Over the past few years there have been sustained efforts by professionals and patient groups to make pain assessment and treatment a priority in medical care, such as has been noted in the International Association for the Study of Pain Declaration of Montreal, which states that access to pain management is a fundamental human right.1 As a result there exist today numerous protocols to guide treatment plans, such as the National Opioid Use Guideline Group guidelines, the Canadian and International Association for the Study of Pain neuropathic guidelines, and the Alberta low back pain guidelines,2–6 as well as an armoury of drugs to help treat pain. However, pain control necessarily involves the patient, and the decision about whether to take medication or to pursue treatment is influenced by the patient’s beliefs about health and illness. In particular, the patient’s beliefs related to medications and their side effects strongly influence adherence to treatment. Several patient factors, such as underreporting, inappropriate expectations, and deficient knowledge of pain and its treatment, can contribute to poor outcomes.7–10
The use of opioids for the treatment of cancer pain, as first proposed in the guidelines released in 1986 by the World Health Organization (WHO), is now supported by more than 27 years of clinical experience, and several new editions of the recommendations have been published.11 The “three-step analgesic ladder,” one of the central components of the guideline, has also been shown to be a safe and beneficial approach to the treatment of patients with chronic noncancer pain.12 It offers a drug-centred approach to the treatment of pain. In 2010 a new adaptation of the analgesic ladder13 promoted its bidirectional use with a “step up, step down” approach.
The 2010 adaptation proposes an upward pathway for the treatment of cancer and chronic pain and a downward pathway for the treatment of intense acute pain, uncontrolled chronic pain, and breakthrough pain.13 The advantage of this adaptation and use of the analgesic ladder is the versatility that it provides the user while maintaining a stepwise progression. An upward pathway can be applied more slowly for chronic and cancer pain, and, conversely, the practitioner can start at the top tier for severe acute pain, uncontrolled chronic pain, and breakthrough pain and quickly come down the ladder as the patient’s pain improves. The 2010 adaptation (Figure 1)13 is appropriate for use in patients with nociceptive pain and combined nociceptive and neuropathic pain, but not for pure neuropathic pain. For pure neuropathic pain, refer to the neuropathic pain guidelines mentioned above.5,6
Figure 1.
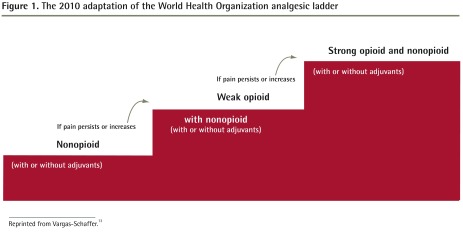
The 2010 adaptation of the World Health Organization analgesic ladder
Reprinted from Vargas-Schaffer.13
The aim of this article is to describe further modifications to the WHO analgesic ladder that will place patients at the centre of their pain care.
Health care practitioners as teachers
Despite the little time allocated in the medical curriculum to pain management outside of palliative care, doctors and health care providers must acquire the ability to transfer knowledge in a format that is easily understood and integrated by the patient.14 Therapeutic patient education is a technique that was developed for the purpose of enabling health care professionals to pass on their knowledge and expertise to patients so that patients can become partners in their own care. According to the WHO document published in 1998,15 therapeutic patient education can be viewed as a set of structured activities that consist of “helping the patient and his family to acquire knowledge and competencies about the disease and its treatment, in order to better collaborate with the caregivers, and to improve his quality of life.”15,16 It encourages the patient to assume a certain level of responsibility for his or her own care.17
Therapeutic patient education is education managed by health care providers trained in the education of patients and it is designed to enable a patient or a group of patients and families to manage the treatment of their conditions and prevent avoidable complications while maintaining or improving quality of life. Its principal purpose is to produce a therapeutic effect in addition to that of all other interventions (pharmacologic, physical therapy, etc). An in-depth discussion of therapeutic patient education is outside the scope of this commentary; however, several extensive publications and useful reviews on the topic have been published.15–18
New element
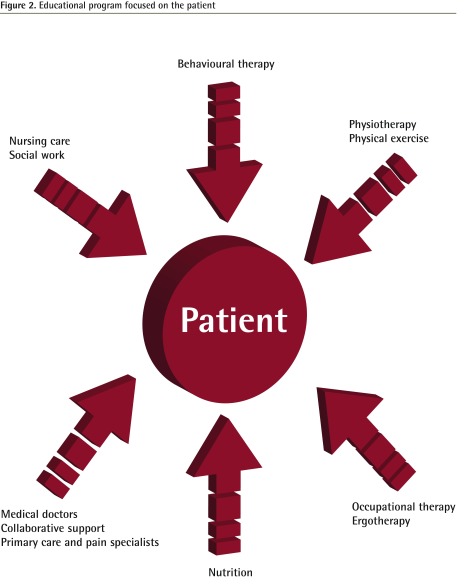
In a recent article Leung19 suggested, once again, that both acute and chronic pain management should include multimodal and nonpharmacologic treatments. We extend this idea by proposing that a therapeutic patient education program be incorporated as the base or foundation of the analgesic ladder (Figure 2). This would transform what is now a purely medically driven, pharmacologic approach to pain management into a patient-centred, multidisciplinary, complementary, and integrative medicine approach, and maintain the patient as an active participant at the centre of the pain management strategy. This format has been adopted, with success, in the authors’ centre.18,20
Figure 2.
Educational program focused on the patient
Revised 4-step model
Step 1: acute and mild pain
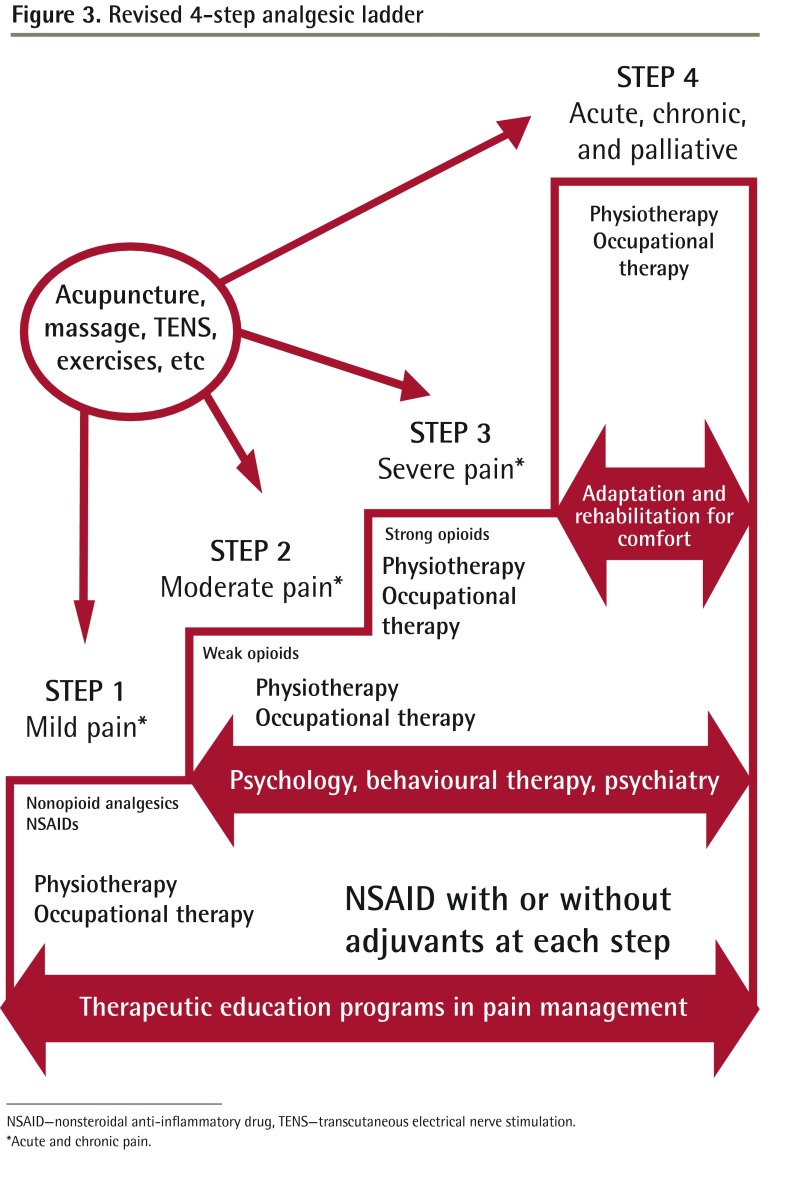
The therapeutic patient education program should be incorporated at the base of the analgesic ladder (Figure 3) and become part of the matrix onto which health care practitioners will add nonopioid analgesics, nonsteroidal anti-inflammatory drugs, physiotherapy, and ergotherapy or occupational therapy, as required by individual patients. Further, at this level and all other levels, additional therapies such as acupuncture, massage, transcutaneous electrical nerve stimulation, and exercise can be added to the treatment plan. The goal of physical therapy and other complementary techniques in this step is to provide the patient with the necessary tools to prevent increased pain and functional limitations. This base is essential because with increased knowledge, patients modify their attitudes, improve their skills, and raise their aspirations in order to adapt their lives to the presence of acute and chronic pain.
Figure 3.
Revised 4-step analgesic ladder
NSAID—nonsteroidal anti-inflammatory drug, TENS—transcutaneous electrical nerve stimulation.
*Acute and chronic pain.
Step 2: chronic and moderate pain
Here, to the existing matrix described in step 1, the health care practitioner will add weak opioids and include a second new element: treatment from a core of consultant therapies as required by each patient.
The addition of a consultation with a physiotherapist, psychologist, or psychiatrist, when necessary, might help maintain physical activity and function and promote the incorporation of social activities that will aid the patient in maintaining a support system.21–23 This is essential in moving toward the acceptance of limitations imposed by pain and adapting to new health conditions.24
Step 2 is highly relevant in the current climate in which issues of addiction and the misuse of prescription medication are raised regularly in the medical literature and media.25–30 Because weak opioids produce less dependence and can be very effective in treating moderate to severe pain,31–34 they are uniquely suited to this step. Three weak opioids—tramadol, the buprenorphine patch, and tapentadol—have demonstrated usefulness in various studies around the world.35–40
Steps 3 and 4: chronic pain, severe pain, and palliative care
At this point all the previous steps are reviewed and care is adapted to the patient’s changing needs at each visit. Strong opioids and interventional treatment might be appropriate at this level. In addition, we suggest a third new element: rehabilitation and adaptation for comfort.
Palliative care should not only apply to cancer patients, but also be implemented for patients with progressive, incurable nonmalignant disease and other life-threatening illnesses. For example, patients with degenerative muscle disease, central nervous system disease, hepatorenal disease, heart failure, and severe respiratory limitation could benefit from increased comfort measures and adequate control of pain, as it would improve their quality of life and that of their relatives and caregivers.41,42 At this stage the aim is to control symptoms and maintain independence as long as possible. Physiotherapy and ergotherapy can also be added.
It is important to remember that for severe, acutely painful states that arise unexpectedly, such as after surgery or for pain flares in the chronic setting, one can begin at the top of the ladder, soothe the patient, and then taper the medication and interventional treatments in subsequent steps. This is in fact the surgical model of care used daily in hospital settings.
Conclusion
In our modern society chronic pain should not be considered a secondary symptom of some other illness but rather a chronic disease in and of itself. Under these circumstances, the key to successful treatment might rest in a paradigm in which patients are at the centre of an individualized, multidisciplinary pain treatment strategy that both requires and empowers them to become dynamic participants in their care and in which they are actively supported in this endeavour through the provision of a patient therapeutic education program.
This adaptation of the analgesic ladder places the family practitioner in the pivotal role of leader and coordinator of a multidisciplinary team focused on the patient. Additional members include a nurse, who is instrumental in ensuring that the patient is well informed; a physiotherapist, an occupational therapist, or a kinesiologist, who can help increase the patient’s level of physical activity while decreasing pain intensity; and a psychologist, who can intervene with issues related to depression and anxiety that are so ubiquitous among patients suffering from chronic pain. The use of integrative therapies might also be encouraged under the supervision of the physician. Many primary care physicians work in clinics and are well connected to networks of allied health care specialists upon whom they can call for information and collaboration. We suggest that this might be the initial stream to follow, as it also frees the family practitioner to care for other aspects of the patient’s health. However, it should be noted that family physicians are amply qualified to provide some of the nonmedical interventions (eg, encourage exercise and simple cognitive strategies, as well as set up self-help groups).
Today, almost 3 decades after it first appeared, the WHO ladder remains a valuable and relevant tool for the care of patients in pain, and its core principles of stepwise progression accommodate the advent of new drugs and treatments with ease. Therapeutic patient education can be integrated so seamlessly at the base of the analgesic ladder that one could almost believe that it was part of the original concept. In fact, the principle purpose of the analgesic ladder, as described in the 1998 WHO document,15 was to provide a therapeutic effect in addition to that of all other interventions—that is, to help patients and their families manage the treatment of their conditions, prevent avoidable complications, and maintain or improve quality of life.
Footnotes
This article has been peer reviewed.
Competing interests
None declared
References
- 1.Cousins MJ, Lynch ME. The Declaration of Montreal: access to pain management is a fundamental human right. Pain. 2011;152(12):2673–4. doi: 10.1016/j.pain.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 2.National Opioid Use Guideline Group . Canadian guideline for safe and effective use of opioids for chronic non-cancer pain. Part A: executive summary and background. Hamilton, ON: McMaster University; 2010. [Google Scholar]
- 3.National Opioid Use Guideline Group . Canadian guideline for safe and effective use of opioids for chronic non-cancer pain. Part B: recommendations for practice. Hamilton, ON: McMaster University; 2010. [Google Scholar]
- 4.Cutforth G, Peter A, Taenzer P. The Alberta Health Technology Assessment (HTA) Ambassador Program: the development of a contextually relevant, multidisciplinary clinical practice guideline for non-specific low back pain: a review. Physiother Can. 2011;63(3):278–86. doi: 10.3138/ptc.2009-39P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, et al. Pharmacological management of chronic neuropathic pain—consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13–21. doi: 10.1155/2007/730785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurley RW, Adams MC, Benzon HT. Neuropathic pain: treatment guidelines and updates. Curr Opin Anaesthesiol. 2013;26(5):580–7. doi: 10.1097/ACO.0b013e328363b4bf. [DOI] [PubMed] [Google Scholar]
- 7.Cogan J, Ouimette MF, Vargas-Schaffer G, Yegin Z, Deschamps A, Denault A. Patient attitudes and beliefs regarding pain medication after cardiac surgery: barriers to adequate pain management. Pain Manag Nurs. 2013 Feb 26; doi: 10.1016/j.pmn.2013.01.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Ward S, Hughes S, Donovan H, Serlin RC. Patient education in pain control. Support Care Cancer. 2001;9(3):148–55. doi: 10.1007/s005200000176. [DOI] [PubMed] [Google Scholar]
- 9.Ward S, Donovan HS, Owen B, Grosen E, Serlin R. An individualized intervention to overcome patient-related barriers to pain management in women with gynecologic cancers. Res Nurs Health. 2000;23(5):393–405. doi: 10.1002/1098-240x(200010)23:5<393::aid-nur6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Lin CC, Chou PL, Wu SL, Chang YC, Lai YL. Long-term effectiveness of a patient and family pain education program on overcoming barriers to management of cancer pain. Pain. 2006;122(3):271–81. doi: 10.1016/j.pain.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Cancer pain relief and palliative care. With a guide to opioid availability. Geneva, Switz: World Health Organization; 1996. [Google Scholar]
- 12.Ventafridda V, Tamburini M, Caraceni A, De Conno F, Naldi F. A validation study of the WHO method for cancer pain relief. Cancer. 1987;59(4):850–6. doi: 10.1002/1097-0142(19870215)59:4<850::aid-cncr2820590432>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician. 2010;56:514–7. (Eng), e202–5 (Fr) [PMC free article] [PubMed] [Google Scholar]
- 14.Watt-Watson J, McGillion M, Hunter J, Choiniere M, Clark AJ, Dewar A, et al. A survey of prelicensure pain curricula in health science faculties in Canadian universities. Pain Res Manag. 2009;14(6):439–44. doi: 10.1155/2009/307932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . Therapeutic patient education. Continuing education programme for healthcare providers in the field of prevention of chronic diseases. Copenhagen, Den: World Health Organization Office for Europe; 1998. [Google Scholar]
- 16.Traynard PY, Grimaldi R. Qu’est-ce que l’éducation thérapeutique? Paris, Fr: Masson; 2007. [Google Scholar]
- 17.D’Ivernois JF, Gagnayre R. Apprendre à éduquer le patient. Approche pédagogique. Paris, Fr: Maloine; 2008. [Google Scholar]
- 18.Vargas-Schaffer G, Cogan J, Jeannotte C, Besner G, Cajac J, Haworth C, et al. First year results of an educational program in chronic pain for French speaking patients. Paper presented at: 7th Congress of the European Federation of IASP Chapters; 2011 Sep 21–24; Hamburg, Germany. [Google Scholar]
- 19.Leung L. From ladder to platform: a new concept for pain management. J Prim Health Care. 2012;4(3):254–8. [PubMed] [Google Scholar]
- 20.Vargas-Schaffer G, Cogan J. Impact of educational program for patients with chronic pain. Results after one year. Poster presented at: 6th Congress of the World Institute of Pain; 2012 Feb 4–6; Miami Beach, FL. [Google Scholar]
- 21.Falla D, Lindstrom R, Rechter L, Boudreau S, Petzke F. Effectiveness of an 8-week exercise programme on pain and specificity of neck muscle activity in patients with chronic neck pain: a randomized controlled study. Eur J Pain. 2013;17(10):1517–28. doi: 10.1002/j.1532-2149.2013.00321.x. Epub 2013 May 6. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen-Barr E, Bohman T, Hallqvist J, Holm LW, Skillgate E. Do physical activity level and body mass index predict recovery from persistent neck pain in men and women of working age? A population-based cohort study. Eur Spine J. 2013;22(9):2077–83. doi: 10.1007/s00586-013-2801-x. Epub 2013 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uebelacker LA, Eaton CB, Weisberg R, Sands M, Williams C, Calhoun D, et al. Social support and physical activity as moderators of life stress in predicting baseline depression and change in depression over time in the Women’s Health Initiative. Soc Psychiatry Psychiatr Epidemiol. 2013;48(12):1971–82. doi: 10.1007/s00127-013-0693-z. Epub 2013 May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCracken LM, Vowles KE, Eccleston C. Acceptance-based treatment for persons with complex, long standing chronic pain: a preliminary analysis of treatment outcome in comparison to a waiting phase. Behav Res Ther. 2005;43(10):1335–46. doi: 10.1016/j.brat.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Kotalik J. Controlling pain and reducing misuse of opioids. Ethical considerations. Can Fam Physician. 2012;58:381–5. (Eng), e190–5 (Fr) [PMC free article] [PubMed] [Google Scholar]
- 26.Dhalla IA, Mamdani MM, Sivilotti ML, Kopp A, Qureshi O, Juurlink DN. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. CMAJ. 2009;181(12):891–6. doi: 10.1503/cmaj.090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.College of Physicians and Surgeons of Ontario . Avoiding abuse, achieving a balance: tackling the opioid public health crisis. Toronto, ON: College of Physicians and Surgeons of Ontario; 2010. [Google Scholar]
- 28.Parker AJ. The appropriate use of opiates in chronic pain. J Clin Psychiatry. 2012;73(8):e26. doi: 10.4088/JCP.11094nr1c. [DOI] [PubMed] [Google Scholar]
- 29.Salinas GD, Susalka D, Burton BS, Roepke N, Evanyo K, Biondi D, et al. Risk assessment and counseling behaviors of healthcare professionals managing patients with chronic pain: a national multifaceted assessment of physicians, pharmacists, and their patients. J Opioid Manag. 2012;8(5):273–84. doi: 10.5055/jom.2012.0127. [DOI] [PubMed] [Google Scholar]
- 30.Benzon HT, Kendall MC, Katz JA, Benzon HA, Malik K, Cox P, et al. Prescription patterns of pain medicine physicians. Pain Pract. 2013;13(6):440–50. doi: 10.1111/papr.12011. Epub 2012 Dec 10. [DOI] [PubMed] [Google Scholar]
- 31.Grond S, Radbruch L, Meuser T, Loick G, Sabatowski R, Lehmann KA. High-dose tramadol in comparison to low-dose morphine for cancer pain relief. J Pain Sympt Manag. 1999;18(3):174–9. doi: 10.1016/s0885-3924(99)00060-3. [DOI] [PubMed] [Google Scholar]
- 32.Mercadante S, Salvaggio L, Dardanoni G, Agnello A, Garofalo S. Dextropropoxyphene versus morphine in opioid-naive cancer patients with pain. J Pain Sympt Manag. 1998;15(2):76–81. [PubMed] [Google Scholar]
- 33.Marinangeli F, Ciccozzi A, Leonardis M, Aloisio L, Mazzei A, Paladini A, et al. Use of strong opioids in advanced cancer pain: a randomized trial. J Pain Sympt Manag. 2004;27(5):409–16. doi: 10.1016/j.jpainsymman.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Tassinari D, Drudi F, Rosati M, Tombesi P, Sartori S, Maltoni M. The second step of the analgesic ladder and oral tramadol in the treatment of mild to moderate cancer pain: a systematic review. Palliat Med. 2011;25(5):410–23. doi: 10.1177/0269216311405090. [DOI] [PubMed] [Google Scholar]
- 35.Dhillon S. Tramadol/paracetamol fixed-dose combination: a review of its use in the management of moderate to severe pain. Clin Drug Investig. 2010;30(10):711–38. doi: 10.2165/11205830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Ballantyne JC, Mao J. Opioid therapy for chronic pain. New Engl J Med. 2003;349(20):1943–53. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 37.Muriel Villoria C, Perez-Castejon Garrote JM, Sanchez Magro I, Neira Alvarez M. Effectiveness and safety of transdermal buprenorphine for chronic pain treatment in the elderly: a prospective observational study [article in Spanish] Med Clin (Barc) 2007;128(6):204–10. doi: 10.1157/13098717. [DOI] [PubMed] [Google Scholar]
- 38.Hoskin PJ, Hanks GW. Opioid agonist-antagonist drugs in acute and chronic pain states. Drugs. 1991;41(3):326–44. doi: 10.2165/00003495-199141030-00002. [DOI] [PubMed] [Google Scholar]
- 39.Afilalo M, Etropolski MS, Kuperwasser B, Kelly K, Okamoto A, Van Hove I, et al. Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo-and active-controlled phase III study. Clin Drug Investig. 2010;30(8):489–505. doi: 10.2165/11533440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Wild JE, Grond S, Kuperwasser B, Gilbert J, McCann B, Lange B, et al. Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract. 2010;10(5):416–27. doi: 10.1111/j.1533-2500.2010.00397.x. [DOI] [PubMed] [Google Scholar]
- 41.Pitcher P. Palliative care in the hospital setting for patients with non-malignant disease. In: Addington-Hall JM, Higginson I, editors. Palliative care for non-cancer patients. New York, NY: Oxford University Press; 2002. pp. 158–71. [Google Scholar]
- 42.Fallon M, Dunn F, Voltz R, Borasio G, George R, Woodruff R. Chronic non-malignant disease. In: Fallon M, Hanks G, editors. ABC of palliative care. 2nd ed. Malden, MA: Blackwell Publishing; 2006. pp. 59–67. [Google Scholar]