Abstract
Human immunodeficiency virus (HIV)–1 and Mycobacterium tuberculosis (M. tuberculosis) both target macrophages, which are key cells in inflammatory responses and their resolution. Therefore, we tested the hypothesis that HIV-1 may modulate macrophage responses to coinfection with M. tuberculosis. HIV-1 caused exaggerated proinflammatory responses to M. tuberculosis that supported enhanced virus replication, and were associated with deficient stimulus-specific induction of anti-inflammatory interleukin (IL)–10 and attenuation of mitogen-activated kinase signaling downstream of Toll-like receptor 2 and dectin-1 stimulation. Our in vitro data were mirrored by lower IL-10 and higher proinflammatory IL-1β in airway samples from HIV-1–infected patients with pulmonary tuberculosis compared with those with non-tuberculous respiratory tract infections. Single-round infection of macrophages with HIV-1 was sufficient to attenuate IL-10 responses, and antiretroviral treatment of replicative virus did not affect this phenotype. We propose that deficient homeostatic IL-10 responses may contribute to the immunopathogenesis of active tuberculosis and propagation of virus infection in HIV-1/M. tuberculosis coinfection.
Keywords: HIV-1, inflammation, interleukin-10, macrophage, tuberculosis
The coincident global distribution of Mycobacterium tuberculosis (M. tuberculosis) and human immunodeficiency virus (HIV)–1 pandemics has generated high rates of M. tuberculosis/HIV-1 coinfection, associated with up to 40-fold greater risk of active tuberculosis and with increased HIV-1 replication [1, 2]. Both of these pathogens have successfully established ecological niches within macrophages. HIV-1 evades innate immune detection by macrophages to establish a foothold in the host from which the virus can efficiently spread to T cells and may contribute to the pathogenesis of AIDS [3, 4]. M. tuberculosis subverts intracellular killing mechanisms to survive and grow within macrophages [5], and to drive inflammatory responses that contribute to tissue destruction in the pathogenesis of active tuberculosis [6, 7].
Macrophages are tissue-resident cells that generate potent inflammatory responses to innate immune stimulation, and regulate anti-inflammatory homeostatic responses to maintain tissue integrity and function [8]. They are the predominant sentinel immune cells within the respiratory tract, which is the principal site of active tuberculosis and the route for acquisition and transmission of M. tuberculosis. Alveolar macrophages are permissive to HIV-1 infection in vitro [9, 10], and HIV-1 can be detected in bronchoalveolar lavage samples and in alveolar macrophages obtained from HIV-1–infected patients [11–13]. These suggest that HIV/M. tuberculosis coinfection of macrophages may take place in vivo. This subject has therefore attracted extensive research interest [14–18], although many questions remain unresolved.
Productive HIV-1 infection of macrophages causes negligible changes to the host cell transcriptome and exerts no cytopathic effect [3]. We have previously shown that HIV-1–infected macrophages exhibit attenuation of classical nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) innate immune signaling pathways in response to specific Toll-like receptor (TLR)4 or TLR2 stimulation but only modest attenuation of downstream transcriptional responses [19]. Herein, we extend the study of this model to test the hypothesis that HIV-1 infection of macrophages modulates host responses to coinfection with M. tuberculosis in such a way that may contribute to the pathogenesis of tuberculosis in HIV-1–infected patients.
MATERIALS AND METHODS
Monocyte-Derived Macrophages
Blood samples were obtained from healthy volunteers or single-donor buffy coats (National Blood Transfusion Service) for production of monocyte-derived macrophages (MDMs) as described previously [19, 20] and in supplementary methods. The study was approved by the University College London Research Ethics Committee, and written informed consent was obtained from participants.
HIV-1 Strains and Cell Culture Infections
Macrophage-tropic HIV-1 strains Ba-L and Yu2 were used to establish uniformly infected MDMs as previously described [3]. Single-round vesicular stomatitis virus G glycoprotein (VSV-G)–pseudotyped HIV-1 was derived from the R9 Ba-L molecular clone [21] by truncating env, and used for cotransfection of producer cell lines with a plasmid-encoding VSV-G envelope. VSV-G–psuedotyped HIV-1Δ env was cotransduced with virus-like particles containing the simian immunodeficiency virus (SIV) accessory protein, Vpx, to increase macrophage permissivity in a single-round infection as previously described [22]. For protease inhibitor experiments, 10 µM indinavir sulphate (Centre for AIDS Reagents, NIBSC) was added to HIV-1–infected macrophages for 3 days before stimulation.
Detection of Extracellular and Intracellular HIV-1 p24
Cell-free HIV-1 p24 concentrations were quantified by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instructions (kit v9.2, AIDS and Cancer Virus Program, National Cancer Institute, Frederick, MD). Intracellular p24 staining was performed as previously described [19].
M. tuberculosis and Streptococcus pneumoniae Culture
M. tuberculosis H37Rv was cultured in Middlebrook 7H9 medium (BD Bioscience) with 10% albumin/dextrose/catalase enrichment medium, 0.2% glycerol and 0.02% Tween 80, and used at mid-log growth (optical density [OD]600 nm 0.6), representing 108 colony forming units (CFU)/mL. M. tuberculosis culture filtrate was generated by centrifugation of M. tuberculosis at 13 000 rpm for 5 minutes followed by filtration through a 0.2-µm filter (Whatman). The S. pneumoniae strain, TIGR4, was cultured in Todd-Hewitt broth with 0.5% yeast extract to OD600 nm0.4 (approximately 108 CFU/mL) and stored at −80°C in 10% glycerol as single-use aliquots.
Stimulation of MDM
Zymosan, lipopolysaccharide (LPS), synthetic diacylated lipopeptide Pam2CSK4, and curdlan were purchased from Invivogen. MDM were stimulated for 4–72 hours with M. tuberculosis (H37Rv) at a multiplicity of infection (MOI) of 1, M. tuberculosis culture filtrate for 4–24 hours or S. pneumoniae (TIGR4) for 4 hours at an MOI of 10. Chemical inhibition of intracellular signaling pathways was performed by preincubation of MDM with inhibitors (10 µM) for 2 hours. The pyridinyl imidazole inhibitor SB203580 was used to inhibit p38 mitogen-activated protein kinase (MAPK). A nonselective mitogen-activated protein kinase kinase (MEK) inhibitor (PD98059) or selective MEK1 inhibitor (U0126) was used to inhibit extracellular-signal-regulated kinases 1/2 (ERK1/2) signaling. The protein tyrosine kinase inhibitor [(3,5-Di-tert-butyl-4-hydroxybenzylidene)-malononitrile] (AG17) was used to inhibit activation of Pyk2 (all from Calbiochem).
Transcriptional Profiling by cDNA Microarray
Total RNA was purified from MDM lysates collected in RLT buffer (Qiagen) or TRIzol (Invitrogen) using the RNeasy Mini kit (Qiagen), and processed for Agilent microarrays as previously described [23]. Principal component analysis was used to compare global gene expression profiles as previously described [24] and paired t tests with Welch approximation and >2 fold-change filter were used to identify significant gene expression differences (P < .05) between samples using the MultiExperiment Viewer v4.6.0 application [25]. DAVID functional annotation clustering (http://david.abcc.ncifcrf.gov) was used to annotate gene lists of interest by gene ontology associations. Microarray data are available in the ArrayExpress database (www.ebi.ac.uk/arrayexpress) under accession number E-TABM-1163.
Quantitative Polymerase Chain Reaction Detection of Gene Transcription
First-strand cDNA was synthesized using the qScript cDNA Supermix kit (Quanta BioSciences) and quantitative polymerase chain reaction reaction (qPCR) of selected genes was performed using TaqMan inventoried assays (Supplementary Table 1) (Applied Biosystems) according to the manufacturer's instructions. Expression levels of target genes were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [3] or hypoxanthine phosphoribosyltransferase 1 (HPRT1) (Supplementary Table 1).
Cytokine and Matrix Metalloproteinase Measurements
MDM culture supernatant cytokine and matrix metalloproteinase (MMP) concentrations were quantified by ELISA (eBioscience) or by Luminex array (R&D Systems) using Luminex beads (Biorad) according to the manufacturer's instructions.
Western Immunoblotting Analysis of HIV-1 gag Expression and Innate Immune Signaling
Cell lysates from MDM cultures were collected in sodium dodecyl sulfate sample buffer containing protease and phosphatase inhibitor cocktails (Sigma-Aldrich) for polyacrylamide gel electrophoresis and immunoblotting as previously described [19]. Primary antibodies used were mouse anti-HIV-1 gag (p24) (E365/366, NIBSC), rabbit antiphosphorylated and antitotal p38 MAPK, rabbit antiphosphorylated and antitotal ERK1/2, rabbit antiphosphorylated Pyk2 (all from Cell Signaling Technology) and mouse antiactin (Abcam).
Induced Sputum and Bronchoalveolar Lavage Fluid Analysis
Induced sputum or bronchoalveolar lavage (BAL) was obtained from HIV-1–positive patients with tuberculosis (n = 18) or other respiratory infections (4 Pneumocystis jirovecii pneumonia and 11 other lower-respiratory-tract infections) (n = 15), median blood CD4 lymphocyte count 259 cells/µL (range, 11–705) and HIV plasma load 4.56 log10 copies/mL (range, 1.70–6.00). Samples were processed as described previously [26, 27] and in Supplementary Methods, and analyzed by Luminex array according to the manufacturer's instructions. Data were normalized to total protein concentrations in each sample. The study was approved by the Royal Free Hospital Ethics committee and written informed consent was obtained from all participants.
Statistical analysis
Parametric data were analyzed by t test or ANOVA, and nonparametric data were analyzed by the Mann–Whitney U test or Spearman's rank correlation, as indicated.
RESULTS
HIV-1 Infection of Macrophages Augments Proinflammatory Responses to M. tuberculosis
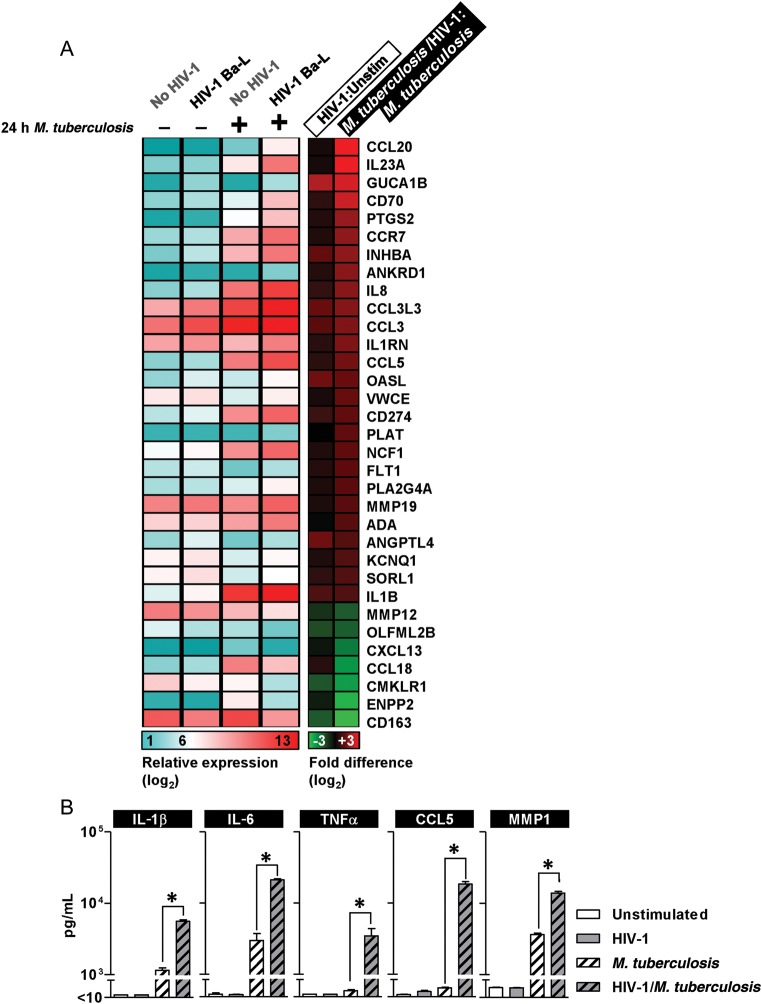
We first studied the effect of HIV-1 infection of MDMs on transcriptional responses to 24 hours coinfection with M. tuberculosis. Gene expression differences in HIV-1–infected and uninfected MDM following M. tuberculosis stimulation were subjected to functional annotation clustering by gene ontology associations and found to be significantly enriched for genes involved in immune and inflammatory responses and for genes related to cytokine and chemokine activity (Table 1). Comparison of relative expression levels for this gene list, in HIV-1–infected and uninfected MDM cultures before and after 24 hour coinfection with M. tuberculosis, showed that many proinflammatory responses to M. tuberculosis were augmented in HIV-1 coinfected cells (Figure 1A). HIV-1 infection alone did not significantly influence their expression. Enhanced expression of IL-23 in response to M. tuberculosis in HIV-1–infected cells was confirmed by qPCR and correlated with protein secretion in cell culture supernatants. These findings were replicated using an alternative HIV-1 strain (Yu2) to confirm that the effect was not virus-strain specific (Supplementary Figure 1A and 1B). Enhanced expression of proinflammatory mediators in HIV-1–infected cells compared with uninfected MDM was still evident at 72 hours after infection of MDM cultures with M. tuberculosis (Figure 1B). We have previously shown that M. tuberculosis also stimulates production of MMP1, implicated in the pathogenesis of tissue destruction in tuberculosis [7]. Like the other proinflammatory molecules assessed here, greater MMP1 production was evident in HIV-1/M. tuberculosis–coinfected macrophages (Figure 1B).
Table 1.
Functional Annotation Clustering Analysis by Gene Ontology Classificationa of Significant Gene Expression Differences Identified by Transcriptional Profiling of HIV-1–Infected and Control MDM Stimulated With M. tuberculosis for 24 hours (in 3 Separate Experiments), Showing the Top 10 Gene Ontology Terms
| Term | % of Gene List | P Value | Fold Enrichment |
|---|---|---|---|
| GO:0006955∼immune response | 32.7 | 3.8 × 10−11 | 7.5 |
| GO:0005125∼cytokine activity | 21.8 | 4.3 × 10−11 | 17.4 |
| GO:0042330∼taxis | 20.0 | 1.1 × 10−10 | 19.8 |
| GO:0006935∼chemotaxis | 20.0 | 1.1 × 10−10 | 19.8 |
| GO:0005615∼extracellular space | 30.9 | 1.2 × 10−09 | 6.6 |
| GO:0044421∼extracellular region part | 34.5 | 2.8 × 10−09 | 5.3 |
| GO:0006954∼inflammatory response | 21.8 | 8.2 × 10−09 | 10.6 |
| GO:0006952∼defense response | 27.3 | 8.7 × 10−09 | 7.0 |
| GO:0008009∼chemokine activity | 12.7 | 1.0 × 10−08 | 42.9 |
| GO:0042379∼chemokine receptor binding | 12.7 | 1.5 × 10−08 | 40.3 |
Abbreviations: GO, gene oncology; HIV, human immunodeficiency virus; MDM, monocyte-derived macrophages; Mtb, M. tuberculosis.
Figure 1.
Augmented proinflammatory responses to M. tuberculosis in HIV-1–infected macrophages. A, Mean gene expression and fold difference matrices are shown for the most highly enriched gene ontology cluster (Table 1) with differential gene expression in HIV-1–infected and uninfected MDMs after stimulation with M. tuberculosis for 24 hours in 3 independent experiments. B, Luminex analysis of HIV-1–infected MDM culture supernatants also showed significantly higher levels of proinflammatory cytokines and chemokines as well as MMP1 after 72-hour stimulation with M. tuberculosis, compared with HIV-1–uninfected cells. Bars represent mean ± SEM for at least 3 separate experiments (*denotes P < .01, t test). Abbreviations: CCL5, Chemokine (C-C motif) ligand 5; HIV, human immunodeficiency virus; IL, interleukin; MDMs, monocyte-derived macrophages; MMP1, matrix metalloproteinase 1; M. tuberculosis, Mycobacterium tuberculosis; SEM, standard error of measurement; TNFα, tumor necrosis factor α.
HIV-1 Infection of Macrophages is Associated With Diminished IL-10 Responses to M. tuberculosis
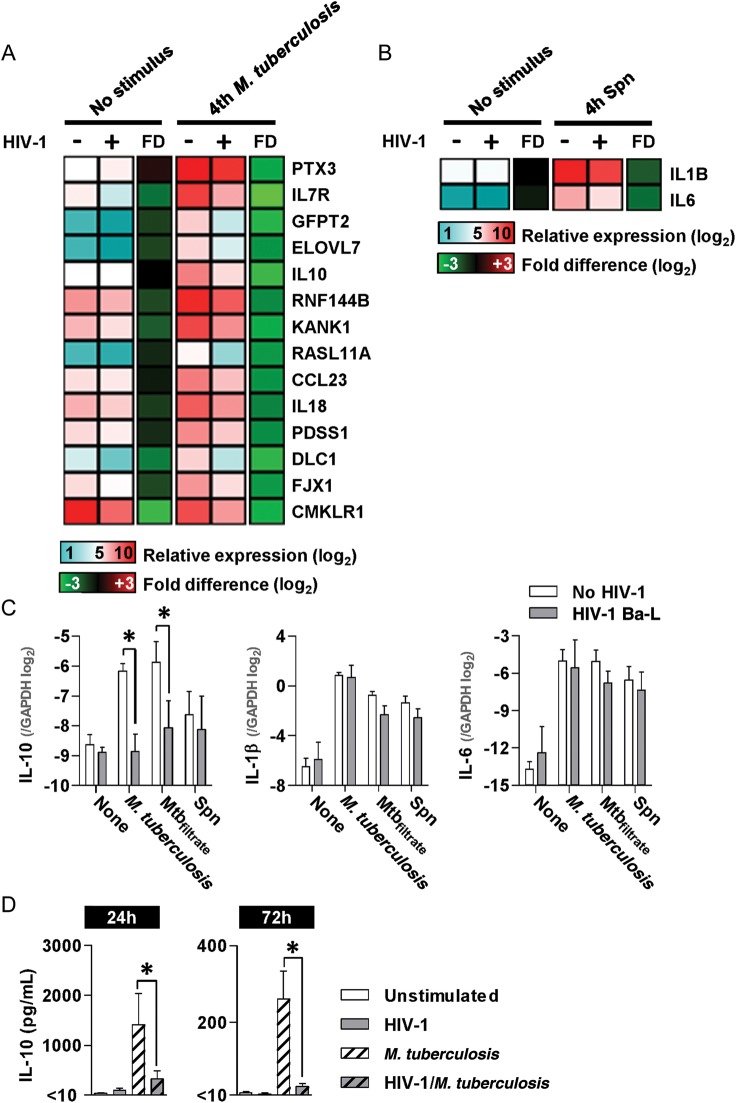
To explore the mechanism leading to enhanced proinflammatory responses to M. tuberculosis in HIV-1–coinfected macrophages, we investigated the effect of HIV-1 infection of MDM on the primary transcriptional response to M. tuberculosis by genome-wide expression arrays, 4 hours after coinfection. Principal component (PC) analysis to visualize changes to gene expression profiles showed similar gene expression profiles in HIV-1–infected and uninfected MDM cultures before and after coinfection with M. tuberculosis, suggesting that the primary transcriptional response to M. tuberculosis was broadly unaffected by HIV-1 (Supplementary Figure 1C). However, paired t tests identified 14 genes whose response to M. tuberculosis at 4 hours was significantly attenuated in HIV-1–infected MDM (Figure 2A). Because HIV-1–infected MDM generate augmented proinflammatory responses to M. tuberculosis at later time points, significant attenuation of IL-10 induction in the early response to M. tuberculosis was of particular interest, given its role in homeostatic regulation of inflammatory responses [28].
Figure 2.
Deficient IL-10 responses to M. tuberculosis in HIV-1 infection. A and B, Heat maps show mean gene expression and mean FD in HIV-1 infected and uninfected MDM cultures following 4-hour stimulation with either M. tuberculosis or Spn in at least 3 separate experiments. Gene-by-gene analysis showed significantly attenuated responses (>2-fold and P < .05, t test) in HIV-1–infected MDM for 14 genes following M. tuberculosis stimulation (A) and 2 genes following Spn stimulation (B). C, Inhibition of IL-10 responses to 4-hour stimulation with M. tuberculosis , and cell-free filtrate from M. tuberculosis cultures (Mtbfiltrate) in HIV-1–infected MDMs, compared with HIV-1–uninfected cells, is confirmed by qPCR. HIV-1 infection did not affect IL-10 responses to Spn or proinflammatory cytokine (IL-1β and IL-6) responses to any stimulus at 4 hours. D, Deficient IL-10 production in HIV-1–infected cells was also evident in cell culture supernatants (quantified by Luminex array) after 24 and 72 hours’ stimulation with M. tuberculosis. Bars represent mean ± SEM for at least 3 separate experiments (*denotes P < .01, t test). Abbreviations: FD, fold differences; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HIV, human immunodeficiency virus; IL, interleukin; MDMs, monocyte-derived macrophages; MMP1, matrix metalloproteinase 1; M. tuberculosis, Mycobacterium tuberculosis; qPCR, quantitative polymerase chain reaction; SEM, standard error of measurement; Spn, S. pneumoniae.
To assess whether early downregulation of macrophage IL-10 was specific to HIV-1/M. tuberculosis coinfection, we made similar comparisons in HIV-1–infected and control MDM cultures after 4 hour stimulation with live S. pneumoniae. The primary transcriptional response to S. pneumoniae generated differences in PC1 only and was comparable in HIV-1–infected and uninfected MDM (Supplementary Figure 1D). Direct comparison of these gene expression profiles revealed statistically significant, albeit modest attenuation, in 2 genes only, IL-1β and IL-6 (Figure 2B). These findings suggest that the effects of HIV-1 infection of MDM on innate immune transcriptional responses to coinfecting pathogens may be stimulus specific.
We validated our array findings by qPCR (Figure 2C), confirming attenuation of IL-10 responses to M. tuberculosis in HIV-1 coinfected MDM cultures, and that the same effect was not evident in HIV-1/S. pneumoniae coinfection. Proinflammatory transcriptional responses to M. tuberculosis, represented by IL-1β and IL-6, were not affected by HIV-1 infection at this time point (Figure 2C).
Innate immune induction of IL-10 by M. tuberculosis stimulation of macrophages is well documented [29–31]. The cell-free filtrate of M. tuberculosis cultures also induced increased IL-10 expression in MDM, which was attenuated in HIV-1–infected cells, suggesting that this effect was independent of live bacterial infection (Figure 2C). Furthermore, IL-10 secretion remained significantly diminished at 24 and 72 hours after stimulation with M. tuberculosis in HIV-1–infected cells (Figure 2D), in contrast to increased production of proinflammatory mediators at these time points (Figure 1B). In order to confirm the anti-inflammatory effects of IL-10 in our model, we showed that the addition of exogenous IL-10 to MDM inhibited selected proinflammatory responses to M. tuberculosis, either at the transcriptional level (measured by qPCR at 24 hours) or the protein level (measured by ELISA at 72 hours) (Supplementary Figure 1E and 1F).
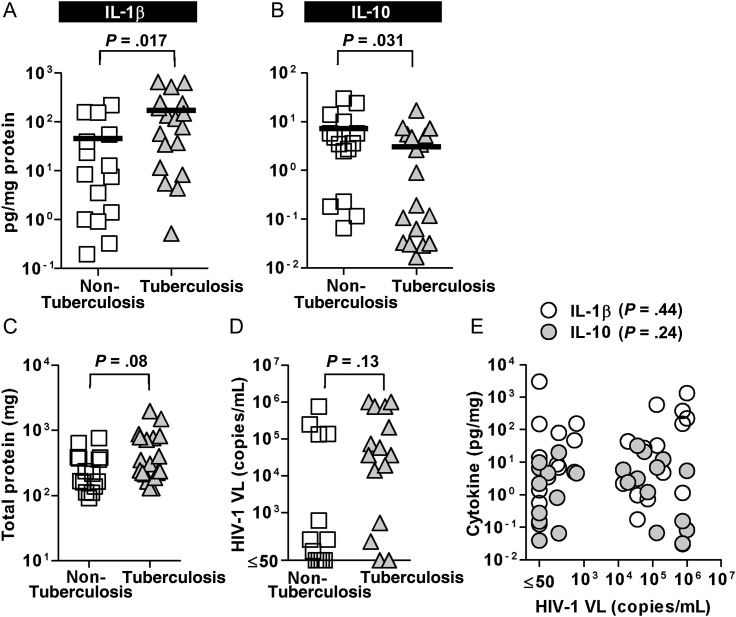
The in vitro experiments detailed above suggested that HIV/M. tuberculosis coinfection in vivo might be associated with diminished IL-10 levels and consequently, enhanced inflammatory cytokine responses. To evaluate whether similar findings may occur in vivo, we measured IL-1β, as a prototypic proinflammatory marker, and IL-10 in induced sputum and BAL samples from HIV-infected patients with tuberculosis or other lower-respiratory-tract infections as previously described [26, 27]. In keeping with our in vitro data, we found that in HIV-infected patients with intercurrent respiratory-tract infections, pulmonary tuberculosis was associated with significantly higher IL-1β levels (Figure 3A) and lower IL-10 levels (Figure 3B). There were no significant differences in total protein concentration in respiratory samples or plasma HIV-1 viral load between tuberculosis and nontuberculosis groups and no correlation between cytokine measurements in respiratory samples and plasma HIV-1 viral load (Figure 3C–E).
Figure 3.
Lower IL-10 and higher IL-1β levels are evident in respiratory samples from HIV-1–infected patients with pulmonary tuberculosis, compared with nontuberculous infections. Induced sputum and BAL fluid samples from HIV-1–infected patients with tuberculosis (n = 18) showed significantly greater IL-1β (A) and lower IL-10 levels (B) (measured by Luminex and normalized to total protein concentration) compared with samples from patients with nontuberculosis respiratory-tract infections (n = 15) (Mann–Whitney U test). Total protein in respiratory samples (C) and plasma HIV-1 viral load were similar (D) in both study groups and there was no significant correlation (Spearman's rank test) between plasma viral load and IL-1β or IL-10 concentrations in respiratory samples (E). Data points indicate individual measurements, and lines indicate the median values. Abbreviations: BAL, bronchoalveolar lavage; HIV, human immunodeficiency virus; IL, interleukin; VL, viral load.
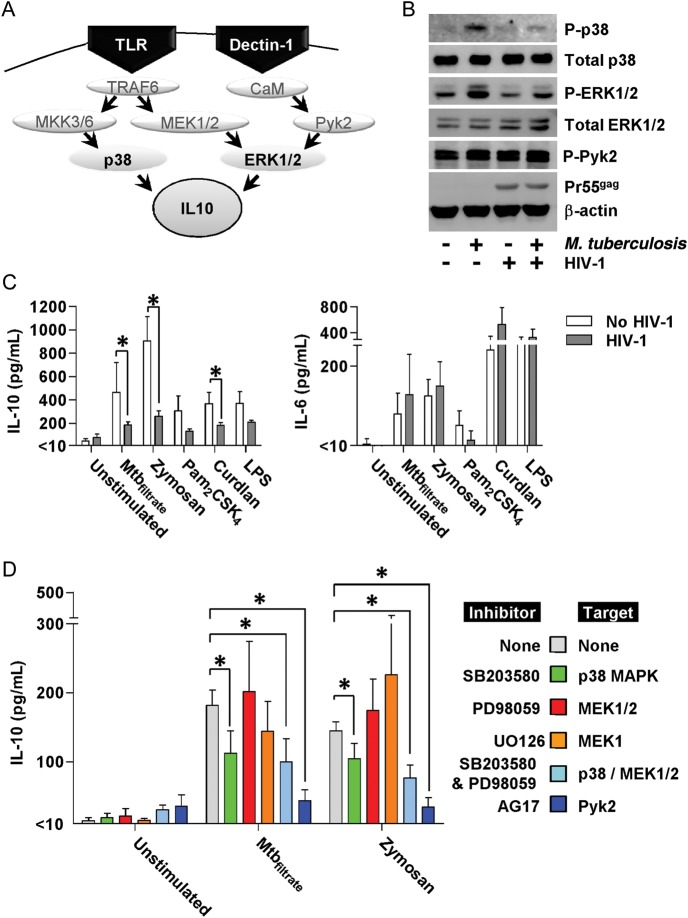
M. tuberculosis–induced p38 and ERK1/2 Activation is Attenuated in HIV-1–Infected Macrophages
Innate immune induction of IL-10 expression in macrophages is principally attributed to Toll-like receptor 2 (TLR2) or dectin-1–mediated signaling pathways that involve activation of p38 and ERK1/2 MAPK [32–34], summarized in Figure 4A. We confirmed activation of these MAPK pathways in MDM stimulated with M. tuberculosis by Western blot detection of phosphorylated p38 and ERK1/2 (Figure 4B). By comparison, activation of these signaling pathways was attenuated in HIV-1–infected MDM. Total p38 and ERK1/2 levels were unaffected by HIV-1 infection as was M. tuberculosis-stimulated phosphorylation of Pyk2, an intermediate in the spleen tyrosine kinase (SYK) signaling pathway, downstream of dectin-1 stimulation [33]. TLR2 and dectin-1 can also be stimulated by the fungal cell wall derivative zymosan [33]. We therefore tested IL-10 responses to zymosan stimulation in our model and also found them to be attenuated in HIV-1 infected MDM (Figure 4C). These data suggest that the effect of HIV-1 is mediated by inhibition of conserved innate immune signaling events in IL-10–induction pathways in response to different stimuli, but lower levels of IL-10 induction by stimulation of specific receptors—dectin-1 by curdlan, TLR2 by Pam2CSK4, and TLR4 by LPS—showed less marked attenuation by HIV-1(Figure 4C). As previously, early IL-6 responses to these stimuli were unaffected by HIV-1.
Figure 4.
HIV-1 inhibition of IL-10 responses is associated with attenuated p38 MAPK signaling. A, Signaling pathways involved in macrophage IL-10 production in response to innate immune activation via TLRs and dectin-1. B, Phosphorylation of p38 and ERK1/2, following 2-hour stimulation with M. tuberculosis, was attenuated in HIV-1–infected MDMs compared with HIV-1–uninfected cells. Total p38 and ERK levels were unaffected by HIV-1 infection. Detection of precursor HIV-1 gag protein confirmed productive virus infection, and β-actin levels show equivalent sample loading. Representative Western blots are shown of experiments using 3 separate donors. C, IL-10 and IL-6 production by MDM cultures ± HIV-1 infection were quantified by ELISA after 4-hour stimulation with Mtbfiltrate, zymosan (0.4 mg/mL), Pam2CSK4 (100 ng/mL), curdlan (0.1 mg/mL), and LPS (100 ng/mL). D, Preincubation of MDMs with inhibitors of p38 signaling (SB203580) and Pyk2 activation (AG17) for 2 hours attenuated IL-10 production in response to 4-hour stimulation with Mtbfiltrate or zymosan, detected by ELISA measurement of IL-10 levels in cell-culture supernatants. Bars represent mean ± SEM of 4 separate experiments.*Denotes significant differences by paired t tests (P < .05). Abbreviations: ELISA, enzyme-linked immunosorbent assay; ERK1/2, extracellular-signal-regulated kinases 1/2; HIV, human immunodeficiency virus; IL, interleukin; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinases; MDMs, monocyte-derived macrophages; M. tuberculosis, Mycobacterium tuberculosis; SEM, standard error of measurement; TLRs, Toll-like receptors.
In order to confirm the functional role of these pathways in the induction of IL-10 responses within our model, we tested the effect of chemical inhibition of components of these pathways on M. tuberculosis and zymosan-induced production of IL-10 (Figure 4D). Inhibition of p38 significantly attenuated IL-10 production by both stimuli. Targeting ERK1/2 signaling alone did not attenuate IL-10 production, but dual p38/ERK1/2 inhibition modestly enhanced the effect of p38 inhibition. Pyk2 inhibition also significantly attenuated IL-10 production. Taken together, our data suggest that HIV-1 attenuation of innate immune IL-10 responses by MDM may be mediated by inhibition of the p38 MAPK pathway with modest additional effect of inhibition of the ERK1/2-dependent pathway.
The Consequence of Attenuated IL-10 Responses to M. tuberculosis on HIV-1 Replication in MDM
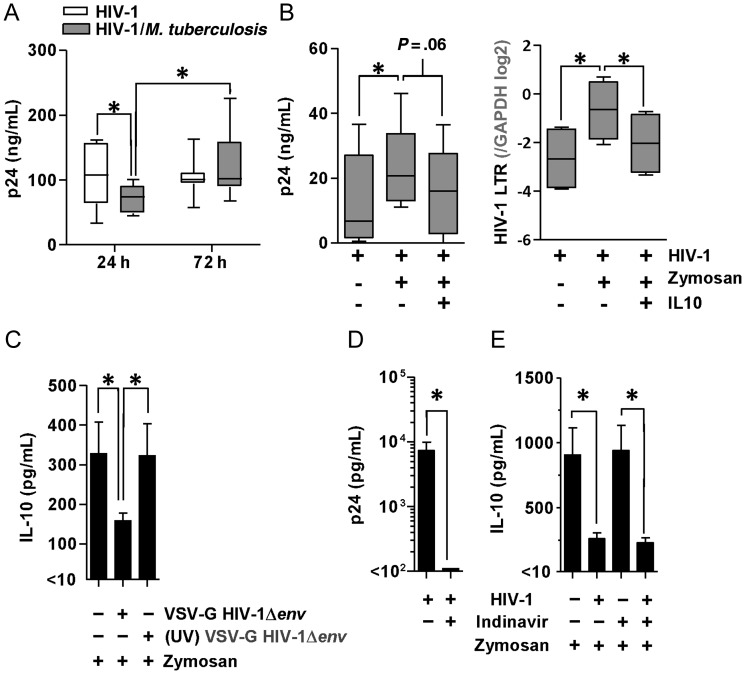
Stimulation of HIV-1–infected macrophages with M. tuberculosis has been reported to inhibit HIV-1 replication as a result of induction of the inhibitory isoform of the C/EBPβ transcription factor [35]. Pro-inflammatory cytokines such as tumor necrosis factor (TNF)α, IL-1β and IL-6 that are induced by M. tuberculosis are known to increase HIV-1 replication by activation of NF-κB pathways and consequent transcriptional activation of the HIV-1 long terminal repeat [36, 37]. We found that HIV-1 release by macrophages, quantified by p24 concentrations in cell-culture supernatants, was significantly attenuated after 24 hours coinfection with M. tuberculosis. However, by 72 hours, p24 levels recovered, and in 5 of 8 experimental replicates exceeded equivalent HIV-1 infected macrophage cultures that were not coinfected with M. tuberculosis (Figure 5A). These data suggest that early suppression of HIV-1 replication, as a result of cellular coinfection with M. tuberculosis, may be reversed at later time points that are associated with enhanced proinflammatory cytokine responses. To extend these observations, we confirmed that stimulation of HIV-1–infected cells with zymosan also led to significantly increased transcription of the viral genome and p24 levels in the cell culture supernatants at 72 hours, and that complementation of deficient IL-10 responses by the addition of recombinant IL-10 suppressed virus production (Figure 5B). Hence, in HIV-1–infected macrophages, inhibition of IL-10 responses to coinfecting pathogens may serve to increase virus replication and propagation.
Figure 5.
HIV-1 replication is sensitive to IL-10 and attenuation of IL-10 responses requires live virus but not virus propagation. A, HIV-1 replication (quantified by HIV-1 p24 ELISA of cell culture supernatants) was initially inhibited in MDMs (P = .034, paired t test) stimulated with M. tuberculosis for 24 hours, but subsequently recovered by 72 hours, in comparison to viral replication in cells that were not co-infected with M. tuberculosis. B, HIV-1 transcription quantified by qPCR of the HIV-1 LTR and p24 levels in cell culture supernatants also increased significantly after 72 hours stimulation with zymosan, but this effect was abrogated by the addition of recombinant IL-10 (10 ng/mL) 4 hours after stimulation to complement the deficient IL-10 responses in HIV-1–infected cells. C, Infection of MDMs with single-round envelope-deficient virus (HIV-1Δenv) pseudotyped with the VSV-G envelope was sufficient to attenuate IL-10 responses to 4-hour zymosan stimulation, but this effect was not evident when using ultraviolet-inactivated virus. D, In MDMs infected with full-length replication-competent HIV-1, treatment with the protease inhibitor Indinavir (10 µM) for 3 days before zymosan stimulation completely abolished the release of any mature virus, reflected in the absence of any detectable p24 in the cell-culture supernatant. E, Early (4-hour) innate immune IL-10 responses were similarly attenuated in the presence and absence of protease inhibitor. Box-and-whisker plots represent the median and range of 5 to 8 experiments in each case. Bars represent mean ± SEM of 5 separate experiments (*denotes P < .05, t test). Abbreviations: ELISA, enzyme-linked immunosorbent assay; HIV, human immunodeficiency virus; IL, interleukin; LTR, long terminal repeat; MDMs, monocyte-derived macrophages; M. tuberculosis, Mycobacterium tuberculosis; qPCR, quantitative polymerase chain reaction; SEM, standard error of measurement; VSV-G, vesicular stomatitis virus G glycoprotein.
HIV-1 Attenuation of Innate Immune IL-10 Induction in MDM Is Dependent on Infection With Replication-Competent Virus but not On-going Virus Propagation
We assessed whether integration or replication were necessary for HIV-1 to inhibit macrophage IL-10 production. Infection of MDM with an envelope-deficient pseudotyped HIV-1, which is only capable of a single round of infection, was sufficient to attenuate IL-10 responses to zymosan. However, UV-inactivated virus, which cannot reverse transcribe and integrate, failed to attenuate IL-10 responses to zymosan (Figure 5C). Inhibition of HIV-1 propagation in MDM by the addition of protease inhibitor (Figure 5D) after infection had no effect on attenuation of IL-10 responses (Figure 5E). These data suggest that HIV-1 integration and transcription, but not on-going virus propagation, mediate the effect on IL-10, and importantly, that this effect of HIV-1 within macrophages may persist in the face of effective antiretroviral therapy that blocks virus propagation.
DISCUSSION
The pathogenesis of active tuberculosis is mediated by excessive proinflammatory responses, highlighted by paradoxical reactions following antituberculous therapy, or immune reconstitution inflammatory syndrome (IRIS) in HIV-1–infected patients following antiretroviral therapy [38]. We sought to evaluate the potential contribution of HIV-1 infection of macrophages to the immunopathogenesis of coinfection with M. tuberculosis. Infection of macrophages with HIV-1 and M. tuberculosis has previously been reported to be dominated by transcriptional responses to M. tuberculosis [17], consistent with our findings that HIV-1 itself does not induce innate immune cellular activation or any significant changes to the host-cell transcriptome [3]. Another study to test the effect of HIV-1 infection in macrophages on responses to M. tuberculosis reported enhanced proinflammatory responses by the measurement of selected cytokines [18]. We found that the early proinflammatory response to M. tuberculosis, like the response to S. pneumoniae and LPS [19], is comparable in HIV-1–infected and uninfected macrophages. However, HIV-1–infected macrophages showed an early attenuation of anti-inflammatory IL-10 transcription, and a more striking reduction in IL-10 protein secretion for up to 72 hours. Diminished early IL-10 responses were associated with exaggerated proinflammatory responses at later time points. Attenuation of IL-10 by HIV-1 is clearly contingent on stimuli that induce IL-10 responses in macrophages. In contrast to M. tuberculosis, infection of MDM with S. pneumoniae did not induce a significant IL-10 response and consequently no effect of HIV-1 coinfection. Therefore, this phenotype would be expected to exhibit pathogen specificity. In keeping with this hypothesis and the possibility that this phenomenon may contribute to in vivo pathogenesis, we found significantly lower IL-10 and higher IL-1β in lung secretions from HIV-1–positive patients with tuberculosis compared with other respiratory-tract infections. Others have reported preserved IL-10 responses to TLR4 stimulation in the U1 leukemia cell line harboring integrated HIV-1 virus [39], increased IL-10 levels in BAL fluid from asymptomatic HIV-infected patients [16], and higher plasma IL-10 levels in patients with HIV/M. tuberculosis coinfection and CD4 lymphocyte counts <200 [40]. The discrepancies with our data are likely to reflect differences in experimental paradigms or context, but merit further consideration, as do potential differences in the biology of distinct macrophage populations.
M. tuberculosis induction of IL-10 requires MAP kinase activity [29, 32, 41]. HIV-1 infection of macrophages was observed to attenuate p38 and ERK1/2 MAPK phosphorylation induced by M. tuberculosis. Alveolar macrophages from HIV-1–infected patients have also been reported to show reduced ERK1/2 phosphorylation [42]. In our model, inhibition of p38 had a greater effect on IL-10 production than selective inhibition of ERK1/2, consistent with the existing literature on a dominant role for p38 in IL-10 production by mononuclear phagocytic cells [30, 41, 43]. We found that HIV-1 infection of macrophages also attenuated IL-10 responses to the fungal cell wall derivative, zymosan, suggesting that the effect of HIV-1 is to inhibit common signaling pathways upstream of IL-10 transcription, although the mycobacterial components that are involved require further investigation.
Interestingly, M. tuberculosis and fungal pathogens are most commonly associated with IRIS in HIV-1–infected patients [38, 44]. Pathogenesis studies of IRIS have focused principally on the role of T cells [45, 46], although a potential role for macrophages has been postulated [47] and supported by recent data that highlight the role of inflammatory cytokines commonly associated with myeloid cells [48]. We show, by using a model of single-round virus replication or protease inhibitors, that on-going virus propagation is not necessary to inhibit IL-10 responses in macrophages. Therefore, given that macrophages are relatively long-lived, HIV-1 attenuation of IL-10 and dysregulation of inflammatory responses may persist despite effective antiretroviral therapy. These responses may be further compounded by lymphocyte recovery and recruitment following antiretroviral therapy and suggest a mechanism by which macrophages may contribute to the pathogenesis of IRIS.
In the absence of antiretrovirals, we initially observed diminished virus production after coinfection with M. tuberculosis, as previously reported [35]. However, this effect was transient and stimulation of HIV-1–infected macrophages with M. tuberculosis or zymosan led to sustained increases in virus production at later time points. Increased virus production was sensitive to complementation of the deficient IL-10 response by addition of recombinant IL-10. These data are in keeping with previous reports that IL-10 can inhibit HIV-1 replication [49, 50], and that proinflammatory cytokines can enhance HIV-1 replication [36, 37]. Therefore, we propose that attenuated IL-10 responses and exaggerated proinflammatory responses to M. tuberculosis may contribute to a cell-autonomous mechanism for increased HIV-1 viral load during coinfection [1]. This effect may also enhance viral propagation by the transfer of HIV-1 to permissive T cells, for which recruitment to the site of infection may be increased as a result of exaggerated proinflammatory cytokine and chemokine responses in HIV-1/M. tuberculosis–coinfected macrophages.
In conclusion, we propose that in vivo, even low-frequency HIV-1–infected macrophages can provide a nidus of exaggerated inflammatory responses to coinfecting pathogens, as a consequence of insufficient induction of homeostatic IL-10. This may contribute to the immunopathogenesis of active tuberculosis in HIV-1–infected patients, and viral propagation as a result of increased virus replication and recruitment of HIV-1–permissive T cells. The pathogenic role of aberrant IL-10 responses in active tuberculosis, and the potential for therapeutic intervention in this pathway, merit further assessment.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by a UK Medical Research Council (MRC) fellowship to G. T. (G0700569), MRC doctoral training award to L. B., Wellcome Trust fellowship to M. N. (WT077161), UK National Institute for Health Research (NIHR) fellowship to P. E., and UK NIHR Comprehensive Biomedical Research Centre funding to UCLH/UCL and Imperial College.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Nakata K, Rom WN, Honda Y, et al. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication in the lung. Am J Respir Crit Care Med. 1997;155:996–1003. doi: 10.1164/ajrccm.155.3.9117038. [DOI] [PubMed] [Google Scholar]
- 2.Toossi Z, Mayanja-Kizza H, Hirsch CS, et al. Impact of tuberculosis (TB) on HIV-1 activity in dually infected patients. Clin Exp Immunol. 2001;123:233–8. doi: 10.1046/j.1365-2249.2001.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsang J, Chain BM, Miller RF, et al. HIV-1 infection of macrophages is dependent on evasion of innate immune cellular activation. AIDS. 2009;23:2255–63. doi: 10.1097/QAD.0b013e328331a4ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noursadeghi M, Katz DR, Miller RF. HIV-1 infection of mononuclear phagocytic cells: the case for bacterial innate immune deficiency in AIDS. Lancet Infect Dis. 2006;6:794–804. doi: 10.1016/S1473-3099(06)70656-9. [DOI] [PubMed] [Google Scholar]
- 5.Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7:355–66. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 6.Dorhoi A, Reece ST, Kaufmann SH. For better or for worse: the immune response against Mycobacterium tuberculosis balances pathology and protection. Immunol Rev. 2011;240:235–51. doi: 10.1111/j.1600-065X.2010.00994.x. [DOI] [PubMed] [Google Scholar]
- 7.Elkington P, Shiomi T, Breen R, et al. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest. 2011;121:1827–33. doi: 10.1172/JCI45666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon S. The macrophage: past, present and future. Eur J Immunol. 2007;37(suppl 1):S9–17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 9.Rich EA, Chen IS, Zack JA, Leonard ML, O'Brien WA. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1) J Clin Invest. 1992;89:176–83. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park IW, Koziel H, Hatch W, Li X, Du B, Groopman JE. CD4 receptor-dependent entry of human immunodeficiency virus type-1 env-pseudotypes into CCR5-, CCR3-, and CXCR4-expressing human alveolar macrophages is preferentially mediated by the CCR5 coreceptor. Am J Respir Cell Mol Biol. 1999;20:864–71. doi: 10.1165/ajrcmb.20.5.3547. [DOI] [PubMed] [Google Scholar]
- 11.Nakata K, Weiden M, Harkin T, Ho D, Rom WN. Low copy number and limited variability of proviral DNA in alveolar macrophages from HIV-1-infected patients: evidence for genetic differences in HIV-1 between lung and blood macrophage populations. Mol Med. 1995;1:744–57. [PMC free article] [PubMed] [Google Scholar]
- 12.Kaner RJ, Santiago F, Rahaghi F, Michaels E, Moore JP, Crystal RG. Adenovirus vectors block human immunodeficiency virus-1 replication in human alveolar macrophages by inhibition of the long terminal repeat. Am J Respir Cell Mol Biol. 2010;43:234–42. doi: 10.1165/rcmb.2008-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood KL, Chaiyarit P, Day RB, et al. Measurements of HIV viral loads from different levels of the respiratory tract. Chest. 2003;124:536–42. doi: 10.1378/chest.124.2.536. [DOI] [PubMed] [Google Scholar]
- 14.Goletti D, Carrara S, Vincenti D, et al. Inhibition of HIV-1 replication in monocyte-derived macrophages by Mycobacterium tuberculosis. J Infect Dis. 2004;189:624–33. doi: 10.1086/381554. [DOI] [PubMed] [Google Scholar]
- 15.Patel NR, Zhu J, Tachado SD, et al. HIV impairs TNF-alpha mediated macrophage apoptotic response to Mycobacterium tuberculosis. J Immunol. 2007;179:6973–80. doi: 10.4049/jimmunol.179.10.6973. [DOI] [PubMed] [Google Scholar]
- 16.Patel NR, Swan K, Li X, Tachado SD, Koziel H. Impaired M. tuberculosis–mediated apoptosis in alveolar macrophages from HIV+ persons: potential role of IL-10 and BCL-3. J Leukoc Biol. 2009;86:53–60. doi: 10.1189/JLB.0908574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maddocks S, Scandurra GM, Nourse C, et al. Gene expression in HIV-1/Mycobacterium tuberculosis co-infected macrophages is dominated by M. tuberculosis. Tuberculosis (Edinb) 2009;89:285–93. doi: 10.1016/j.tube.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Pathak S, Wentzel-Larsen T, Asjo B. Effects of in vitro HIV-1 infection on mycobacterial growth in peripheral blood monocyte-derived macrophages. Infect Immun. 2010;78:4022–32. doi: 10.1128/IAI.00106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noursadeghi M, Tsang J, Miller RF, et al. Genome-wide innate immune responses in HIV-1-infected macrophages are preserved despite attenuation of the NF-kappa B activation pathway. J Immunol. 2009;182:319–28. doi: 10.4049/jimmunol.182.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elkington PT, Nuttall RK, Boyle JJ, et al. Mycobacterium tuberculosis, but not vaccine BCG, specifically upregulates matrix metalloproteinase-1. Am J Respir Crit Care Med. 2005;172:1596–604. doi: 10.1164/rccm.200505-753OC. [DOI] [PubMed] [Google Scholar]
- 21.Swingler S, Gallay P, Camaur D, Song J, Abo A, Trono D. The Nef protein of human immunodeficiency virus type 1 enhances serine phosphorylation of the viral matrix. J Virol. 1997;71:4372–7. doi: 10.1128/jvi.71.6.4372-4377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger G, Durand S, Goujon C, et al. A simple, versatile and efficient method to genetically modify human monocyte-derived dendritic cells with HIV-1-derived lentiviral vectors. Nat Protoc. 2011;6:806–16. doi: 10.1038/nprot.2011.327. [DOI] [PubMed] [Google Scholar]
- 23.Chain B, Bowen H, Hammond J, et al. Error, reproducibility and sensitivity: a pipeline for data processing of Agilent oligonucleotide expression arrays. BMC Bioinformatics. 2010;11:344. doi: 10.1186/1471-2105-11-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomlinson GS, Cashmore TJ, Elkington PT, et al. Transcriptional profiling of innate and adaptive human immune responses to mycobacteria in the tuberculin skin test. Eur J Immunol. 2011;41:3253–60. doi: 10.1002/eji.201141841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saeed AI, Sharov V, White J, et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–8. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 26.Breen RA, Hardy GA, Perrin FM, et al. Rapid diagnosis of smear-negative tuberculosis using immunology and microbiology with induced sputum in HIV-infected and uninfected individuals. PLOS One. 2007;2:e1335. doi: 10.1371/journal.pone.0001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breen RA, Barry SM, Smith CJ, et al. Clinical application of a rapid lung-orientated immunoassay in individuals with possible tuberculosis. Thorax. 2008;63:67–71. doi: 10.1136/thx.2007.078857. [DOI] [PubMed] [Google Scholar]
- 28.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 29.Redford PS, Murray PJ, O'Garra A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 2011;4:261–70. doi: 10.1038/mi.2011.7. [DOI] [PubMed] [Google Scholar]
- 30.Nair S, Ramaswamy PA, Ghosh S, et al. The PPE18 of Mycobacterium tuberculosis interacts with TLR2 and activates IL-10 induction in macrophage. J Immunol. 2009;183:6269–81. doi: 10.4049/jimmunol.0901367. [DOI] [PubMed] [Google Scholar]
- 31.Barnes PF, Lu S, Abrams JS, Wang E, Yamamura M, Modlin RL. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993;61:3482–9. doi: 10.1128/iai.61.8.3482-3489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 33.Kelly EK, Wang L, Ivashkiv LB. Calcium-activated pathways and oxidative burst mediate zymosan-induced signaling and IL-10 production in human macrophages. J Immunol. 2010;184:5545–52. doi: 10.4049/jimmunol.0901293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10:2058–66. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 35.Weiden M, Tanaka N, Qiao Y, et al. Differentiation of monocytes to macrophages switches the Mycobacterium tuberculosis effect on HIV-1 replication from stimulation to inhibition: modulation of interferon response and CCAAT/enhancer binding protein beta expression. J Immunol. 2000;165:2028–39. doi: 10.4049/jimmunol.165.4.2028. [DOI] [PubMed] [Google Scholar]
- 36.Poli G, Kinter A, Justement JS, et al. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci USA. 1990;87:782–5. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poli G, Kinter AL, Fauci AS. Interleukin 1 induces expression of the human immunodeficiency virus alone and in synergy with interleukin 6 in chronically infected U1 cells: inhibition of inductive effects by the interleukin 1 receptor antagonist. Proc Natl Acad Sci USA. 1994;91:108–12. doi: 10.1073/pnas.91.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and “unmasking" of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177:680–5. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tachado SD, Li X, Bole M, et al. MyD88-dependent TLR4 signaling is selectively impaired in alveolar macrophages from asymptomatic HIV+ persons. Blood. 2010;115:3606–15. doi: 10.1182/blood-2009-10-250787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.da Silva TP, Giacoia-Gripp CB, Schmaltz CA, Sant Anna FM, Rolla V, Morgado MG. T Cell activation and cytokine profile of tuberculosis and HIV-positive individuals during antituberculous treatment and Efavirenz-based regimens. PLOS One. 2013;8:e66095. doi: 10.1371/journal.pone.0066095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song CH, Lee JS, Lee SH, et al. Role of mitogen-activated protein kinase pathways in the production of tumor necrosis factor-alpha, interleukin-10, and monocyte chemotactic protein-1 by Mycobacterium tuberculosis H37Rv-infected human monocytes. J Clin Immunol. 2003;23:194–201. doi: 10.1023/a:1023309928879. [DOI] [PubMed] [Google Scholar]
- 42.Tachado SD, Zhang J, Zhu J, Patel N, Koziel H. HIV impairs TNF-alpha release in response to Toll-like receptor 4 stimulation in human macrophages in vitro. Am J Respir Cell Mol Biol. 2005;33:610–21. doi: 10.1165/rcmb.2004-0341OC. [DOI] [PubMed] [Google Scholar]
- 43.O'Leary S, O'Sullivan MP, Keane J. IL-10 blocks phagosome maturation in Mycobacterium tuberculosis–infected human macrophages. Am J Respir Cell Mol Biol. 2011;45:172–80. doi: 10.1165/rcmb.2010-0319OC. [DOI] [PubMed] [Google Scholar]
- 44.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–73. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 45.Bourgarit A, Carcelain G, Martinez V, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 46.Meintjes G, Wilkinson KA, Rangaka MX, et al. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med. 2008;178:1083–9. doi: 10.1164/rccm.200806-858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van den Bergh R, Vanham G, Raes G, De BP, Colebunders R. Mycobacterium-associated immune reconstitution disease: macrophages running wild? Lancet Infect Dis. 2006;6:2–3. doi: 10.1016/S1473-3099(05)70302-9. [DOI] [PubMed] [Google Scholar]
- 48.Tadokera R, Meintjes G, Skolimowska KH, et al. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur Respir J. 2010;37:1248–59. doi: 10.1183/09031936.00091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka N, Hoshino Y, Gold J, et al. Interleukin-10 induces inhibitory C/EBPbeta through STAT-3 and represses HIV-1 transcription in macrophages. Am J Respir Cell Mol Biol. 2005;33:406–11. doi: 10.1165/rcmb.2005-0140OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Rice AP. Interleukin-10 inhibits HIV-1 LTR-directed gene expression in human macrophages through the induction of cyclin T1 proteolysis. Virology. 2006;352:485–92. doi: 10.1016/j.virol.2006.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.