Abstract
GPRC6A (GPCR, class C, group 6, subtype A) is a class C GPCR that has been cloned from human, mouse and rat. Several groups have shown that the receptor is activated by a range of basic and small aliphatic L-α-amino acids of which L-arginine, L-lysine and L-ornithine are the most potent compounds with EC50 values in the mid-micromolar range. In addition, several groups have shown that the receptor is either directly activated or positively modulated by divalent cations such as Ca2+ albeit in concentrations above 5 mM, which is above the physiological concentration in most tissues. More recently, the peptide osteocalcin and the steroid testosterone have also been suggested to be endogenous GPRC6A agonists. The receptor is widely expressed in all three species which, along with the omnipresence of the amino acids and divalent cation ligands, suggest that the receptor could be involved in a broad range of physiological functions. So far, this has mainly been addressed by analyses of genetically modified mice where the GPRC6A receptor has been ablated. Although there has been some discrepancies among results reported from different groups, there is increasing evidence that the receptor is involved in regulation of inflammation, metabolism and endocrine functions. GPRC6A could thus be an interesting target for new drugs in these therapeutic areas.
Linked ArticlesThis article is part of a themed section on Molecular Pharmacology of GPCRs. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-5
Keywords: amino acid sensing, divalent cation sensing, 7TM receptor, GPRC6A, knockout mouse
Molecular pharmacology
Cloning and sequence analyses
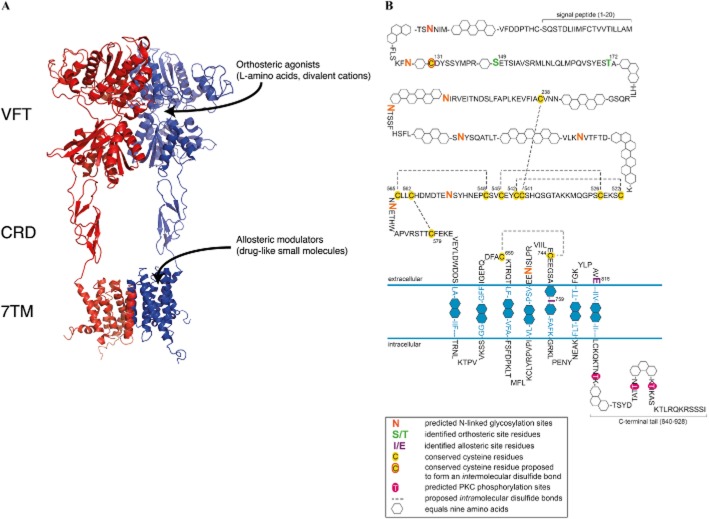
Using the protein sequences of class C GPCRs as a query, we identified the GPRC6A (GPCR, class C, group 6, subtype A) sequence in the Ensembl human genome database and subsequently cloned it from a human kidney cDNA library in 2004 (Wellendorph and Bräuner-Osborne, 2004). The human and mouse genes consist of six exons, which can be alternatively spliced leading to truncations in the Venus flytrap (VFT) domain and presumably inactive variants of the receptor (Figure 1) (Wellendorph and Bräuner-Osborne, 2004). Phylogenetic analyses revealed that the receptor indeed belongs to class C GPCRs, which consist of eight metabotropic glutamate receptors (mGlu1–8), two γ-amino butyric acid type B receptors (GABAB1,B2), the calcium-sensing receptor (CaSR), three taste receptors (T1R1-3) and seven orphan receptors (GPR156, GPR158, GPR179 and GPRC5A-D) (Figure 2) (Wellendorph and Bräuner-Osborne, 2004; Bräuner-Osborne et al., 2007; Rondard et al., 2011; Orlandi et al., 2012) [receptor nomenclature according to (Alexander et al., 2013)]. Expanding the homology search to non-mammalian species revealed that GPRC6A displays the highest amino acid sequence identity (44%) with the goldfish 5.24 receptor (Figure 2) (Speca et al., 1999; Wellendorph and Bräuner-Osborne, 2004; Kuang et al., 2005). Further analyses of the protein sequence revealed that the receptor contains a VFT domain, cysteine-rich domain (CRD) and seven transmembrane (7TM) domain (see modelled class C receptor in Figure 3A), which are hallmarks of class C GPCRs (Wellendorph and Bräuner-Osborne, 2004; Bräuner-Osborne et al., 2007; Rondard et al., 2011). The VFT domain bears homology to bacterial periplasmic binding proteins (O'Hara et al., 1993) and has been shown to contain the orthosteric amino acid and calcium binding sites for mGlu, CaSR, GABAB1 and T1R1 receptors (Figure 3A) (Wellendorph and Bräuner-Osborne, 2009a).
Figure 1.
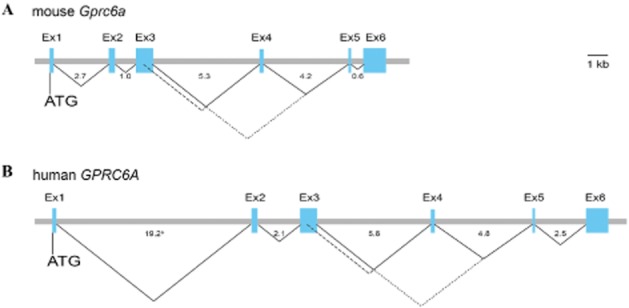
Genomic organization of the mouse and human GPRC6A genes. Exons (Ex1-6) are shown by boxes and introns by horizontal lines. The number of nucleotides in each intron is indicated (kb). Putative splicing to generate three receptor variants (Wellendorph and Bräuner-Osborne, 2004; Kuang et al., 2005) is indicated by the connecting lines; isoform 1 (_____), 2 (- - - -), and 3 (⋅ ⋅ ⋅.).aThe size of intron 1 in human GPRC6A is 2 kb per unit due to its large size.
Figure 2.
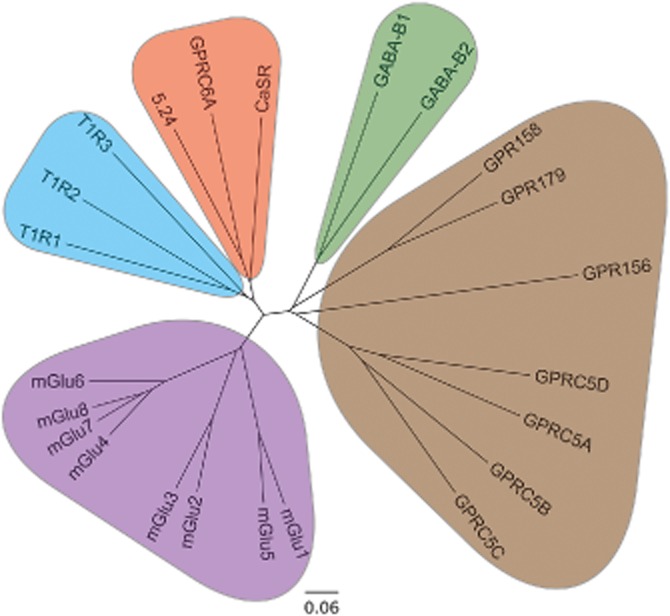
Phylogenetic analysis of GPRC6A with all presently known human family C receptors and the goldfish 5.24 odorant receptor. The predicted transmembrane spanning domains were used for generating a multiple alignment using ClustalW2 (http://www.ebi.ac.uk/), and a midpoint rooted tree was constructed using the program FigTree 1.4.0 (http://tree.bio.ed.ac.uk/). The scale bar is a function of amino acid substitutions based on the Blosum series substitution weight matrix.
Figure 3.
(A) Model of an active conformation of a dimeric family C GPCR based on 3D X-ray structures of domains from mGlu1, mGlu3 and β2-adrenergic receptors. The localizations of the Venus flytrap (VFT) domain, cysteine-rich domain (CRD) and seven transmembrane domain (7TM), orthosteric and allosteric ligand binding sites are indicated. The models were constructed with the program MacPyMol using coordinates from PDB files 1EWK (mGlu1 open–closed/active VFT), 2E4U (mGlu3 CRD) and 2R4S (β2-adrenergic receptor 7TM). (B) Schematic representation of key structural features of the predicted mGPRC6A protein. Symbols are provided in the key. Orthosteric and allosteric site residues were reported previously in human GPRC6A (Wellendorph et al., 2005) and mouse GPRC6A (Faure et al., 2009; Gloriam et al., 2011) respectively. Cysteine residues predicted to be involved in intra- and intermolecular bond formation are conserved in class C ATDs (Bräuner-Osborne et al., 2007), and in the CRDs of mGlu receptors (Rondard et al., 2011). Predictions were made using the following servers at http://www.cbs.dtu.dk: Signal peptide, SignalP 4.1; transmembrane domains, TMHMM 2.0; N-linked glycosylations, NetNGlyc 1.0; kinase phosphorylation, NetPhosK 1.0.
The GPRC6A protein sequence has been cloned from human, mouse and rat and predicted in the genomes of dog, chicken, chimpanzee and several other simians. Mouse and rat receptor proteins display a notable higher degree of sequence identity (93%) than murine and human proteins (80%), based on alignments of the full-length sequences (Table 1). Instead human GPRC6A is more closely related to chimpanzee (98%) and dog (86%) orthologues (Table 1). GPRC6A has also been predicted to be conserved through evolution to goldfish, zebrafish and pufferfish (Luu et al., 2004; Bjarnadóttir et al., 2005; Christiansen et al., 2006a), but not to the invertebrates fruit fly, mosquito and roundworm (Bjarnadóttir et al., 2005). Within the different domains of the receptor, the sequence identity is highest in the VFT compared to the 7TM domain, for example human and mouse sequence identities are 82% between the VFTs but only 50% between the 7TMs (Wellendorph et al., 2005). This suggests that the orthosteric VFT binding site is harder to discriminate between species than allosteric sites in the 7TM domain.
Table 1.
Amino acid sequence identities among GPRC6A orthologues from selected species. Numbers indicate percentages for the overall identities of amino acids between pairwisely aligned sequences using the Blosum62 matrix. Protein sequences of chimpanzee, dog and chicken are based on the conceptualized translated mRNA from predicted gene products
| Human | Mouse | Rat | Chimpanzee | Dog | Chicken | Goldfish 5.24 | |
|---|---|---|---|---|---|---|---|
| Human | 100 | 80 | 80 | 98 | 86 | 84 | 43 |
| Mouse | – | 100 | 93 | 79 | 80 | 63 | 42 |
| Rat | – | – | 100 | 79 | 80 | 63 | 42 |
| Chimpanzee | – | – | – | 100 | 85 | 65 | 43 |
| Dog | – | – | – | – | 100 | 66 | 44 |
| Chicken | – | – | – | – | – | 100 | 44 |
| Goldfish 5.24 | – | – | – | – | – | – | 100 |
In 1996, it was shown by Romano et al. that the mGlu5 receptor is a disulfide-linked dimer as a Western blot band, corresponding to the size of a dimer, was converted to a monomer size band upon reduction with dithiothreitol (DTT) (Romano et al., 1996). A few years later, it was shown by mutagenesis that a cysteine residue in a loop in the VFT of mGlu1 [C140, (Ray and Hauschild, 2000)], mGlu5 [C129, (Romano et al., 2001)] and CaSR [C129/C131, (Ray et al., 1999)] makes this intramolecular disulfide bond. This cysteine is conserved in GPRC6A (C131 in mouse) and it is thus likely that this receptor is also a covalently linked dimer (Figure 3B). This is further substantiated by the observation of a dimer size Western blot band of mouse (Kuang et al., 2005) and rat (Wellendorph et al., 2007) GPRC6A expressed in HEK293 cells, which is converted to monomer size upon reduction with DTT (Kuang et al., 2005). In addition, the VFT domain contains several potential N-glycosylation sites (Figure 3B) whereas no O-glycosylation sites are predicted (using NetOGlyc at http://www.cbs.dtu.dk). Albeit none of these glutamine residues have been confirmed experimentally to be glycosylated, we and the group of Hampson have previously shown by Western blotting that treatment with the N-linked deglycosylating enzyme PNGase F reduces the size of receptor, strongly indicating the receptor is N-glycosylated (Kuang et al., 2005; Wellendorph et al., 2007).
The CRD contains nine cysteine residues, which are known to be 100% conserved in all class C receptors (except the GABAB1,B2 and the orphan receptors that do not contain a CRD) (Bräuner-Osborne et al., 2007; Rondard et al., 2011). Analyses of mGlu receptors have shown that eight of the cysteine residues form intra-CRD disulfide bonds (Muto et al., 2007), whereas the ninth cysteine residue form a disulfide bond with a cysteine residue in the VFT (Rondard et al., 2006; Muto et al., 2007) thus promoting a very rigid connection between the VFT and CRD. All of these residues are conserved in the GPRC6A receptor and it is thus conceivable that the disulfide bonds are also present in this receptor subtype (Figure 3B). Furthermore, a disulfide bond between cysteine residues in the first and second extracellular loop, analogous to the disulfide bond shown in class A receptors (Cherezov et al., 2007), is predicted (Figure 3B). Finally, the C-terminal contains three potential PKC phosphorylation sites, which might regulate receptor desensitization, internalization and/or interaction with scaffolding proteins (Figure 3B).
Activation mechanism, dimerization and cell surface expression
The activation mechanism of class C receptors including GPRC6A has not yet been fully elucidated, but the level of our current understanding has recently been reviewed in detail by the Pin group (Rondard et al., 2011). Briefly, it is assumed that agonist binding to the cleft of the VFT promotes closure of either one or both of the clefts, which in turn leads to a twist of the dimer interface (Jensen et al., 2002). This conformational twist is then relayed to the 7TM domains via the rigid VFT-CRD interaction, which leads to a reorientation of the 7TMs in the dimer and/or a conformation change within the 7TM domains (Rondard et al., 2011). Most of this knowledge has been obtained using mGlu receptors as model systems, but it is reasonable to believe that the activation mechanism is conserved to the GPRC6A receptor given that it is also activated by L-α-amino acids like the mGlu model receptors.
It is evident from the information above, that the dimerization of class C receptors is essential for their function. mGlu and CaSR receptors form homodimers, whereas GABAB1 has to form a heterodimer with GABAB2 in order to traffic to the cell surface and become functional (Bräuner-Osborne et al., 2007; Rondard et al., 2011). Likewise, T1R1 and T1R2 have to form heterodimers with T1R3 to become functional (Nelson et al., 2001; 2002). Recently, it was also shown by fluorescence resonance energy transfer that mGlu1,5 and mGlu2,3,4,6,7,8 receptors can form heterodimers within these two subgroups when expressed in HEK293 cells (Doumazane et al., 2011), but whether they also form functional heterodimers in vivo remains to be demonstrated. In 2001, the CaSR was also suggested to be able to form heterodimers with mGlu1 and mGlu5 (Gama et al., 2001), but the lack of follow-up reports on this observation suggest that it is not of widespread importance compared to the homomeric receptors. It is worth noting that the highly confirmed GABAB and T1R heterodimers and the proposed mGlu heterodimers each group together with their heterodimeric partner within branches of the phylogenetic tree (Figure 2). The closest mammalian homolog of GPRC6A is the CaSR. Both receptors are widely expressed and have overlapping expression patterns (Wellendorph et al., 2009c; Rossol et al., 2012). It is thus possible that the receptors could either directly form heterodimers or, if expressed as homodimers in the same cell, have synergistic effects on intracellular signalling. Future experiments will have to address these questions.
When we cloned the human GPRC6A receptor, we observed that albeit the protein was expressed intracellularly in tsA cells (HEK293 variant), only very low amounts trafficked to the cell membrane (Wellendorph and Bräuner-Osborne, 2004). Later, we and the group of Hampson demonstrated that both mouse (Kuang et al., 2005; Wellendorph et al., 2005) and rat (Wellendorph et al., 2007) GPRC6A traffic to the cell membrane where they are able to form functional homomeric receptors. In analogy with the GABAB1 receptor, human GPRC6A contains a potential endoplasmic retention motif, RKR, in the C-terminal tail, which could cause the observed intracellular retention. However, mutation of the motif does not promote cell surface expression (Kuang et al., 2005; Wellendorph et al., 2005). It is also possible that activation of the receptor could lead to phosphorylation of the C-terminal tail (Figure 3B), which in turn could lead to internalization of the receptor. However, inactivating mutations of two highly conserved agonist binding residues in the VFT cleft did not lead to cell surface expression (Wellendorph et al., 2005). It is possible that, for example, a scaffolding protein or a heterodimerization partner is necessary to promote cell surface expression of particularly the human orthologue, which is absent in the cell lines used for recombinant expression. The finding that endogenously expressed and functionally active GPRC6A receptor exist in the human 22RV1 and PC-3 cell lines (Pi and Quarles, 2012a), indicates that the human receptor can be functionally expressed at the cell surface and thus lacks a partner in the heterologous cell lines used.
Ligands and signalling
In early 2005, we were the first to deorphanize the GPRC6A receptor. Given the lack of cell surface expression and function of the human orthologue expressed in tsA, COS and CHO cells, we hypothesized that the intracellular retention was caused by the 7TM domain and/or C-terminal tail, and thus generated a chimeric receptor consisting of the VFT and CRD from human GPRC6A and the 7TM domain of the goldfish 5.24 receptor (Wellendorph et al., 2005). This chimeric receptor trafficked to the cell surface and was functional in a Fluo-4 based intracellular calcium assay in agreement with the observed Gq pathway coupling of the 5.24 receptor (Speca et al., 1999; Christiansen et al., 2006a). These experiments showed that the receptor is a promiscuous L-α-amino acid receptor most potently activated by the basic amino acids L-arginine, L-lysine and L-ornithine (mid μM EC50 values) and less potently by small aliphatic amino acids (high μM EC50 values) (Table 2). We also expressed the chimeric human GPRC6A/goldfish 5.24 receptor and wild-type (WT) mouse GPRC6A in Xenopus oocytes where we could show that receptor activation by these L-α-amino acids led to increases in intracellular calcium and subsequent activation of an endogenously expressed calcium-activated chloride channel (Wellendorph et al., 2005). A few months later these results were confirmed by the group of Hampson who also used two-electrode voltage clamp electrophysiology of mouse GPRC6A expressed in Xenopus oocytes to show that the receptor is activated by basic and small aliphatic L-α-amino acids (Kuang et al., 2005). The activation of recombinantly expressed GPRC6A by basic L-α-amino acid has later also been confirmed by the groups of Ruat (Faure et al., 2009) and Quarles (Pi and Quarles, 2012a). Furthermore, using site-directed mutagenesis, we have shown that two highly conserved serine/threonine residues in the orthosteric VFT binding site are mediating L-α-amino acid recognition as has been observed for a number of other class C GPCRs (Figure 3B) (Wellendorph et al., 2005; Wellendorph and Bräuner-Osborne, 2009a).
Table 2.
Agonist potencies of L-α-amino acids at mouse, human and rat GPRC6A expressed in tsA cells compared to concentrations found in mouse plasma
| Mousea | Humanb | Rata | Concentration of L-α-amino acid (mean ± SD) in mouse plasmac | |
|---|---|---|---|---|
| L-α-amino acid | EC50 (μM) [% relative efficacy]d | (μM) | ||
| L-Orn | 63.6 | 112 | 264 [100] | 86 |
| L-Lys | 135 | 169 | >1000 [44] | 366 |
| L-Arg | 284 [100] | 44.1 | >1000 [51] | 137 |
| L-Cyse | 356 | >1000 | >1000 [73] | Not determined |
| L-Ala | 486 | 173 | >1000 [50] | 431 |
| Gly | 538 [49] | 263 | 455 [74] | 340 |
| L-Ser | 1160 | 623 | 859 [63] | 181 |
| L-Cit | >1000 | 287 | 615 [77] | 106 |
| L-Met | >1000 | 854 | >1000 [72] | 71 |
| L-Gln | >1000 | 590 | >1000 [76] | 661 |
Results from mouse and rat GPRC6A were derived from agonist-induced inositol phosphate accumulation in tsA cells co-expressing Gαq(G66D) (Christiansen et al., 2007; Wellendorph et al., 2007).
Results from human GPRC6A were obtained using a chimeric receptor containing the human VFT/CRD domains and the goldfish 5.24 7TM domain using a Fluo-4 based intracellular calcium assay (Wellendorph et al., 2005).
Data from (Komarov and Reddy, 1998).
Relative efficacies were calculated as a percentage of maximum response to that observed for 10 mM L-Arg (mouse) or 10 mM L-Orn (rat) in the same experiment (set to 100%). For compounds with low activities (EC50 values >1000 μM), relative efficacies were calculated as a percentage of response at 10 mM for the compound divided by the response for L-Arg/L-Orn at 10 mM.
L-Cys displayed a small but significant effect on mock-transfected cells at high concentrations (≥ 1 mM). The EC50 values might thus be overestimated.
Whereas we did not observe any direct agonist activity of 4 mM Ca2+ on mouse GPRC6A expressed in Xenopus oocytes (Wellendorph et al., 2005), the group of Hampson interestingly demonstrated that 5 mM Ca2+ potentiated L-α-amino acid responses (Kuang et al., 2005) indicating that divalent cations could act as positive modulators as had previously been observed for mGlu and GABAB receptors (Kubo et al., 1998; Galvez et al., 2000). At the end of 2005, this observation was further substantiated by the group of Quarles who reported that GPRC6A expressed in HEK293 cells could be directly activated by high concentrations of Ca2+ (> 5 mM) and other cations (Pi et al., 2005). This report prompted us to return to test higher Ca2+ concentrations on mouse GPRC6A expressed in the Xenopus oocyte where we were able to confirm the positive modulatory effect observed by the group of Hampson, but unable to detect a direct agonist activity of up to 50 mM Ca2+ (Christiansen et al., 2007). More recently, we have indeed confirmed an apparent direct activity of Ca2+ on mouse GPRC6A stably expressed in a CHO cell line (Jacobsen et al., 2013). In both our and Quarles pharmacological experiments using endpoint assays with >1-h ligand incubations, it is likely that there will be a build up of amino acids released from the cells (Thomsen et al., 1994). It is thus our hypothesis that the direct Ca2+ activity seen in these assays (in contrast to the electrophysiological experiments in Xenopus oocytes using continuous ligand perfusion) is in fact positive modulation of L-α-amino acids excreted from the cells during the experiment. However, regardless of whether Ca2+, Mg2+ and other cations are direct agonists or positive modulators of basic/small aliphatic L-α-amino acids, it is evident that the GPRC6A receptor is able to sense fluctuations in concentrations of both ligand classes in physiological relevant concentrations (Table 2) and they could thus represent endogenous signalling molecules for the receptor.
More recently, the group of Quarles has suggested that GPRC6A mediates some of the observed physiological responses of the steroid testosterone (Pi et al., 2010a) and the peptide osteocalcin (Pi et al., 2011). The latter ligand has also been suggested by the Karsenty group who showed that osteocalcin elicited bell-shaped concentration-dependent cAMP increases, suggesting Gs coupling, but no osteocalcin-mediated activation of the Gq or ERK pathways in TM3 Leydig cells. These osteocalcin responses were, however, not shown to be specifically mediated by GPCR6A (Oury et al., 2011) and the lack of ERK activation contrast findings in HEK293 cells expressing mouse GPRC6A reported by the Quarles group (Pi et al., 2011). GPCRs are highly versatile proteins activated by a broad range of ligands (Granier and Kobilka, 2012). Nevertheless, it is not precedented that the same receptor should have evolved to be activated by four very different classes of endogenous ligands: cations, L-α-amino acids, a steroid and a 49 amino acid peptide. Phylogenetically, class C GPCRs are viewed as nutrient receptors derived from bacterial periplasmic binding proteins, which transport amino acids and other small nutrients (Conklin and Bourne, 1994; Kuang et al., 2006). Accordingly, all other human class C GPCRs with a VFT domain (Figures 2 and 3) sense amino acids, cations and/or sugars and no other subtype in class C has previously been suggested to be activated by endogenous steroids or peptides of the size of osteocalcin (Bräuner-Osborne et al., 2007). In analogy with this reasoning based on phylogenetic relationships, we have been unable to confirm the activity of either testosterone or osteocalcin on mouse GPRC6A stably expressed in CHO cells when measuring Gq, Gi, Gs and ERK pathways (Jacobsen et al., 2013). More research is thus warranted to understand the molecular basis for the reported GPRC6A mediated effects of testosterone and osteocalcin.
All the previously mentioned putative endogenous ligands have multiple other targets in mammals, which severely limit their use as pharmacological tool compounds in tissue preparations and whole animals to investigate the physiological function and therapeutic prospect of the GPRC6A receptor. There is thus a great need for development of potent and selective agonists and antagonists. To this end, we initially screened a collection of L-Arg, L-Lys and L-ornithine analogues at the chimeric human GPRC6A/goldfish 5.24 receptor. Several compounds were equipotent with the natural basic amino acids as agonists at this receptor. However, these analogues are also known to be regulators of nitric oxide synthase and arginase and thus not sufficiently selective to be used as pharmacological tools to probe GPRC6A function (Christiansen et al., 2006b). The group of Ruat were the first to demonstrate that the CaSR allosteric modulators calindol and NPS2143 were antagonists at mouse GPRC6A (IC50∼10 μM) (Faure et al., 2009). However, both these compounds are ∼30 fold more potent at CaSR and thus not very useful as pharmacological tools to probe GPRC6A function. This led us to use a chemogenomic approach to identify novel negative allosteric modulators based on a 2-phenyl-indole scaffold (Gloriam et al., 2011). The resulting compounds are the most selective GPRC6A antagonists reported so far, but still need optimization of potency, solubility and selectivity for in vivo use. Using site-directed mutagenesis, it has been shown that calindol and the 2-phenyl-indole derivative ‘compound 3’ bind to E816 and I759 in transmembrane helix 5 and 7, respectively (Figure 3B) (Faure et al., 2009; Gloriam et al., 2011). This is in analogy with other class C GPCRs where most ‘drug-like’ allosteric modulators have been demonstrated to bind in the 7TM domain (Figure 3A) (Bräuner-Osborne et al., 2007).
As previously mentioned, we and the group of Hampson initially demonstrated L-α-amino acid-mediated activation of a calcium activated chloride channel in Xenopus oocytes indicative of Gq coupling (Kuang et al., 2005; Wellendorph et al., 2005; Christiansen et al., 2007). Furthermore, the Hampson group generated a chimeric receptor consisting of the VFT domain from the goldfish 5.24 receptor and the 7TM domain from mouse GPRC6A and demonstrated that this chimeric receptor expressed in HEK293 cells led to increased intracellular calcium levels upon activation by L-α-amino acids, which is also indicative of Gq coupling (Kuang et al., 2005). Most recently, we have used a CHO cell line stably expressing mouse GPRC6A to show that L-α-amino acids and Ca2+ activate the Gq but not Gi or Gs pathways and that the former response can be blocked by the Gq inhibitor UBO-QIC (Jacobsen et al., 2013). Using mGPRC6A-transfected HEK293 cells and pathway selective inhibitors, the Quarles group has shown that downstream serum-response element and/or ERK are activated by divalent cations [Gq and Gi pathways (Pi et al., 2005; 2010a; Pi and Quarles, 2012a)], L-arginine [pathway not investigated (Pi et al., 2011)], testosterone [Gi pathway, Gq not investigated (Pi et al., 2010a)] and osteocalcin [Gq pathway, Gi not investigated (Pi et al., 2011)]. In addition, the group has shown that all four agonist classes lead to cAMP accumulation in the GPRC6A-HEK293 cell line indicating Gs coupling (Dreaden et al., 2012; Pi et al., 2012b). It thus appears that GPRC6A can couple to several G protein pathways depending on the cell line and agonist used, albeit it is our opinion that the Gq pathway is the predominating signalling pathway. This is in analogy with the homologous CaSR, which is also preferentially coupled to Gq but also able to activate Gi, Gs and G12 pathways in some cells (Thomsen et al., 2012).
Expression pattern
Information of expression pattern and tissue distribution of the GPRC6A receptor primarily originates from real time (RT)-PCR analyses that is qualitative/semi-quantitative mRNA expression levels. Several studies have examined the tissue expression in human, mouse and rat, and a number of tissues evidently express the GPRC6A receptor, although with some discrepancies and differences between species (Wellendorph and Bräuner-Osborne, 2004; Kuang et al., 2005; Pi et al., 2005; Regard et al., 2007; Wellendorph et al., 2007; Bystrova et al., 2010; Luo et al., 2010; Haid et al., 2011; 2012). Generally, these studies have shown that the receptor is widely expressed albeit at relatively low levels.
Human GPRC6A mRNA has been identified in brain, lung, liver, heart, kidney, pancreas, skeletal muscle, placenta, spleen, ovary, testis, prostate, leukocyte and monocyte (Table 3) (Wellendorph and Bräuner-Osborne, 2004; Kuang et al., 2005; Pi and Quarles, 2012a; Rossol et al., 2012; ). So far, only a few studies have examined the mRNA expression of GPRC6A in the rat (Wellendorph et al., 2007; Harno et al., 2008). We identified the receptor in rat kidney, lung, liver, brain and the so-called ‘geschmacksstreifen’ rich in taste buds, thus proposing a role for the receptor in taste sensation (Table 3) (Wellendorph et al., 2007). Another research article demonstrated expression of GPRC6A in rat mesenteric artery endothelial cells and myocytes, suggesting a role for the receptor in regulating blood flow (Harno et al., 2008). Several studies have examined expression pattern of GPRC6A in mice and the receptor has been identified in lung, pancreas, salivary gland, mammary gland, taste cells, aorta, heart, stomach, kidney, liver, skeletal muscle, eye, spleen, tongue, small intestine, large intestine, white adipose tissue, brown adipose tissue, thymus, testis, Leydig cells, bone marrow, brain and embryonic tissue (Table 3) (Kuang et al., 2005; Regard et al., 2007; Wellendorph et al., 2009b; Bystrova et al., 2010; Luo et al., 2010; Pi et al., 2010a; 2011; 2012b; Oury et al., 2011; Oya et al., 2013; Smajilovic et al., 2013).
Table 3.
Expression pattern of the GPRC6A receptor in human, rat and mouse tissues
| Species | Tissues/Cells | References |
|---|---|---|
| Human | Brain Lung Liver Heart Kidney Pancreas Skeletal muscle Placenta Spleen Ovary Testis Prostate Leukocyte Monocyte |
(Wellendorph and Bräuner-Osborne, 2004; Pi and Quarles, 2012a; Rossol et al., 2012; ) |
| Rat | Kidney Lung Liver Brain Tongue (‘geschmacksstreifen’) Mesenteric artery |
(Wellendorph et al., 2007; Harno et al., 2008) |
| Mouse | Lung Pancreas Salivary gland Mammary gland Taste cells Aorta Heart Stomach Kidney Liver Skeletal muscle Eye Spleen Tongue Small intestine Large intestine White adipose tissue Brown adipose tissue Thymus Testis Bone marrow Brain Embryonic tissue |
(Kuang et al., 2005; Regard et al., 2007; Wellendorph et al., 2009b; Bystrova et al., 2010; Luo et al., 2010; Pi et al., 2010a; 2011; 2012b; Oury et al., 2011; Oya et al., 2013; Smajilovic et al., 2013) |
In addition, GPRC6A mRNA is endogenously expressed in the mouse insulin secreting cell line TC-6 as well as in three cell lines of human prostate cancer (Pi et al., 2011; Pi and Quarles, 2012a).
However, discrepant reports about the presence of GPRC6A in islets of Langerhans from mice exist. Regard et al. reported in 2007, using a quantitative RT-PCR study, expression of GPRC6A mRNA in mouse islets of Langerhans, and proposed a possible role of the receptor in regulation of insulin secretion (Regard et al., 2007). In contrast, a study of tissue expression of GPRC6A mRNA investigated by in situ hybridization showed the location of the expression to be mainly in the exocrine tissue of the pancreas and not in the islets of Langerhans (Luo et al., 2010). However, using RT-PCR, we have recently confirmed the expression of GPRC6A in the islet of Langerhans (Smajilovic et al., 2013).
Very recently, GPRC6A mRNA was detected in the intestinal L cell lines, GLUTag and STC-1 cells, and in the mouse small intestine (Oya et al., 2013). Moreover, expression of the receptor was also detected in fluorescence-activated cell sorting (FACS)-purified mouse primary intestinal cells by quantitative RT-PCR, and was enriched in the L cell population compared with non-L cell population.
Identification of the GPRC6A receptor at the protein level has so far been hampered by the lack of specific and well-functioning antibodies. A few studies have reported GPRC6A protein expression and identified the receptor in rat mesenteric and porcine coronary artery, mouse kidney, 15-day-old mouse embryo, testis from both mouse and human monocytes (Harno et al., 2008; Pi et al., 2008; Oury et al., 2011; Rossol et al., 2012). However, only Pi et al. (2008) used GPRC6A deficient mice to validate antibody specificity (Pi et al., 2008), hence well-founded knowledge on GPRC6A translation and protein expression remains to be established. Furthermore, there is an urgent need to obtain more detailed knowledge on which cells in the aforementioned tissues that express the GPRC6A receptor.
Physiology and pathophysiology
Knowledge pertaining to the expression pattern and ligand preferences of GPRC6A gave rise to early speculations on the physiological role of the receptor such as: monitor of the urea cycle, endocrine modulator, neuroreceptor and a detector of cell death (Civelli, 2005; Kuang et al., 2005; Pi et al., 2005; Wellendorph et al., 2005; Christiansen et al., 2006b). However, it required the engineering of genetically modified mouse models to make significant headway in understanding GPRC6A biology. Nonetheless, basic research has thus far only scratched the surface and truly uncovering the function of GPRC6A in physiology and pathophysiology requires a close synthesis of molecular and pharmacological approaches with whole-animal physiology. In the following, literature exploring the physiological function(s) of the GPRC6A receptor will be reviewed.
Role of GPRC6A in bone metabolism
By selectively deleting exon II of the mouse GPRC6A gene, which encodes for a minor part of the extracellular domain of the receptor, the group of Quarles generated in 2008 the first global GPRC6A knockout (KO) mouse model (Pi et al., 2008). Phenotypic analyses of this GPRC6A KO model identified multiple physiological abnormalities associate with ablation of the receptor, including a disruption in bone metabolism (Pi et al., 2008) (Figure 4). In a follow-up paper, these observations were further strengthened as direct function of GPRC6A in osteoblasts was reported along with an indication of a biological role for the receptor in humans (Pi et al., 2010b). Thus, the authors described an association between two single-nucleotide polymorphisms in the vicinity of the GPRC6A gene and a reduction in bone mineral density and consequently proposed that GPRC6A polymorphisms may play a role in human osteopenia (Pi et al., 2010b). By targeted disruption of the GPRC6A exon VI, containing the entire 7TM domain and C-terminal of the gene, we generated in 2009, another global GPRC6A mouse model. However, in contrast to the phenotype reported by the group of Quarles, we failed to identify bone abnormalities in our GPRC6A KO mouse model under standard physiological conditions (Wellendorph et al., 2009b), thus leaving it unresolved if GPRC6A is causally involved in regulating bone metabolism. Whether the differences between the two KO models relate to their origin remains to be uncovered but these data indicate that a more subtle phenotype is associated with deletion of exon VI than with deletion of exon II of the GPRC6A gene.
Figure 4.
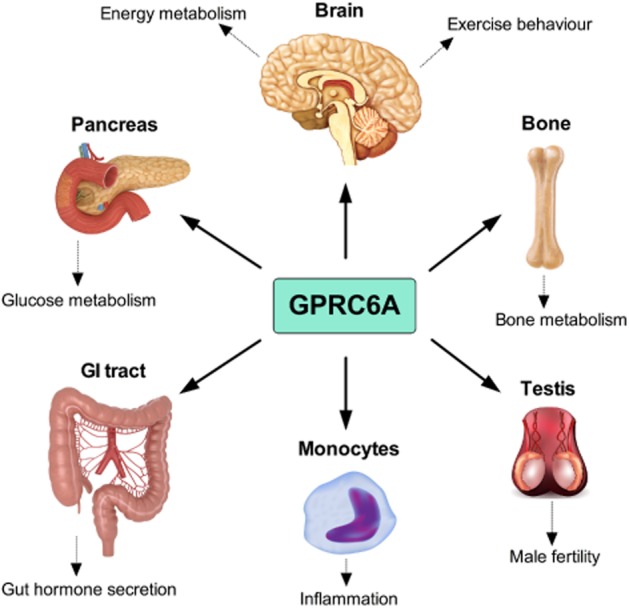
Cartoon of the major physiological systems in which the GPRC6A receptor plays a role.
Role of GPRC6A in male reproduction
It has been observed that ablation of GPRC6A in male mice results in feminization characteristics including reduced weight of the testis and decreased circulating testosterone levels (Pi et al., 2008). These observations were recently supported, as a direct role for GPRC6A in male reproduction was discovered (Oury et al., 2011) (Figure 4). GPRC6A is expressed in the Leydig cells of the testis where it serves to regulate testosterone secretion and subsequently spermatogenesis in male mice (Oury et al., 2011). Further, the authors showed that GPRC6ALeydig exon II conditional KO mice exhibit some of the same characteristics as the global GPRC6A exon II KO mice; hence ablation of GPRC6A in the Leydig cells results in reduced size and weight of the testis, decreased sperm counts and reduced circulating testosterone levels (Oury et al., 2011). This was the first study to employ a tissue-specific GPRC6A KO model and to elegantly demonstrate that 75% knock-down of GPRC6A in a specific cell type potently affects mouse physiology. Based on these findings, the group of Karsenty thus hypothesized a novel bone-testis endocrine loop in which bone-derived osteocalcin functions to regulate male reproduction via the GPRC6A receptor. Conversely, GPRC6A has also been proposed to act as a steroid sensor mediating non-genomic effects of testosterone (Pi et al., 2010b). GPRC6A deficient mice administered with testosterone were reported to exhibit a dampened phosphorylation of the MAP kinase ERK and reduced mRNA levels of the early growth response protein 1 in bone marrow and testis relative to WT controls (Pi et al., 2010b). Also, it was observed that testosterone highly induces luteinizing hormone secretion in GPRC6A KO but not in WT mice. Thus, despite that these data support a role for GPRC6A in male reproduction central questions concerning the biological mechanisms are left unanswered. Whereas the genetically modified mouse models clearly imply that GPRC6A is involved in fertility, the pharmacological evidence supporting that GPRC6A is a steroid and/or osteocalcin receptor is scarce, and further studies are therefore urgently needed to gain insight into the molecular details underlying GPRC6A's role in reproduction.
Role of GPRC6A in energy homeostasis
Several studies have shown that ablation of GPRC6A has no impact on body weight progression in mice with ad libitum access to a chow diet (Pi et al., 2008; Wellendorph et al., 2009b; Clemmensen et al., 2013a), implying that the receptor is not vital for energy balance regulation under standard physiological conditions. This is underscored by the fact that parameters such as food intake, oxygen consumption and substrate metabolism are similar between GPRC6A KO and WT control mice (Clemmensen et al., 2013a). Noteworthy, whereas we repeatedly have failed to identify any differences in body composition between chow fed KO and WT mice (Smajilovic et al., 2013; Clemmensen et al., 2013a, 2013b), Pi et al. found increased adiposity in GPRC6A deficient mice on a chow diet (Pi et al., 2008). However, we recently identified an increased sensitivity to high-fat diet induced hyperphagia in GPRC6A deficient relative to WT mice (Clemmensen et al., 2013b). The obese phenotype was associated with reduced locomotion, highly increased circulating leptin levels and multiple disruptions in glucose metabolic parameters (Clemmensen et al., 2013b). Thus, despite that the different GPRC6A KO models exhibit differences in energy homeostatic sensitivity, the overall evidence points to a direct role for GPRC6A in energy metabolism (Figure 4). Worthy of note, we have very recently identified an exercise phenotype in our GPRC6A deficient mouse model. Ablation of the receptor results in a 50% increase in voluntary wheel running when compared to WT littermate mice (Clemmensen et al., 2013a). We are currently working to uncover if the exercise phenotype and the susceptibility to diet-induced obesity are interrelated and thus controlled by a common biological pathway. Giving that eating palatable foods and exercising are both rewarding and evoke hedonic responses (Garland et al., 2011), GPRC6A may serve a function in the mesolimbic system controlling rewarding behaviour. Important to note, the biological pathways involved in eating behaviour, reward and exercise are also highly interconnected to physiological stress responses (Adam and Epel, 2007; Garland et al., 2011), hence the identified phenotypes may arise as a consequence of complex gene-by-environment interactions. Exercise is also known to differentially lower adiposity in diet-induced obese animals versus diet-resistant animals (Levin and Dunn-Meynell, 2006), proposing that exercise behaviour may represent an energy homeostatic corrective response. Interestingly, it was recently discovered that hypothalamic orexin neurons, which are known to regulate reward and energy balance, are highly influenced by mixtures of dietary amino acids (Karnani et al., 2011). Therefore, it should be interesting for future studies to explore if GPRC6A plays a role in the central orexin network and thus regulates foraging behaviour based on circulating amino acid availability.
Role of GPRC6A in glucose metabolism
The GPRC6A receptor is perhaps most studied for its potential involvement in regulating glucose metabolism (Figure 4) (Pi et al., 2011; 2012b; Oya et al., 2013; Smajilovic et al., 2013; Clemmensen et al., 2013b). But, despite a growing bulk of research on the subject, it is still elusive whether GPRC6A plays a direct role in glucose homeostatic control. Whereas the group of Quarles observes glucose intolerance and insulin resistance in GPRC6A deficient mice administered a chow diet (Pi et al., 2008), our global GPRC6A KO mice neither suffer from glucose intolerance, nor from insulin resistance (Smajilovic et al., 2013). However, disruptions in glucose metabolism surface when the animals are exposed to an obesogenic environment, and may thus appear secondary to the obese phenotype (Clemmensen et al., 2013b). The metabolic phenotype identified by the group of Quarles also exhibits coexistence of adiposity and worsened glucose metabolism (Pi et al., 2008); hence the disturbances in glucose homeostasis might likewise be a consequence of obesity and not directly linked to ablation of GPRC6A.
It has been known for decades that the GPRC6A agonist L-arginine impacts multiple glucose regulating hormones and that it is a potent insulin secretagogue (Floyd et al., 1966; Palmer et al., 1975). Further, it is known that dietary supplementation with L-arginine potently improves glucose metabolism in mice (Clemmensen et al., 2012). Interestingly, we and others have identified expression of the GPRC6A receptor in the endocrine pancreas (Regard et al., 2007; Pi et al., 2011; Smajilovic et al., 2013), which, combined with the fact that the pharmacological data clearly classify L-arginine as a potent GPRC6A agonist, prompted us to explore the hypothesis that GPRC6A is mediating L-arginine-induced insulin secretion. We thus assessed a role for GPRC6A in L-arginine-induced insulin release in vivo and ex vivo (Smajilovic et al., 2013). Both intravenous and oral administration of L-arginine potently induced insulin release equally in our GPRC6A deficient and WT mice, implying that the receptor serves no key function in L-arginine-induced insulin release in vivo. These findings were confirmed in isolated islets of Langerhans incubated with L-arginine, as both genotypes responded similarly to the insulinotropic effects of the amino acid (Smajilovic et al., 2013). The group of Quarles more recently explored a parallel hypothesis and found that isolated islets from GPRC6A KO mice exhibit a reduced response to multiple insulin secretagogues (L-arginine and glucose) due to an elevated basal insulin level in the KO mice compared to WT (Pi et al., 2012b). Interestingly, the group of Quarles previously discovered that osteocalcin injected into the peritoneal cavity increase circulating insulin levels in WT but not in GPRC6A KO mice (Pi et al., 2011). Together, these data underline that the global GPRC6A mouse model, in which exon 2 from the GPRC6A gene is deleted, suffers from glucose metabolic disturbances in comparison with our exon 6 deleted KO mice (Figure 1). Nonetheless, additional data is required to confirm a specific and direct role for GPRC6A in amino acid- and/or osteocalcin-induced insulin release.
GPRC6A has also been proposed to serve a physiological relevant role as an amino acid sensor in the gastrointestinal tract (Haid et al., 2011; Haid et al., 2012; Janssen and Depoortere, 2013), which may consequently affect glucose homeostasis. In the intestinal GLUTag cell line, GPRC6A mediates L-ornithine-induced secretion of the glucagon-like peptide 1 (GLP-1) (Oya et al., 2013) (Figure 4). Albeit these data show a direct role for GPRC6A in L-ornithine-induced GLP-1 secretion, the findings should be interpreted with caution, as GPRC6A was hardly detectable in FACS-sorted intestinal endocrine cells (but highly expressed in the GLUTag cells). Also, a role for the CaSR in amino acid-induced incretin hormone release ex vivo has been identified (Mace et al., 2012), thus, follow-up studies are needed to uncover if GPRC6A – potentially in concert with the CaSR – is involved in amino acid-induced GLP-1 secretion in vivo.
Role of GPRC6A in inflammation
Recently, using our global exon VI GPRC6A KO mice, data from the group of Wagner elegantly illustrated a dual role for GPRC6A and the CaSR in mediating extracellular calcium-induced inflammatory responses (Rossol et al., 2012). GPRC6A is expressed in monocytes, and primary cells isolated from our GPRC6A deficient mice exhibits a reduction in calcium-induced secretion of the proinflammatory cytokine IL-1β. This discovery was corroborated in vivo, thus proposing a central role for GPRC6A in inflammation (Figure 4) (Rossol et al., 2012). In line with this, a genome-wide association study (GWAS) identified GPRC6A as a novel loci associated with circulating C-reactive protein (CRP) levels (Dehghan et al., 2011). CRP is a general biomarker for systemic inflammation and increased CRP levels are associated with an array of disorders, including human obesity (Visser et al., 1999). Although not straightforward, it should be interesting for future studies to explore a potential link between the metabolic and the inflammatory phenotypes associated with GPRC6A deficiency and further down the road assess if this knowledge holds any human translational value.
GPRC6A in prostate cancer
Somewhat in line with the animal studies revealing a role for GPRC6A in male reproduction, GPRC6A has been associated with human prostate cancer. GWAS has identified GPRC6A as a genetic loci associated with prostate cancer in Japanese and Chinese populations (Takata et al., 2010; Long et al., 2012; Wang et al., 2012). To study the role of GPRC6A in the progression of prostate cancer, the group of Quarles crossed the GPRC6A KO mouse model with the transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse model, which is known to mirror the pathogenesis of human prostate cancer (Pi and Quarles, 2012a). Deletion of GPRC6A in the TRAMP mouse retarded prostate cancer progression and improved survival relative to TRAMP null mice expressing GPRC6A. Moreover, molecular biological experiments identified increased GPRC6A expression in cancer-derived prostate cells compared to healthy prostate tissue (Pi and Quarles, 2012a), supporting a role for the receptor in prostate cancer. Future studies may decipher if pharmacological tools designed to antagonise GPRC6A could be beneficial in halting the progression of and/or treating human prostate cancer.
Conclusion and future perspectives
Since the GPRC6A receptor was cloned in 2004, a lot of knowledge regarding its pharmacology and physiological function has been obtained. The data have shown that the receptor belongs to a growing family of promiscuous GPCRs (Wellendorph et al., 2009c), albeit it remains a matter of debate which of the suggested ligands are the most important physiologically. Several studies have delineated that the receptor mRNA is widely expressed at the tissue level, but the lack of well-functioning antibodies have so far prevented a much needed global mapping of the expression pattern of the receptor protein at the subtissue level. Due to the lack of receptor-specific pharmacological tool compounds, the physiological function of the receptor has so far mainly been addressed by phenotype assessment of KO mice. Results from three different KO mice strains have been surrounded by discrepancies, but taken together suggest that the receptor is involved in regulation of inflammation, metabolism and endocrine functions. GPRC6A could thus be a new interesting target for novel drugs in these therapeutic areas. However, more studies and development of novel tools such as selective agonists, antagonists and antibodies are needed to fully uncover the physiological function and therapeutic potential of the receptor.
Acknowledgments
This work was supported by the UNIK: Food, Fitness & Pharma for Health and Disease research programme, the Novo Nordisk Foundation, the Carlsberg Foundation, the Danish Council for Independent Research - Medical Sciences, the Lundbeck Foundation and the Drug Research Academy.
Glossary
- 7TM
seven transmembrane
- CaSR
calcium-sensing receptor
- CRD
cysteine rich domain
- CRP
C-reactive protein
- GABAB
GABA type B
- GLP-1
glucagon-like peptide 1
- GPRC6A
GPCR, class C, group 6, subtype A
- GWAS
genome-wide association study
- KO
knockout
- mGlu
metabotropic glutamate
- T1R
taste receptors
- TRAMP
transgenic adenocarcinoma of the mouse prostate
- VFT
Venus flytrap
- WT
wild type
Conflict of interest
None.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnadóttir TK, Fredriksson R, Schiöth HB. The gene repertoire and the common evolutionary history of glutamate, pheromone (V2R), taste(1) and other related G protein-coupled receptors. Gene. 2005;362:70–84. doi: 10.1016/j.gene.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Bräuner-Osborne H, Wellendorph P, Jensen AA. Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets. 2007;8:169–184. doi: 10.2174/138945007779315614. [DOI] [PubMed] [Google Scholar]
- Bystrova MF, Romanov RA, Rogachevskaja OA, Churbanov GD, Kolesnikov SS. Functional expression of the extracellular-Ca2+-sensing receptor in mouse taste cells. J Cell Sci. 2010;123:972–982. doi: 10.1242/jcs.061879. [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, et al. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen B, Wellendorph P, Bräuner-Osborne H. Activity of L-α-amino acids at the promiscuous goldfish odorant receptor 5.24. Eur J Pharmacol. 2006a;536:98–101. doi: 10.1016/j.ejphar.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Christiansen B, Wellendorph P, Bräuner-Osborne H. Known regulators of nitric oxide synthase and arginase are agonists at the human G-protein-coupled receptor GPRC6A. Br J Pharmacol. 2006b;147:855–863. doi: 10.1038/sj.bjp.0706682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen B, Hansen KB, Wellendorph P, Bräuner-Osborne H. Pharmacological characterization of mouse GPRC6A, an L-α-amino acid receptor with ability to sense divalent cations. Br J Pharmacol. 2007;150:798–807. doi: 10.1038/sj.bjp.0707121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O. An orphan receptor adopted by a family of transmitters. Mol Pharmacol. 2005;67:583–584. doi: 10.1124/mol.104.010348. [DOI] [PubMed] [Google Scholar]
- Clemmensen C, Madsen A, Smajilovic S, Holst B, Bräuner-Osborne H. L-Arginine improves multiple physiological parameters in mice exposed to diet-induced metabolic disturbances. Amino Acids. 2012;43:1265–1275. doi: 10.1007/s00726-011-1199-1. [DOI] [PubMed] [Google Scholar]
- Clemmensen C, Pehmøller C, Klein AB, Ratner C, Wojtaszewski JFP, Bräuner-Osborne H. Enhanced voluntary wheel running in GPRC6A receptor knockout mice. Physiol Behav. 2013a;118:144–151. doi: 10.1016/j.physbeh.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Clemmensen C, Smajilovic S, Madsen AN, Klein AB, Holst B, Bräuner-Osborne H. Increased susceptibility to diet-induced obesity in male GPRC6A receptor knockout mice. J Endocrinol. 2013b;217:151–160. doi: 10.1530/JOE-12-0550. [DOI] [PubMed] [Google Scholar]
- Conklin BR, Bourne HR. Homeostatic signals. Marriage of the flytrap and the serpent. Nature. 1994;367:22. doi: 10.1038/367022a0. [DOI] [PubMed] [Google Scholar]
- Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation. 2011;123:731–738. doi: 10.1161/CIRCULATIONAHA.110.948570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, Pin J-P. A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J. 2011;25:66–77. doi: 10.1096/fj.10-163147. [DOI] [PubMed] [Google Scholar]
- Dreaden EC, Gryder BE, Austin LA, Tene Defo BA, Hayden SC, Pi M, et al. Antiandrogen gold nanoparticles dual-target and overcome treatment resistance in hormone-insensitive prostate cancer cells. Bioconjug Chem. 2012;23:1507–1512. doi: 10.1021/bc300158k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure H, Gorojankina T, Rice N, Dauban P, Dodd RH, Bräuner-Osborne H, et al. Molecular determinants of non-competitive antagonist binding to the mouse GPRC6A receptor. Cell Calcium. 2009;46:323–332. doi: 10.1016/j.ceca.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Floyd JC, Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Insulin secretion in response to protein ingestion. J Clin Invest. 1966;45:1479–1486. doi: 10.1172/JCI105455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez T, Urwyler S, Prézeau L, Mosbacher J, Joly C, Malitschek B, et al. Ca2+ requirement for high-affinity γ-aminobutyric acid (GABA) binding at GABAB receptors: involvement of serine 269 of the GABABR1 subunit. Mol Pharmacol. 2000;57:419–426. doi: 10.1124/mol.57.3.419. [DOI] [PubMed] [Google Scholar]
- Gama L, Wilt SG, Breitwieser GE. Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J Biol Chem. 2001;276:39053–39059. doi: 10.1074/jbc.M105662200. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Med. 2011;214:206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloriam DE, Wellendorph P, Johansen LD, Thomsen ARB, Phonekeo K, Pedersen DS, et al. Chemogenomic discovery of allosteric antagonists at the GPRC6A receptor. Chem Biol. 2011;18:1489–1498. doi: 10.1016/j.chembiol.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Granier S, Kobilka B. A new era of GPCR structural and chemical biology. Nat Chem Biol. 2012;8:670–673. doi: 10.1038/nchembio.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haid D, Widmayer P, Breer H. Nutrient sensing receptors in gastric endocrine cells. J Mol Histol. 2011;42:355–364. doi: 10.1007/s10735-011-9339-1. [DOI] [PubMed] [Google Scholar]
- Haid DC, Jordan-Biegger C, Widmayer P, Breer H. Receptors responsive to protein breakdown products in G-cells and d-cells of mouse, swine and human. Front Physiol. 2012;3:65. doi: 10.3389/fphys.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harno E, Edwards G, Geraghty AR, Ward DT, Dodd RH, Dauban P, et al. Evidence for the presence of GPRC6A receptors in rat mesenteric arteries. Cell Calcium. 2008;44:210–219. doi: 10.1016/j.ceca.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Jacobsen SE, Nørskov-Lauritsen L, Thomsen ARB, Smajilovic S, Wellendorph P, Larsson NHP, et al. Delineation of the GPRC6A receptor signaling pathways using a mammalian cell line stably expressing the receptor. J Pharmacol Exp Ther. 2013 doi: 10.1124/jpet.113.206276. doi: 10.1124/jpet.113.206276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen S, Depoortere I. Nutrient sensing in the gut: new roads to therapeutics? Trends Endocrinol Metab. 2013;24:92–100. doi: 10.1016/j.tem.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Greenwood JR, Bräuner-Osborne H. The dance of the clams: twists and turns in the family C GPCR homodimer. Trends Pharmacol Sci. 2002;23:491–493. doi: 10.1016/s0165-6147(02)02107-7. [DOI] [PubMed] [Google Scholar]
- Karnani MM, Apergis-Schoute J, Adamantidis A, Jensen LT, de Lecea L, Fugger L, et al. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron. 2011;72:616–629. doi: 10.1016/j.neuron.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Komarov AM, Reddy MN. Effect of septic shock on nitrate, free amino acids, and urea in murine plasma and urine. Clin Biochem. 1998;31:107–111. doi: 10.1016/s0009-9120(97)00168-9. [DOI] [PubMed] [Google Scholar]
- Kuang D, Yao Y, Lam J, Tsushima RG, Hampson DR. Cloning and characterization of a family C orphan G-protein coupled receptor. J Neurochem. 2005;93:383–391. doi: 10.1111/j.1471-4159.2005.03025.x. [DOI] [PubMed] [Google Scholar]
- Kuang D, Yao Y, Maclean D, Wang M, Hampson DR, Chang BS. Ancestral reconstruction of the ligand-binding pocket of Family C G protein-coupled receptors. Proc Natl Acad Sci U S A. 2006;103:14050–14055. doi: 10.1073/pnas.0604717103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y, Miyashita T, Murata Y. Structural basis for a Ca2+-sensing function of the metabotropic glutamate receptors. Science. 1998;279:1722–1725. doi: 10.1126/science.279.5357.1722. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA. Differential effects of exercise on body weight gain and adiposity in obesity-prone and -resistant rats. Int J Obes. 2006;30:722–727. doi: 10.1038/sj.ijo.0803192. [DOI] [PubMed] [Google Scholar]
- Long QZ, Du YF, Ding XY, Li X, Song WB, Yang Y, et al. Replication and fine mapping for association of the C2orf43, FOXP4, GPRC6A and RFX6 genes with prostate cancer in the Chinese population. PLoS ONE. 2012;7:e37866. doi: 10.1371/journal.pone.0037866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Liu Z, Liu J, Eugene CY. Distribution pattern of GPRC6A mRNA in mouse tissue by in situ hybridization. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:1–10. doi: 10.3969/j.issn.1672-7347.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Luu P, Acher F, Bertrand HO, Fan J, Ngai J. Molecular determinants of ligand selectivity in a vertebrate odorant receptor. J Neurosci. 2004;24:10128–10137. doi: 10.1523/JNEUROSCI.3117-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace OJ, Schindler M, Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J Physiol. 2012;590:2917–2936. doi: 10.1113/jphysiol.2011.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto T, Tsuchiya D, Morikawa K, Jingami H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc Natl Acad Sci U S A. 2007;104:3759–3764. doi: 10.1073/pnas.0611577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJP, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- O'Hara PJ, Sheppard PO, Thøgersen H, Venezia D, Haldeman BA, McGrane V, et al. The ligand-binding domain in metabotropic glutamate receptors is related to bacterial periplasmic binding proteins. Neuron. 1993;11:41–52. doi: 10.1016/0896-6273(93)90269-w. [DOI] [PubMed] [Google Scholar]
- Orlandi C, Posokhova E, Masuho I, Ray TA, Hasan N, Gregg RG, et al. GPR158/179 regulate G protein signaling by controlling localization and activity of the RGS7 complexes. J Cell Biol. 2012;197:711–719. doi: 10.1083/jcb.201202123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya M, Kitaguchi T, Pais R, Reimann F, Gribble F, Tsuboi T. The G protein-coupled receptor family C group 6 subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J Biol Chem. 2013;288:4513–4521. doi: 10.1074/jbc.M112.402677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JP, Walter RM, Ensinck JW. Arginine-stimulated acute phase of insulin and glucagon secretion. I. in normal man. Diabetes. 1975;24:735–740. doi: 10.2337/diab.24.8.735. [DOI] [PubMed] [Google Scholar]
- Pi M, Quarles LD. GPRC6A regulates prostate cancer progression. Prostate. 2012a;72:399–409. doi: 10.1002/pros.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem. 2005;280:40201–40209. doi: 10.1074/jbc.M505186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. Plos ONE. 2008;3::e3858. doi: 10.1371/journal.pone.0003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Parrill AL, Quarles LD. GPRC6A mediates the non-genomic effects of steroids. J Biol Chem. 2010a;285:39953–39964. doi: 10.1074/jbc.M110.158063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Zhang L, Lei SF, Huang MZ, Zhu W, Zhang J, et al. Impaired osteoblast function in GPRC6A null mice. J Bone Miner Res. 2010b;25:1092–1102. doi: 10.1359/jbmr.091037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in β-cells in vitro and pancreas in vivo. J Bone Miner Res. 2011;26:1680–1683. doi: 10.1002/jbmr.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Wu Y, Lenchik NI, Gerling I, Quarles LD. GPRC6A mediates the effects of L-arginine on insulin secretion in mouse pancreatic islets. Endocrinology. 2012b;153:4608–4615. doi: 10.1210/en.2012-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray K, Hauschild BC. Cys-140 is critical for metabotropic glutamate receptor-1 dimerization. J Biol Chem. 2000;275:34245–34251. doi: 10.1074/jbc.M005581200. [DOI] [PubMed] [Google Scholar]
- Ray K, Hauschild BC, Steinbach PJ, Goldsmith PK, Hauache O, Spiegel AM. Identification of the cysteine residues in the amino-terminal extracellular domain of the human Ca2+ receptor critical for dimerization. Implications for function of monomeric Ca2+ receptor. J Biol Chem. 1999;274:27642–27650. doi: 10.1074/jbc.274.39.27642. [DOI] [PubMed] [Google Scholar]
- Regard JB, Kataoka H, Cano DA, Camerer E, Yin L, Zheng Y-W, et al. Probing cell type specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J Clin Invest. 2007;117:4034–4043. doi: 10.1172/JCI32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Yang WL, Omalley KL. Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J Biol Chem. 1996;271:28612–28616. doi: 10.1074/jbc.271.45.28612. [DOI] [PubMed] [Google Scholar]
- Romano C, Miller JK, Hyrc K, Dikranian S, Mennerick S, Takeuchi Y, et al. Covalent and noncovalent interactions mediate metabotropic glutamate receptor mGlu5 dimerization. Mol Pharmacol. 2001;59:46–53. [PubMed] [Google Scholar]
- Rondard P, Liu J, Huang S, Malhaire F, Vol C, Pinault A, et al. Coupling of agonist binding to effector domain activation in metabotropic glutamate-like receptors. J Biol Chem. 2006;281:24653–24661. doi: 10.1074/jbc.M602277200. [DOI] [PubMed] [Google Scholar]
- Rondard P, Goudet C, Kniazeff J, Pin JP, Prezeau L. The complexity of their activation mechanism opens new possibilities for the modulation of mGlu and GABAB class C G protein-coupled receptors. Neuropharmacology. 2011;60:82–92. doi: 10.1016/j.neuropharm.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Rossol M, Pierer M, Raulien N, Quandt D, Meusch U, Rothe K, et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun. 2012;3:1329. doi: 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smajilovic S, Clemmensen C, Johansen LD, Wellendorph P, Holst JJ, Thams PG, et al. The L-alpha-amino acid receptor GPRC6A is expressed in the islets of Langerhans but is not involved in L-arginine-induced insulin release. Amino Acids. 2013;44:383–390. doi: 10.1007/s00726-012-1341-8. [DOI] [PubMed] [Google Scholar]
- Speca DJ, Lin DM, Sorensen PW, Isacoff EY, Ngai J, Dittman AH. Functional identification of a goldfish odorant receptor. Neuron. 1999;23:487–498. doi: 10.1016/s0896-6273(00)80802-8. [DOI] [PubMed] [Google Scholar]
- Takata R, Akamatsu S, Kubo M, Takahashi A, Hosono N, Kawaguchi T, et al. Genome-wide association study identifies five new susceptibility loci for prostate cancer in the Japanese population. Nat Genet. 2010;42:751–754. doi: 10.1038/ng.635. [DOI] [PubMed] [Google Scholar]
- Thomsen AR, Smajilovic S, Bräuner-Osborne H. Novel strategies in drug discovery of the calcium-sensing receptor based on biased signaling. Curr Drug Targets. 2012;13:1324–1335. doi: 10.2174/138945012802429642. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Hansen L, Suzdak PD. L-glutamate uptake inhibitors may stimulate phosphoinositide hydrolysis in baby hamster kidney cells expressing mGluR1a via heteroexchange with L-glutamate without direct activation of mGluR1a. J Neurochem. 1994;63:2038–2047. doi: 10.1046/j.1471-4159.1994.63062038.x. [DOI] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Wang M, Liu F, Hsing AW, Wang X, Shao Q, Qi J, et al. Replication and cumulative effects of GWAS-identified genetic variations for prostate cancer in Asians: a case-control study in the ChinaPCa consortium. Carcinogenesis. 2012;33:356–360. doi: 10.1093/carcin/bgr279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellendorph P, Bräuner-Osborne H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene. 2004;335:37–46. doi: 10.1016/j.gene.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Wellendorph P, Bräuner-Osborne H. Molecular basis for amino acid sensing by family C G protein-coupled receptors. Br J Pharmacol. 2009a;156:869–884. doi: 10.1111/j.1476-5381.2008.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellendorph P, Hansen KB, Balsgaard A, Greenwood JR, Egebjerg J, Bräuner-Osborne H. Deorphanization of GPRC6A: a promiscuous L-α-amino acid receptor with preference for basic amino acids. Mol Pharmacol. 2005;67:589–597. doi: 10.1124/mol.104.007559. [DOI] [PubMed] [Google Scholar]
- Wellendorph P, Burhenne N, Christiansen B, Walter B, Schmale H, Bräuner-Osborne H. The rat GPRC6A: cloning and characterization. Gene. 2007;396:257–267. doi: 10.1016/j.gene.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Wellendorph P, Johansen L, Jensen A, Casanova E, Gassmann M, Deprez P, et al. No evidence for a bone phenotype in GPRC6A knockout mice under normal physiological conditions. J Mol Endocrinol. 2009b;42:215–223. doi: 10.1677/JME-08-0149. [DOI] [PubMed] [Google Scholar]
- Wellendorph P, Johansen LD, Bräuner-Osborne H. Molecular pharmacology of promiscuous seven transmembrane receptors sensing organic nutrients. Mol Pharmacol. 2009c;76:453–465. doi: 10.1124/mol.109.055244. [DOI] [PubMed] [Google Scholar]