Abstract
Objective
To determine the proportion of patients with atrial fibrillation (AF) in primary care achieving guideline-concordant stroke prevention treatment based on both the previous (2010) and the updated (2012) Canadian guideline recommendations.
Design
Retrospective chart review.
Participants
Primary care patients (N = 204) with AF. The mean age was 71.3 years and 53.4% were women.
Setting
Large urban community family practice in Toronto, Ont.
Main outcome measures
Patient demographic characteristics such as sex and age; a list of current cardiac medications including anticoagulants and antiplatelets; the total number of medications; relevant current and past medical history including presence of diabetes, stroke or transient ischemic attack, hypertension, and vascular disease; number of visits to the family physician and cardiologist in the past year and past 5 years, and how many of these were for AF; the number of visits to the emergency department or hospitalizations for AF, congestive heart failure, or stroke; if patients were taking warfarin, how often their international normalized ratios were recorded, and how many times they were in the reference range; CHADS2 (congestive heart failure, hypertension, age ≥ 75, diabetes mellitus, and stroke or transient ischemic attack) score, if recorded; and reason for not taking oral anticoagulants when they should have been, if recorded.
Results
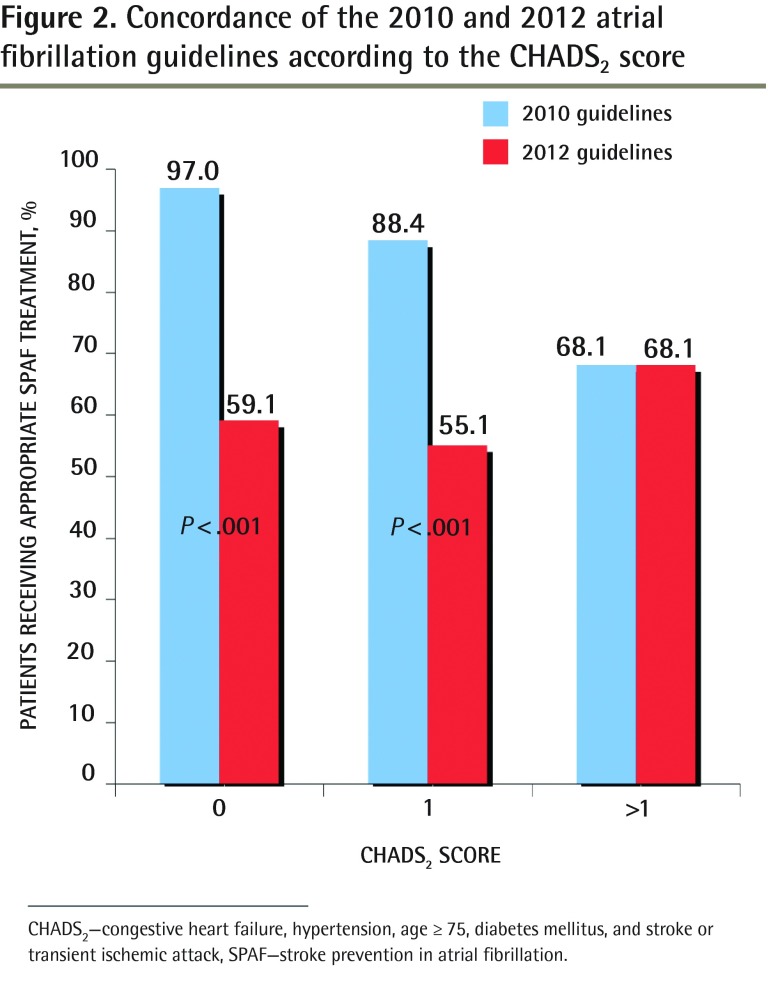
Among those who had CHADS2 scores of 0, 64 patients (97.0%) were receiving appropriate stroke prevention in AF (SPAF) treatment according to the 2010 guidelines. When the 2012 guidelines were applied, 39 patients (59.1%) were receiving appropriate SPAF treatment (P < .001). For those with CHADS2 scores of 1, 88.4% of patients had appropriate SPAF treatment according to the 2010 guidelines, but only 55.1% were adequately treated according to the 2012 guidelines (P < .001). Of the patients at the highest risk (CHADS2 score > 1), 68.1% were adequately treated with anticoagulation and an additional 8.7% (6 of 69) had documented reasons why they were not taking anticoagulants.
Conclusion
When assessed using the 2012 Canadian Cardiovascular Society AF guidelines, the proportion of patients receiving appropriate SPAF therapy in this primary care setting decreased substantially. All patients with CHADS2 scores of 0 or 1 should be reassessed to ensure that they are receiving optimal stroke prevention treatment.
Résumé
Objectif
Déterminer la proportion des patients souffrant de fibrillation auriculaire (FA) en milieu de soins primaires qui reçoivent un traitement de prévention des accidents vasculaires cérébraux conforme aux directives de la Société canadienne de cardiologie, et ce, en utilisant les directives précédentes (2010) ou les directives mises à jour de 2012.
Type d’étude
Revue rétrospective de dossiers.
Participants
Patients des soins primaires (N = 204) souffrant de fibrillation auriculaire. L’âge moyen était de 71,3 ans et 53,4 % étaient des femmes.
Contexte
Une grande clinique communautaire urbaine de médecine familiale à Toronto, Ontario.
Principaux paramètres à l’étude
Caractéristiques démographiques des patients, p. ex. sexe et âge; liste des médicaments actuels pour le cœur, incluant les anticoagulants et les antiplaquettaires; nombre total de médicaments; histoire pertinente, passée et présente, incluant la présence de diabète, d’accident vasculaire cérébral ou d’ischémie cérébrale transitoire, d’hypertension et de maladie vasculaire; nombre de visites au médecin de famille et au cardiologue au cours de l’année précédente et des 5 dernières années, et combien de ces visites étaient pour de la FA; nombre de visites à l’urgence ou d’hospitalisations pour de la FA, de l’insuffisance cardiaque ou pour un accident vasculaire cérébral; le patient prenait-il de la warfarine, à quelle fréquence les INR ont été enregistrés et combien de fois les valeurs étaient à l’intérieur de la normale; le score CHADS2 (pour : Congestive heart failure, Hypertension, Age > 75, Diabetus mellitus, et Stroke ou ischémie cérébrale transitoire) s’il a été noté; et les raisons pour ne pas prendre des anticoagulants oraux lorsque c’était indiqué, si elles ont été notées.
Résultats
Parmi les patients qui avaient un score de 0 au CHADS2, 64 (97,0 %) recevaient le traitement approprié pour prévenir un accident vasculaire cérébral secondaire à la FA d’après les directives de 2010. Lorsqu’on utilisait plutôt les directives de 2012, 39 patients (59,1 %) recevaient le traitement préventif approprié (P < ,001). Parmi les patients qui avaient un score de 1 au CHADS2, 88,4 % avaient le traitement préventif approprié selon les directives de 2010, alors que seulement 55,1 % d’entre eux étaient traités adéquatement d’après les directives de 2012 (P < ,001). Dans le cas des patients présentant le risque le plus élevé (score > 1 au CHADS2), 68,1 % recevaient une anticoagulation correcte, tandis qu’un autre 8,7 % (6 patients sur 69) avaient des raisons documentées pour ne pas prendre d’anticoagulants.
Conclusion
Lorsqu’on utilise les directives de 2012 de la Société canadienne de cardiologie pour la FA pour déterminer la proportion des patients de ce milieu de soins primaires qui reçoivent un traitement adéquat pour prévenir les accidents vasculaires cérébraux, on observe que cette proportion est beaucoup moindre. Tous les patients qui ont un score de 0 ou de 1 au CHADS2 devraient être réévalués pour s’assurer qu’ils reçoivent le traitement optimal pour prévenir les accidents vasculaires cérébraux.
Atrial fibrillation (AF) is the most common cardiac arrhythmia, the incidence of which is growing as the population ages.1,2 Patients with AF account for 15% of all stroke patients, leaving them with an increased risk of death, or a new disability in 60% of cases.3 Fortunately, stroke prevention therapy (eg, anticoagulation) can reduce the risk of stroke in patients with AF by almost two-thirds.4,5 However, systematic reviews have found that of all patients at high risk of stroke due to AF, only 51% receive any anticoagulation, and of these patients, their international normalized ratios (INRs) are within the target range only half the time.6,7
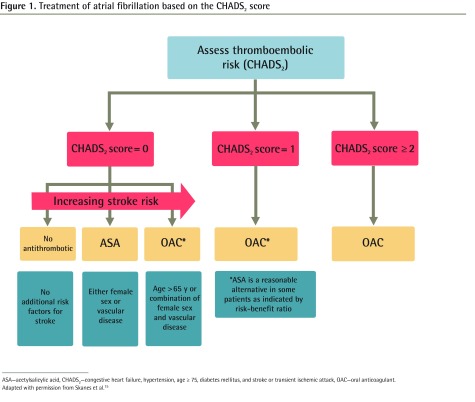
Guidelines recommend identification of specific risk factors for stroke in patients with AF.1–8 Although formal risk assessment tools incorporating these risk factors have been available for some time,9 there is evidence that these are not used consistently to inform selection of treatment.10–13 Based on the CHA2DS2-VASc (congestive heart failure; hypertension; age ≥ 75; diabetes mellitus; stroke or transient ischemic attack; vascular disease [previous myocardial infarction, peripheral artery disease, or aortic plaque]; age 65 to 74 years; sex category [ie, female]) model,14 the updated 2012 Canadian Cardiovascular Society guidelines recommend use of a modified CHADS2 (congestive heart failure, hypertension, age ≥ 75, diabetes mellitus, and stroke or transient ischemic attack) scoring tool to assess stroke risk (Figure 1).15 Most notably, this tool further refines the risk stratification of patients with a CHADS2 score of 0 using age (> 65 years), female sex, and presence of vascular disease to identify patients who should be taking anticoagulants despite their CHADS2 scores of 0. These recommendations are in contrast to previous guidelines, which suggested use of a less-precise risk assessment tool (the original CHADS2) and indicated that acetylsalicylic acid was an appropriate choice for a greater proportion of patients (Table 1).15,16 In addition to this refinement of risk stratification, the new guidelines also recommend preferential use of new oral anticoagulants (OACs) (eg, dabigatran, rivaroxaban, apixaban) over warfarin.15 These newer agents have been shown to be at least as good as, and in some cases better than, warfarin with respect to stroke prevention, with less intracranial bleeding. However, there has been more documented gastrointestinal bleeding with dabigatran and rivaroxaban than with warfarin.17–19 Despite their relative convenience for patients,20 these medications have had slow uptake for a variety of reasons, including high price, the lack of post-marketing surveillance data, and concern about the lack of treatments to manage bleeding.21
Figure 1.
Treatment of atrial fibrillation based on the CHADS2 score
ASA—acetylsalicylic acid, CHADS2—congestive heart failure, hypertension, age ≥ 75, diabetes mellitus, and stroke or transient ischemic attack, OAC—oral anticoagulant.
Adapted with permission from Skanes et al.15
Table 1.
Comparison of treatment recommendations from the Canadian Cardiovascular Society 2010 and 2012 atrial fibrillation guidelines
| CHADS2 SCORE | RECOMMENDATION |
|---|---|
| 2010 guidelines | |
| • 0 | No treatment or ASA |
| • 1 | ASA or OAC |
| • ≥ 2 | OAC |
| 2012 guidelines | |
| • 0 (age ≤ 65 y, male sex, and no vascular disease) | No treatment |
| • 0 (either female sex or vascular disease) | ASA |
| • 0 (age > 65 y or female sex with vascular disease) | OAC |
| • 1 | OAC |
| • ≥ 2 | OAC |
Previous studies have found long delays in the implementation of new guideline recommendations in practice.22–24 In this study, we sought to determine the proportion of patients with AF in primary care achieving guideline-concordant stroke prevention based on both the previous (2010)16 and updated (2012)15 guideline recommendations. Although cognizant that the 2012 guidelines were published around the time of our chart review, we wondered how many patients would be affected by these new guidelines. We hypothesized that the newer recommendations would increase the measurable “care gap” in stroke prevention therapy in primary care.
METHODS
Study design
This study was a retrospective cohort chart review conducted in March 2012 using electronic medical record (EMR) data from a primary care practice. The study was approved by the research ethics board at the University of Toronto in Ontario (protocol no. 27059).
Context
The study was performed in a large urban community family practice in downtown Toronto spread across 2 locations, with 14 family physicians and more than 18 000 rostered patients. Through Ontario’s primary care reform initiatives, providers are remunerated by capitation when patients are rostered to the practice. In addition, the practice receives salary support from the Ontario Ministry of Health for allied care providers such as nurses, nurse practitioners, pharmacists, dietitians, diabetes educators, and social workers (known as a family health team). As part of routine care, patients taking warfarin had the opportunity to see the practice pharmacist or trained nurse for anticoagulation management.
This practice used the Practice Solutions EMR for all clinical notes, billing, and scheduling for all physicians and allied health professionals. Most of the data entry is free text; structured coding of diagnoses is optional. When viewing a patient chart, the current medical concerns, past medical history, and active treatments are easily visible. Currently, there are no standardized decision support tools embedded in the practice’s EMR.
In Ontario, the provincial government is the single payer for physician and hospital services. Patients do not copay for hospitalizations or for visits to their primary care practices. Laboratory tests and other investigations are also covered under the health plan. For those older than 65 years and those on social assistance, many prescription medications are covered under the Ontario Drug Benefit plan. At the time of this study, new antithrombotics such as dabigatran and rivaroxaban were not yet covered by the Ontario Drug Benefit plan. Acetylsalicylic acid (325-mg dose) was included in the formulary, as were warfarin and clopidogrel.
Identification of patient cohort
Two approaches were used to identify patients with AF. First, all electrocardiograms (ECGs) conducted between January 1, 2006, and January 1, 2012, were examined by an abstractor looking for ECGs that were identified as having AF. Next, an automated search of the practice’s EMR system was conducted, scanning the problem list, past medical history list, consultation notes, physician notes, and billing codes. As the practice’s EMR did not use structured data in these sections, a free-text search was developed to cover most probable permutations for describing AF, including variations in nomenclature (Table 2).
Table 2.
Search terms for identifying atrial fibrillation in the electronic medical record
| SCANNED ITEMS | SEARCH TERMS |
|---|---|
| Problem list | a fib, afib, a-fib, atrial fib, 427, a. fib, a. fib, fibrillation, a.f., flutter, parox, AF-, AF: |
| Past medical history | a fib, afib, a-fib, atrial fib, a. fib, a.fib, fibrillation, a.f., flutter, parox, AF-, AF: |
| Text notes | afib, a-fib, atrial fib, a. fib, a.fib, fibrillation, a.f., atrial flutter |
| Billing code | 427 |
Data collection
A multidisciplinary team consisting of pharmacists, internists, cardiologists, family physicians, and nurses collaborated to develop an abstraction manual. The data elements included demographic characteristics such as sex and age; a list of current cardiac medications including anticoagulants and antiplatelets; the total number of medications; relevant current and past medical history including presence of diabetes, stroke or transient ischemic attack, hypertension, and vascular disease; number of visits to the family physician and cardiologist in the past year and past 5 years, and how many of these were for AF; the number of visits to the emergency department or hospitalizations for AF, congestive heart failure, or stroke; if patients were taking warfarin, how often their INRs were recorded, and how many times they were in the reference range; CHADS2 or CHA2DS2-VASc score, if recorded; and reason for not taking OACs when they should have been, if recorded.
An abstractor was trained in use of the EMR system and was made aware of the various ways data were entered and could therefore be extracted. A family physician investigator and member of the practice (A.V.) double-checked the relevant data for determining whether stroke prevention treatment was concordant with guidelines. A sample of 15 charts was reviewed by a family physician for accuracy of data extraction, with 100% concordance. In addition to any CHADS2 scores reported in the charts, the family physician used clinical data available at the time of abstraction to determine up-to-date CHADS2 scores.
Analysis
Categorical variables for patient factors were compared using McNemar tests or
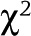 tests as appropriate.
tests as appropriate.
RESULTS
There were 204 patients identified as having AF, representing 1.1% of the total practice (Table 3). Of patients with AF, 92.4% visited their family physicians within the past year and 69.1% visited their cardiologists during this time. When assessing the reason for their visits in the past year, 30.6% of all visits were for AF, and during the past 5 years, 62.4% visits were for AF. Of all patients taking OACs, 86.3% were taking warfarin, 12.6% were taking the newer OAC dabigatran, and 1.1% were taking rivaroxaban.
Table 3.
Population characteristics: N = 204.
| CHARACTERISTIC | VALUE |
|---|---|
| Female sex, % | 53.4 |
| Mean age (range), y | 71.3 (37–103) |
| Mean medications (range) | 6.6 (0–25) |
| Mean active medical problems (range) | 5.1 (0–17) |
| Rate control medications, % | 61.3 |
| Diabetes, % | 13.2 |
| Coronary artery disease, % | 14.7 |
| Hypertension, % | 37.3 |
| History of CVA or TIA, % | 8.3 |
| Congestive heart failure, % | 10.8 |
| Have cardiologists, % | 78.9 |
| CHADS2 score, n (%) | |
| • 0 | 66 (32.4) |
| • 1 | 69 (33.8) |
| • 2 | 44 (21.6) |
| • 3 | 15 (7.4) |
| • 4 | 7 (3.4) |
| • 5 | 2 (1.0) |
| • 6 | 1 (0.5) |
CHADS2—congestive heart failure, hypertension, age ≥ 75, diabetes mellitus, and stroke or TIA, CVA—cerebrovascular accident, TIA—transient ischemic attack.
Sixty-six patients (32.4%) had a CHADS2 score of 0, 69 patients (33.8%) had a CHADS2 score of 1, and 69 patients (33.8%) had a CHADS2 score of 2 or greater.
Of the highest-risk patients (CHADS2 score > 1), 68.1% were taking OACs, which is concordant with both the 2010 and 2012 guidelines and an additional 8.7% (6 of 69) had documented reasons why they were not taking OACs. The main reasons for patients not taking OACs were high bleeding risk and patient preference. For patients who had CHADS2 scores of 0, 64 patients (97.0%) were receiving appropriate stroke prevention in AF (SPAF) treatment according to the 2010 guidelines (Figure 2). When the 2012 guidelines were applied, 39 patients (59.1%) were receiving appropriate SPAF treatment (P < .001). For those with CHADS2 scores of 1, the change in guidelines meant going from 88.4% receiving appropriate SPAF treatment to only 55.1% being adequately treated (P < .001).
Figure 2.
Concordance of the 2010 and 2012 atrial fibrillation guidelines according to the CHADS2 score
CHADS2—congestive heart failure, hypertension, age ≥ 75, diabetes mellitus, and stroke or transient ischemic attack, SPAF—stroke prevention in atrial fibrillation.
Of all patients with AF, 48 (23.5%) had documented CHADS2 scores. Of all patients with AF, 153 (75.0%) had AF documented on the problem list. Overall, having AF on the problem list or a documented CHADS2 score was not associated with improved processes of care or optimal prescribing. However, there was a trend toward significance when only the highest-risk patients were assessed (ie, CHADS2 score ≥ 2). In this population, 76.6% of patients with documented AF were taking appropriate therapy versus 42.9% who did not have documented AF (P = .08).
DISCUSSION
In this primary care setting that uses EMRs and point-of-care anticoagulation clinics, we found that a high proportion of patients with AF had adequate stroke prevention therapy. In particular, the proportion of patients taking appropriate anticoagulation therapy according to the 2010 guidelines was substantially higher than in other studies.6,7,24,25 However, we also found that 39.2% of patients were not receiving treatment in accordance with the new guidelines.
Family physicians often make the diagnosis of AF and have most frequent contact with patients with AF; therefore, they are the most likely to initiate anticoagulation treatment and provide ongoing monitoring and periodic reassessment of stroke risk.26 Educational efforts aimed at primary care practitioners appear to increase adherence to OACs.27,28 There might also be a specific need for non-biased educational efforts regarding the new OACs. Given that several studies have shown poor uptake of guidelines,22,23 carefully designed and implemented knowledge translation initiatives will likely be necessary to further improve guideline concordance. Knowledge translation initiatives that simultaneously address the patient, physician, and practice levels while engaging interdisciplinary teams29–31 seem to be effective. Other initiatives that have been successful include provincial health databases to notify physicians and patients that follow-up care is needed,32 use of electronic data management systems to issue guideline-specific reminders to physicians,31,33 and dashboard assistance within EMRs (ie, electronic clinical decision support).34 Finally, identifying the reason—clinical or otherwise—that a patient might not be receiving the correct anticoagulation therapy might also shrink the care gap.35
Similar to other EMR chart reviews,36 we found that a quarter of patients with AF were not identified by the problem list or the past medical history as having AF. We did not find that such patients were less likely to have adequate stroke prevention therapy, possibly because these patients had long-standing, well-controlled AF that the physicians did not transfer to their EMRs, as it was not an active issue. Nevertheless, to facilitate future chart audits and quality assurance or improvement, continued efforts to improve EMR data entry are necessary. Specifically, use of a search engine optimized for assessing free text in EMRs would increase the accuracy of identifying at-risk patients.37 Also, it has been shown that the use of algorithms or rules for inferring patient problems is a more accurate method for identifying patients with certain medical conditions compared with relying on the EMR problem list.38
Limitations
The results might not be generalizable, given that the population studied was relatively young and part of a large urban family practice with ready access to cardiologists. These factors likely contributed to the higher guideline concordance found in this practice compared with what has been previously reported. Although substantial effort was made to identify patients with AF in this practice, it is possible that some patients with AF were missed. It is also possible that documentation was poor for contraindications to OACs, which would falsely increase the apparent discrepancy between guideline recommendations and observed practices. For instance, although the 2012 guidelines recommend use of a bleeding risk score such as HAS-BLED (hypertension [systolic blood pressure > 160 mm Hg], abnormal renal or liver function, stroke [caused by bleeding], bleeding, labile INR, elderly [age > 65 years], drugs [acetylsalicylic acid or nonsteroidal anti-inflammatory drugs] or alcohol [≥ 8 drinks per week]), we were unable to abstract this information.
Conclusion
In an urban family practice setting, we applied the 2010 and 2012 Canadian Cardiovascular Society guidelines to 204 patients with AF. We found that in patients with CHADS2 scores of 0 and 1, the rates of guideline-concordant management were significantly lower when applying the 2012 guidelines (P < .001). All patients with CHADS2 scores of 0 and 1 should be reassessed to ensure that they are receiving optimal stroke prevention treatment according to their individualized risk, using new risk stratification tools. Knowledge translation activities targeting primary care providers to address the new quality-of-care gap generated by more stringent recommendations are needed.
Acknowledgments
This research was supported by an unrestricted grant from Boehringer Ingelheim. Filomena Valle-Leutri was the research analyst. Kaye Benson, Leslie Beard Ashley, and Christine Plaza all contributed to the concept and design. Dr Ivers is supported by Fellowship Awards from the Canadian Institutes of Health Research and from the Department of Family and Community Medicine at the University of Toronto.
EDITOR’S KEY POINTS
In patients with CHADS2 (congestive heart failure, hypertension, age ≥ 75, diabetes mellitus, and stroke or transient ischemic attack) scores of 0 and 1, the rates of Canadian Cardiovascular Society guideline-concordant atrial fibrillation management were significantly lower when applying the 2012 guidelines than when applying the 2010 guidelines (P < .001).
All patients with CHADS2 scores of 0 and 1 should be reassessed to ensure that they are receiving optimal stroke prevention treatment according to their individualized risk, using new risk stratification tools.
Knowledge translation activities targeting primary care providers to address the new quality-of-care gap generated by more stringent recommendations are needed.
POINTS DE REPÈRE DU RÉDACTEUR
Chez les patients qui ont un score de 0 et de 1 au CHADS2 (pour : Congestive heart failure, Hypertension, Age > 75, Diabetus mellitus, et Stroke ou ischémie cérébrale transitoire), la proportion des traitements de la fibrillation auriculaire qui étaient conformes aux directives de la Société canadienne de cardiologie était significativement plus basse lorsqu’on utilisait les directives de 2012 par rapport à celles de 2010 (P < ,001).
Tous les patients qui ont des scores de 0 ou de 1 au CHADS2 devraient être réévalués de façon à s’assurer qu’ils reçoivent le traitement optimal pour prévenir les accidents vasculaires cérébraux, et ce, selon leur risque individuel et en utilisant les nouveaux outils de stratification du risque.
Il y a lieu d’instaurer des activités de formation à l’intention des soignants de première ligne pour qu’ils prennent connaissance de la nouvelle brèche dans la qualité des soins qui résulte des recommandations plus strictes.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
All authors have contributed to the concept and design of the protocol and to drafting the manuscript, and have read the manuscript and given final approval for publication.
Competing interests
None declared
References
- 1.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation. 2006;114(7):e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. Erratum in: Circulation 2007;116(6):e138. [DOI] [PubMed] [Google Scholar]
- 2.Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study) Am J Cardiol. 1994;74(3):236–41. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285(18):2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 4.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154(13):1449–57. Erratum in: Arch Intern Med 1994;154(19):2254. [PubMed] [Google Scholar]
- 5.Albers GW, Dalen JE, Laupacis A, Manning WJ, Petersen P, Singer DE. Antithrombotic therapy in atrial fibrillation. Chest. 2001;119(1 Suppl):194S–206S. doi: 10.1378/chest.119.1_suppl.194s. [DOI] [PubMed] [Google Scholar]
- 6.Hylek EM, D’Antonio J, Evans-Molina C, Shea C, Henault LE, Regan S. Translating the results of randomized trials into clinical practice. The challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37(4):1075–80. doi: 10.1161/01.STR.0000209239.71702.ce. Epub 2006 Mar 9. [DOI] [PubMed] [Google Scholar]
- 7.Samsa GP, Matchar DB, Goldstein LB, Bonito AJ, Lux LJ, Witter DM, et al. Quality of anticoagulation management among patients with atrial fibrillation: results of a review of medical records from 2 communities. Arch Intern Med. 2000;160(7):967–73. doi: 10.1001/archinte.160.7.967. [DOI] [PubMed] [Google Scholar]
- 8.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation. Europace. 2010;12(10):1360–420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 9.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke. Results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 10.Sandhu RK, Bakal JA, Ezekowitz JA, McAlister FA. Risk stratification schemes, anticoagulation use and outcomes: the risk-treatment paradox in patients with newly diagnosed non-valvular atrial fibrillation. Heart. 2011;97(24):2046–50. doi: 10.1136/heartjnl-2011-300901. Epub 2011 Nov 10. [DOI] [PubMed] [Google Scholar]
- 11.Zimetbaum PJ, Thosani A, Yu HT, Xiong Y, Lin J, Kothawala P, et al. Are atrial fibrillation patients receiving warfarin in accordance with stroke risk? Am J Med. 2010;123(5):446–53. doi: 10.1016/j.amjmed.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Friberg L, Hammar N, Ringh M, Pettersson H, Rosenqvist M. Stroke prophylaxis in atrial fibrillation: who gets it and who does not? Report from the Stockholm Cohort-study on Atrial Fibrillation (SCAF-study) Eur Heart J. 2006;27(16):1954–64. doi: 10.1093/eurheartj/ehl146. Epub 2006 Jul 17. [DOI] [PubMed] [Google Scholar]
- 13.Klein D, Levine M. Are family physicians using the CHADS2 score? Is it useful for assessing risk of stroke in patients with atrial fibrillation? Can Fam Physician. 2011;57:e305–9. Available from: www.cfp.ca/content/57/8/e305.full.pdf+html. Accessed 2014 Feb 19. [PMC free article] [PubMed] [Google Scholar]
- 14.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137(2):263–72. doi: 10.1378/chest.09-1584. Epub 2009 Sep 17. [DOI] [PubMed] [Google Scholar]
- 15.Skanes AC, Healey JS, Cairns JA, Dorian P, Gillis AM, McMurtry MS, et al. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol. 2012;28(2):125–36. doi: 10.1016/j.cjca.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Cairns JA, Connolly S, McMurtry S, Stephenson M, Talajic M, CCS Atrial Fibrillation Guidelines Committee Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention of stroke and systemic thromboembolism in atrial fibrillation and flutter. Can J Cardiol. 2011;27(1):74–90. doi: 10.1016/j.cjca.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91. doi: 10.1056/NEJMoa1009638. Epub 2011 Aug 10. [DOI] [PubMed] [Google Scholar]
- 18.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92. doi: 10.1056/NEJMoa1107039. Epub 2011 Aug 27. [DOI] [PubMed] [Google Scholar]
- 19.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51. doi: 10.1056/NEJMoa0905561. Epub 2009 Aug 30. [DOI] [PubMed] [Google Scholar]
- 20.Garcia D, Libby E, Crowther MA. The new oral anticoagulants. Blood. 2010;115(1):15–20. doi: 10.1182/blood-2009-09-241851. Epub 2009 Oct 30. [DOI] [PubMed] [Google Scholar]
- 21.Van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103(6):1116–27. doi: 10.1160/TH09-11-0758. Epub 2010 Mar 29. [DOI] [PubMed] [Google Scholar]
- 22.Leone M, Ragonnet B, Alonso S, Allaouchiche B, Constantin JM, Jaber S, et al. Variable compliance with clinical practice guidelines identified in a 1-day audit at 66 French adult intensive care units. Crit Care Med. 2012;40(12):3189–95. doi: 10.1097/CCM.0b013e31826571f2. [DOI] [PubMed] [Google Scholar]
- 23.Dainty KN, Brooks SC, Morrison LJ. Are the 2010 guidelines on cardiopulmonary resuscitation lost in translation? A call for increased focus on implementation science. Resuscitation. 2013;84(4):422–5. doi: 10.1016/j.resuscitation.2012.08.336. Epub 2012 Sep 11. [DOI] [PubMed] [Google Scholar]
- 24.Stafford RS, Singer DE. Recent national patterns of warfarin use in atrial fibrillation. Circulation. 1998;97(13):1231–3. doi: 10.1161/01.cir.97.13.1231. [DOI] [PubMed] [Google Scholar]
- 25.Ceresne L, Upshur RE. Atrial fibrillation in a primary care practice: prevalence and management. BMC Fam Pract. 2002;3:11. doi: 10.1186/1471-2296-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King DE, Dickerson LM, Sack JL. Acute management of atrial fibrillation: part II. Prevention of thromboembolic complications. Am Fam Physician. 2002;66(2):261–4. [PubMed] [Google Scholar]
- 27.Modig S, Höglund P, Troein M, Midlöv P. GP’s adherence to guidelines for cardiovascular disease among elderly: a quality development study. ScientificWorldJournal. 2012;2012:767892. doi: 10.1100/2012/767892. Epub 2012 May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray S, Lazure P, Pullen C, Maltais P, Dorian P. Atrial fibrillation care: challenges in clinical practice and educational needs assessment. Can J Cardiol. 2011;27(1):98–104. doi: 10.1016/j.cjca.2010.12.006. Erratum in: Can J Cardiol 2011:27(3):388. [DOI] [PubMed] [Google Scholar]
- 29.Licskai C, Sands T, Ong M, Paolatto L, Nicoletti I. Using a knowledge translation framework to implement asthma clinical practice guidelines in primary care. Int J Qual Health Care. 2012;24(5):538–46. doi: 10.1093/intqhc/mzs043. Epub 2012 Aug 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haaland DA, Cohen DR, Kennedy CC, Khalidi NA, Adachi JD, Papaioannou A. Closing the osteoporosis care gap: increased osteoporosis awareness among geriatrics and rehabilitation teams. BMC Geriatr. 2009;9:28. doi: 10.1186/1471-2318-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liddy C, Hogg W, Russell G, Wells G, Armstrong CD, Akbari A, et al. Improved delivery of cardiovascular care (IDOCC) through outreach facilitation: study protocol and implementation details of a cluster randomized controlled trial in primary care. Implement Sci. 2011;6:110. doi: 10.1186/1748-5908-6-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caetano PA, Labine L, Klassen P, Dreilich D, Leslie WD. Closing the postfracture care gap using administrative health databases: design and implementation of a randomized controlled trial. J Clin Densitom. 2011;14(4):422–7. doi: 10.1016/j.jocd.2011.04.008. Epub 2011 Jul 1. [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin VV, Langer A, Tan M, Clements PJ, Oudiz RJ, Tapson VF, et al. Contemporary trends in the diagnosis and management of pulmonary arterial hypertension: an initiative to close the care gap. Chest. 2013;143(2):324–32. doi: 10.1378/chest.11-3060. [DOI] [PubMed] [Google Scholar]
- 34.Koopman RJ, Kochendorfer KM, Moore JL, Mehr DR, Wakefield DS, Yadamsuren B, et al. A diabetes dashboard and physician efficiency and accuracy in accessing data needed for high-quality diabetes care. Ann Fam Med. 2011;9(5):398–405. doi: 10.1370/afm.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Putnam W, Nicol K, Anderson D, Brownell B, Chiasson M, Burge FI, et al. Anticoagulation in atrial fibrillation. Is there a gap in care for ambulatory patients? Can Fam Physician. 2004;50:1244–50. [PMC free article] [PubMed] [Google Scholar]
- 36.Hazelhurst B, McBurnie MA, Mularski RA, Puro JE, Chauvie SL. Automating care quality measurement with health information technology. Am J Manag Care. 2012;18(6):313–9. [PubMed] [Google Scholar]
- 37.Seyfried L, Hanauer DA, Nease D, Albeiruti R, Kavanagh J, Kales HC. Enhanced identification of eligibility for depression research using an electronic medical record search engine. Int J Med Inform. 2009;78(12):e13–8. doi: 10.1016/j.ijmedinf.2009.05.002. Epub 2009 Jun 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright A, Pang J, Feblowitz JC, Maloney FL, Wilcox AR, Ramelson HZ, et al. A method and knowledge base for automated inference of patient problems from structured data in an electronic medical record. J Am Med Inform Assoc. 2011;18(6):859–67. doi: 10.1136/amiajnl-2011-000121. Epub 2011 May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]