analysis. Thus, any observed association between immune response and clinical and parasitological outcome may not necessarily be causal.
One alternative to measuring immune responses and attempting to correlate these with protection is simply to observe how the characteristics of infection itself change with age. That is, if we can identify how the dynamics of infection change with age, we can potentially infer what immune mechanisms might have been responsible for these changes. A number of recent studies have used an approach of prior treatment to cure existing infections and subsequent close monitoring of cohorts living in malaria-endemic areas [9–15]. This allows observation and comparison of the dynamics of infection in individuals of different ages. Early studies of treatment and infection found no effect of levels of circumsporozoite antibodies in patients experiencing reinfection by 98 days posttreatment [16]. However, recent studies show an increasing delay in the timing of first infection with age, and a decrease in the prevalence and level of parasitemia with age. The central question is then whether the observed dynamics can be best explained by changes in liver-stage immunity (reducing the number of emergent blood-stage infections), or blood-stage immunity (slowing the growth of parasites in blood). Using a combination of statistical analysis and modeling of the changes in time-to-first-infection with age, we have recently demonstrated that the dynamics of infection could be best explained by a decrease in parasite multiplication rate with age [17].
In the present study, we aim to estimate parasite growth rate in blood using a number of different approaches to compare growth rates in individuals of different ages. Using a combination of microscopy and polymerase chain reaction (PCR) detection of parasites over time, we show a significant slowing of parasite growth rate with increasing age. We argue that the major age-associated changes in infection dynamics can be explained, at least in part, by changes in parasite multiplication rate with age.
METHODS AND MATERIALS
Field Study
The field study data were obtained from a cohort study of 201 patients in a holoendemic region of western Kenya, performed in 2003 [8]. Subjects were treated with Coartem, which is effective against blood-stage infection but does not affect liver-stage parasites [18]. After treatment, blood smears were monitored weekly for 11 weeks for the presence of P. falciparum parasites by light microscopy. Patients were removed from the study if they were found microscopy-positive by week 2 after treatment (due to presumed treatment failure), or if weekly samples were not collected after the second week after treatment, thus leaving 197 individuals for analysis. The cohort was divided into 4 age groups for analysis (1–4, 5–9, 10–14, >15 years old). The groups are referred in the study as C1, C2, C3 (children of increasing age), and A (adults), respectively. The results of the microscopy study [19] as well as an analysis of the dynamics of infection using the microscopy data [17] have been previously published.
The present study extends this work by the analysis of whole-blood samples by PCR to increase the sensitivity of detection of P. falciparum parasitemia. The nested PCR method that was used in analyzing the data is described in [20]. This analysis was performed post-hoc, and thus did not affect the inclusion criteria for the field study.
Estimating the Delay Between PCR and Microscopy Detection
In order to estimate the delay between PCR and microscopy detection, we tracked each patient longitudinally from the time of treatment and identified the first week when parasites were detected by PCR, and then the delay until parasites were detected by microscopy. If both were detected first in the same week, then the delay was zero. In the case where a patient became PCR positive but did not subsequently become microscopy positive before the end of the study period, we simply estimated the minimum delay from PCR to microscopy positive as being the time from PCR detection to the end of the study.
Estimating Growth Rate Using PCR and Microscopy Data
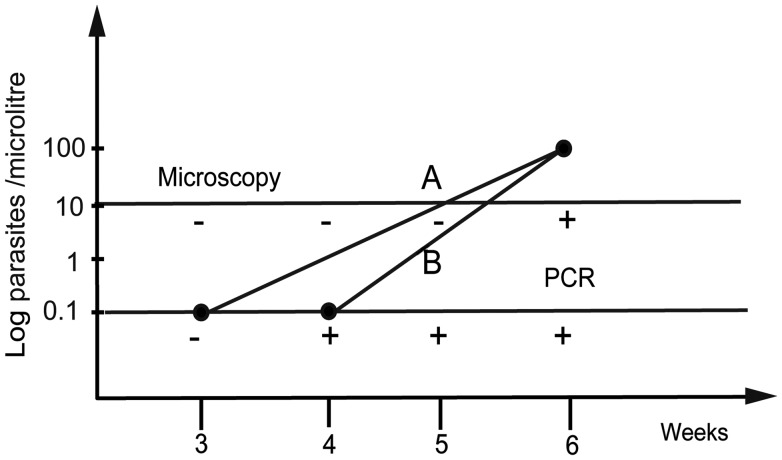
We also can assess the growth rate of parasites (expressed as the mean parasite multiplication rate [PMR]) in individuals of different ages using the time between PCR and microscopy detection for each study participant. Because the PCR measurement shows only the presence or absence of parasites above a threshold rather than the concentration of parasites, we cannot estimate the growth rate precisely. However, we can estimate the minimal growth rate, being the rate that would allow for growth from the last PCR-negative time point to the level of parasitemia at the first positive microscopy time point. Similarly, the maximal growth rate can be estimated from the first PCR positive to the first microscopy positive time point. The scheme of estimation of growth rate is shown in Figure 1. Using a geometric growth assumption, we can calculate the growth rate between 2 measurements as
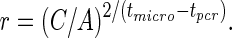 |
(1) |
Figure 1.
Method for estimated growth using PCR and microscopy measurements in each patient. Minimal and maximal growth rates (incline lines) are estimated from the last PCR negative (A) or first positive (B) week of PCR detection through to the observed level of parasitemia on the week of microscopy detection. Horizontal lines indicate microscopy and PCR detection thresholds. Minus or plus denotes negative or positive measurement, respectively. Abbreviation: PCR, polymerase chain reaction.
Formula (1) was derived from 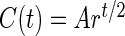 , where r is the growth rate (PMR); A is the concentration of parasites detected by PCR; C is the concentration of parasites at the microscopy detection point; t the time passed after the initiation of blood-stage infection; and tPCR and tmicro the time of detection by PCR and by microscopy, respectively.
, where r is the growth rate (PMR); A is the concentration of parasites detected by PCR; C is the concentration of parasites at the microscopy detection point; t the time passed after the initiation of blood-stage infection; and tPCR and tmicro the time of detection by PCR and by microscopy, respectively.
We note that the minimal PMRs calculated will tend to exclude patients with very low PMR who do not become microscopy positive before the end of the study. Therefore, for these patients, we estimate the maximal PMR given the data (ie, assuming the study participant was at the PCR-detection threshold on the first PCR-detection day, and at the microscopy-detection threshold at the last day of the study).
Modeling the Dynamics of Infection
The analysis of the infection curves from the field study data indicates a delay in the timing of infection between the youngest children and older children and adults in the study using microscopy detection. However, in the PCR detection data, the curves are much more similar (Figure 2). We have previously applied a mathematical modeling approach to “reverse engineer” the infection curves derived from the microscopy infection data [17]. In that study, we showed that the microscopy infection curves in the different age groups are consistent with the reduction in the PMR with age, leading to a delay in the time until the detection of infection, as well as a reduced peak of parasitemia. We could not obtain the observed infection dynamics by modeling liver-stage immunity alone. In the current paper, we extended mathematical models that were developed in [17] for both PCR and microscopy data to find if our previous conclusion is consistent with the more sensitive PCR detection method.
Figure 2.
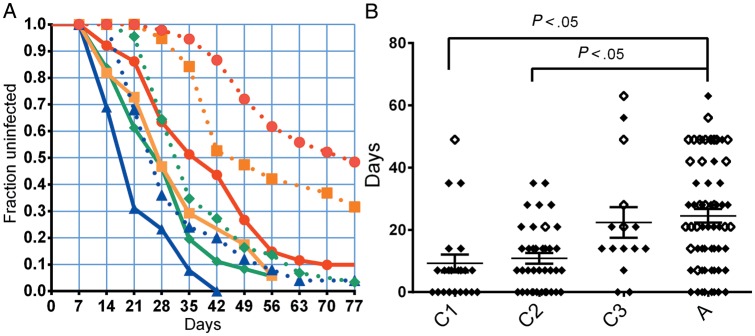
The dynamics of infection. A, The “survival curve,” indicating the fraction of uninfected patients for different age groups detected by microscopy (dashed lines), or by PCR (solid lines). Different colors indicate different age groups; C1 (1–4 years old), blue triangles and blue lines; C2 (5–9), green diamonds and green lines; C3 (10–14), orange squares and orange lines; A (>15), red circles and red lines. B, The delay between PCR and microscopy detection is indicated for each patient. Solid diamonds are patients where both PCR and microscopy measurements are present. Open diamonds are patients where only PCR detection occurs before the end of follow-up; in this case, the delay indicated is from PCR detection to the final follow-up time (week 11). Median growth rates and interquartile range are indicated. Horizontal bars above indicate groups that are significantly different (P < .05, Kruskal–Wallis test). Abbreviations: A, adults; C1, C2, C3, children of increasing age; PCR, polymerase chain reaction.
In this study, we first repeated our fitting of the infection curves for different age groups, changing only the average growth rate between groups. However, we fitted the model to the infection curves of both PCR and microscopy detection (detailed in Supplementary Text 1, Part 1). We also used alternative models to explore whether liver-stage immunity, or combinations of liver- and blood-stage immunity, may also explain the observed infection curves. The detailed descriptions of these models are in Supplementary Text 1, Parts 2, 3, and 4.
RESULTS
Detection of Infection by PCR Changes Observed Infection Dynamics
We and others have previously reported that the infection dynamics of the different age groups show an unusual pattern of infection when infection is defined by detection using microscopy [17]. That is, for the youngest age group, the infection curve conforms nicely to a simple exponential curve, where the rate of initiation of new blood-stage infection is around 0.5 per week. However, the curves in adults show a much more complex pattern, with an apparent delay until the first detection of infection by microscopy. The curves observed for PCR detection are quite different from that for microscopy. For all age groups, infection is first detected earlier by PCR, and looks much more like the simple exponential curves observed for microscopy among children (Figure 2A). This change in infection curves is consistent with altered parasite growth being the major difference between age groups; the lower threshold for parasite detection by PCR minimizes any delay caused by slower parasite growth that we previously observed in the microscopy data.
A number of previous studies have observed a delay in time-to-first-infection after treatment with age, and in this study, such an association was previously observed using microscopy data [14, 17]. Comparison of the rate of infection obtained by microscopy with that observed using PCR shows that a higher proportion of individuals were observed to be infected earlier after treatment using PCR, as might be expected from a more sensitive test. Figure 2A shows the microscopy and PCR infection curves for each age group over the 11 weeks of the study. The curves were estimated using the Kaplan–Meier method, which is suitable for the processing of censored data.
Increasing Delay From PCR Detection to Microscopy Detection With Increasing Age
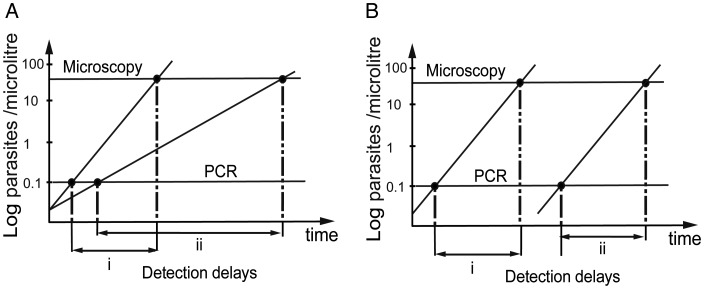
In order to determine the reason for the delay in detection of parasites by microscopy in the older cohorts, we studied the time taken to go from becoming PCR positive to become microscopy positive. That is, if delayed detection in adults is due to slower parasite growth, then we expect that there should be a greater delay between PCR detection and microscopy detection the slower the parasite growth (Figure 3A). However, if delayed detection of adults were due to a decrease in the rate of initiation of blood-stage infection, but parasites all grew at the same rate after infection, then we would expect no change in the delay between PCR detection and microscopy detection with age (Figure 3B). Consistent with slower growth of parasitemia with age, we saw a significantly greater delay between becoming PCR positive and microscopy positive in older individuals (Figure 2B). Children aged <5 years had a mean delay of 9.23 days. By contrast, adults (>15 years) showed a mean delay of 24.5 days, which was significantly slower (P < .05, Kruskal–Wallis with posttest).
Figure 3.
Schematic of the possible mechanisms for delay in detection of infection. If parasite growth is reduced by blood stage immunity (A), the delay between the PCR and microscopy detections will be shorter in individuals with high growth (i) compared to patients with low PMR (ii). If infection is delayed because liver-stage immunity blocks some infective bites (B), then the delay between the PCR and microscopy detection is the same in individuals with low immunity (i) and in individuals with stronger immunity (ii). Incline lines indicate schematic parasite growth curves. Horizontal lines indicate the PCR and microscopy detection thresholds. Abbreviations: PCR, polymerase chain reaction; PMR, mean parasite multiplication rate.
Slower Growth of P. falciparum With Age
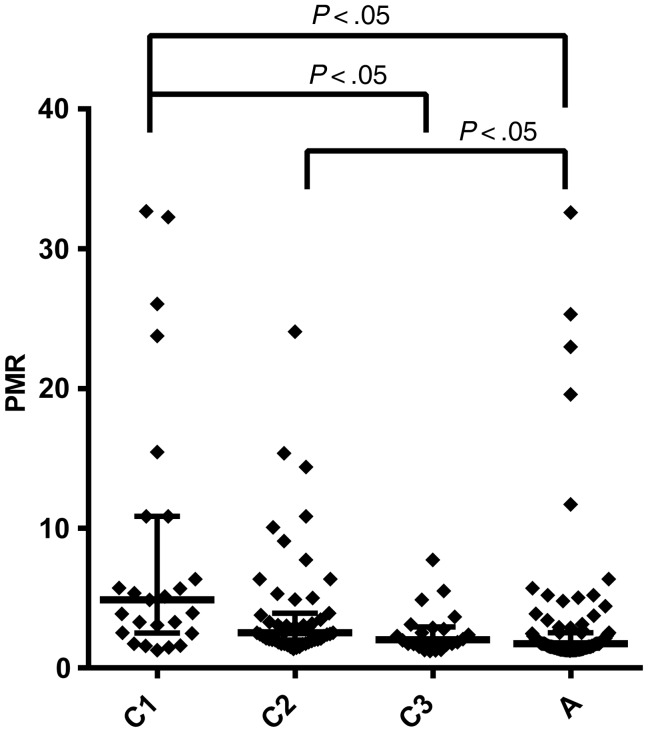
The delay between PCR detection and microscopy detection discussed above ignores the data we have on parasitemia levels by microscopy, and instead merely asks if the microscopy was positive or negative. However, it is also possible to directly estimate parasite growth from the timing of initial PCR detection (which is not quantitative) and the level of parasitemia when first observed by microscopy (which is quantitative). Our PCR detection approach gives only a “plus” or “minus” level—whether the parasitemia exceeded the threshold for detection or not. Moreover, the threshold for detection is also an estimate. Therefore, our measurement of PMR must frequently rely on estimating the minimal growth rate that could have produced the observed data. However, any inaccuracies in the threshold or issues with estimating growth rate should be minimized by using the same approach to estimate growth in adults and children. Analysis of PMR estimates from the different age groups using this approach again demonstrates significantly slower growth of parasites in adults compared with children (Figure 4).
Figure 4.
Reduced parasite growth rate with age. The estimated parasite growth rates for individuals in the different age groups are indicated. Median growth rates and interquartile range are indicated. Horizontal bars above indicate groups that are significantly different (P < .05, Kruskal–Wallis test). Abbreviations: A, adults; C1, C2, C3, children of increasing age; PMR, mean parasite multiplication rate.
Modeling Slower Parasite Growth and Delayed Time to Detection With Age
In our previous study, we analyzed the curves of “time to detection” of first microscopic detection of P. falciparum infection of the different age groups, and used a deterministic model of parasite growth to argue that the dynamics of infection are explained by a model of decreasing PMR with age, with a distribution of PMR within age groups [17]. After generating “survival curves” of time to first detection, we showed that a model of decreasing mean PMR can fit the field study microscopy data on time to infection extremely well, with only 1 parameter (mean PMR) changing between age groups. If this mechanism and modeling is correct, then we should be able to use the same model to simultaneously fit both the microscopy and PCR data on time to detection, using only 1 additional parameter: the threshold of detection by PCR.
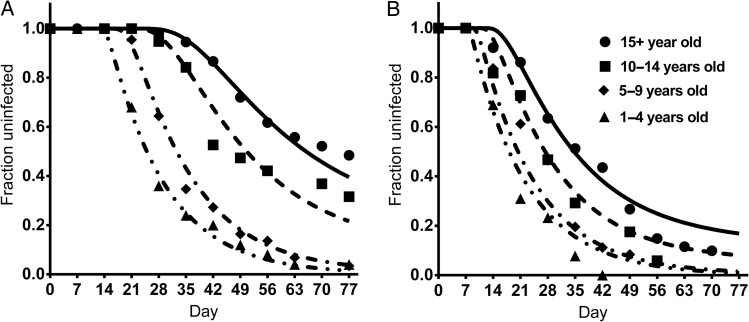
We used a step-wise approach of first fitting a model with the same PMR and rate of initiation on new infections for all age groups. This produced a poor fit to the data, as shown in Supplementary Text 1, Part 3. We then allowed the PMR to differ with age group, and also to have a distribution within age groups. In this case, we fitted 7 parameters. Figure 5 shows the simultaneous fitting of the microscopy and PCR-derived survival curves for the different ages, fitting the optimal PMR for each age group. This illustrates how a simple change in mean growth rates in different age groups can explain features such as the unusual sigmoid shape of the adult microscopy reinfection curve, as well as the shift to the left and change in shape observed with the PCR detection curve (Figure 5). The best-fit parameters are shown in Table 1.
Figure. 5.
Modeling reinfection simultaneouly fitting to PCR and microscopy curves. The “survival curves” until detection of P. falciparum infection by microscopy (left) or PCR (right) of the different age groups are indicated for both the experimental data (solid shapes) and the model fit (solid and dashed lines). The model shows that both the shapes and delay in the curves can be explained simply by reduced parasite growth rate with age. Abbreviation: PCR, polymerase chain reaction.
Table 1.
Thе Best Fit Parameters and the Goodness of Fit Statistic for the Blood-Stage Immunity Model
| Parameter | Estimate | Units | 95% CI |
|---|---|---|---|
| Mean growth rate (PMR) | |||
| 1–4 age group | 6.03 | Per cycle | (2.90, 9.17) |
| 5–9 age group | 2.90 | “ | (2.44, 3.35) |
| 10–14 age group | 1.73 | “ | (1.64, 1.83) |
| ≥15 age group | 1.48 | “ | (1.42, 1.53) |
| Rate of initiation of blood-stage infections | 0.069 | Blood inf./day | (.060, .079) |
| Standard deviation of growth rate (as proportion of mean) | 0.25 | (.204, .299) | |
| PCR detection threshold | 0.12 | Parasites/μL | (.070, .178) |
| Akaike information criterion | −197.7 | ||
Abbreviations: CI, confidence interval; inf., infection; PCR, polymerase chain reaction; PMR, mean parasite multiplication rate.
The estimated PMR differed significantly between age groups, varying from approximately 6.0 per day in the youngest age groups to approximately 1.5 per day in the adults. The estimated rate of initiation of new blood-stage infections was consistent with that estimated by fitting of an exponential to the survival curve of the youngest age group (0.48 bites per week). The estimated PCR detection threshold was 0.12 parasites per microliter, which conforms to estimates in the published data [21–28]. This lower detection threshold reduces the time from emergence of the liver stage until detection, and makes the overall infection curve much more exponential (Figure 5B). The figures of distributions of PMR and delays to detection are in Supplementary Text 1, Part 1.
We also fitted models assuming liver-stage immunity, or combinations of liver- and blood-stage immunity, and found that either liver-stage immunity alone, or combinations of liver- and blood-stage immunity, did not give a better fit to the reinfection data (Supplementary Text 1, Parts 2–4).
DISCUSSION
Older individuals in holoendemic malaria regions show reduced rates of clinical malaria and reduced prevalence and levels of parasitemia. The mechanisms of this age- and exposure-associated protection are unclear, but may provide significant insights to aid the development of vaccines and immune therapeutics. Here, we have analyzed the dynamics of infection of a cohort of individuals of different ages living in a holoendemic setting in western Kenya in order to assess the changes in parasite infection dynamics with age. We look at the rate of detectable infection that was observed using both microscopy detection of parasitemia (previously reported [17]) and PCR detection of parasitemia performed on the same samples. We observed that there was a significantly greater delay between PCR detection and microscopy detection in older children and adults than in younger children (age <10 years), consistent with slower parasite growth associated with increasing age and cumulative exposure to malaria. Direct estimates of the growth rate of parasites and modeling of the dynamics of reinfection confirm a reduction in parasite growth rate with age in this cohort. Our estimates of parasite multiplication rate are in keeping with previous estimates in the literature [29, 30], and significantly lower than estimates derived from studies of unexposed volunteers [22, 31]. This suggests that even in our youngest age group, there is already likely to be a significant reduction in parasite growth rate compared with unexposed individuals.
Our observations suggest that the rate of initiation of new blood-stage infections may not change significantly with age, because the reinfection curves are well explained by a model that uses the same rate of infection in each age group and that invokes only differences in parasite growth rate with age. This does not preclude some effects of liver-stage immunity, because it could be that adults receive more infected bites but more effectively reduce the number of those that progress through the liver stage, thus leading to the same observed rate of new blood-stage infections. However, the most parsimonious explanation seems to be that there was the same infection rate in different age groups. A more sensitive study, able to detect the first release of blood-stage parasites from the liver, would be ideal to resolve this issue. If our conclusion that growth rate differences are the major discriminator between age groups is true, then we should expect to see the infection curves in different age groups become overlapping as the sensitivity of detection is reduced.
One limitation of our study is that our sampling was limited to parasites present in peripheral blood. Thus, in principle, a higher level of parasite sequestration in adults could lead to an apparent reduction in peripheral parasitemia, and subsequently an underestimation of the growth rate. However, because sequestration is transient during the infection cycle and also more likely to affect younger individuals, this seems unlikely to play a major role in our observations. Studies of P. falciparum histidine-rich protein (PfHRP2) permit some estimation of the total body burden of parasites, avoiding the potential issue of measuring only parasites in peripheral blood with no estimate of those that sequestered in various vascular beds. However, we note that these estimates are themselves strongly affected by assumptions about the underlying parasite growth rate [32].
A number of studies have shown that either mosquito infection or injection with live or attenuated sporozoites is able to elicit sterilizing immunity from mosquito infection in vaccinated volunteers [33–35]. It is therefore somewhat surprising that we find no evidence for liver-stage immunity in this heavily exposed population. This difference of immunity may be explained by factors such as the low dose of sporozoites inoculated during natural infection, or the effects of subsequent blood-stage replication of the parasite on liver-stage immunity [36]. Understanding whether the lack of liver-stage immunity in the field is due to lack of priming, lack of memory, or lack of effector function (due to factors such as antigenic variation) is important to predicting the efficacy and longevity of liver-stage vaccines in the field.
The present study suggests that the reduction in parasite growth rate due to blood-stage immunity may be the major change occurring with age and exposure to infection, and that this may explain the relative protection from clinical disease observed in adults. This suggests, in turn, that vaccines aimed at blood-stage infection may be able to achieve similar levels of protection by reducing parasite growth rates even if they are unable to induce sterile immunity that would prevent blood-stage infections. This does not preclude a role for liver-stage-targeted vaccines, as it may be that vaccination can achieve a level of immunity not seen in natural exposure. However, our studies of natural infection do suggest that blood-stage immunity is achievable and is a major determinant of the different infection dynamics seen in different age groups. A major goal of future work should be to identify the molecular and cellular mechanisms of such immunity and to include reductions in parasite growth rate as a secondary outcome in malaria vaccine trials.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the parents and guardians for enrolling their children in this study and our team of community health workers without whom this study would not have been possible. We also would like to acknowledge Jennifer Stoia Vetter, who performed all the DNA extractions and PCR assays. This work is published with the permission of the Director of the Kenya Medical Research Institute.
Financial support. This work is supported by the National Health and Medical Research Council (NHMRC, Australia) (#630542), the Australian Research Council (DP120100064), and National Institutes of Health (NIH, USA), National Institute of Allergy and Infectious Diseases (NIAID), R01 AI043906 (J. K. and A. M. M.), and Fogarty International Center (FIC) 1D43TW006576 (K. C.). M. P. D. is an NHMRC Senior Research Fellow. This study is published with permission from the Director, Kenya Medial Research Institute.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Murray CJL, Rosenfeld LC, Lim SS, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–31. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 2.Sarr J, Remoue F, Samb B, et al. Evaluation of antibody response to Plasmodium falciparum in children according to exposure of Anopheles gambiae s.l or Anopheles funestus vectors. Malar J. 2007;6:117. doi: 10.1186/1475-2875-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray JC, Corran PH, Mangia E, et al. Profiling the antibody immune response against blood stage malaria vaccine candidates. Clin Chem. 2007;53:1244–53. doi: 10.1373/clinchem.2006.081695. [DOI] [PubMed] [Google Scholar]
- 4.Yone CLRP, Kremsner PG, Luty AJF. Immunoglobulin G isotype responses to erythrocyte surface-expressed variant antigens of Plasmodium falciparum predict protection from malaria in African children. Infect Immun. 2005;73:2281–7. doi: 10.1128/IAI.73.4.2281-2287.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tongren JE, Drakeley CJ, McDonald SLR, et al. Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect Immun. 2005;74:257–64. doi: 10.1128/IAI.74.1.257-264.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCallum FJ, Persson KEM, Mugyenyi CK, et al. Acquisition of growth-inhibitory antibodies against blood-stage Plasmodium falciparum. PLOS ONE. 2008;3:e3571. doi: 10.1371/journal.pone.0003571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roussilhon C, Oeuvray C, Muller-Graf C, et al. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLOS Med. 2007;4:e320. doi: 10.1371/journal.pmed.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moormann AM, Sumba PO, Chelimo K, et al. Humoral and cellular immunity to Plasmodium falciparum merozoite surface protein 1 and protection from infection with blood-stage parasites. J Infect Dis. 2013;208:149–58. doi: 10.1093/infdis/jit134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beadle C, McElroy PD, Oster CN, et al. Impact of transmission intensity and age on Plasmodium falciparum density and associated fever: implications for malaria vaccine trial design. J Infect Dis. 1995;172:1047–54. doi: 10.1093/infdis/172.4.1047. [DOI] [PubMed] [Google Scholar]
- 10.John CC, Moormann AM, Pregibon DC, et al. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am J Trop Med Hyg. 2005;73:222–8. [PubMed] [Google Scholar]
- 11.Okech BA, Corran PH, Todd J, et al. Fine specificity of serum antibodies to Plasmodium falciparum merozoite surface protein, PfMSP-1(19), predicts protection from malaria infection and high-density parasitemia. Infect Immun. 2004;72:1557–67. doi: 10.1128/IAI.72.3.1557-1567.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tall A, Sokhna C, Perraut R, et al. Assessment of the relative success of sporozoite inoculations in individuals exposed to moderate seasonal transmission. Malar J. 2009;8:161. doi: 10.1186/1475-2875-8-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beier JC, Oster CN, Onyango FK, et al. Plasmodium falciparum incidence relative to entomologic inoculation rates at a site proposed for testing malaria vaccines in western Kenya. Am J Trop Med Hyg. 1994;50:529–36. doi: 10.4269/ajtmh.1994.50.529. [DOI] [PubMed] [Google Scholar]
- 14.Sokhna CS, Rogier C, Dieye A, Trape JF. Host factors affecting the delay of reappearance of Plasmodium falciparum after radical treatment among a semi-immune population exposed to intense perennial transmission. Am J Trop Med Hyg. 2000;62:266–70. doi: 10.4269/ajtmh.2000.62.266. [DOI] [PubMed] [Google Scholar]
- 15.Sokhna CS, Faye FBK, Spiegel A, Dieng H, Trape JF. Rapid reappearance of Plasmodium falciparum after drug treatment among Senegalese adults exposed to moderate seasonal transmission. Am J Trop Med Hyg. 2001;65:167–70. doi: 10.4269/ajtmh.2001.65.167. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman SL, Oster CN, Plowe CV, et al. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science. 1987;237:639–42. doi: 10.1126/science.3299709. [DOI] [PubMed] [Google Scholar]
- 17.Pinkevych M, Petravic J, Chelimo K, Kazura JW, Moormann AM, Davenport MP. The dynamics of naturally acquired immunity to Plasmodium falciparum infection. PLOS Comput Biol. 2012;8:e1002729. doi: 10.1371/journal.pcbi.1002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golenser J, Waknine JH, Krugliak M, Hunt NH, Grau GE. Current perspectives on the mechanism of action of artemisinins. Int J Parasitol. 2006;36:1427–41. doi: 10.1016/j.ijpara.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Dent AE, Bergmann-Leitner ES, Wilson DW, et al. Antibody-mediated growth inhibition of Plasmodium falciparum: relationship to age and protection from parasitemia in Kenyan children and adults. PLOS ONE. 2008;3:e3557. doi: 10.1371/journal.pone.0003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ofulla AV, Moormann AM, Embury PE, Kazura JW, Sumba PO, John CC. Age-related differences in the detection of Plasmodium falciparum infection by PCR and microscopy, in an area of Kenya with holo-endemic malaria. Ann Trop Med Parasitol. 2005;99:431–5. doi: 10.1179/136485905X36316. [DOI] [PubMed] [Google Scholar]
- 21.Coleman RE, Sattabongkot J, Promstaporm S, et al. Comparison of PCR and microscopy for the detection of asymptomatic malaria in a Plasmodium falciparum/vivax endemic area in Thailand. Malar J. 2006;5:121. doi: 10.1186/1475-2875-5-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bejon P, Andrews L, Andersen RF, et al. Calculation of liver-to-blood inocula, parasite growth rates, and preerythrocytic vaccine efficacy, from serial quantitative polymerase chain reaction studies of volunteers challenged with malaria sporozoites. J Infect Dis. 2005;191:619–26. doi: 10.1086/427243. [DOI] [PubMed] [Google Scholar]
- 23.Witney AA, Doolan DL, Anthony RM, Weiss WR, Hoffman SL, Carucci DJ. Determining liver stage parasite burden by real time quantitative PCR as a method for evaluating pre-erythrocytic malaria vaccine efficacy. Mol Biochem Parasitol. 2001;118:233–45. doi: 10.1016/s0166-6851(01)00372-3. [DOI] [PubMed] [Google Scholar]
- 24.Veron V, Simon S, Carme B. Multiplex real-time PCR detection of P. falciparum, P. vivax and P. malariae in human blood samples. Exp Parasitol. 2009;121:346–51. doi: 10.1016/j.exppara.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Rider MA, Byrd BD, Keating J, Wesson DM, Caillouet KA. PCR detection of malaria parasites in desiccated Anopheles mosquitoes is uninhibited by storage time and temperature. Malar J. 2012;11:193. doi: 10.1186/1475-2875-11-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adam A, Witney RMA, Daniel J. Carucci. Quantitation of Liver-Stage Parasites by Automated TaqMan® Real-Time PCR. In: Doolan DL, editor. Malaria Methods and Protocols. Totowa, New Jersey 07512: Humana Press Inc.; 2002. pp. 137–40. [DOI] [PubMed] [Google Scholar]
- 27.Andrews L, Andersen RF, Webster D, et al. Quantitative real-time polymerase chain reaction for malaria diagnosis and its use in malaria vaccine clinical trials. Am J Trop Med Hyg. 2005;73:191–8. [PubMed] [Google Scholar]
- 28.Taylor BJ, Martin KA, Arango E, Agudelo OM, Maestre A, Yanow SK. Real-time PCR detection of Plasmodium directly from whole blood and filter paper samples. Malar J. 2011;10:244. doi: 10.1186/1475-2875-10-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deans AM, Lyke KE, Thera MA, et al. Low multiplication rates of African Plasmodium falciparum isolates and lack of association of multiplication rate and red blood cell selectivity with malaria virulence. Am J Trop Med Hyg. 2006;74:554–63. [PMC free article] [PubMed] [Google Scholar]
- 30.Douglas AD, Andrews L, Draper SJ, et al. Substantially reduced pre-patent parasite multiplication rates are associated with naturally acquired immunity to Plasmodium falciparum. J Infect Dis. 2011;203:1337–40. doi: 10.1093/infdis/jir033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson JA, Aarons L, Collins WE, Jeffery GM, White NJ. Population dynamics of untreated Plasmodium falciparum malaria within the adult human host during the expansion phase of the infection. Parasitology. 2002;124:247–63. doi: 10.1017/s0031182001001202. [DOI] [PubMed] [Google Scholar]
- 32.Dondorp AM, Desakorn V, Pongtavornpinyo W, et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLOS Med. 2005;2:e204. doi: 10.1371/journal.pmed.0020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman SL, Goh LM, Luke TC, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–64. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 34.Bijker EM, Bastiaens GJ, Teirlinck AC, et al. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci USA. 2013;110:7862–7. doi: 10.1073/pnas.1220360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seder RA, Chang LJ, Enama ME, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341:1359–65. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 36.Ocana-Morgner C, Mota MM, Rodriguez A. Malaria blood stage suppression of liver stage immunity by dendritic cells. J Exp Med. 2003;197:143–51. doi: 10.1084/jem.20021072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.