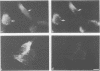
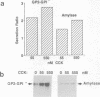
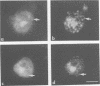
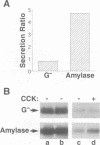
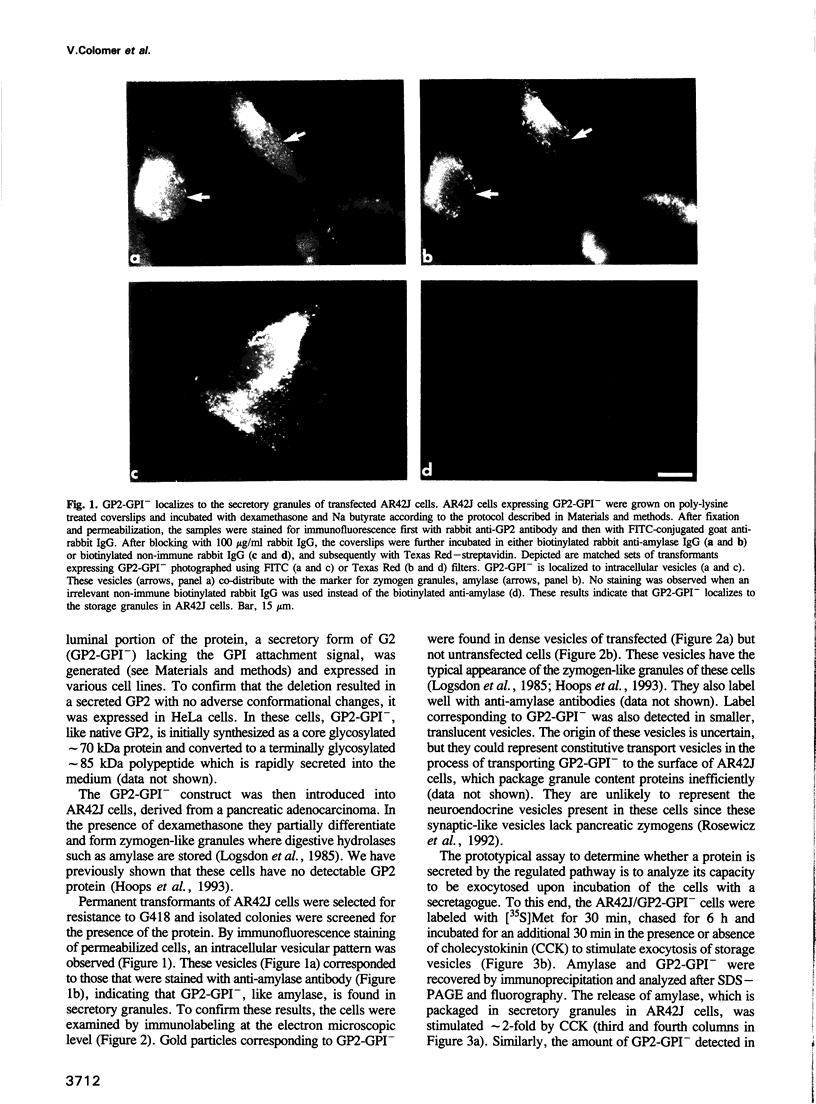
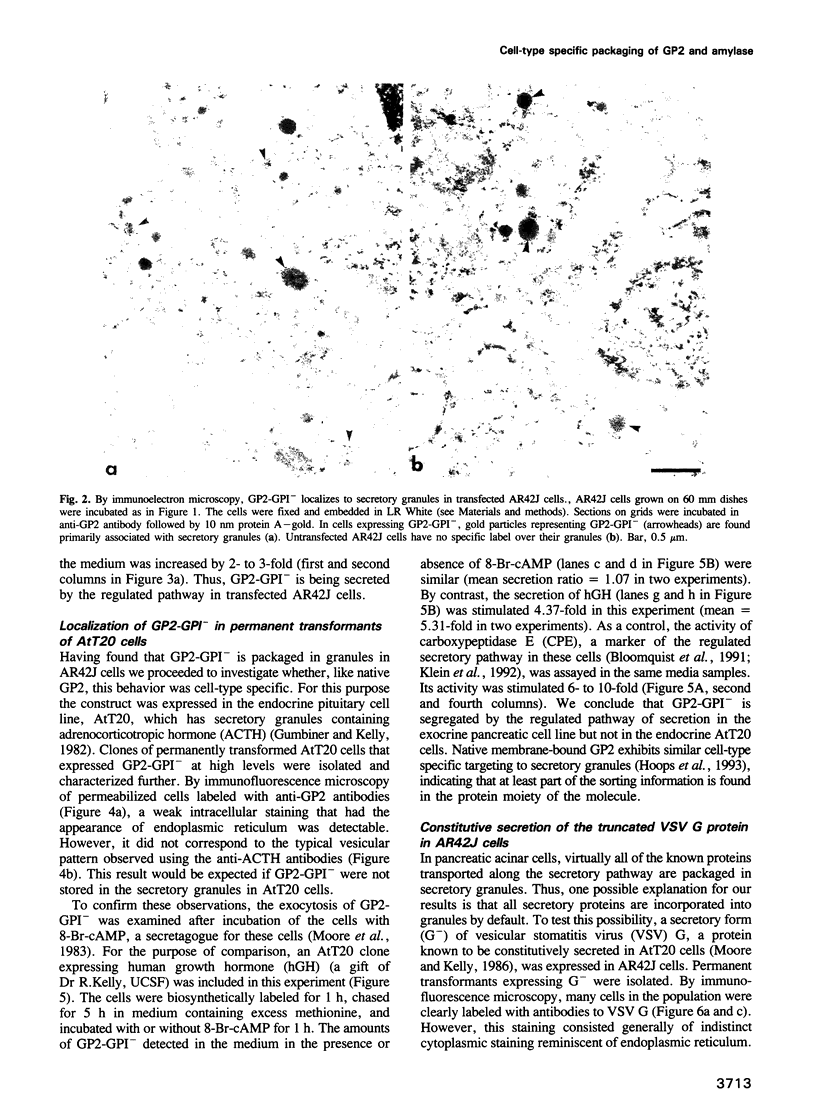
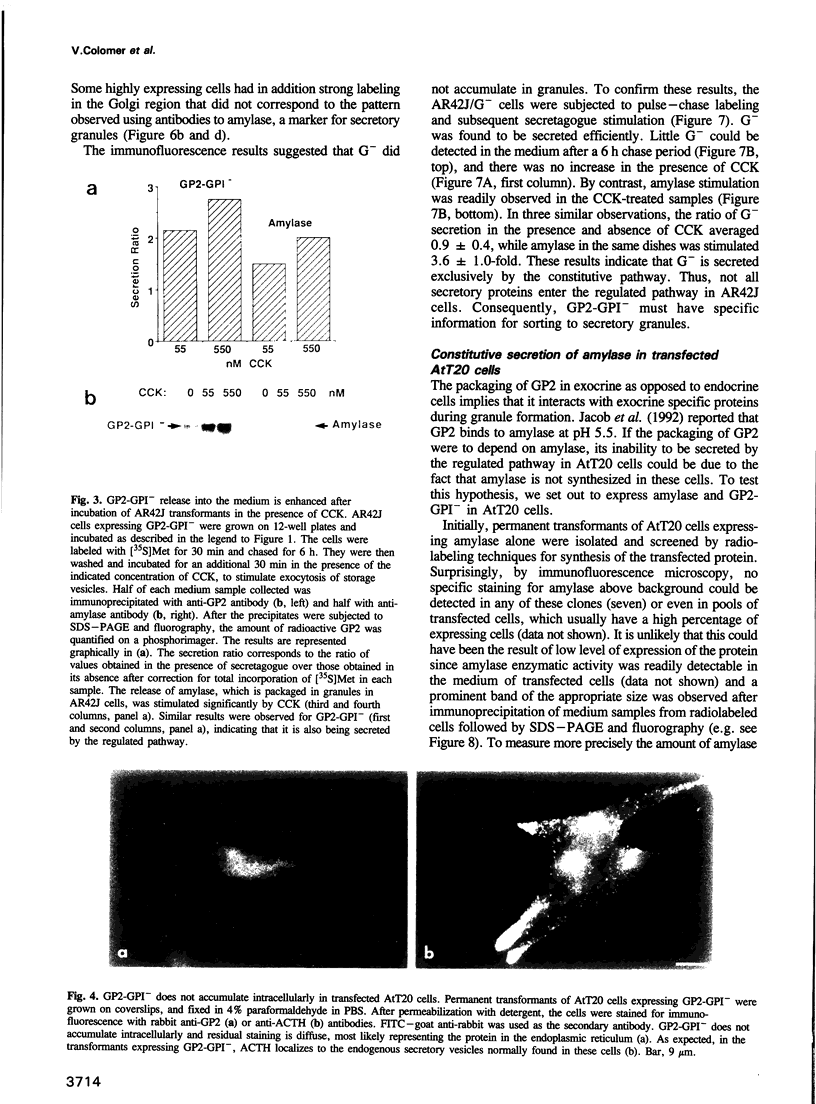
Abstract
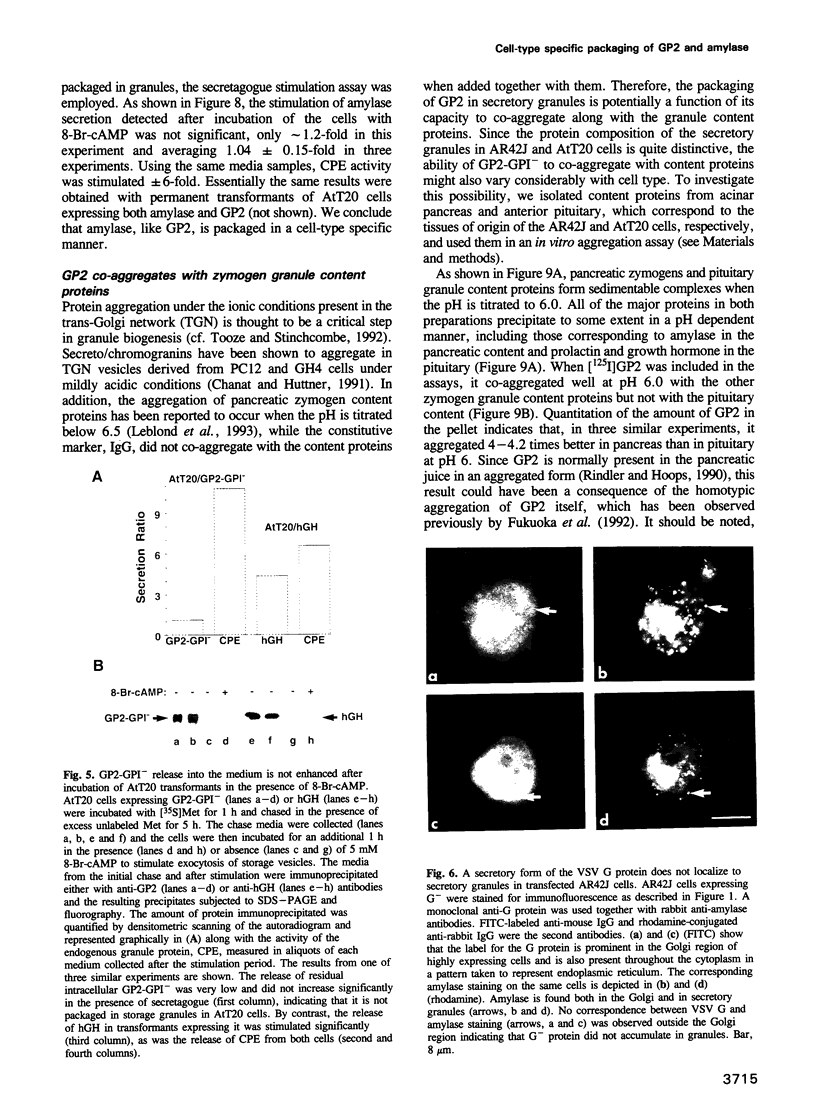
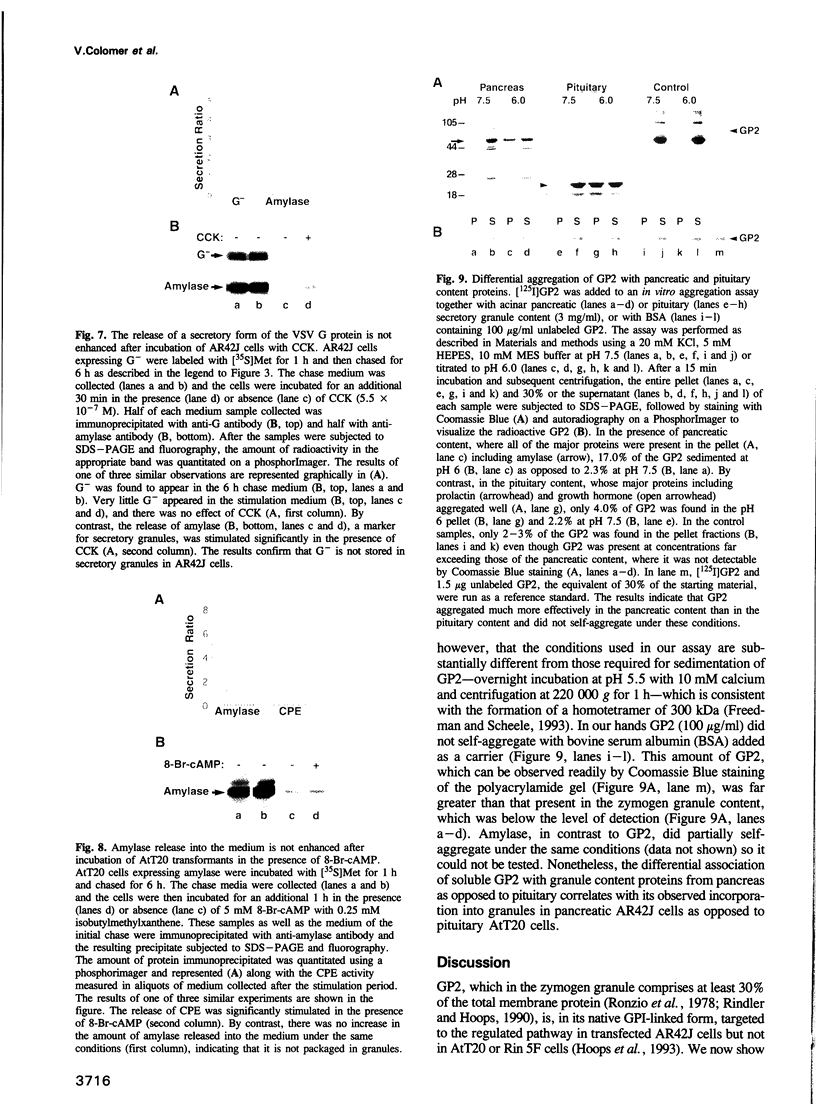
The mechanisms for segregation of secretory and membrane proteins incorporated into storage granules from those transported constitutively have been thought to be conserved in diverse cell types, including exocrine and endocrine cells. However, GP2, the major protein of pancreatic zymogen granule membranes, in its native glycosyl phosphatidylinositol (GPI)-linked form, is incorporated into secretory granules when expressed in exocrine pancreatic AR42J cells, but not in the endocrine cells such as pituitary AtT20. To determine whether the protein moiety of GP2 contains the cell-type specific information for packaging into granules, a secretory form of GP2 (GP2-GPI-), with the GPI attachment site deleted, was generated and introduced into AR42J and AtT20 cells. Like native GP2, GP2-GPI- localized to the zymogen-like granules of AR42J cells and underwent regulated secretion. In AtT20 cells expressing GP2-GPI-, however, the protein was secreted by the constitutive pathway. Thus, a granule packaging signal is present in the luminal portion of GP2 that is functional only in the exocrine cells. However, this cell-type dependent sorting process is not limited to GP2 or membrane proteins. Amylase, a major content protein of pancreatic acinar and serous salivary gland granules, was also secreted exclusively by the constitutive pathway when expressed in AtT20 cells. The cell-type specific targeting of GP2 to granules correlated with its behavior in an in vitro aggregation assay where it co-aggregated more effectively with content proteins from pancreatic zymogen granules than with those from pituitary granules.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloomquist B. T., Eipper B. A., Mains R. E. Prohormone-converting enzymes: regulation and evaluation of function using antisense RNA. Mol Endocrinol. 1991 Dec;5(12):2014–2024. doi: 10.1210/mend-5-12-2014. [DOI] [PubMed] [Google Scholar]
- Braas K. M., Stoffers D. A., Eipper B. A., May V. Tissue specific expression of rat peptidylglycine alpha-amidating monooxygenase activity and mRNA. Mol Endocrinol. 1989 Sep;3(9):1387–1398. doi: 10.1210/mend-3-9-1387. [DOI] [PubMed] [Google Scholar]
- Burgess T. L., Craik C. S., Kelly R. B. The exocrine protein trypsinogen is targeted into the secretory granules of an endocrine cell line: studies by gene transfer. J Cell Biol. 1985 Aug;101(2):639–645. doi: 10.1083/jcb.101.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron R. S., Castle J. D. Isolation and compositional analysis of secretion granules and their membrane subfraction from the rat parotid gland. J Membr Biol. 1984;79(2):127–144. doi: 10.1007/BF01872117. [DOI] [PubMed] [Google Scholar]
- Castle A. M., Castle J. D. Novel secretory proline-rich proteoglycans from rat parotid. Cloning and characterization by expression in AtT-20 cells. J Biol Chem. 1993 Sep 25;268(27):20490–20496. [PubMed] [Google Scholar]
- Castle A. M., Stahl L. E., Castle J. D. A 13-amino acid n-terminal domain of a basic proline-rich protein is necessary for storage in secretory granules and facilitates exit from the endoplasmic reticulum. J Biol Chem. 1992 Jun 25;267(18):13093–13100. [PubMed] [Google Scholar]
- Chanat E., Huttner W. B. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol. 1991 Dec;115(6):1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K. N., Walter P., Aponte G. W., Moore H. P. Molecular sorting in the secretory pathway. Science. 1989 Jan 13;243(4888):192–197. doi: 10.1126/science.2911732. [DOI] [PubMed] [Google Scholar]
- Dickinson C. J., Takeuchi T., Guo Y. J., Stadler B. T., Yamada T. Expression and processing of prohormones in nonendocrine cells. Am J Physiol. 1993 Mar;264(3 Pt 1):G553–G560. doi: 10.1152/ajpgi.1993.264.3.G553. [DOI] [PubMed] [Google Scholar]
- Disdier M., Morrissey J. H., Fugate R. D., Bainton D. F., McEver R. P. Cytoplasmic domain of P-selectin (CD62) contains the signal for sorting into the regulated secretory pathway. Mol Biol Cell. 1992 Mar;3(3):309–321. doi: 10.1091/mbc.3.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A., Green C. B., Campbell T. A., Stoffers D. A., Keutmann H. T., Mains R. E., Ouafik L. Alternative splicing and endoproteolytic processing generate tissue-specific forms of pituitary peptidylglycine alpha-amidating monooxygenase (PAM). J Biol Chem. 1992 Feb 25;267(6):4008–4015. [PubMed] [Google Scholar]
- Fisher J. M., Sossin W., Newcomb R., Scheller R. H. Multiple neuropeptides derived from a common precursor are differentially packaged and transported. Cell. 1988 Sep 9;54(6):813–822. doi: 10.1016/s0092-8674(88)91131-2. [DOI] [PubMed] [Google Scholar]
- Freedman S. D., Scheele G. A. Reversible pH-induced homophilic binding of GP2, a glycosyl-phosphatidylinositol-anchored protein in pancreatic zymogen granule membranes. Eur J Cell Biol. 1993 Aug;61(2):229–238. [PubMed] [Google Scholar]
- Fricker L. D., Devi L. Comparison of a spectrophotometric, a fluorometric, and a novel radiometric assay for carboxypeptidase E (EC 3.4.17.10) and other carboxypeptidase B-like enzymes. Anal Biochem. 1990 Jan;184(1):21–27. doi: 10.1016/0003-2697(90)90005-t. [DOI] [PubMed] [Google Scholar]
- Fukuoka S., Freedman S. D., Scheele G. A. A single gene encodes membrane-bound and free forms of GP-2, the major glycoprotein in pancreatic secretory (zymogen) granule membranes. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2898–2902. doi: 10.1073/pnas.88.7.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka S., Freedman S. D., Yu H., Sukhatme V. P., Scheele G. A. GP-2/THP gene family encodes self-binding glycosylphosphatidylinositol-anchored proteins in apical secretory compartments of pancreas and kidney. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1189–1193. doi: 10.1073/pnas.89.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorr S. U., Dean W. L., Radley T. L., Cohn D. V. Calcium-binding and aggregation properties of parathyroid secretory protein-I (chromogranin A). Bone Miner. 1988 Apr;4(1):17–25. [PubMed] [Google Scholar]
- Gorr S. U., Hamilton J. W., Cohn D. V. Regulated, but not constitutive, secretory proteins bind porcine chymotrypsinogen. J Biol Chem. 1992 Oct 25;267(30):21595–21600. [PubMed] [Google Scholar]
- Gottlieb T. A., Beaudry G., Rizzolo L., Colman A., Rindler M., Adesnik M., Sabatini D. D. Secretion of endogenous and exogenous proteins from polarized MDCK cell monolayers. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2100–2104. doi: 10.1073/pnas.83.7.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb T. A., Ivanov I. E., Adesnik M., Sabatini D. D. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993 Feb;120(3):695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Kelly R. B. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982 Jan;28(1):51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Fumagalli G., Zanini A., Meldolesi J. Sorting of three secretory proteins to distinct secretory granules in acidophilic cells of cow anterior pituitary. J Cell Biol. 1987 Oct;105(4):1579–1586. doi: 10.1083/jcb.105.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havinga J. R., Slot J. W., Strous G. J. Membrane detachment and release of the major membrane glycoprotein of secretory granules in rat pancreatic exocrine cells. Eur J Cell Biol. 1985 Nov;39(1):70–76. [PubMed] [Google Scholar]
- Hoops T. C., Ivanov I., Cui Z., Colomer-Gould V., Rindler M. J. Incorporation of the pancreatic membrane protein GP-2 into secretory granules in exocrine but not endocrine cells. J Biol Chem. 1993 Dec 5;268(34):25694–25705. [PubMed] [Google Scholar]
- Hoops T. C., Rindler M. J. Isolation of the cDNA encoding glycoprotein-2 (GP-2), the major zymogen granule membrane protein. Homology to uromodulin/Tamm-Horsfall protein. J Biol Chem. 1991 Mar 5;266(7):4257–4263. [PubMed] [Google Scholar]
- Jacob M., Lainé J., LeBel D. Specific interactions of pancreatic amylase at acidic pH. Amylase and the major protein of the zymogen granule membrane (GP-2) bind to immobilized or polymerized amylase. Biochem Cell Biol. 1992 Oct-Nov;70(10-11):1105–1114. doi: 10.1139/o92-156. [DOI] [PubMed] [Google Scholar]
- Jung L. J., Kreiner T., Scheller R. H. Expression of mutant ELH prohormones in AtT-20 cells: the relationship between prohormone processing and sorting. J Cell Biol. 1993 Apr;121(1):11–21. doi: 10.1083/jcb.121.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner S. S., Katz J., Berliner B. A., Levitz M., Finlay T. H. Hormonally sensitive esterase activity in the mouse uterus results from uptake of plasma esterase. Endocrinology. 1984 Dec;115(6):2406–2417. doi: 10.1210/endo-115-6-2406. [DOI] [PubMed] [Google Scholar]
- Kelly R. B. Pathways of protein secretion in eukaryotes. Science. 1985 Oct 4;230(4721):25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Klein R. S., Das B., Fricker L. D. Secretion of carboxypeptidase E from cultured astrocytes and from AtT-20 cells, a neuroendocrine cell line: implications for neuropeptide biosynthesis. J Neurochem. 1992 Jun;58(6):2011–2018. doi: 10.1111/j.1471-4159.1992.tb10941.x. [DOI] [PubMed] [Google Scholar]
- LeBel D., Beattie M. The major protein of pancreatic zymogen granule membranes (GP-2) is anchored via covalent bonds to phosphatidylinositol. Biochem Biophys Res Commun. 1988 Jul 29;154(2):818–823. doi: 10.1016/0006-291x(88)90213-6. [DOI] [PubMed] [Google Scholar]
- Leblond F. A., Viau G., Lainé J., Lebel D. Reconstitution in vitro of the pH-dependent aggregation of pancreatic zymogens en route to the secretory granule: implication of GP-2. Biochem J. 1993 Apr 1;291(Pt 1):289–296. doi: 10.1042/bj2910289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon C. D., Moessner J., Williams J. A., Goldfine I. D. Glucocorticoids increase amylase mRNA levels, secretory organelles, and secretion in pancreatic acinar AR42J cells. J Cell Biol. 1985 Apr;100(4):1200–1208. doi: 10.1083/jcb.100.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Milgram S. L., Johnson R. C., Mains R. E. Expression of individual forms of peptidylglycine alpha-amidating monooxygenase in AtT-20 cells: endoproteolytic processing and routing to secretory granules. J Cell Biol. 1992 May;117(4):717–728. doi: 10.1083/jcb.117.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Moore H. H., Kelly R. B. Re-routing of a secretory protein by fusion with human growth hormone sequences. Nature. 1986 May 22;321(6068):443–446. doi: 10.1038/321443a0. [DOI] [PubMed] [Google Scholar]
- Moore H. P., Gumbiner B., Kelly R. B. A subclass of proteins and sulfated macromolecules secreted by AtT-20 (mouse pituitary tumor) cells is sorted with adrenocorticotropin into dense secretory granules. J Cell Biol. 1983 Sep;97(3):810–817. doi: 10.1083/jcb.97.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H. P., Kelly R. B. Secretory protein targeting in a pituitary cell line: differential transport of foreign secretory proteins to distinct secretory pathways. J Cell Biol. 1985 Nov;101(5 Pt 1):1773–1781. doi: 10.1083/jcb.101.5.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D. M., Palmiter R. D., Hammer R. E., Brinster R. L., Swift G. H., MacDonald R. J. Specific expression of an elastase-human growth hormone fusion gene in pancreatic acinar cells of transgenic mice. Nature. 1985 Feb 14;313(6003):600–602. doi: 10.1038/313600a0. [DOI] [PubMed] [Google Scholar]
- Rindler M. J., Hoops T. C. The pancreatic membrane protein GP-2 localizes specifically to secretory granules and is shed into the pancreatic juice as a protein aggregate. Eur J Cell Biol. 1990 Oct;53(1):154–163. [PubMed] [Google Scholar]
- Ronzio R. A., Kronquist K. E., Lewis D. S., MacDonald R. J., Mohrlok S. H., O'Donnell J. J., Jr Glycoprotein synthesis in the adult rat pancreas. IV. Subcellular distribution of membrane glycoproteins. Biochim Biophys Acta. 1978 Mar 21;508(1):65–84. doi: 10.1016/0005-2736(78)90189-x. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983 Sep;34(2):513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- Rosewicz S., Vogt D., Harth N., Grund C., Franke W. W., Ruppert S., Schweitzer E., Riecken E. O., Wiedenmann B. An amphicrine pancreatic cell line: AR42J cells combine exocrine and neuroendocrine properties. Eur J Cell Biol. 1992 Oct;59(1):80–91. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Frommer J. Localization of amylase in major salivary glands of the guinea pig. J Dent Res. 1975 Sep-Oct;54(5):1027–1030. doi: 10.1177/00220345750540050401. [DOI] [PubMed] [Google Scholar]
- Tooze J., Tooze S. A. Clathrin-coated vesicular transport of secretory proteins during the formation of ACTH-containing secretory granules in AtT20 cells. J Cell Biol. 1986 Sep;103(3):839–850. doi: 10.1083/jcb.103.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
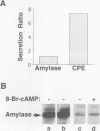
- Tooze S. A., Stinchcombe J. C. Biogenesis of secretory granules. Semin Cell Biol. 1992 Oct;3(5):357–366. doi: 10.1016/1043-4682(92)90021-m. [DOI] [PubMed] [Google Scholar]
- Wagner D. D., Saffaripour S., Bonfanti R., Sadler J. E., Cramer E. M., Chapman B., Mayadas T. N. Induction of specific storage organelles by von Willebrand factor propolypeptide. Cell. 1991 Jan 25;64(2):403–413. doi: 10.1016/0092-8674(91)90648-i. [DOI] [PubMed] [Google Scholar]
- Wolf D., Richter-Landsberg C., Short M. P., Cepko C., Breakefield X. O. Retrovirus-mediated gene transfer of beta-nerve growth factor into mouse pituitary line AtT-20. Mol Biol Med. 1988 Feb;5(1):43–59. [PubMed] [Google Scholar]
- Yoo S. H. pH-dependent association of chromogranin A with secretory vesicle membrane and a putative membrane binding region of chromogranin A. Biochemistry. 1993 Aug 17;32(32):8213–8219. doi: 10.1021/bi00083a023. [DOI] [PubMed] [Google Scholar]