Abstract
Aim
The PlA1/A2 polymorphism of glycoprotein IIIa (GPIIIa) has been associated with both antiplatelet drug resistance and increased cardiovascular events. The aim of this study was to conduct the first meta-analysis investigating the association between carriage of the PlA2 allele and resistance to currently licensed antiplatelet drugs.
Methods
Electronic databases (MEDLINE and EMBASE) were searched for all articles evaluating genetic polymorphisms of GPIIIa. For studies where antiplatelet resistance was measured using validated techniques, pooled odds ratios (ORs) were calculated using fixed effects and random effects models.
Results
Sixteen studies were eligible for statistical analysis and included 1650 PlA1 homozygous subjects and 668 carriers of the PlA2 allele. For carriers of the PlA2 allele, OR 0.924 (n = 2318; 95% CI 0.743, 1.151; P = 0.481) was observed for resistance to any antiplatelet drug, OR 0.862 (n = 2085; 95% CI 0.685, 1.086; P = 0.208) for resistance to aspirin and OR 1.429 (n = 233; 95% CI 0.791, 2.582; P = 0.237) for resistance to clopidogrel. In the aspirin cohort, sub-group analysis revealed no statistical association in either healthy subjects or those with cardiovascular disease. PlA2 carriage was marginally associated with aspirin sensitivity using the fixed effects model when identified by the PFA-100 assay (n = 1151; OR 0.743, 95% CI 0.558, 0.989; P = 0.041) but with significant heterogeneity (I2 = 55%; P = 0.002). Significance was lost with analysis using a random effects model.
Conclusions
The totality of published data does not support an association between carriage of the PlA2 allele and antiplatelet drug resistance. Significant heterogeneity indicates the need for larger studies using validated and standardized assays.
Keywords: antiplatelet drugs, drug resistance, genetic
What is Already Known about this Subject.
Resistance to antiplatelet drugs is associated with adverse cardiovascular outcomes.
The PlA1/A2 polymorphism of glycoprotein IIIa has been identified as a possible biomarker for antiplatelet resistance.
There exists poor inter-study agreement in relation to the impact of carriage of the PlA2 allele.
What this Study Adds.
Carriage of the PlA2 allele does not appear to be a biomarker for resistance to aspirin, P2Y12 antagonists or glycoprotein IIb/IIIa inhibitors.
Calculated odds ratios are dependent on the method used for defining resistance.
Significant inter-study heterogeneity prevents detailed sub-group analysis and the need for further studies remains.
Introduction
The central role of platelets is to terminate haemorrhage following vascular injury [1]. The fibrinogen receptor is integral to this process as it represents the final common pathway of platelet activation, adhesion and aggregation, and binds a combination of fibrinogen, von Willebrand factor (vWF) and fibronectin [2]. The fibrinogen receptor is the most abundant integrin on the platelet surface [3] and is formed from two subunits: glycoprotein IIb (GPIIb, integrin αIIb) and glycoprotein IIIa (GPIIIa, integrin β3). The GPIIIa subunit is polymorphic with single amino acid substitutions resulting in a number of stable allelic variants [4]. The PlA1/A2 diallelic antigen system is one of the more heavily studied due to its involvement in alloimmunity [4] and is subject to ongoing controversy relating to its possible association with cardiovascular disease and resistance to antiplatelet agents. The prevalence of the PlA2 allele is dependent on ethnicity, with a frequency of approximately 15 per 100 in Caucasian populations [5] falling to 1 per 100 in Oriental populations [6].
Lying within the extracellular domain, the PlA1 epitope is transformed into that of the PlA2 allelic variant by a single amino acid substitution of proline for leucine at position 33 [7]. The proximity of the epitope to the ligand binding site stimulated investigation into the impact of the polymorphism on the binding of fibrinogen and vWF, with results in static systems showing no significant differences in maximal binding (Bmax) or dissociation constant (Kd) [8,9]. In cell culture under conditions of shear stress, enhanced adhesion to both ligands was subsequently observed in the presence of the PlA2 allele, and this was attributed to enhancement of outside-in signalling [10,11]. These disagreements between studies extend into observations regarding possible differences in basal level of platelet activation in the resting state and in the activation of platelets in response to various agonists [8,12].
Despite this lack of clarity into the molecular impact of the single nucleotide polymorphism, carriage of the PlA2 allele showed early promise as a biomarker for cardiovascular risk when its prevalence was noted to be higher in those with acute coronary thrombosis, especially in patients with a primary event occurring under the age of 60 years [13]. Meta-analyses have since yielded inconsistent results, with no association found with myocardial infarction [14] but a positive association found with coronary artery disease more generally (odds ratio (OR) 1.10; 95% CI 1.03, 1.18) [15]. A possible mechanism for increased cardiovascular risk is resistance to the antiplatelet drug aspirin (acetylsalicylic acid), with a meta-analysis in 2008 finding a significant association between carriage of the PlA2 allele and aspirin resistance in healthy subjects (OR 2.36; 95% CI 1.24, 4.49; P = 0.009) [16]. However in the same study, no such difference was identified in subjects with cardiovascular disease (OR 0.92; 95% CI 0.65, 1.30; P = 0.64).
The heterogeneity in the findings observed in these meta-analyses are a reflection of the heterogeneity of the populations studied and the diverse methods used for assessing endpoints. There remains a need to assess definitively the PlA2 allele as a biomarker for cardiovascular disease and, if confirmed as a useful biomarker, to refine current hypotheses into the underlying mechanism. To this end, we present here the first systematic review and meta-analysis investigating the impact of carriage of the PlA2 allele on the efficacy of all currently licensed antiplatelet agents.
Methods
Data sources
Electronic databases (MEDLINE and EMBASE) were searched up until 1 April 2013 for all articles evaluating genetic polymorphisms in the platelet GpIIIa receptor. The Medical Subject Headings terms and text words used for the primary search were ‘genetic’, ‘gene’, ‘polymorphism’, ‘mutation’ and ‘genotype’ in combination with ‘glycoprotein IIIa’, ‘glycoprotein IIb/IIIa’, ‘GP IIIa’, ‘GP IIb/IIIa’, ‘integrin beta3’ and ‘ITGB3’. All languages were searched and included. A secondary search was performed on all potentially relevant articles for any additional articles. The inclusion criteria for the systematic review were the use of a licensed antiplatelet drug (either in vivo or in vitro) and measurement of platelet function. Inclusion into the meta-analysis required the definition and measurement of antiplatelet resistance using validated laboratory techniques.
Statistical analysis
Data were analyzed using Comprehensive Meta-analysis software, version 2 (Biostat, USA). For each antiplatelet drug for which data were available for at least two studies, a meta-analysis was performed. For each antiplatelet drug, a pooled OR was calculated using fixed and random effects models, along with the 95% CI to measure the strength of association. Fixed effects summary ORs were calculated using the Mantel–Haenszel method [17] and the DerSimonian method was used to calculate random effects summary ORs [18]. For data where an individual was assessed for drug sensitivity by multiple methods, combined effects were calculated [19].
Tests for heterogeneity were performed for each meta-analysis, with significance set at P < 0.05 [20]. For assessment of publication bias, we utilized a funnel plot and the Egger regression asymmetry test [21]. In addition, the effect of individual studies on the summary OR was evaluated by re-estimating and plotting the summary OR in the absence of each study.
Results
The primary search yielded 2288 articles of which 45 were identified as being potentially relevant. Following the addition of articles from the secondary search, a total of 36 articles met the inclusion criteria for the review, of which 16 contained suitable data for statistical analysis. Table 1 summarizes the number of studies identified based on individual antiplatelet drugs. Studies that were not eligible for inclusion in the statistical analysis are summarized in Tables 2–4.
Table 1.
Summary of number of studies included in the systematic review and meta-analysis
| Antiplatelet drug | Number of studies included in systematic review | Number of studies included in meta-analysis |
|---|---|---|
| Aspirin | 28 | 14 |
| Clopidogrel | 8 | 3 |
| Ticagrelor | 1 | 0 |
| Abciximab | 5 | 0* |
| Tirofiban | 3 | 0* |
| Eptifabide | 4 | 0* |
There are no accepted definitions of GPIIb/IIIa inhibitor resistance and so this drug class was not eligible for inclusion in the meta-analysis.
Table 2.
Summary of studies investigating the association of the PlA1/A2 polymorphism and resistance to aspirin which could not be included in the statistical analysis
| Study | Subject characteristics | Assessment methods | Comment |
|---|---|---|---|
| Andrioli et al. (2000) [36] | Healthy subjects (n = 16) | LTA (AA agonist) | PlA2 carriers demonstrated increased sensitivity to antiplatelet action of in vitro aspirin (1–100 μmol l−1) |
| Boudoulas et al. (2001) [37] | Healthy subjects (n = 30) | LTA (epinephrine agonist) | PlA2 carriers more sensitive to antiplatelet action of low dose in vitro aspirin in response to epinephrine agonist |
| Cooke et al. (1998) [38] | Healthy subjects (n = 26) | LTA (ADP and epinephrine agonist) | PlA1 homozygotes associated with a reduced response to in vitro aspirin (0.053–53 μmol l−1) |
| Cooke et al. (2006) [39] | Stable coronary artery disease (n = 20) | LTA (ADP, collagen and epinephrine agonists) | PlA2 carriers significantly were less inhibited by in vivo aspirin (325 mg once daily for 10 days) when collagen used as agonist |
| GPIIb/IIIa activation and α-granule release measured by flow cytometry (ADP, collagen and epinephrine agonists) | |||
| Dropinski et al. (2007) [40] | Male coronary artery disease (n = 28) | Thrombin generation and bleeding time | PlA2 carriers associated with reduced response to in vivo aspirin (300 mg once daily for 2 weeks) |
| Isordia-Salas et al. (2012) [41] | Emergency percutaneous coronary intervention for acute coronary syndrome (n = 60) | LTA (AA agonist) | No association between PlA2 carriers and non-response to in vivo aspirin (300 mg) |
| Lepantalo et al. (2006) [42] | Elective percutaneous coronary intervention (n = 101) | LTA (AA agonist) | No association between PlA2 carriers and response to in vivo aspirin (mean dose 100 mg once daily) |
| PFA-100 (collagen/epinephrine cartridge) | |||
| Lim et al. (2007) [43] | Postoperative period following coronary artery bypass grafting (n = 63) | LTA (ADP, collagen and epinephrine agonists) | PlA2 carriers had more impaired response to in vivo aspirin (325 mg or 100 mg once daily) but did not reach statistical significance |
| Michelson et al. (2000) [12] | Healthy subjects (n = 56) | LTA (epinephrine agonist) | PlA1/A2 more sensitive to in vitro aspirin than homozygous PlA1 and homozygous PlA2 |
| Morawski et al. (2005) [45] | Patients undergoing coronary artery bypass grafting (n = 51) | PFA-100 (collagen/ADP and collagen/epinephrine cartridges) | PlA2 carriers more sensitive to in vivo aspirin (150 mg) |
| Bleeding time | |||
| Stepien et al. (2007) [44] | Elective percutaneous coronary intervention (n = 31) | Thrombin generation | PlA2 carriers had statistically higher thrombin generation but no difference if sCD40L generation in response to in vivo aspirin |
| Soluble CD40 ligand (sCD40L) | |||
| Szczeklik et al. (2000) [46] | Healthy male subjects (n = 80) | Bleeding time and thrombin generation | Carriers of PlA2 allele demonstrated significantly reduced antiplatelet effect following in vivo aspirin (300 mg) |
| Undas et al. (1999) [47] | Healthy male subjects (n = 40) | Thrombin generation | PlA2 carriers had significantly impaired depression of thrombin generation in response in vivo aspirin (75 mg once daily for 7 days) |
| Undas et al. (2001) [48] | Healthy male subjects (n = 24) | Various measures of thrombin generation in bleeding time blood | PlA2 carriers had significantly impaired antithrombotic action in response to in vivo aspirin (75 mg once daily for 7 days) |
AA, arachidonic acid; ADP, adenosine diphosphate; GPIIb/IIIa, glycoprotein IIb/IIIa; LTA, light transmission aggregometry.
Table 4.
Summary of studies investigation the association of the PlA1/A2 polymorphism and resistance to GPIIb/IIIa inhibitors
| Study | Subject characteristics | Drug exposure | Assessment methods | Comment |
|---|---|---|---|---|
| Aalto-Setala et al. (2005) [54] | Healthy subjects (n = 28) | Abciximab in vitro (0–3 μg ml−1) | PFA-100 (ADP/epinephrine cartridges) | PlA2 carriers more sensitive to antiplatelet effects of tirofiban with sodium citrate as anticoagulant |
| Tirofiban in vitro (0-70 ng ml−1) | Effect abolished if PPACK anticoagulant used | |||
| Eptifabide in vitro (0–1 μg ml−1) | ||||
| Michelson et al. (2000) [12] | Healthy subjects (n = 56) | Abciximab in vitro (0.5–2.5 μg ml−1) | LTA (ADP agonist) | PlA2 allele conferred increased sensitiviy |
| Sirotkina et al. (2007) [55] | Healthy subjects (n = 35) | Eptifabide in vitro (50–150 mg ml−1) | LTA (ADP agonist) | No significant difference in aggregation between genotypes |
| Verdoia et al. (2013) [56] | Elective percutaneous coronary intervention (n = 40) | Abciximab in vivo (0.25 mg kg−1 bolus, 0.125 μg kg−1 min−1 infusion) | Impedance aggregometry (AA, ADP, collagen, prostaglandin E1 and TRAP agonists) | The PlA2 allele did not influence platelet response to either abciximab or the small molecule inhibitors |
| Elective percutaneous coronary intervention (n = 40) | Tirofiban in vivo (25 μg kg−1 bolus, 0.1 μg kg−1 min−1 infusion) | |||
| or | ||||
| Eptifabide in vivo (double bolus 180 μg kg−1, 2 μg kg−1 min−1 infusion) | ||||
| Weber et al. (2002) [57] | Healthy subjects (n = 62) and Coronary artery disease (n = 177) | Abciximab in vitro (0.03–3 μg ml−1) | Fibrinogen binding measured by flow cytometry (ADP agonist) | The PlA2 allele did not significantly influence fibrinogen binding in either healthy subjects or patients with coronary artery disease |
| Tirofiban in vitro (0.3–30 nmol l−1) | ||||
| Eptifabide in vitro (0.01–1 μg ml−1) | ||||
| Wheeler et al. (2002) [58] | Patients undergoing percutaneous coronary intervention (n = 87) | Abciximab in vivo (0.25 mg kg−1 bolus, 10 μg min−1 infusion) | LTA (ADP agonist) | Less completely inhibited at 1 h as assessed by LTA |
| Ultegra rapid platelet function assay (TRAP agonist) | Less completely inhibited at 1 h and 24 h as assessed by Ultegra | |||
| Abciximab binding assay | PlA2 carriers had less receptor occupancy at 24 h |
AA, arachidonic acid; ADP, adenosine diphosphate; LTA, light transmission aggregometry; PPACK, Phe-Pro-Arg-chloromethylketone; TRAP, thrombin receptor activating peptide.
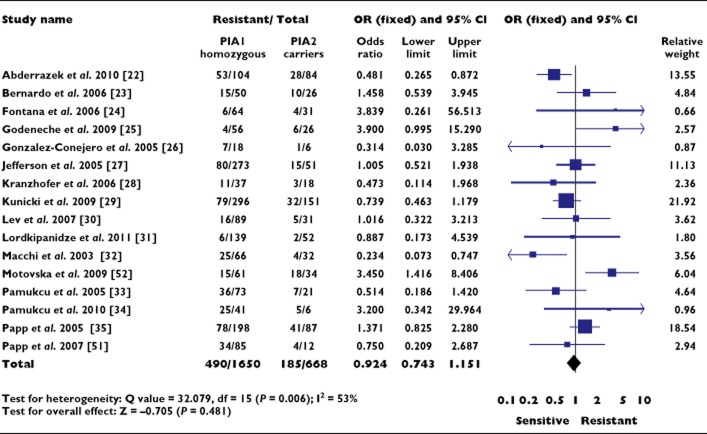
The 16 studies analyzed statistically included 1650 subjects who were homozygous for PlA1 and 668 carriers of the PlA2 allele. An OR of 0.924 (95% CI 0.743, 1.151; P = 0.481) was observed for resistance to any antiplatelet drug in subjects carrying the PlA2 allele (Figure 1). Significant heterogeneity existed between studies (P = 0.006) and analysis using the random effects model did not lead to a significant association (OR 0.955, 95% CI 0.663, 1.376; P = 0.804). The distribution of the log OR in relation to precision was symmetrical with a non-significant Egger regression test (P = 0.675), suggesting low probability of publication bias.
Figure 1.
Association between the PlA1/A2 polymorphism and resistance to all antiplatelet drugs. OR, odds ratio; CI, confidence interval
Aspirin
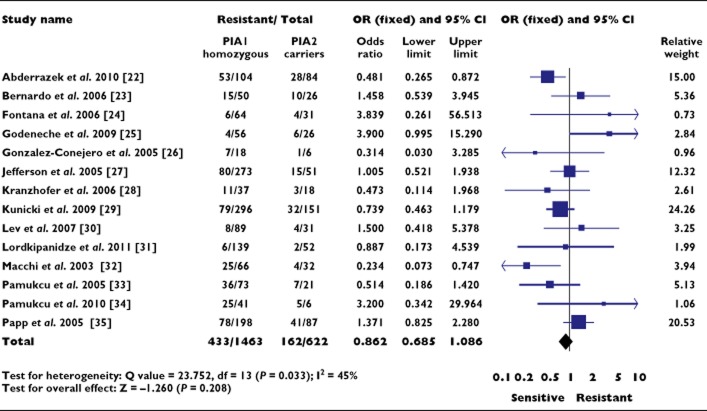
Fourteen of the 28 studies investigating aspirin resistance were eligible for inclusion in the statistical analysis and included 1463 subjects who were homozygous for the PlA1 allele and 622 who carried the PlA2 allele [22–35]. The remaining 14 studies are summarized in Table 2.
An OR of 0.862 (95% CI 0.685, 1.086; P = 0.208) was observed for aspirin resistance in subjects carrying the PlA2 allele (Figure 2). Significant heterogeneity was again present (P = 0.033) with a low probability of publication bias (P = 0.663).
Figure 2.
Association between the PlA1/A2 polymorphism and aspirin resistance. OR, odds ratio; CI, confidence interval
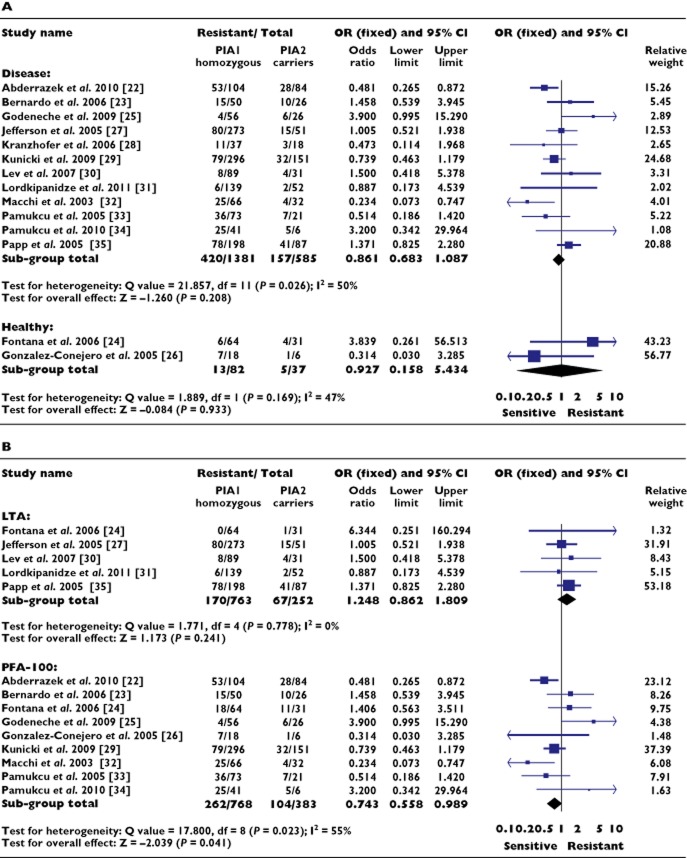
Initial sub-group analysis of the aspirin cohort considered whether sensitivity to the drug in relation to the polymorphism was dependent on the presence or absence of cardiovascular disease, since such a dependence was previously reported [16]. No such association was identified either in patients with cardiovascular disease (OR 0.861, 95% CI 0.683, 1.087; P = 0.208) or in healthy subjects (OR 0.927, 95% CI 0.158, 5.434; P = 0.933) (Figure 3A).
Figure 3.
Association between the PlA1/A2 polymorphism and aspirin resistance, sub-group analyses. (A) Cardiovascular disease vs. healthy subjects. (B) Light transmission aggregometry vs. PFA-100. OR, odds ratio; CI, confidence interval
A further sub-group analysis of the aspirin cohort considered how the methodology used to assess the presence of aspirin resistance might influence any association. The available data permitted the comparison of two methods: light transmission aggregometry (LTA) and the point-of-care assay PFA-100 (Figure 3B).
The use of LTA did not reveal a significant association between carriage of the PlA2 allele and aspirin resistance (OR 1.248, 95% CI 0.862, 1.809; P = 0.241), but did show significant homogeneity between studies (P = 0.778). This homogeneity is despite variation in agonists used to identify aspirin resistance, with three studies using the conventional cut-off value of 20% aggregation in response to arachidonic acid (AA) [24,27,31], and the remaining two studies assessing aggregation in response to two independently administered agonists [30,35]. Sub-group analysis of the three studies using AA as the sole agonist did not increase homogeneity when compared with the entire aspirin LTA cohort (P = 0.535), nor result in a significant association with aspirin resistance (OR 1.053, 95% CI 0.579, 1.919; P = 0.886).
Use of the PFA-100 assay revealed a significant association between aspirin sensitivity and carriage of the PlA2 allele (OR 0.743, 95% CI 0.558, 0.989; P = 0.041), but with significant heterogeneity between studies (P = 0.023); significance was lost with use of the random effects model (OR 0.793, 95% CI 0.522, 1.204; P = 0.277). As with LTA, the PFA-100 assay enables the testing of different agonists, with the choice of either a collagen and epinephrine (CEPI) cartridge or a collagen and adenosine diphosphate (CADP) cartridge. In all studies, the CEPI cartridge was used to define aspirin resistance with a mean closure time of <198 ± 37 s. In four of these studies, platelet reactivity in response to the CADP cartridge was compared between the CEPI-defined aspirin resistant and aspirin sensitive subjects. A significant (P < 0.01) reduction in CADP closure time was seen in those who had CEPI-defined aspirin resistance, suggesting that these patients had an increase in global platelet reactivity independent of changes related solely to the COX-1 pathway [23,25,29,32].
Studies that were not suitable for inclusion in the meta-analysis included 300 healthy subjects and 354 with cardiovascular disease (Table 2) [12,36–48]. Conclusions from the studies were similarly heterogeneous, with half of the studies suggesting an association between carriage of the PlA2 allele and aspirin resistance [39,40,43,44,46–48].
P2Y12 receptor antagonists
Nine studies were identified that investigated the impact of the PlA1/PlA2 polymorphism on the efficacy of P2Y12 antagonists [24,30,39,41,49–53]. All participants in these studies received in vivo clopidogrel except for a single study investigating in vivo ticagrelor [53]. No studies were identified that considered the efficacy of prasugrel in association with carriage of the PlA2 allele. Three studies were analyzed and included 100 clopidogrel-resistant and 212 clopidogrel-sensitive patients with coronary artery disease [30,51,52]. An OR of 1.429 (95% CI 0.791, 2.582; P = 0.237) was observed for clopidogrel resistance in subjects carrying the PlA2 allele (Figure 4). Significant heterogeneity existed between studies (P = 0.034). Despite the small number of studies, the distribution of the log OR in relation to precision was symmetrical with a non-significant Egger regression test (P = 0.48), suggesting low probability of publication bias.
Figure 4.
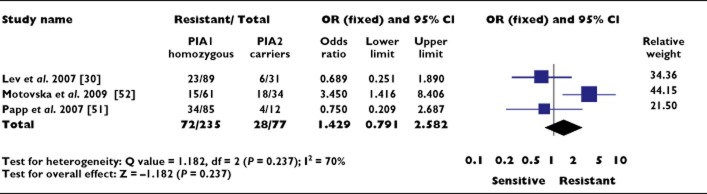
Association between the PlA1/A2 polymorphism and clopidogrel resistance. OR, odds ratio; CI, confidence interval
Sub-group analysis of the two studies utilizing LTA decreased the OR to below unity, suggesting a possible association of clopidogrel sensitivity with PlA2 carriage, but this did not achieve significance (OR 0.712, 95% CI 0.322, 1.571; P = 0.400) [30,51].
Six studies did not contain adequate data for statistical analysis and are summarized in Table 3 [24,39,41,49,50,53]. Of these studies, one reported a positive association between carriage of the PlA2 allele and clopidogrel resistance [49], one reported a negative association [50] and the remainder failed to identify any association. The single study considering the influence that carriage of the PlA2 allele might have on the efficacy of in vivo ticagrelor found no significant association in patients with cardiovascular disease [53]. Within all of the above studies no trend in response was identified in relation to subject characteristics, assessment methods or drug dosing schedules.
Table 3.
Summary of studies investigating the association of the PlA1/A2 polymorphism and resistance to P2Y12 antagonists which could not be included in the statistical analysis
| Study | Subject characteristics | Assessment methods | Comment |
|---|---|---|---|
| Angiolillo et al. (2004) [49] | Elective percutaneous coronary intervention (n = 38) | GPIIb/IIIa activation and P-selectin expression measured by flow cytometry (ADP agonist) | PlA2 carriers had significantly higher GPIIb/IIIa activation in response to in vivo clopidogrel (single 300 mg dose) |
| Cooke et al. (2006) [39] | Stable coronary artery disease (n = 20) | LTA (ADP, collagen and epinephrine agonists) | PlA1/A2 genotype significantly more sensitive to ADP and trending to be less sensitive to collagen when compared to PlA1/A1 following in vivo clopidogrel (75 mg once daily for 10 days) |
| GPIIb/IIIa activation and α-granule release measured by flow cytometry (ADP, collagen and epinephrine agonists) | |||
| Dropinski et al. (2005) [50] | Male coronary artery disease or myocardial infarction >6 months previously (n = 48) | Bleeding time and thrombin generation | Greater increase in bleeding time and PFA-100 closure time for PlA2 carriers, with a greater reduction of P-selectin expression |
| PFA-100 (ADP/collagen cartridge) | PlA2 carriers demonstrated superior antithrombotic response to in vivo clopidogrel (75 mg once daily for 14 days) | ||
| GPIIb/IIIa activation and P-selectin expression measured by flow cytometry (ADP agonist) | |||
| Fontana et al. (2006) [24] | Healthy males (n = 94) | LTA (ADP agonist) | No association between PlA2 carriers and response to in vivo clopidogrel (75 mg once daily for 1 week) |
| Isordia-Salas et al. (2012) [41] | Emergency percutaneous coronary intervention for acute coronary syndrome (n = 60) | LTA (ADP agonist) | No association between PlA2 carriers and non-response to in vivo clopidogrel (300 mg loading dose) |
| Storey et al. (2009) [53] | Stable atherosclerotic disease or non-ST segment elevation acute coronary syndrome (n = 147) | LTA (ADP agonist) | PlA2 allele did not influence response to in vivo ticagrelor (100 mg once daily for 28 days) |
ADP, adenosine diphosphate; GPIIb/IIIa, glycoprotein IIb/IIIa; LTA, light transmission aggregometry.
Glycoprotein IIb/IIIa inhibitors
Six studies were identified which examined the three licensed GPIIb/IIIa inhibitors (GPIs) abciximab, tirofiban and eptifabide [12,54–58]. None of these studies was suitable for inclusion into the meta-analysis due to the lack of existing criteria defining resistance within this drug class. Table 4 summarizes the findings of these studies.
Of the six studies, a significant difference in drug efficacy related to PlA2 carriage was observed in two studies but with opposite effects. Carriage of the PlA2 allele was observed to exhibit increased sensitivity to in vitro abciximab in 56 healthy subjects, but a statistically significant difference was seen only between PlA1/A2 vs. PlA2/A2 genotypes (P = 0.046) [12]. The converse effect was observed in vivo where, in the presence of the PlA2 allele, aggregation was found to be less completely inhibited following a bolus of abciximab during percutaneous coronary intervention [58]. In this clinical study, the observed effect was assay-dependent as significance was no longer present at 24 h when assessed by LTA, but remained present when assessed by the Ultegra rapid platelet function assay. Using impedance aggregometry neither of these findings has been replicated in vivo or in vitro [56,57].
The findings of studies investigating the small molecule GPIs (eptifabide and tirofiban) concur in their results, irrespective of subject cohort or method of drug exposure. In four studies comprising a total of 125 healthy subjects and 217 patients, no differences were detected between PlA2 carriers and non-carriers using a variety of assessment methods [54–57].
Discussion
In this systematic review and meta-analysis of 36 studies totalling 3539 subjects we have found no consistent association between the PlA1/A2 polymorphism and resistance to antiplatelet drugs, either overall or for individual drugs. Given the heterogeneous nature of the studies in relation to subject recruitment, antiplatelet drug used and methods of measurement, sub-group analyses were performed according to the parameters described above.
Results from analysis of the aspirin cohort broadly concur with those of a meta-analysis into the genetics of aspirin resistance performed in 2007 [16]. However, in healthy subjects, Goodman et al. observed that carriage of the PlA2 allele was significantly associated with aspirin resistance (OR 2.26, 95% CI 1.24, 4.49; P = 0.009) as opposed to the non-significant result presented here (OR 1.102, 95% CI 0.480, 2.533; P = 0.229). Our exclusion of two studies based on a lack of appropriate definition of aspirin resistance and non-standard measures of platelet function accounts for this discrepancy [46,47].
LTA in response to AA and PFA-100 (using the CEPI cartridge) are the assays most commonly used to define aspirin resistance, as they are designed to challenge the COX-1 pathway of platelet activation which aspirin specifically inhibits. Despite both assays targeting the same pathway, they gave rise to apparently diverging results, with the ‘gold standard’ assay LTA favouring resistance albeit non-significantly (OR 1.248, 95% CI 0.862, 1.809; P = 0.241) and the point-of-care test PFA-100 favouring sensitivity (OR 0.743, 95% CI 0.558, 0.989; P = 0.041) to aspirin in the presence of the PlA2 allele. The discrepancy in the results of the two assays is perhaps unsurprising given the significant intra-and inter-individual variability associated with platelet function testing. In fact, the PFA-100 correlates especially poorly with LTA, as demonstrated by the two techniques identifying a prevalence of aspirin resistance of 59.6% and 4.0%, respectively, in a cohort of 201 patients with stable coronary artery disease [59]. This variation in assay sensitivity and specificity is also clearly highlighted by the range of intra-study results that are dependent on method of assessment [24,26,39,44,58]. Additionally, whilst the criteria for aspirin resistance by LTA were relatively uniform, the closure time identifying aspirin resistance by PFA-100 varied greatly between studies (<198 ± 37 s).
The choice of assay with which to measure aspirin resistance is generally guided by the wish to investigate COX-1 dependent responses, with the assumption that failure to suppress COX-1 activity by aspirin will give rise to increased cardiovascular events. Indeed, a correlation between residual COX-1 activity and cardiovascular events has been demonstrated in a number of studies [60,61], but should not be assumed to be the sole determinant of platelet aggregation in aspirin-treated patients. In a study of 700 aspirin-treated patients undergoing cardiac catheterization, it was response as assessed by the PFA-100 CADP cartridge rather than the CEPI cartridge that was significantly associated with major adverse cardiovascular outcomes [62]. It is interesting to note that, in the aspirin cohort, all who were investigated using the PFA-100 CADP cartridge demonstrated a significantly shortened closure time if defined aspirin resistant by the CEPI cartridge. This observation suggests an increased underlying level of platelet reactivity in such patients, independent of COX-1.
One limitation of this systematic review is the paucity of studies considering drugs other than aspirin, with clopidogrel being the only other drug eligible for statistical analysis. The weight of studies not included in the meta-analysis do however support the statistical results in demonstrating no significant association between the PlA1/A2 polymorphism and clopidogrel resistance.
Similarly, there were few studies considering the impact of the PlA2 allele on GPI efficacy, with the additional limitation that, due to there being no defined criteria for resistance to these drugs, statistical analysis was not possible. Once again however, no consistent pattern was seen between PlA2 carriage and response to the three licensed GPIs, in vitro or in vivo.
These technical issues and study limitations likely contribute to the significant heterogeneity observed within our analyses. Publication bias was not identified to be a contributing factor. Such heterogeneity indicates that, if more data were available in the future, more refined sub-group analyses considering parameters such as gender, age, co-morbidities and biochemical indices may reveal a possible effect of the PlA1/A2 polymorphism on antiplatelet drug resistance.
In conclusion, the data presented do not support an association between carriage of the PlA2 allele of GPIIIa and resistance to antiplatelet drugs, in either healthy subjects or those with cardiovascular disease, irrespective of the method of assessment. Significant heterogeneity was observed throughout the analyses and underlines the necessity for larger studies using validated and standardized assays to assess accurately any potential impact of this polymorphism.
Competing Interests
Both authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Pinniger JL, Prunty FT. Some observations on the blood-clotting mechanism; the role of fibrinogen and platelets, with reference to a case of congenital afibrinogenaemia. Br J Exp Pathol. 1946;27:200–210. [PMC free article] [PubMed] [Google Scholar]
- 2.Floyd C, Ferro A. The platelet fibrinogen receptor: from megakaryocyte to the mortuary. J R Soc Med Cardiovasc Dis. 2012;1:7. doi: 10.1258/cvd.2012.012007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner CL, Mascelli MA, Neblock DS, Weisman HF, Coller BS, Jordan RE. Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood. 1996;88:907–914. [PubMed] [Google Scholar]
- 4.Newman PJ, Valentin N. Human platelet alloantigens: recent findings, new perspectives. Thromb Haemost. 1995;74:234–239. [PubMed] [Google Scholar]
- 5.Simsek S, Faber NM, Bleeker PM, Vlekke AB, Huiskes E, Goldschmeding R, von dem Borne AE. Determination of human platelet antigen frequencies in the Dutch population by immunophenotyping and DNA (allele-specific restriction enzyme) analysis. Blood. 1993;81:835–840. [PubMed] [Google Scholar]
- 6.Lim J, Lal S, Ng KC, Ng KS, Saha N, Heng CK. Variation of the platelet glycoprotein IIIa PI(A1/A2) allele frequencies in the three ethnic groups of Singapore. Int J Cardiol. 2003;90:269–273. doi: 10.1016/s0167-5273(02)00567-3. [DOI] [PubMed] [Google Scholar]
- 7.Newman PJ, Derbes RS, Aster RH. The human platelet alloantigens, PlA1 and PlA2, are associated with a leucine33/proline33 amino acid polymorphism in membrane glycoprotein IIIa, and are distinguishable by DNA typing. J Clin Invest. 1989;83:1778–1781. doi: 10.1172/JCI114082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corral J, Gonzalez-Conejero R, Rivera J, Iniesta JA, Lozano ML, Vicente V. HPA-1 genotype in arterial thrombosis – role of HPA-1b polymorphism in platelet function. Blood Coagul Fibrinolysis. 1997;8:284–290. doi: 10.1097/00001721-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Bennett JS, Catella-Lawson F, Rut AR, Vilaire G, Qi W, Kapoor SC, Murphy S, FitzGerald GA. Effect of the Pl(A2) alloantigen on the function of beta(3)-integrins in platelets. Blood. 2001;97:3093–3099. doi: 10.1182/blood.v97.10.3093. [DOI] [PubMed] [Google Scholar]
- 10.Vijayan KV, Huang TC, Liu Y, Bernardo A, Dong JF, Goldschmidt-Clermont PJ, Alevriadou BR, Bray PF. Shear stress augments the enhanced adhesive phenotype of cells expressing the Pro33 isoform of integrin beta3. FEBS Lett. 2003;540:41–46. doi: 10.1016/s0014-5793(03)00170-4. [DOI] [PubMed] [Google Scholar]
- 11.Vijayan KV, Liu Y, Sun W, Ito M, Bray PF. The Pro33 isoform of integrin beta3 enhances outside-in signaling in human platelets by regulating the activation of serine/threonine phosphatases. J Biol Chem. 2005;280:21756–21762. doi: 10.1074/jbc.M500872200. [DOI] [PubMed] [Google Scholar]
- 12.Michelson AD, Furman MI, Goldschmidt-Clermont P, Mascelli MA, Hendrix C, Coleman L, Hamlington J, Barnard MR, Kickler T, Christie DJ, Kundu S, Bray PF. Platelet GP IIIa Pl(A) polymorphisms display different sensitivities to agonists. Circulation. 2000;101:1013–1018. doi: 10.1161/01.cir.101.9.1013. [DOI] [PubMed] [Google Scholar]
- 13.Weiss EJ, Bray PF, Tayback M, Schulman SP, Kickler TS, Becker LC, Weiss JL, Gerstenblith G, Goldschmidt-Clermont PJ. A polymorphism of a platelet glycoprotein receptor as an inherited risk factor for coronary thrombosis. N Engl J Med. 1996;334:1090–1094. doi: 10.1056/NEJM199604253341703. [DOI] [PubMed] [Google Scholar]
- 14.Zhu MM, Weedon J, Clark LT. Meta-analysis of the association of platelet glycoprotein IIIa PlA1/A2 polymorphism with myocardial infarction. Am J Cardiol. 2000;86:1000–1005. doi: 10.1016/s0002-9149(00)01136-x. A8. [DOI] [PubMed] [Google Scholar]
- 15.Di Castelnuovo A, de Gaetano G, Donati MB, Iacoviello L. Platelet glycoprotein receptor IIIa polymorphism PLA1/PLA2 and coronary risk: a meta-analysis. Thromb Haemost. 2001;85:626–633. [PubMed] [Google Scholar]
- 16.Goodman T, Ferro A, Sharma P. Pharmacogenetics of aspirin resistance: a comprehensive systematic review. Br J Clin Pharmacol. 2008;66:222–232. doi: 10.1111/j.1365-2125.2008.03183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester, West Sussex, UK: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 20.Deeks JJ. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001;323:157–162. doi: 10.1136/bmj.323.7305.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abderrazek F, Chakroun T, Addad F, Dridi Z, Gerotziafas G, Gamra H, Hassine M, Elalamy I. The GPIIIa PlA polymorphism and the platelet hyperactivity in Tunisian patients with stable coronary artery disease treated with aspirin. Thromb Res. 2010;125:e265–268. doi: 10.1016/j.thromres.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Bernardo E, Angiolillo DJ, Ramirez C, Cavallari U, Trabetti E, Sabate M, Hernandez R, Moreno R, Escaned J, Alfonso F, Banuelos C, Costa MA, Bass TA, Pignatti PF, Macaya C, Fernandez-Ortiz A. Lack of association between gene sequence variations of platelet membrane receptors and aspirin responsiveness detected by the PFA-100 system in patients with coronary artery disease. Platelets. 2006;17:586–590. doi: 10.1080/09537100600881412. [DOI] [PubMed] [Google Scholar]
- 24.Fontana P, Nolli S, Reber G, De Moerloose P. Biological effects of aspirin and clopidogrel in a randomized cross-over study in 96 healthy volunteers. J Thromb Haemost. 2006;4:813–819. doi: 10.1111/j.1538-7836.2006.01867.x. [DOI] [PubMed] [Google Scholar]
- 25.Godeneche G, Sorel N, Ragot S, Chomel JC, Neau JP, Macchi L. Stroke and aspirin non-responder patients: relation with hypertension and platelet response to adenosine diphosphate. Platelets. 2009;20:471–477. doi: 10.3109/09537100903171404. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Conejero R, Rivera J, Corral J, Acuna C, Guerrero JA, Vicente V. Biological assessment of aspirin efficacy on healthy individuals: heterogeneous response or aspirin failure? Stroke. 2005;36:276–280. doi: 10.1161/01.STR.0000151362.65339.f9. [DOI] [PubMed] [Google Scholar]
- 27.Jefferson BK, Foster JH, McCarthy JJ, Ginsburg G, Parker A, Kottke-Marchant K, Topol EJ. Aspirin resistance and a single gene. Am J Cardiol. 2005;95:805–808. doi: 10.1016/j.amjcard.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 28.Kranzhofer R, Ruef J. Aspirin resistance in coronary artery disease is correlated to elevated markers for oxidative stress but not to the expression of cyclooxygenase (COX) 1/2, a novel COX-1 polymorphism or the PlA(1/2) polymorphism. Platelets. 2006;17:163–169. doi: 10.1080/09537100500441101. [DOI] [PubMed] [Google Scholar]
- 29.Kunicki TJ, Williams SA, Nugent DJ, Harrison P, Segal HC, Syed A, Rothwell PM. Lack of association between aspirin responsiveness and seven candidate gene haplotypes in patients with symptomatic vascular disease. Thromb Haemost. 2009;101:123–133. [PubMed] [Google Scholar]
- 30.Lev EI, Patel RT, Guthikonda S, Lopez D, Bray PF, Kleiman NS. Genetic polymorphisms of the platelet receptors P2Y(12), P2Y(1) and GP IIIa and response to aspirin and clopidogrel. Thromb Res. 2007;119:355–360. doi: 10.1016/j.thromres.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Lordkipanidze M, Diodati JG, Palisaitis DA, Schampaert E, Turgeon J, Pharand C. Genetic determinants of response to aspirin: appraisal of 4 candidate genes. Thromb Res. 2011;128:47–53. doi: 10.1016/j.thromres.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 32.Macchi L, Christiaens L, Brabant S, Sorel N, Ragot S, Allal J, Mauco G, Brizard A. Resistance in vitro to low-dose aspirin is associated with platelet PlA1 (GP IIIa) polymorphism but not with C807T(GP Ia/IIa) and C-5T Kozak (GP Ibalpha) polymorphisms. J Am Coll Cardiol. 2003;42:1115–1119. doi: 10.1016/s0735-1097(03)00921-5. [DOI] [PubMed] [Google Scholar]
- 33.Pamukcu B, Oflaz H, Nisanci Y. The role of platelet glycoprotein IIIa polymorphism in the high prevalence of in vitro aspirin resistance in patients with intracoronary stent restenosis. Am Heart J. 2005;149:675–680. doi: 10.1016/j.ahj.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Pamukcu B, Oflaz H, Onur I, Hancer V, Yavuz S, Nisanci Y. Impact of genetic polymorphisms on platelet function and aspirin resistance. Blood Coagul Fibrinolysis. 2010;21:53–56. doi: 10.1097/MBC.0b013e328332ef66. [DOI] [PubMed] [Google Scholar]
- 35.Papp E, Havasi V, Bene J, Komlosi K, Czopf L, Magyar E, Feher C, Feher G, Horvath B, Marton Z, Alexy T, Habon T, Szabo L, Toth K, Melegh B. Glycoprotein IIIA (PlA) polymorphism aspirin resistance: Is there any correlation? Ann Pharmacother. 2005;39:1013–1018. doi: 10.1345/aph.1E227. [DOI] [PubMed] [Google Scholar]
- 36.Andrioli G, Minuz P, Solero P, Pincelli S, Ortolani R, Lussignoli S, Bellavite P. Defective platelet response to arachidonic acid and thromboxane A(2) in subjects with Pl(A2) polymorphism of beta(3) subunit (glycoprotein IIIa) Br J Haematol. 2000;110:911–918. doi: 10.1046/j.1365-2141.2000.02300.x. [DOI] [PubMed] [Google Scholar]
- 37.Boudoulas KD, Cooke GE, Roos CM, Bray PF, Goldschmidt-Clermont PJ. The PlA polymorphism of glycoprotein IIIa functions as a modifier for the effect of estrogen on platelet aggregation. Arch Pathol Lab Med. 2001;125:112–115. doi: 10.5858/2001-125-0112-TPAPOG. [DOI] [PubMed] [Google Scholar]
- 38.Cooke GE, Bray PF, Hamlington JD, Pham DM, Goldschmidt-Clermont PJ. PI (A2) polymorphism and efficacy of aspirin. Lancet. 1998;351:1253. doi: 10.1016/S0140-6736(05)79320-X. [DOI] [PubMed] [Google Scholar]
- 39.Cooke GE, Liu-Stratton Y, Ferketich AK, Moeschberger ML, Frid DJ, Magorien RD, Bray PF, Binkley PF, Goldschmidt-Clermont PJ. Effect of platelet antigen polymorphism on platelet inhibition by aspirin, clopidogrel, or their combination. J Am Coll Cardiol. 2006;47:541–546. doi: 10.1016/j.jacc.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 40.Dropinski J, Musial J, Sanak M, Wegrzyn W, Nizankowski R, Szczeklik A. Antithrombotic effects of aspirin based on PLA1/A2 glycoprotein IIIa polymorphism in patients with coronary artery disease. Thromb Res. 2007;119:301–303. doi: 10.1016/j.thromres.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Isordia-Salas I, Olalde-Roman MJ, Santiago-German D, De La Pena NC, Valencia-Sanchez JS. The impact of CYP3A5*1/*3, PIA1/A2 and T744C polymorphisms on clopidogrel and acetylsalicylic acid response variability in Mexican population. Thromb Res. 2012;130:e67–e72. doi: 10.1016/j.thromres.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 42.Lepantalo A, Mikkelsson J, Resendiz JC, Viiri L, Backman JT, Kankuri E, Karhunen PJ, Lassila R. Polymorphisms of COX-1 and GPVI associate with the antiplatelet effect of aspirin in coronary artery disease patients. Thromb Haemost. 2006;95:253–259. doi: 10.1160/TH05-07-0516. [DOI] [PubMed] [Google Scholar]
- 43.Lim E, Carballo S, Cornelissen J, Ali ZA, Grignani R, Bellm S, Large S. Dose-related efficacy of aspirin after coronary surgery in patients with PlA2 polymorphism ( NCT00262275) Ann Thorac Surg. 2007;83:134–138. doi: 10.1016/j.athoracsur.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Stepien E, Szuldrzynski K, Branicka A, Stankiewicz E, Pazdan A, Zielinski L, Bozek M, Tomala M, Guzik B, Godlewski J, Pawelec T, Zmudka K. The thrombin generation is associated with the PIA1/A2 beta3, integrin polymorphism in aspirin-treated patients with coronary artery disease: a role of statins. Pol Arch Med Wewn. 2007;117:33–40. [PubMed] [Google Scholar]
- 45.Morawski W, Sanak M, Cisowski M, Szczeklik M, Szczeklik W, Dropinski J, Waclawczyk T, Ulczok R, Bochenek A. Prediction of the excessive perioperative bleeding in patients undergoing coronary artery bypass grafting: role of aspirin and platelet glycoprotein IIIa polymorphism. J Thorac Cardiovasc Surg. 2005;130:791–796. doi: 10.1016/j.jtcvs.2005.02.041. [DOI] [PubMed] [Google Scholar]
- 46.Szczeklik A, Undas A, Sanak M, Frolow M, Wegrzyn W. Relationship between bleeding time, aspirin and the PlA1/A2 polymorphism of platelet glycoprotein IIIa. Br J Haematol. 2000;110:965–967. doi: 10.1046/j.1365-2141.2000.02267.x. [DOI] [PubMed] [Google Scholar]
- 47.Undas A, Sanak M, Musial J, Szczeklik A. Platelet glycoprotein IIIa polymorphism, aspirin, and thrombin generation. Lancet. 1999;353:982–983. doi: 10.1016/S0140-6736(98)05054-5. [DOI] [PubMed] [Google Scholar]
- 48.Undas A, Brummel K, Musial J, Mann KG, Szczeklik A. Pl (A2) polymorphism of beta(3) integrins is associated with enhanced thrombin generation and impaired antithrombotic action of aspirin at the site of microvascular injury. Circulation. 2001;104:2666–2672. doi: 10.1161/hc4701.099787. [DOI] [PubMed] [Google Scholar]
- 49.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Sabate M, Fernandez C, Stranieri C, Trabetti E, Pignatti PF, Macaya C. PlA polymorphism and platelet reactivity following clopidogrel loading dose in patients undergoing coronary stent implantation. Blood Coagul Fibrinolysis. 2004;15:89–93. doi: 10.1097/00001721-200401000-00014. [DOI] [PubMed] [Google Scholar]
- 50.Dropinski J, Musial J, Jakiela B, Wegrzyn W, Sanak M, Szczeklik A. Anti-thrombotic action of clopidogrel and P1(A1/A2) polymorphism of beta3 integrin in patients with coronary artery disease not being treated with aspirin. Thromb Haemost. 2005;94:1300–1305. [PubMed] [Google Scholar]
- 51.Papp E, Havasi V, Bene J, Komlosi K, Talian G, Feher G, Horvath B, Szapary L, Toth K, Melegh B. Does glycoprotein IIIa gene (Pl(A)) polymorphism influence clopidogrel resistance?: a study in older patients. Drugs Aging. 2007;24:345–350. doi: 10.2165/00002512-200724040-00006. [DOI] [PubMed] [Google Scholar]
- 52.Motovska Z, Widimsky P, Kvasnicka J, Petr R, Bilkova D, Hajkova J, Marinov L, Simek S, Kala P. High loading dose of clopidogrel is unable to satisfactorily inhibit platelet reactivity in patients with glycoprotein IMA gene polymorphism: A genetic substudy of PRAGUE-8 trial. Blood Coagul Fibrinolysis. 2009;20:257–262. doi: 10.1097/mbc.0b013e328325455b. [DOI] [PubMed] [Google Scholar]
- 53.Storey RF, Melissa Thornton S, Lawrance R, Husted S, Wickens M, Emanuelsson H, Cannon CP, Heptinstall S, Armstrong M. Ticagrelor yields consistent dose-dependent inhibition of ADP-induced platelet aggregation in patients with atherosclerotic disease regardless of genotypic variations in P2RY12, P2RY1, and ITGB3. Platelets. 2009;20:341–348. doi: 10.1080/09537100903075324. [DOI] [PubMed] [Google Scholar]
- 54.Aalto-Setala K, Karhunen PJ, Mikkelsson J, Niemela K. The effect of glycoprotein IIIa PIA 1/A2 polymorphism on the PFA-100 response to GP IIb IIa receptor inhibitors-the importance of anticoagulants used. J Thromb Thrombolysis. 2005;20:57–63. doi: 10.1007/s11239-005-2912-2. [DOI] [PubMed] [Google Scholar]
- 55.Sirotkina OV, Khaspekova SG, Zabotina AM, Shimanova YV, Mazurov AV. Effects of platelet glycoprotein IIb-IIIa number and glycoprotein IIIa Leu33Pro polymorphism on platelet aggregation and sensitivity to glycoprotein IIb-IIIa antagonists. Platelets. 2007;18:506–514. doi: 10.1080/09537100701326739. [DOI] [PubMed] [Google Scholar]
- 56.Verdoia M, Pergolini P, Camaro C, Restifo M, Rolla R, Schaffer A, Di Giovine G, Marino P, Bellomo G, Suryapranata H, De Luca G. PlA1/PlA2 polymorphism does not influence response to Gp IIb-IIIa inhibitors in patients undergoing coronary angioplasty. Blood Coagul Fibrinolysis. 2013;24:411–418. doi: 10.1097/MBC.0b013e32835d546e. [DOI] [PubMed] [Google Scholar]
- 57.Weber AA, Jacobs C, Meila D, Weber S, Zotz RB, Scharf RE, Kelm M, Strauer BE, Schror K. No evidence for an influence of the human platelet antigen-1 polymorphism on the antiplatelet effects of glycoprotein IIb/IIIa inhibitors. Pharmacogenetics. 2002;12:581–583. doi: 10.1097/00008571-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Wheeler GL, Braden GA, Bray PF, Marciniak SJ, Mascelli MA, Sane DC. Reduced inhibition by abciximab in platelets with the PlA2 polymorphism. Am Heart J. 2002;143:76–82. doi: 10.1067/mhj.2002.119763. [DOI] [PubMed] [Google Scholar]
- 59.Lordkipanidze M, Pharand C, Schampaert E, Turgeon J, Palisaitis DA, Diodati JG. A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease. Eur Heart J. 2007;28:1702–1708. doi: 10.1093/eurheartj/ehm226. [DOI] [PubMed] [Google Scholar]
- 60.Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol. 2003;41:961–965. doi: 10.1016/s0735-1097(02)03014-0. [DOI] [PubMed] [Google Scholar]
- 61.Wenaweser P, Dorffler-Melly J, Imboden K, Windecker S, Togni M, Meier B, Haeberli A, Hess OM. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol. 2005;45:1748–1752. doi: 10.1016/j.jacc.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 62.Frelinger AL, III, Li Y, Linden MD, Barnard MR, Fox ML, Christie DJ, Furman MI, Michelson AD. Association of cyclooxygenase-1-dependent and-independent platelet function assays with adverse clinical outcomes in aspirin-treated patients presenting for cardiac catheterization. Circulation. 2009;120:2586–2596. doi: 10.1161/CIRCULATIONAHA.109.900589. [DOI] [PubMed] [Google Scholar]