Abstract
AIM: To evaluate the efficacy of magnesium sulfate (MGS) in comparison with remifentanil for induction of relative hypotension in posterior fusion of spine (PSF).
METHODS: In this randomized clinical trial, 40 patients with the American Society of Anesthesiologists I and II physical status undergoing lumbar PSF were randomized to receive remifentanil (REM) 0.15 μg/kg or MGS 50 mg/kg for controlled hypotension. The administering anesthesiologist was blinded to the medication. Continuous infusion was maintained at a fixed volume rate to deliver precalculated doses of either study drugs. All other aspects of anesthesia and surgery were similar in the two groups. The target mean arterial pressure (MAP) range used in this study was 60-70 mmHg. In the course of surgery, the hemodynamic variables, volume of blood loss, urine output, fluid intake and surgeon’s satisfaction were recorded. Data was analyzed with SPSS version 13.0 and P values less than 0.05 were considered significant.
RESULTS: Twenty patients in the MGS group and 19 patients in the REM group were studied. There was no difference between the two groups in the hemodynamic variables, blood loss, urine output, fluid requirement and surgeon’s satisfaction for exposure. The target MAP was achieved in 75% of Mg and 58% of remifentanil groups. Although a higher number of patients in the REM group required nitroglycerin (42.1%) to reach the target MAP than those in the MGS group (25%), this difference was not statistically significant (P = 0.32).
CONCLUSION: Our findings showed that in patients undergoing lumbar PSF surgery, remifentanil and MGS have a similar hypotensive effect and comparable amount of blood loss without any significant adverse effects.
Keywords: Controlled hypotension, Magnesium sulfate, Remifentanil, Spine surgery, Blood loss
Core tip: We conducted a relatively small sized prospective randomized clinical trial comparing intravenous infusion of remifentanil with magnesium in controlling blood pressure during posterior spine fusion in order to decrease the intraoperative blood loss. Our experiments showed no difference between the two administered regimens in reducing mean arterial blood pressure and intraoperative blood loss, and satisfaction of the operating surgeons.
INTRODUCTION
In lumbar spine surgery, especially in posterior spinal fusion, a dry surgical field not only results in convenience for the surgeon, but also better hemostasis, reducing the likelihood of transfusion and its complications. Also, it decreases operation time and thereby avoids complications of prolonged surgery, particularly in the prone position, such as eyes injuries and nerve damage due to pressure, tongue edema and airway inflammation[1,2].
Induced hypotension is one of the most effective methods for reduction of bleeding during surgery, known as controlled hypotension. Using this method during anesthesia for major spinal surgeries results in less bleeding and less need for blood transfusion[3]. Different methods for controlled hypotension have been described that can be divided into two main groups. First, the traditional style that keeps the patient in a light anesthesia with a secure airway and complete monitoring under a ganglion blocker or a vasodilator with a direct effect and, using this way, a beta blocker being used simultaneously is preferred[4]. The second method tries to control blood pressure without hypotensive drugs by increasing the depth of anesthesia, adequate ventilation and optimal position[5].
Reduction in bleeding is not only important to maintain the hemodynamic balance, but also creates better visibility for the surgeon during surgery; this issue is especially important (significant) in spinal cord surgery because important neuronal structures are located in the field. In major surgical procedures such as scoliosis correction or posterior fusion, excessive bleeding occurs during or after surgery, so controlled hypotension is considered a way to reduce bleeding and improve surgical conditions[2,3].
Various drugs have been used to reduce blood pressure, including vasodilators (nitroprusside, nicardipine), alpha-2 receptor agonists (clonidine, dexmedetomidine), beta-adrenergic antagonists (propranolol, esmolol) and alpha and beta antagonists (labetalol)[6-10]. However, hypotensive agents have several disadvantages, such as reflex tachycardia and tachyphylaxis[9]. Therefore, it is necessary to use compounds with dose-response effects that are predictable[11]. In recent years, remifentanil as a short acting opioid receptor agonist has been used for this purpose in order for a mild to moderate reduction of blood pressure and as an effective agent for controlled hypotension[12]. Nevertheless, high doses of remifentanil during surgery have its limits, with pain management after surgery and hyperalgesia in these patients[13]. Currently, remifentanil with propofol is used for total intravenous anesthesia. Compared with other opioid drugs such as fentanyl and alfentanil, remifentanil can provide better hemodynamic stability in the stressful events of surgery and can minimize changes in cerebral blood flow[14-17].
Magnesium sulfate (MGS) is a noncompetitive antagonist of N-methyl, D-aspartate (NMDA) receptors with an analgesic effect and is essential for release of acetylcholine from the presynaptic terminals[18,19] and, similar to calcium channel blockers (CCB), can prevent the entry of calcium into the cell. According to previous studies, magnesium can cause hypotension with a vasodilatory effect. The vasodilatory effects of this ion are due to increased construction of prostacyclins and inhibition of angiotensin converting enzyme. So it seems that this product could be applied for lowering blood pressure during various surgical procedures[20,21].
To the best of our knowledge, there have been no studies comparing controlled hypotension by magnesium and remifentanil in lumbar spine fusion surgery. So, we decided to compare their efficacy, blood loss and likely hemodynamic complications in a prospective study in candidates for lumbar spine fusion surgery. We hypothesized that magnesium is as effective as remifentanil in deliberate induction of hypotension used to reduce surgical blood loss.
MATERIALS AND METHODS
The study was designed as a prospective double-blind randomized trial. The experimental design and complete study protocol were reviewed and approved by the Medical Research Ethics Committee at Tehran University of Medical Sciences. The study was also registered with the national Iranian Registry of Clinical Trials (http://www.irct.ir), IRCT201111138091N1 in accordance with World Health Organization’s requirements and the International Committee of Medical Journal Editors’ initiative.
The study population included orthopedic and neurological patients admitted to Tehran’s University Hospital (Firoozgar center) from 2010 to 2011. Patients between 20 and 60 years old undergoing posterior lumbar spine fusion were surveyed based on inclusion and exclusion criteria. Eligible patients [American Society of Anesthesiologists (ASA) physical status 1 or 2, operation time of 3 to 5 h and mentally competent] were approached and signed informed consent after complete explanation of the details of the research project and its potential risks.
Exclusion criteria were as follows: operation time more than 5 h, allergic reaction to drugs, and patients with a history of liver, renal, heart and vascular failure, cardiac conduction disturbance, opium addiction, any drug or substance abuse and chronic treatment with opium, non-steroidal anti-inflammatory drugs and CCB.
Patients were randomized with block randomization (four piece blocks) into two groups: MGS and receive remifentanil (REM). Someone who was uninformed of the study developed a list of replacement and then according to the entry of each sample to study, treatment groups of patients were determined. Upon arrival to the operating room, standard monitoring, including end-tidal carbon dioxide, pulse oximetry, non-invasive blood pressure and 5-lead electrocardiography, was started and baseline hemodynamic variables were recorded. After establishing an IV line and administration of 500 cc normal saline before induction of anesthesia, patients were preoxygenated with 100% oxygen for 5 min. All patients in both groups received midazolam 0.03 mg/kg and fentanyl 3 μg/kg upon arrival in the operating room. For induction of anesthesia, patients received 1.5-2.0 mg/kg propofol and atracurium 0.5 mg/kg was used for muscle relaxation and intubation. After induction of anesthesia, a radial arterial line from the non-dominant hand for continuous monitoring of mean arterial pressure (MAP) was obtained. All patients were mechanically ventilated with a mixture of oxygen and nitrous oxide (fraction of inspired oxygen was 50%) at a tidal volume of 8 mL/kg (ideal body weight), I/E ratio of 1/3 and the respiratory rates were adjusted in the 9-14 breaths/min range to maintain end-tidal concentration of carbon dioxide between 30 and 40 mmHg. Continuous infusion of propofol with a dose of 100-120 μg/kg per minute and bolus doses of atracurium every 20 to 30 min were used for maintenance of anesthesia, similar in both groups. Also, in the first 30 min after the position, morphine sulfate was infused 0.1 mg/kg within 20 min.
After establishing a prone position and ensuring hemodynamic stability, for the MGS group, a loading dose of 50 mg/kg magnesium diluted in 100 cc saline infused within 10 min and then the magnesium infusion continued with dose of 15 mg/kg per hour throughout surgery. In the REM group, remifentanil (loading dose 1 μg/kg; maintenance infusion, 0.25 μg/kg per minute) was administered during anesthesia. A research pharmacist prepared both magnesium and remifentanil solutions to make sure the volumes of infusion were the same for each group in order to maintain the blinding process for the administering anesthesiology team.
Our goal was to maintain MAP between 60 to 70 mmHg. Using the above method, if the optimum MAP was not reached in 10 min after skin incision, nitroglycerin (NTG) 10 to 20 μg/min was applied and increased if needed. Duration and frequency of NTG use was recorded as a measurement of failure in the primary goal for comparison between groups. If the blood pressure dropped below the target range, the infusion rate of propofol was reduced to half and in cases where hypotension continued, ephedrine was injected in bolus doses of 5 mg. Possible complications such as arrhythmia, hypotension, hypertension and bradycardia and measures necessary to treat these complications were recorded in both groups. Operation time, approximate amount of bleeding, urine output, volume of fluids and the need for transfusion were recorded on special forms. Blood transfusion was determined based on primary hemoglobin and calculation of maximum allowable blood loss (target hemoglobin = 10 g/dL).
For reducing the chance of prolonged muscle relaxation by magnesium, the study drug infusion was stopped about half an hour before the end of surgery (after fixation of rods and starting repair of wounds) and propofol and remifentanil infusion was reduced to half. Finally, after surgery and changing the patient’s position from a prone to supine position, muscle relaxant effects was reversed and, in order to ensure adequate reversal of muscle relaxation, nerve stimulator and double burst stimulation (DBS) were applied (DBS > 90%).
After removing the endotracheal tube, patients were transferred to the post-anesthesia care unit where they were monitored for signs of hypermagnesemia (respiratory depression, decreased deep tendon reflexes, electrocardiographic changes) every 15 min in addition to routine cardiorespiratory monitoring. Serum magnesium concentrations were obtained if there was any clinical sign of hypermagnesemia.
Surgeries were performed in two centers with the two almost constant teams. Surgeon’s satisfaction was recorded using the Likert technique or scale at the end of surgery (1 = very dissatisfied to 5 = completely satisfied). The research team member who recorded data during surgery, the person administering the infusion and the surgeon were blinded to the type of intervention.
Statistical analysis
The data was analyzed with SPSS 18.0 software. Quantitative data was given as the mean and standard deviation and qualitative data presented as frequency. Categorical data were analyzed using the χ2 test and Fisher’s exact test; continuous variables were analyzed by the Student t test when data complied with a normal distribution and were expressed as mean ± SD. Otherwise, non-parametric tests were used (Wilcoxon Rank test) and data were expressed as median and interquartile range. analysis of variance with repeated measures was used to analyze hemodynamic variables at different time points. Null hypotheses were rejected if P values were less than 0.05.
RESULTS
The study was completed with 39 patients. One patient from the REM group was excluded from the study because of an unusual type of surgery and prolonged duration of operation. The average age was 41.2 ± 14.4 years old and body mass index was 30.8 ± 9.2 kg/m2. Demographic variables (age, gender, ASA physical status and body habitus) were comparable in the MGS and REM groups (Table 1).
Table 1.
Demographic data
| REM | MGS | P value | |
| n = 19 | n = 20 | ||
| Age (yr) | 43.1 ± 15.6 | 39.3 ± 13.2 | 0.42 |
| Gender; male (%) | 10 (52%) | 11 (55%) | 0.98 |
| Body mass index (kg/m2) | 31.3 ± 12.4 | 30.2 ± 4.6 | 0.72 |
| ASA PS-1 | 5 (27%) | 5 (25%) | 0.86 |
| ASA PS-2 | 14 (73%) | 15 (75%) | |
| Satisfaction score | 4.2 ± 0.6 | 4.3 ± 1.1 | 0.89 |
MGS: Magnesium sulfate; REM: Remifentanil; ASA: American Society of Anesthesiologists.
Total intra-operative fluid administration was 3.6 ± 0.9 L in the MGS group and 3.3 ± 1.0 L in the REM group (P = 0.39) (Table 2). Average bleeding in the MGS and REM groups was 650 ± 335.9 mL and 681 ± 312.8 mL, respectively (P = 0.77). Three patients in the MGS group (15%) and 5 patients in the REM group (25%) required blood transfusion but it was not significant statistically (P = 0.21). Maximum blood transfusion in patients was 2 bags of packed cells. There was no difference in urine output between the two study arms (P = 0.56). The satisfaction scores from the surgeons regarding exposure of the surgical field between the groups were similar. In both groups, these values were higher than 3 (good satisfaction).
Table 2.
Perioperative variables
| MGS | REM | P value | |
| n = 20 | n = 19 | ||
| Duration of surgery (min) | 168 ± 54 | 162 ± 42 | 0.32 |
| Lowest recorded MAP (mmHg) | 52 ± 13 | 55 ± 15 | 0.72 |
| Intravenous fluids (L) | 3.6 ± 0.9 | 3.3 ± 1.02 | 0.39 |
| Urine output (mL) | 959 ± 368 | 1043 ± 508 | 0.56 |
| Intraoperative blood loss (mL) | 650 ± 336 | 681 ± 313 | 0.77 |
| Transfusion frequency (%) | 3 (15%) | 5 (26%) | 0.21 |
| PRBC transfused (units) | 1.75 ± 0.46 | 1.50 ± 0.54 | 0.33 |
| Preop. serum magnesium (mEq/L) | 2.16 ± 0.21 | 2.18 ± 0.19 | 0.76 |
MGS: Magnesium sulfate; REM: Remifentanil; MAP: Mean arterial pressure; PRBC: Packed red blood cells; Preop.: Preoperative.
The baseline heart rate was 84 ± 18 bpm in the MGS group and 81 ± 13 bpm in the REM group (P = 0.67). The mean heart rate changes at different time-points during surgery are shown in Figure 1. These changes were similar in both groups. The baseline MAP was 102 ± 12 mmHg and 99 ± 11 mmHg in the MGS and REM groups, respectively. Changes in MAP were similar in both groups (Figure 2). Five patients in the MGS group (25%) and 8 patients in the REM group (42.1%) failed to reach the target blood pressure and required NTG infusion at doses ranging from 0.5-1 μg/kg per minute (P = 0.32).
Figure 1.
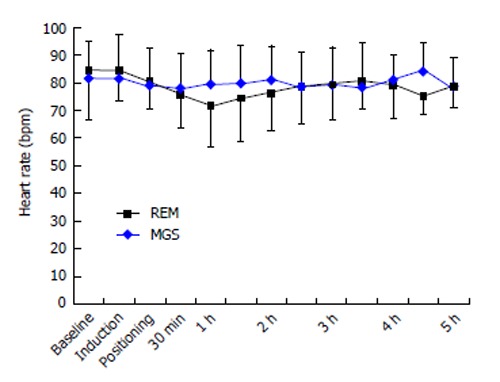
Temporal changes of the heart rate in both groups at the baseline, the induction of anesthesia, positioning, following 30 min and every hour thereafter. MGS: Magnesium sulfate; REM: Remifentanil.
Figure 2.
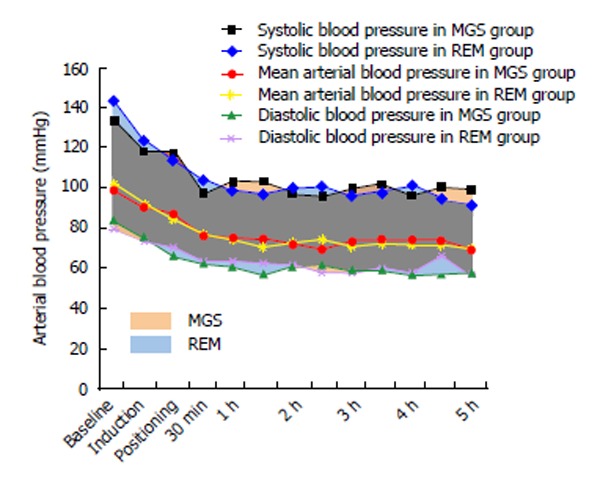
Temporal changes of the systolic, diastolic and mean arterial pressure in both groups at the baseline, the induction of anesthesia, positioning, following 30 min and every hour thereafter. MGS: Magnesium sulfate; REM: Remifentanil.
There were 4 episodes of hypotension (MAP ≤ 60) in the MGS group and 3 episodes in the REM group requiring pharmacological intervention (ephedrine boluses) (P = 0.732). There was no reported arrhythmias/tachycardia in either group. A total of 3 patients in the MGS group (15%) and 1 patient in the REM group received atropine due to bradycardia (HR ≤ 50 b/min), which was not significant (P = 0.61). Clinical assessment and neuromuscular monitoring of patients revealed a full recovery from the remaining effects of neuromuscular blocking drugs in all patients. Additionally, no patients had signs and symptoms of hypermagnesemia post-operatively.
DISCUSSION
We have shown that magnesium is as effective as remifentanil in inducing controlled hypotension during lumbar fusion surgery. Magnesium was able to reduce MAP (60-70 mmHg), similar to remifentanil. Equal numbers of patients needed supplemental nitroglycerine infusion to decrease the blood pressure to preset target levels. There was no life-threatening bradycardia, arrhythmias or hypotension in either group.
Controlled circulation, formerly known as deliberate hypotension, is used to reduce bleeding during surgery and to reduce blood transfusion. With surgical procedures on bone and some other vessel-rich areas where direct compression of the blood vessels is not feasible due to anatomical reasons, significant bleeding may occur. Therefore, a controlled hypotension may reduce bleeding and improve the surgical exposure in these patients. Our findings showed that magnesium has a similar efficacy in reducing bleeding as remifentanil in lumbar fusion surgeries. Additionally, surgeon satisfaction scores were used to subjectively assess the surgical bleeding and exposure. A five point Likert scale was used for this assessment. Likert scales may be subject to central tendency bias, where the respondents agree to neutral answers; acquiescence bias, where they agree with statements as presented; or social desirability bias, where they try to portray themselves or their organization in a more favorable light.
Previously, Ryu et al[22] compared the efficacy of remifentanil with magnesium to induce controlled hypotension in middle ear surgery. These authors demonstrated that magnesium and remifentanil were comparable in inducing controlled hypotension; however, the patients reported less post-operative pain in the magnesium group. Additionally, magnesium was associated with a lower incidence of postoperative nausea and/or vomiting and shivering during emergence from anesthesia when compared to those receiving remifentanil. Our findings confirmed the results reported by these investigators. Analgesic effects of magnesium were attributed to its ability to inhibit NMDA receptors in a non-competitive way. Although we did not study the analgesic effects of magnesium particularly, the intraoperative pain profile and requirement for morphine supplement was similar to remifentanil. Several other investigators also showed that the use of magnesium reduced the analgesic requirement after surgery[19,23,24].
Magnesium is used to induce controlled hypotension due to its inherent vasodilatory effects. Yosry et al[25] successfully used magnesium to induce controlled hypotension for choroid melanoma (a highly vascular tumor with a tendency to bleed) surgery. They compared magnesium to nitroprusside and eloquently demonstrated that choroidal blood flow as measured by laser Doppler flowmetry was reduced with magnesium, comparable to nitroprusside. There was no need for a pharmacological supplement in either group in order to decrease the blood pressure to the target levels. Although we needed to supplement our patients with intravenous NTG in 25% of patients to attain the preset target MAPs down to the 60-70 mmHg range, there was no difference between the REM and MGS groups in the amount of surgical bleeding. Similarly, Göral et al[26] demonstrated that magnesium significantly decreased the amount of surgical bleeding following lumbar discectomy surgery and improved the surgical exposure compared to normal saline controls. These authors used normal saline as a control, a strategy that we could not establish for both therapeutic and ethical reasons.
Cerebral perfusion is the main point of concern when deliberate hypotension is used in clinical settings. Although an intact auto-regulation is supposed to maintain a constant blood flow to the brain as long as MAPs are maintained equal to or greater than 50 mmHg, the adequacy of perfusion depends on cerebral metabolic rates. Controversy remains regarding the effects of magnesium on cerebral blood flow. While magnesium has been shown to increase cerebral blood flow in patients with preeclampsia, Wong et al[27] were unable to show any increase in regional cerebral blood flow using perfusion magnetic resonance imagings among 12 patients following acute subarachnoid hemorrhage following intravenous MGS infusion. In experimental settings, magnesium treatment preserves the ischemia-induced reduction in S-100 proteins, possibly by restoration of blood brain permeability during hypoxia[28]. Additionally, Mori et al[29] have described neuro-protective effects following intrathecal infusion of magnesium in a rat model of subarachnoid hemorrhage. Neuroprotective effects of magnesium are considered advantageous during controlled hypotension.
Cardiovascular complications associated with magnesium infusion, such as arrhythmia, hypotension, bradycardia and tachycardia, were similar between the MGS and REM groups in our study. Although the number of patients studied in our series was not powered to comment about the safety of magnesium, the incidence of these complications in patients after a similar dose of magnesium was rare in the literature. Moreover, previous research showed that magnesium might in fact be beneficial in reducing post myocardial infarction incidence of dysrhythmias, pump dysfunction and death[30]. Even the issue of retention of muscle relaxant drugs after surgery was not very concerning[31].
COMMENTS
Background
Bleeding becomes an important intraoperative issue when surgery is being performed on bone and other non-compressible tissues. Lumbar spine surgery, especially posterior spinal fusion, is not an exception to this complication. While other methods of hemostasis are rendered ineffective, deliberate reduction of the blood pressure has been effective in decreasing surgical bleeding and the likelihood of transfusion.
Research frontiers
Various drugs have been used to reduce blood pressure, including vasodilators, alpha-2 receptor agonists, alpha-1 antagonists and beta-adrenergic antagonists. However, hypotensive agents have several disadvantages, such as reflex tachycardia and tachyphylaxis. Therefore, it is necessary to use compounds with dose-response effects that are predictable. In recent years, remifentanil as a short acting opioid receptor agonist has been used for this purpose. This agent was used as a treatment arm in this study.
Innovations and breakthroughs
The authors concluded that administration of intravenous magnesium could be used as a good alternative to induce controlled hypotension, decrease surgical bleeding and provide a better surgical exposure in spinal surgeries. Although a pharmacoeconomic comparison was not the authors main objective in this study, the lower cost of magnesium compared to the other pharmacological alternatives may make it more cost effective.
Applications
The lack of a control group that received no magnesium and/or remifentanil may be a limitation of this study. The authors have shown that magnesium is as effective as remifentanil in inducing controlled hypotension during lumbar fusion surgery. Magnesium has been able to reduce mean arterial pressure (60-70 mmHg), similar to remifentanil. There was no life-threatening bradycardia, arrhythmias or hypotension in either group.
Peer review
The manuscript is well prepared and this is a well designed clinical trial. The study has been reviewed and approved by the institutional review board, following the ethical guidelines of human research. The only possible deficiency is the choice of remifentanil for induced hypotension because this is not a commonly used hypotensive agent.
Footnotes
P- Reviewers: Do SH, Nader ND, Peng BG S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Liu SQ
References
- 1.Sollevi A. Hypotensive anesthesia and blood loss. Acta Anaesthesiol Scand Suppl. 1988;89:39–43. doi: 10.1111/j.1399-6576.1988.tb02841.x. [DOI] [PubMed] [Google Scholar]
- 2.Patel NJ, Patel BS, Paskin S, Laufer S. Induced moderate hypotensive anesthesia for spinal fusion and Harrington-rod instrumentation. J Bone Joint Surg Am. 1985;67:1384–1387. [PubMed] [Google Scholar]
- 3.Malcolm-Smith NA, McMaster MJ. The use of induced hypotension to control bleeding during posterior fusion for scoliosis. J Bone Joint Surg Br. 1983;65:255–258. doi: 10.1302/0301-620X.65B3.6841391. [DOI] [PubMed] [Google Scholar]
- 4.Salem MR, Ivankovic AD. The place of beta-adrenergic blocking drugs in the deliberate induction of hypotension. Anesth Analg. 1970;49:427–434. [PubMed] [Google Scholar]
- 5.Larson AG. Deliberate Hypotension. Anesthesiology. 1964;25:682–706. doi: 10.1097/00000542-196409000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Blau WS, Kafer ER, Anderson JA. Esmolol is more effective than sodium nitroprusside in reducing blood loss during orthognathic surgery. Anesth Analg. 1992;75:172–178. doi: 10.1213/00000539-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Hersey SL, O’Dell NE, Lowe S, Rasmussen G, Tobias JD, Deshpande JK, Mencio G, Green N. Nicardipine versus nitroprusside for controlled hypotension during spinal surgery in adolescents. Anesth Analg. 1997;84:1239–1244. doi: 10.1097/00000539-199706000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Pilli G, Güzeldemir ME, Bayhan N. Esmolol for hypotensive anesthesia in middle ear surgery. Acta Anaesthesiol Belg. 1996;47:85–91. [PubMed] [Google Scholar]
- 9.Porter SS, Asher M, Fox DK. Comparison of intravenous nitroprusside, nitroprusside-captopril, and nitroglycerin for deliberate hypotension during posterior spine fusion in adults. J Clin Anesth. 1988;1:87–95. doi: 10.1016/0952-8180(88)90027-x. [DOI] [PubMed] [Google Scholar]
- 10.Saarnivaara L, Klemola UM, Lindgren L. Labetalol as a hypotensive agent for middle ear microsurgery. Acta Anaesthesiol Scand. 1987;31:196–201. doi: 10.1111/j.1399-6576.1987.tb02549.x. [DOI] [PubMed] [Google Scholar]
- 11.Degoute CS. Controlled hypotension: a guide to drug choice. Drugs. 2007;67:1053–1076. doi: 10.2165/00003495-200767070-00007. [DOI] [PubMed] [Google Scholar]
- 12.Degoute CS, Ray MJ, Manchon M, Dubreuil C, Banssillon V. Remifentanil and controlled hypotension; comparison with nitroprusside or esmolol during tympanoplasty. Can J Anaesth. 2001;48:20–27. doi: 10.1007/BF03019809. [DOI] [PubMed] [Google Scholar]
- 13.Guy J, Hindman BJ, Baker KZ, Borel CO, Maktabi M, Ostapkovich N, Kirchner J, Todd MM, Fogarty-Mack P, Yancy V, et al. Comparison of remifentanil and fentanyl in patients undergoing craniotomy for supratentorial space-occupying lesions. Anesthesiology. 1997;86:514–524. doi: 10.1097/00000542-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Davis PJ, Lerman J, Suresh S, McGowan FX, Coté CJ, Landsman I, Henson LG. A randomized multicenter study of remifentanil compared with alfentanil, isoflurane, or propofol in anesthetized pediatric patients undergoing elective strabismus surgery. Anesth Analg. 1997;84:982–989. doi: 10.1097/00000539-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Fodale V, Schifilliti D, Praticò C, Santamaria LB. Remifentanil and the brain. Acta Anaesthesiol Scand. 2008;52:319–326. doi: 10.1111/j.1399-6576.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- 16.Philip BK, Scuderi PE, Chung F, Conahan TJ, Maurer W, Angel JJ, Kallar SK, Skinner EP, Jamerson BD. Remifentanil compared with alfentanil for ambulatory surgery using total intravenous anesthesia. The Remifentanil/Alfentanil Outpatient TIVA Group. Anesth Analg. 1997;84:515–521. doi: 10.1097/00000539-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Schüttler J, Albrecht S, Breivik H, Osnes S, Prys-Roberts C, Holder K, Chauvin M, Viby-Mogensen J, Mogensen T, Gustafson I, et al. A comparison of remifentanil and alfentanil in patients undergoing major abdominal surgery. Anaesthesia. 1997;52:307–317. doi: 10.1111/j.1365-2044.1997.24-az0051.x. [DOI] [PubMed] [Google Scholar]
- 18.Koinig H, Wallner T, Marhofer P, Andel H, Hörauf K, Mayer N. Magnesium sulfate reduces intra- and postoperative analgesic requirements. Anesth Analg. 1998;87:206–210. doi: 10.1097/00000539-199807000-00042. [DOI] [PubMed] [Google Scholar]
- 19.Tramer MR, Schneider J, Marti RA, Rifat K. Role of magnesium sulfate in postoperative analgesia. Anesthesiology. 1996;84:340–347. doi: 10.1097/00000542-199602000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Elsharnouby NM, Elsharnouby MM. Magnesium sulphate as a technique of hypotensive anaesthesia. Br J Anaesth. 2006;96:727–731. doi: 10.1093/bja/ael085. [DOI] [PubMed] [Google Scholar]
- 21.James MF, Beer RE, Esser JD. Intravenous magnesium sulfate inhibits catecholamine release associated with tracheal intubation. Anesth Analg. 1989;68:772–776. [PubMed] [Google Scholar]
- 22.Ryu JH, Sohn IS, Do SH. Controlled hypotension for middle ear surgery: a comparison between remifentanil and magnesium sulphate. Br J Anaesth. 2009;103:490–495. doi: 10.1093/bja/aep229. [DOI] [PubMed] [Google Scholar]
- 23.Levaux Ch, Bonhomme V, Dewandre PY, Brichant JF, Hans P. Effect of intra-operative magnesium sulphate on pain relief and patient comfort after major lumbar orthopaedic surgery. Anaesthesia. 2003;58:131–135. doi: 10.1046/j.1365-2044.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- 24.Telci L, Esen F, Akcora D, Erden T, Canbolat AT, Akpir K. Evaluation of effects of magnesium sulphate in reducing intraoperative anaesthetic requirements. Br J Anaesth. 2002;89:594–598. doi: 10.1093/bja/aef238. [DOI] [PubMed] [Google Scholar]
- 25.Yosry M, Othman IS. Controlled hypotension in adults undergoing choroidal melanoma resection: comparison between the efficacy of nitroprusside and magnesium sulphate. Eur J Anaesthesiol. 2008;25:891–896. doi: 10.1017/S0265021508004584. [DOI] [PubMed] [Google Scholar]
- 26.Göral N, Ergil J, Alptekin A, Ozkan D, Gürer B, Dolgun H, Gümüs H. Effect of magnesium sulphate on bleeding during lumbar discectomy. Anaesthesia. 2011;66:1140–1145. doi: 10.1111/j.1365-2044.2011.06898.x. [DOI] [PubMed] [Google Scholar]
- 27.Wong GK, Kwok R, Tang K, Yeung D, Ahuja A, King AD, Poon WS. Effects of magnesium sulfate infusion on cerebral perfusion in patients after aneurysmal SAH. Acta Neurochir Suppl. 2010;106:133–135. doi: 10.1007/978-3-211-98811-4_23. [DOI] [PubMed] [Google Scholar]
- 28.Goñi-de-Cerio F, Alvarez A, Alvarez FJ, Rey-Santano MC, Alonso-Alconada D, Mielgo VE, Gastiasoro E, Hilario E. MgSO4 treatment preserves the ischemia-induced reduction in S-100 protein without modification of the expression of endothelial tight junction molecules. Histol Histopathol. 2009;24:1129–1138. doi: 10.14670/HH-24.1129. [DOI] [PubMed] [Google Scholar]
- 29.Mori K, Yamamoto T, Miyazaki M, Hara Y, Aiko Y, Koike N, Sakamoto S, Nakao Y, Esaki T. Effect of intrathecal magnesium sulfate solution injection via a microcatheter in the cisterna magna on cerebral vasospasm in the canine subarachnoid haemorrhage model. Br J Neurosurg. 2012;26:64–68. doi: 10.3109/02688697.2011.591948. [DOI] [PubMed] [Google Scholar]
- 30.Gyamlani G, Parikh C, Kulkarni AG. Benefits of magnesium in acute myocardial infarction: timing is crucial. Am Heart J. 2000;139:703. doi: 10.1016/s0002-8703(00)90051-6. [DOI] [PubMed] [Google Scholar]
- 31.Ghodraty MR, Saif AA, Kholdebarin AR, Rokhtabnak F, Pournajafian AR, Nikzad-Jamnani AR, Shah A, Nader ND. The effects of magnesium sulfate on neuromuscular blockade by cisatracurium during induction of anesthesia. J Anesth. 2012;26:858–863. doi: 10.1007/s00540-012-1439-x. [DOI] [PubMed] [Google Scholar]


