Abstract
In vitro synthesis of firefly luciferase and its folding into an enzymatically active conformation were studied in a wheat germ cell-free translation system. A novel method is described by which the enzymatic activity of newly synthesized luciferase can be monitored continuously in the cell-free system while this protein is being translated from its mRNA. It is shown that ribosome-bound polypeptide chains have no detectable enzymatic activity, but that this activity appears within a few seconds after luciferase has been released from the ribosome. In contrast, the renaturation of denatured luciferase under identical conditions occurs with a half-time of 14 min. These results support the cotranslational folding hypothesis which states that the nascent peptides start to attain their native tertiary structure during protein synthesis on the ribosome.
Full text
PDF
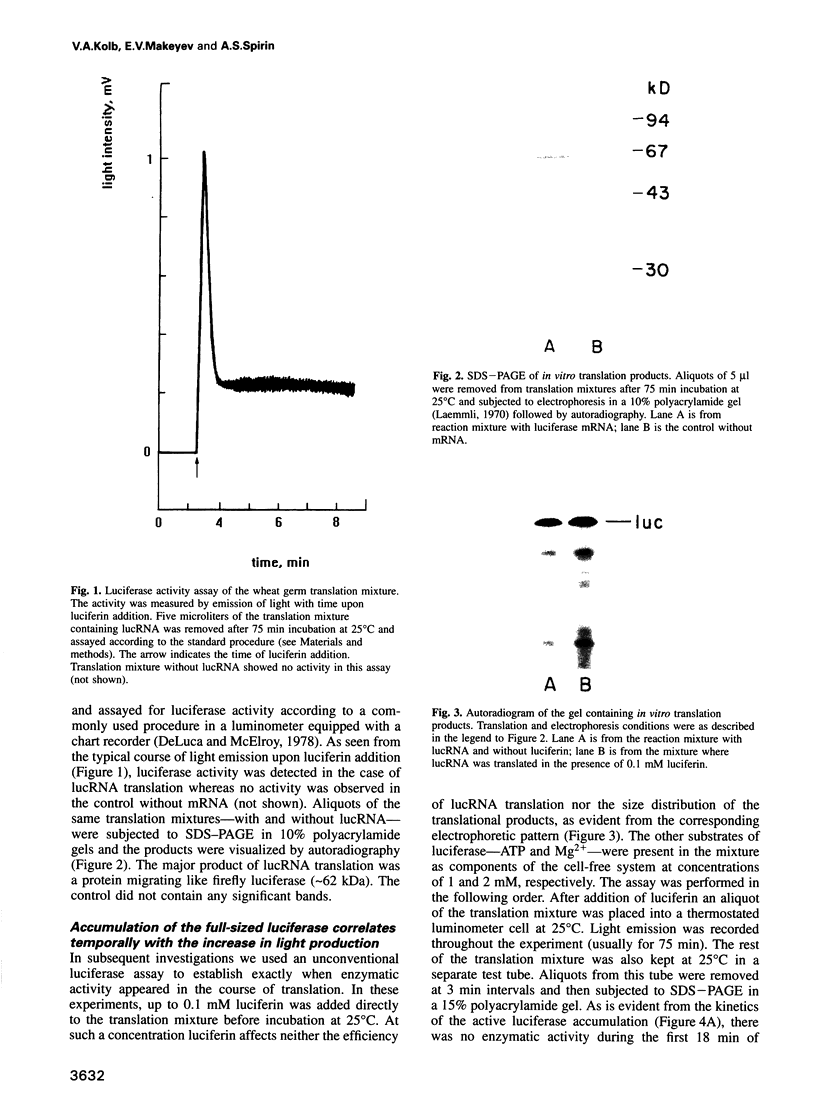
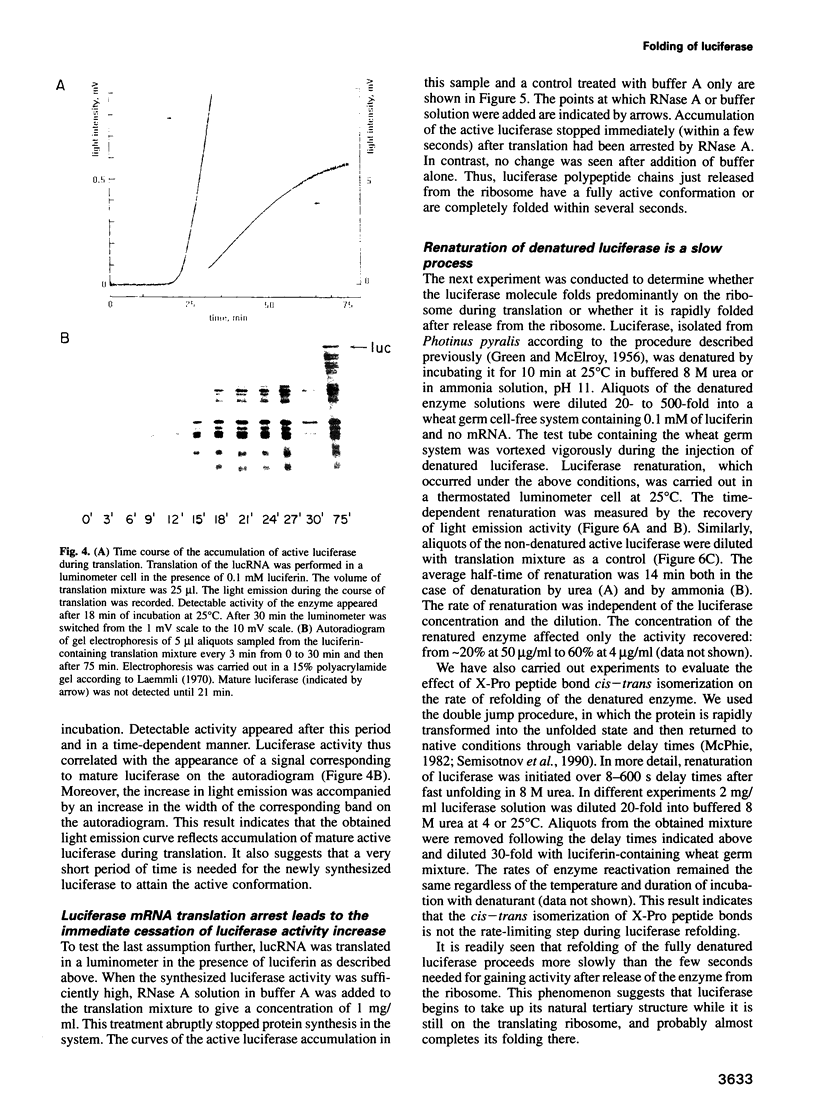
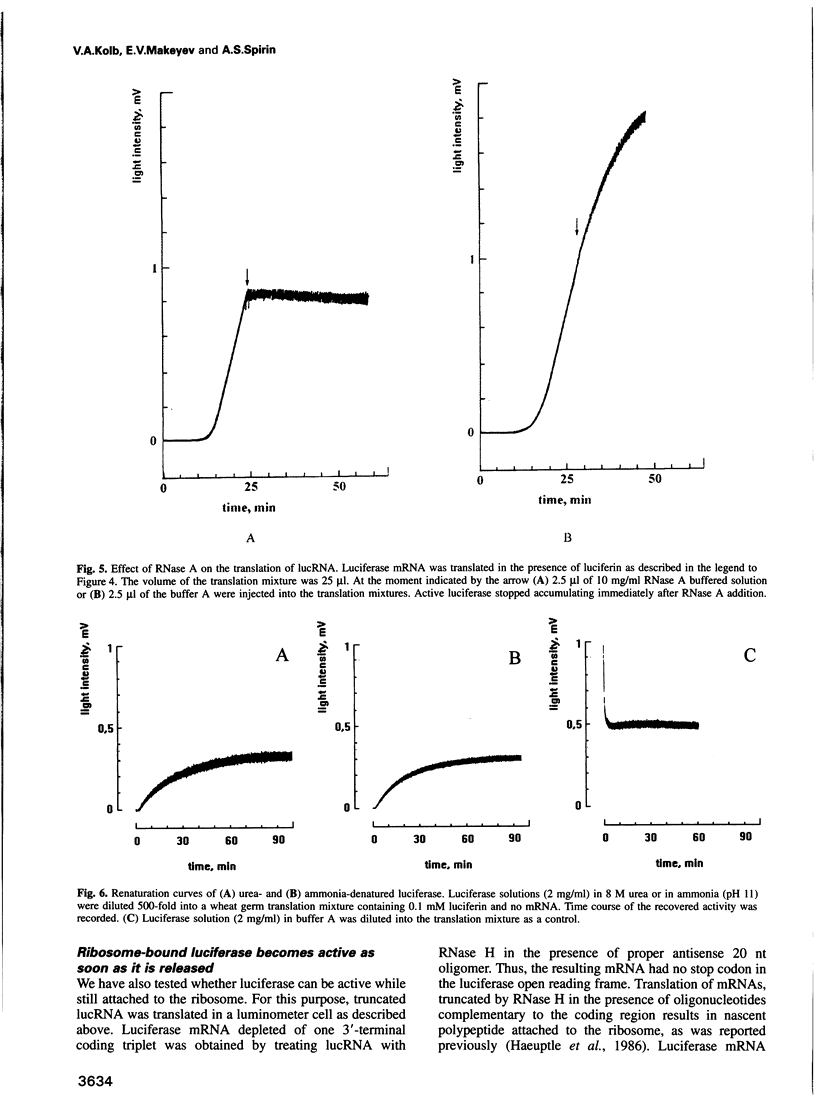
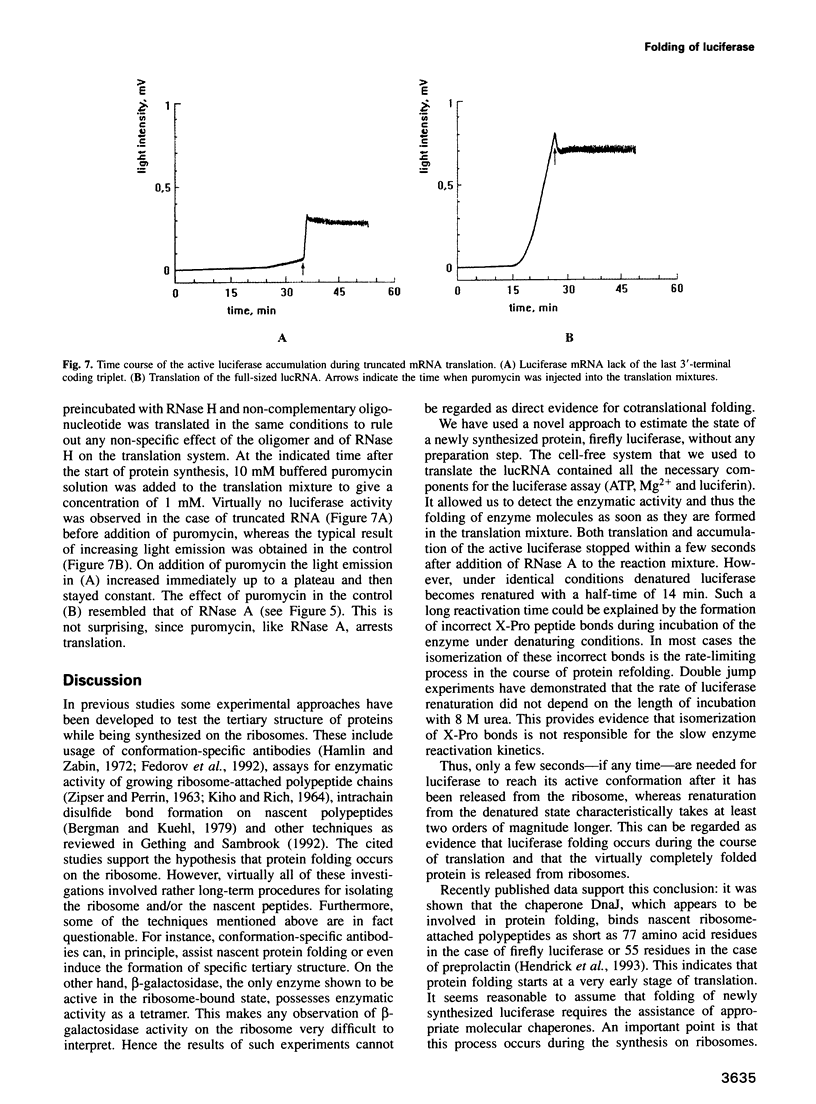


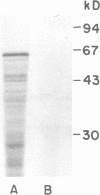
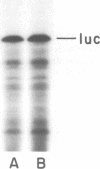
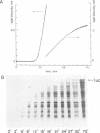
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANFINSEN C. B., HABER E. Studies on the reduction and re-formation of protein disulfide bonds. J Biol Chem. 1961 May;236:1361–1363. [PubMed] [Google Scholar]
- Anfinsen C. B. Principles that govern the folding of protein chains. Science. 1973 Jul 20;181(4096):223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- Anfinsen C. B., Scheraga H. A. Experimental and theoretical aspects of protein folding. Adv Protein Chem. 1975;29:205–300. doi: 10.1016/s0065-3233(08)60413-1. [DOI] [PubMed] [Google Scholar]
- Bergman L. W., Kuehl W. M. Formation of an intrachain disulfide bond on nascent immunoglobulin light chains. J Biol Chem. 1979 Sep 25;254(18):8869–8876. [PubMed] [Google Scholar]
- Blobel G., Sabatini D. D. Controlled proteolysis of nascent polypeptides in rat liver cell fractions. I. Location of the polypeptides within ribosomes. J Cell Biol. 1970 Apr;45(1):130–145. doi: 10.1083/jcb.45.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. Pathways and mechanisms of protein folding. Adv Biophys. 1984;18:1–20. doi: 10.1016/0065-227x(84)90004-2. [DOI] [PubMed] [Google Scholar]
- De Coen J. L. Folding of the polypeptide chain during biosynthesis. J Mol Biol. 1970 Apr 28;49(2):405–414. doi: 10.1016/0022-2836(70)90253-6. [DOI] [PubMed] [Google Scholar]
- Dice J. F., Goldberg A. L. Relationship between in vivo degradative rates and isoelectric points of proteins. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3893–3897. doi: 10.1073/pnas.72.10.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H. F., Schechter A. N., Chen R. F., Anfinsen C. B. Folding of staphylococcal nuclease: kinetic studies of two processes in acid renaturation. J Mol Biol. 1971 Sep 28;60(3):499–508. doi: 10.1016/0022-2836(71)90184-7. [DOI] [PubMed] [Google Scholar]
- Fedorov A. N., Friguet B., Djavadi-Ohaniance L., Alakhov Y. B., Goldberg M. E. Folding on the ribosome of Escherichia coli tryptophan synthase beta subunit nascent chains probed with a conformation-dependent monoclonal antibody. J Mol Biol. 1992 Nov 20;228(2):351–358. doi: 10.1016/0022-2836(92)90825-5. [DOI] [PubMed] [Google Scholar]
- GREEN A. A., MCELROY W. D. Crystalline firefly luciferase. Biochim Biophys Acta. 1956 Apr;20(1):170–176. doi: 10.1016/0006-3002(56)90275-x. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gurevich V. V., Pokrovskaya I. D., Obukhova T. A., Zozulya S. A. Preparative in vitro mRNA synthesis using SP6 and T7 RNA polymerases. Anal Biochem. 1991 Jun;195(2):207–213. doi: 10.1016/0003-2697(91)90318-n. [DOI] [PubMed] [Google Scholar]
- Haeuptle M. T., Frank R., Dobberstein B. Translation arrest by oligodeoxynucleotides complementary to mRNA coding sequences yields polypeptides of predetermined length. Nucleic Acids Res. 1986 Feb 11;14(3):1427–1448. doi: 10.1093/nar/14.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J., Zabin I. -Galactosidase: immunological activity of ribosome-bound, growing polypeptide chains. Proc Natl Acad Sci U S A. 1972 Feb;69(2):412–416. doi: 10.1073/pnas.69.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick J. P., Langer T., Davis T. A., Hartl F. U., Wiedmann M. Control of folding and membrane translocation by binding of the chaperone DnaJ to nascent polypeptides. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10216–10220. doi: 10.1073/pnas.90.21.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikai A., Tanford C. Kinetic evidence for incorrectly folded intermediate states in the refolding of denatured proteins. Nature. 1971 Mar 12;230(5289):100–102. doi: 10.1038/230100a0. [DOI] [PubMed] [Google Scholar]
- KIHO Y., RICH A. INDUCED ENZYME FORMED ON BACTERIAL POLYRIBOSOMES. Proc Natl Acad Sci U S A. 1964 Jan;51:111–118. doi: 10.1073/pnas.51.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. Intermediates in the folding reactions of small proteins. Annu Rev Biochem. 1990;59:631–660. doi: 10.1146/annurev.bi.59.070190.003215. [DOI] [PubMed] [Google Scholar]
- Kuwajima K. The molten globule state as a clue for understanding the folding and cooperativity of globular-protein structure. Proteins. 1989;6(2):87–103. doi: 10.1002/prot.340060202. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lim V. I., Spirin A. S. Stereochemical analysis of ribosomal transpeptidation. Conformation of nascent peptide. J Mol Biol. 1986 Apr 20;188(4):565–574. doi: 10.1016/s0022-2836(86)80006-7. [DOI] [PubMed] [Google Scholar]
- Malkin L. I., Rich A. Partial resistance of nascent polypeptide chains to proteolytic digestion due to ribosomal shielding. J Mol Biol. 1967 Jun 14;26(2):329–346. doi: 10.1016/0022-2836(67)90301-4. [DOI] [PubMed] [Google Scholar]
- McPhie P. Swine pepsinogen folding intermediates are highly structured, motile molecules. Biochemistry. 1982 Oct 26;21(22):5509–5515. doi: 10.1021/bi00265a020. [DOI] [PubMed] [Google Scholar]
- Sala-Newby G., Kalsheker N., Campbell A. K. Removal of twelve C-terminal amino acids from firefly luciferase abolishes activity. Biochem Biophys Res Commun. 1990 Oct 30;172(2):477–482. doi: 10.1016/0006-291x(90)90697-l. [DOI] [PubMed] [Google Scholar]
- Tanford C., Aune K. C., Ikai A. Kinetics of unfolding and refolding of proteins. 3. Results for lysozyme. J Mol Biol. 1973 Jan 10;73(2):185–197. doi: 10.1016/0022-2836(73)90322-7. [DOI] [PubMed] [Google Scholar]
- Teale J. M., Benjamin D. C. Antibody as immunological probe for studying refolding of bovine serum albumin. Refolding within each domain. J Biol Chem. 1977 Jul 10;252(13):4521–4526. [PubMed] [Google Scholar]
- Tsou C. L. Folding of the nascent peptide chain into a biologically active protein. Biochemistry. 1988 Mar 22;27(6):1809–1812. doi: 10.1021/bi00406a001. [DOI] [PubMed] [Google Scholar]
- Wetlaufer D. B. Folding of protein fragments. Adv Protein Chem. 1981;34:61–92. doi: 10.1016/s0065-3233(08)60518-5. [DOI] [PubMed] [Google Scholar]
- Wright P. E., Dyson H. J., Lerner R. A. Conformation of peptide fragments of proteins in aqueous solution: implications for initiation of protein folding. Biochemistry. 1988 Sep 20;27(19):7167–7175. doi: 10.1021/bi00419a001. [DOI] [PubMed] [Google Scholar]