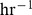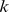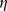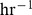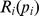Abstract
Predicting the dynamic behavior of a large network from that of the composing modules is a central problem in systems and synthetic biology. Yet, this predictive ability is still largely missing because modules display context-dependent behavior. One cause of context-dependence is retroactivity, a phenomenon similar to loading that influences in non-trivial ways the dynamic performance of a module upon connection to other modules. Here, we establish an analysis framework for gene transcription networks that explicitly accounts for retroactivity. Specifically, a module's key properties are encoded by three retroactivity matrices: internal, scaling, and mixing retroactivity. All of them have a physical interpretation and can be computed from macroscopic parameters (dissociation constants and promoter concentrations) and from the modules' topology. The internal retroactivity quantifies the effect of intramodular connections on an isolated module's dynamics. The scaling and mixing retroactivity establish how intermodular connections change the dynamics of connected modules. Based on these matrices and on the dynamics of modules in isolation, we can accurately predict how loading will affect the behavior of an arbitrary interconnection of modules. We illustrate implications of internal, scaling, and mixing retroactivity on the performance of recurrent network motifs, including negative autoregulation, combinatorial regulation, two-gene clocks, the toggle switch, and the single-input motif. We further provide a quantitative metric that determines how robust the dynamic behavior of a module is to interconnection with other modules. This metric can be employed both to evaluate the extent of modularity of natural networks and to establish concrete design guidelines to minimize retroactivity between modules in synthetic systems.
Author Summary
Biological modules are inherently context-dependent as the input/output behavior of a module often changes upon connection with other modules. One source of context-dependence is retroactivity, a loading phenomenon by which a downstream system affects the behavior of an upstream system upon interconnection. This fact renders it difficult to predict how modules will behave once connected to each other. In this paper, we propose a general modeling framework for gene transcription networks to accurately predict how retroactivity affects the dynamic behavior of interconnected modules, based on salient physical properties of the same modules in isolation. We illustrate how our framework predicts surprising and counter-intuitive dynamic properties of naturally occurring network structures, which cannot be captured by existing models of the same dimension. We describe implications of our findings on the bottom-up approach to designing synthetic circuits, and on the top-down approach to identifying functional modules in natural networks, revealing trade-offs between robustness to interconnection and dynamic performance. Our framework carries substantial conceptual analogies with electrical network theory based on equivalent representations. We believe that the framework we have proposed, also based on equivalent network representations, can be similarly useful for the analysis and design of biological networks.
Introduction
The ability to accurately predict the behavior of a complex system from that of the composing modules has been instrumental to the development of engineering systems. It has been proposed that biological networks may have a modular organization similar to that of engineered systems and that core processes, or motifs, have been conserved through the course of evolution and across different contexts [1], [2], [3], [4], [5]. In addition to having profound consequences from an evolutionary perspective, this view implies that biology can be understood, just like engineering, in a modular fashion [6]. To predict the behavior of a network from that of its composing modules, it is certainly desirable that the salient properties of modules do not change upon connection with other modules. This modularity property is especially important in a bottom-up approach to engineer biological systems, in which small systems are combined to create larger ones [7], [8].
Unfortunately, despite the fact that biological networks are rich of frequently repeated motifs, suggesting a modular organization, a module's behavior is often affected by its context [9]. Context-dependence is due to a number of different factors. These include unknown regulatory interactions between the module and its surrounding systems; various effects that the module has on the cell network, such as metabolic burden [10], effects on cell growth [11], and competition for shared resources [12]; and loading effects associated with known regulatory linkages between the module and the surrounding systems, a phenomenon known as retroactivity [13], [14]. As a result, our current ability of predicting the emergent behavior of a network from that of the composing modules remains limited. This inability is a central problem in systems biology and especially daunting for synthetic biology, in which circuits need to be re-designed through a lengthy and ad hoc process every time they are inserted in a different context [15].
In the phenomenon known as retroactivity, a downstream module perturbs the dynamic state of its upstream module in the process of receiving information from the latter [13], [14]. These effects are due to the fact that, upon interconnection, a species of the upstream module becomes temporarily unavailable for the reactions that make up the upstream module, changing the upstream module's dynamics. The resulting perturbations can have dramatic effects on the upstream module's behavior. For example, in experiments in gene circuits in Escherichia coli, a few fold ratio in gene copy number between the upstream module and the downstream target results in more than 40% change in the upstream module's response time [16]. More intriguing effects take place when the upstream module is a complex dynamical system such as an oscillator. In particular, experiments in transcriptional circuits in vitro showed that the frequency and amplitude of a clock's oscillations can be largely affected by a load [17] and computational studies on the genetic activator-repressor clock of [18] further revealed that just a few additional targets for the activator impose enough load to quench oscillations. Surprisingly, adding a few targets for the repressor can restore the stable limit cycle [19]. Retroactivity has also been experimentally demonstrated in signaling networks in vitro [20] and in the MAPK cascade in vivo [21]. In particular, it was shown in [19] that a few fold ratio between the amounts of the upstream and downstream system's proteins can lead to more than triple the response time of the upstream system.
In this paper, we provide a quantitative framework to accurately predict how and the extent to which retroactivity will change a module's temporal dynamics for general gene transcription networks and illustrate the implications on a number of recurrent network motifs. We demonstrate that the dynamic effects of loading due to interconnections can be fully captured by three retroactivity matrices. The first is the internal retroactivity, which accounts for loading due to intramodular connections. We illustrate that due to internal retroactivity, negative autoregulation can surprisingly slow down the temporal response of a gene as opposed to speeding it up, as previously reported [22]; perturbations applied at one node can lead to a response at another node even in the absence of a regulatory path from the first node to the second, having consequences relevant for network identification techniques (e.g., reviewed in [23]); and an oscillator design can fail even in the presence of small retroactivity. The other two matrices, which we call scaling and mixing retroactivity, account for loading due to intermodular connections. We illustrate that because of the scaling retroactivity, the switching characteristics of a genetic toggle switch can be substantially affected when the toggle switch is inserted in a multi-module system such as that proposed for artificial tissue homeostasis in [24]. The interplay between scaling and internal retroactivity plays a role in performance/robustness trade-offs, which we illustrate considering the single-input motif [5]. Using these retroactivities, we further provide a metric establishing the robustness of a module's behavior to interconnection. This metric can be explicitly calculated as a function of measurable biochemical parameters, and it can be used both for evaluating the extent of modularity of natural networks and for designing synthetic circuits modularly.
Our work is complementary to but different from studies focusing on partitioning large transcription networks into modules using graph-theoretic approaches [13], [25], [26]. Instead, our main objective is to develop a general framework to accurately predict both the quantitative and the qualitative behavior of interconnected modules from their behavior in isolation and from key physical properties (internal, scaling, and mixing retroactivity). In this sense, our approach is closer to that of disciplines in biochemical systems analysis, such as metabolic control analysis (MCA) [27], [28]. However, while MCA is primarily focused on steady state and near-equilibrium behavior, our approach considers global nonlinear dynamics evolving possibly far from equilibrium situations.
This paper is organized as follows. We first introduce a general mechanistic model for gene transcription networks to explain the physical origin of retroactivity and to formulate the main question of the paper (System Model and Problem Formulation). We then provide the two main results of the paper (Results). These are obtained by reducing the mechanistic model through the use of time scale separation (leading to models of the same dimension as those based on Hill functions), in which only macroscopic parameters and protein concentrations appear. In these reduced models, the retroactivity matrices naturally arise, whose practical implications are illustrated on five different application examples.
System Model and Problem Formulation
We begin by introducing a standard mechanistic model for gene transcription networks, which includes protein production, decay, and reversible binding reactions between transcription factors (TFs) and promoter sites, required for transcriptional regulation. Specifically, transcription networks are usually viewed as the input/output interconnection of fundamental building blocks called transcriptional components. A transcriptional component takes a number of TFs as inputs, and produces a single TF as an output. The input TFs form complexes with promoter sites in the transcriptional component through reversible binding reactions to regulate the production of the output TF, through the process of gene expression (for details, see Methods). To simplify the notation, we treat gene expression as a one-step process, neglecting mRNA dynamics. This assumption is based on the fact that mRNA dynamics occur on a time scale much faster than protein production/decay [1]. In addition to this, including mRNA dynamics is not relevant for the study of retroactivity, and would yield only minor changes in our results (see Methods).
Within a transcription network, we identify a transcriptional component with a node. Consequently, a transcription network is a set of interconnected nodes in which node 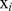 represents the transcriptional component producing TF
represents the transcriptional component producing TF  . There is a directed edge from node
. There is a directed edge from node 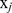 to
to 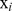 if
if  is a TF regulating the activity of the promoter controlling the expression of
is a TF regulating the activity of the promoter controlling the expression of 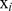 [29], in which case we call
[29], in which case we call 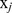 a parent of
a parent of  . Activation and repression are denoted by
. Activation and repression are denoted by  and
and  , respectively. Modules are a set of connected nodes. Modules communicate with each other by having TFs produced in one module regulate the expression of TFs produced in a different module. When a node
, respectively. Modules are a set of connected nodes. Modules communicate with each other by having TFs produced in one module regulate the expression of TFs produced in a different module. When a node 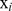 is inside the module, we call the corresponding TF
is inside the module, we call the corresponding TF 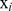 an internal TF, while when node
an internal TF, while when node 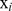 is outside the module we call the corresponding TF
is outside the module we call the corresponding TF  an external TF. Further, we identify external TFs that are parents to internal TFs as inputs to the module. Let
an external TF. Further, we identify external TFs that are parents to internal TFs as inputs to the module. Let  ,
,  and
and  denote the concentration vector of internal TFs, inputs and TF-promoter complexes, respectively. According to [30], we can write the dynamics of the module as
denote the concentration vector of internal TFs, inputs and TF-promoter complexes, respectively. According to [30], we can write the dynamics of the module as
| (1) |
where  is the stoichiometry matrix and
is the stoichiometry matrix and  is the reaction flux vector. The reactions are either protein production/decay or binding/unbinding reactions. Therefore, we partition
is the reaction flux vector. The reactions are either protein production/decay or binding/unbinding reactions. Therefore, we partition  into
into 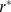 and
and  , representing the reaction flux vectors corresponding to production/decay and binding/unbinding reactions, respectively (see Methods). We assume that the DNA copy number is conserved, therefore, we can rewrite (1) as
, representing the reaction flux vectors corresponding to production/decay and binding/unbinding reactions, respectively (see Methods). We assume that the DNA copy number is conserved, therefore, we can rewrite (1) as
 |
where the upper left block matrix in  is the zero matrix as DNA is not produced/degraded. As a result, with
is the zero matrix as DNA is not produced/degraded. As a result, with  we obtain
we obtain
| (2) |
which we call the isolated dynamics of a module.
Next, consider the case when the module is inserted into a network, which we call the context of the module. We represent all the quantities related to the context with an overbar. Let  and
and  denote the concentration vector of TFs and promoter complexes of the context, respectively. Furthermore, denote by
denote the concentration vector of TFs and promoter complexes of the context, respectively. Furthermore, denote by  and
and  the reaction flux vectors corresponding to production/decay and binding/unbinding reactions between TFs and promoters in the context of the module, respectively. Then, the dynamics of the species in the module (
the reaction flux vectors corresponding to production/decay and binding/unbinding reactions between TFs and promoters in the context of the module, respectively. Then, the dynamics of the species in the module ( and
and  ) and in the context (
) and in the context ( and
and  ) can be written as
) can be written as
 |
(3) |
where the upper left block matrix is zero as DNA is assumed to be a conserved species. Furthermore, since  and
and  encapsulate the binding/unbinding reactions in the module and in its context, respectively, the off-diagonal block matrices in the upper right block matrix are zero. Similarly, as
encapsulate the binding/unbinding reactions in the module and in its context, respectively, the off-diagonal block matrices in the upper right block matrix are zero. Similarly, as  and
and  encapsulate the production/decay reactions in the module and its context, respectively, the off-diagonal block matrices in the lower left block matrix are zero. Finally, the stoichiometry matrix
encapsulate the production/decay reactions in the module and its context, respectively, the off-diagonal block matrices in the lower left block matrix are zero. Finally, the stoichiometry matrix  represents how internal TFs of the module participate in binding/unbinding reactions in the context of the module (
represents how internal TFs of the module participate in binding/unbinding reactions in the context of the module ( can be interpreted similarly).
can be interpreted similarly).
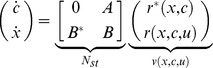
With  describing the effective rate of change of
describing the effective rate of change of  due to intermodular binding reactions, we obtain
due to intermodular binding reactions, we obtain
| (4) |
which we call the connected dynamics of a module. We refer to  as the retroactivity to the output of the module, encompassing retroactivity applied to the module due to the context of the module. Similarly, we call
as the retroactivity to the output of the module, encompassing retroactivity applied to the module due to the context of the module. Similarly, we call  the retroactivity to the input of a module, representing retroactivity originating inside the module. The general interconnection of a group of modules can be treated similarly (Figure 1).
the retroactivity to the input of a module, representing retroactivity originating inside the module. The general interconnection of a group of modules can be treated similarly (Figure 1).
Figure 1. The dynamics of a module depend on the module's context.

Downstream modules change the dynamics of an upstream module by applying a load. The effect of this load is captured by the retroactivity to the output  of the upstream module, which is the weighted sum of the retroactivity to the input
of the upstream module, which is the weighted sum of the retroactivity to the input  of the downstream modules.
of the downstream modules.
As an example of the implications of retroactivity  on the module's dynamic behavior, consider Figure 2. For the purpose of illustration, assume that
on the module's dynamic behavior, consider Figure 2. For the purpose of illustration, assume that  and
and  , external inputs to
, external inputs to  and
and  (see Methods), are periodic (in general, they can be arbitrary time-varying signals). When the module is not connected to its context (Figure 2A), its output is periodic (Figure 2B). Upon interconnection with its context (Figure 2C), due to the retroactivity to the output
(see Methods), are periodic (in general, they can be arbitrary time-varying signals). When the module is not connected to its context (Figure 2A), its output is periodic (Figure 2B). Upon interconnection with its context (Figure 2C), due to the retroactivity to the output  applied by the context, the output of the module changes significantly (Figure 2D). Hence, connection with the context leads to a dramatic departure of the dynamics of the module from its behavior in isolation. This example illustrates that retroactivity
applied by the context, the output of the module changes significantly (Figure 2D). Hence, connection with the context leads to a dramatic departure of the dynamics of the module from its behavior in isolation. This example illustrates that retroactivity  significantly alters the dynamic behavior of modules after interconnection, therefore, it cannot be neglected if accurate prediction of temporal dynamics is required. Unfortunately, model (4) provides little analytical insight into how measurable parameters and interconnection topology affect retroactivity.
significantly alters the dynamic behavior of modules after interconnection, therefore, it cannot be neglected if accurate prediction of temporal dynamics is required. Unfortunately, model (4) provides little analytical insight into how measurable parameters and interconnection topology affect retroactivity.
Figure 2. The context (downstream system) affects the behavior of the module (upstream system).
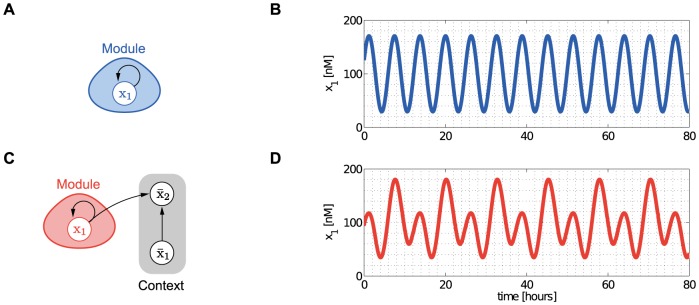
(A) The module in isolation. (B) The module in isolation displays sustained oscillations. (C) The module connected to its context. (D) Upon interconnection with its context, the dynamics of the module change due to the retroactivity  from its context, since some of the molecules of
from its context, since some of the molecules of  are involved in binding reactions at node
are involved in binding reactions at node  . As a result, those molecules are not available for reactions in the module, and the output of the module is severely changed. For details on the system and parameters, see Supporting Text S1.
. As a result, those molecules are not available for reactions in the module, and the output of the module is severely changed. For details on the system and parameters, see Supporting Text S1.
The aim of this paper is to provide a model that captures the effects of retroactivity, unlike standard regulatory network models of the same dimension based on Hill functions [1]. Specifically, we seek a model that explicitly describes the change in the dynamics of a module once it is arbitrarily connected to other modules in the network. This model is only a function of measurable biochemical parameters, TF concentrations, and interconnection topology.
Results
We first characterize the effect of intramodular connections on an isolated module's dynamics. We then analytically quantify the effects of intermodular connections on a module's behavior. Finally, we determine a metric of robustness to interconnection quantifying the extent by which the dynamics of a module are affected by its context. We demonstrate the use of our framework and its implications on network motifs taken from the literature.
The main technical assumptions in what follows are that (a) there is a separation of time scale between production/degradation of proteins and the reversible binding reactions between TFs and DNA, and that (b) the corresponding quasi-steady state is locally exponentially stable. Assumption (a) is justified by the fact that gene expression is on the time scale of minutes to hours while binding reactions are on the second to subsecond time scale [3]. Assumption (b) is implicitly made any time Hill function-based models are used in gene regulatory networks. In addition to these technical assumptions, to simplify notation, we model gene expression as a one-step process, however, a more detailed description of transcription/translation would not yield any changes to the main results (see Methods).
Effect of Intramodular Connections
Here, we focus on a single module without inputs and describe how retroactivity among nodes, modeled by 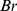 in (2), affects the module's dynamics. To this end, we provide a model that well approximates the isolated module dynamics, in which only measurable macroscopic parameters appear, such as dissociation constants and TF concentrations. We then present implications of this model for negative autoregulation, combinatorial regulation and the activator-repressor clock of [18].
in (2), affects the module's dynamics. To this end, we provide a model that well approximates the isolated module dynamics, in which only measurable macroscopic parameters appear, such as dissociation constants and TF concentrations. We then present implications of this model for negative autoregulation, combinatorial regulation and the activator-repressor clock of [18].
Employing assumptions (a)–(b), we obtain the first main result of the paper as follows. Let  denote the vector of concentrations of internal TFs, then the dynamics
denote the vector of concentrations of internal TFs, then the dynamics
| (5) |
well approximate the dynamics of  in (2) in the isolated module with
in (2) in the isolated module with
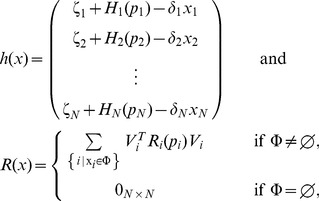 |
(6) |
where  represents external perturbations to
represents external perturbations to  (inducer, noise, or disturbance,
(inducer, noise, or disturbance, 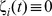 unless specified otherwise),
unless specified otherwise), 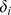 is the decay rate of
is the decay rate of  , and
, and  is the Hill function modeling the production rate of
is the Hill function modeling the production rate of  , regulated by the parents
, regulated by the parents  of
of 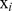 . We call
. We call 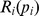 the retroactivity of node
the retroactivity of node  . For the most common binding types, Figure 3 shows the expressions of
. For the most common binding types, Figure 3 shows the expressions of 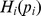 and
and  (for their definition, see Methods). The binary matrix
(for their definition, see Methods). The binary matrix  has as many columns as the number of nodes in the module, and as many rows as the number of parents of
has as many columns as the number of nodes in the module, and as many rows as the number of parents of  , such that its
, such that its  element is 1 if the
element is 1 if the  parent of
parent of  is
is  , otherwise the entry is zero. That is, an entry in the following matrix
, otherwise the entry is zero. That is, an entry in the following matrix
 |
is 1 if the species indexing the corresponding row and column are the same, otherwise the entry is zero, yielding 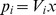 . Finally,
. Finally, 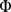 is the set of nodes having parents from inside the module. For the derivation of this result, see Theorem 1 in Supporting Text S2.
is the set of nodes having parents from inside the module. For the derivation of this result, see Theorem 1 in Supporting Text S2.
Figure 3. Hill function and retroactivity of node  for the most common binding types.
for the most common binding types.
If node  has no parents, its node retroactivity is not defined. In the single parent case, node
has no parents, its node retroactivity is not defined. In the single parent case, node 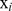 has one parent,
has one parent,  binding as an
binding as an  -multimer with dissociation constant
-multimer with dissociation constant  . In the case of independent, competitive and cooperative binding, node
. In the case of independent, competitive and cooperative binding, node 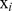 has two parents,
has two parents, 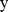 and
and  , binding as multimers with multimerization factors
, binding as multimers with multimerization factors  and
and  , respectively, together with dissociation constants
, respectively, together with dissociation constants  and
and 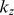 , respectively. The total concentration of the promoter of
, respectively. The total concentration of the promoter of 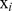 is denoted by
is denoted by  . The production rates
. The production rates  ,
,  ,
, 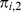 and
and 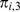 correspond to the promoter complexes without parents, with
correspond to the promoter complexes without parents, with 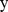 only, with
only, with  only, and with both
only, and with both  and
and  , respectively. For details, see Supporting Text S3.
, respectively. For details, see Supporting Text S3.
We call  the internal retroactivity of the module as it describes how retroactivity among the nodes internal to the module affects the isolated module dynamics. When
the internal retroactivity of the module as it describes how retroactivity among the nodes internal to the module affects the isolated module dynamics. When  , we have
, we have 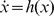 , the commonly used Hill function-based model for gene transcription networks [3]. It is possible to show that
, the commonly used Hill function-based model for gene transcription networks [3]. It is possible to show that  represents the rate of change of total (free and bound) TFs (see Supporting Text S2). Hence, (6) describes how changes in the total concentration of TFs
represents the rate of change of total (free and bound) TFs (see Supporting Text S2). Hence, (6) describes how changes in the total concentration of TFs  relate to changes
relate to changes  in the concentration of free TFs. Specifically, to change the concentration of free TFs by one unit, the module has to change the total concentration of TFs by
in the concentration of free TFs. Specifically, to change the concentration of free TFs by one unit, the module has to change the total concentration of TFs by  units, as
units, as  units are “spent on” changing the concentration of bound TFs. Having
units are “spent on” changing the concentration of bound TFs. Having  implies that the module's effort on affecting the total concentration of TFs is entirely spent on changing the concentration of free TFs. By contrast,
implies that the module's effort on affecting the total concentration of TFs is entirely spent on changing the concentration of free TFs. By contrast, 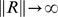 implies that no matter how much the total concentration of TFs changes, it is not possible to achieve any changes in the free concentration of some of the TFs. Therefore, the internal retroactivity
implies that no matter how much the total concentration of TFs changes, it is not possible to achieve any changes in the free concentration of some of the TFs. Therefore, the internal retroactivity  describes how “stiff” the module is against changes in
describes how “stiff” the module is against changes in  due to loading applied by internal connections.
due to loading applied by internal connections.
The entries of  have the following physical interpretation. Consider first a module with the autoregulated node
have the following physical interpretation. Consider first a module with the autoregulated node  , that is,
, that is, 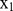 has a single parent: itself. The retroactivity of node
has a single parent: itself. The retroactivity of node  is
is  , where
, where  is given in Figure 3. In this case, we obtain
is given in Figure 3. In this case, we obtain 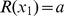 by (6), so that (5) yields
by (6), so that (5) yields 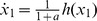 . Hence, the greater
. Hence, the greater  , the harder to change the concentration of free
, the harder to change the concentration of free 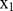 by changing its total concentration (the “stiffer” the node), and the temporal dynamics of
by changing its total concentration (the “stiffer” the node), and the temporal dynamics of  become slower. The retroactivity
become slower. The retroactivity  of node
of node  can be increased by increasing its DNA copy number
can be increased by increasing its DNA copy number 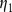 or by decreasing the dissociation constant
or by decreasing the dissociation constant  of
of  . For a node with two parents, we provide the explicit formula for
. For a node with two parents, we provide the explicit formula for  in Figure 3 in the case of the most frequent binding types, so that here we simply write
in Figure 3 in the case of the most frequent binding types, so that here we simply write
| (7) |
The diagonal entries 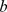 and
and  in (7) can be interpreted similarly to
in (7) can be interpreted similarly to  , while the off-diagonal entries can be interpreted as follows. Having
, while the off-diagonal entries can be interpreted as follows. Having  means that the second parent facilitates the binding of the first, whereas
means that the second parent facilitates the binding of the first, whereas 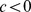 represents blockage (
represents blockage ( can be interpreted similarly with the parents having reverse roles). Therefore, we have
can be interpreted similarly with the parents having reverse roles). Therefore, we have 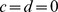 in the case of independent binding (Figure 3), as the parents bind to different sites. By contrast, we have
in the case of independent binding (Figure 3), as the parents bind to different sites. By contrast, we have  in the case of competitive binding (Figure 3), since the parents are competing for the same binding sites, forcing each other to unbind. Following a similar reasoning, we obtain
in the case of competitive binding (Figure 3), since the parents are competing for the same binding sites, forcing each other to unbind. Following a similar reasoning, we obtain  in the case of cooperative binding (Figure 3). Notice that
in the case of cooperative binding (Figure 3). Notice that  is scaled by the total concentration of promoter
is scaled by the total concentration of promoter  , which can be changed, for example, in synthetic circuits by changing the plasmid copy number.
, which can be changed, for example, in synthetic circuits by changing the plasmid copy number.
Practical Implications of Intramodular Connections
In order to illustrate the effects of intramodular connections, we consider three recurrent network motifs in gene transcription networks: (i) negative autoregulation of a gene, (ii) combinatorial regulation of a gene by two TFs, and (iii) the activator-repressor clock of [18].
Negative autoregulation
One of the most frequent network motifs in gene transcription networks is negative autoregulation, as over 40% of known Escherichia coli TFs are autorepressed [29]. Earlier studies concluded that negative autoregulation makes the response of a gene faster [22]. Here, we demonstrate that in the case of significant retroactivity, negative autoregulation can actually slow down the response of a gene. To this end, consider a module consisting of the single node 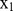 , and analyze first the case when its production is constitutive with promoter concentration
, and analyze first the case when its production is constitutive with promoter concentration 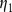 , production rate constant
, production rate constant  and decay rate
and decay rate  . Then, the dynamics of
. Then, the dynamics of 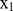 are given by
are given by  .
.
In the case of negative autoregulation, 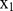 has itself as the only parent. Let
has itself as the only parent. Let 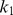 denote the dissociation constant of
denote the dissociation constant of 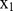 and assume it binds as a monomer repressing its own production (so that
and assume it binds as a monomer repressing its own production (so that  and
and 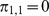 in Figure 3). According to Figure 3, we have
in Figure 3). According to Figure 3, we have 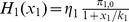 and
and  together with
together with  and
and  , yielding from (6)
, yielding from (6)  and
and 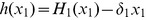 , so that (5) results in
, so that (5) results in
| (8) |
This expression indicates that negative autoregulation yields two changes in the dynamics. First, protein production changes from 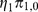 to the Hill function
to the Hill function  . Second, the dynamics are premultiplied by
. Second, the dynamics are premultiplied by 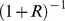 , which is the effect of internal retroactivity.
, which is the effect of internal retroactivity.
As it was shown in [22], the response time of the regulated system without retroactivity is smaller than that of the unregulated system. When considering internal retroactivity, however, the response time increases, as the absolute value of  decreases with increased
decreases with increased  according to (8). Specifically, the response time with
according to (8). Specifically, the response time with  is greater than without
is greater than without  since
since 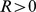 . That is, while the Hill function makes the response faster, internal retroactivity has an antagonistic effect, so that negative autoregulation can render the response slower than that of the unregulated system, as illustrated in Figure 4. Furthermore, if
. That is, while the Hill function makes the response faster, internal retroactivity has an antagonistic effect, so that negative autoregulation can render the response slower than that of the unregulated system, as illustrated in Figure 4. Furthermore, if 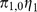 is kept constant, the response time of both the unregulated (blue) and the regulated system without retroactivity (green) remain the same, together with the steady states. By contrast, increasing
is kept constant, the response time of both the unregulated (blue) and the regulated system without retroactivity (green) remain the same, together with the steady states. By contrast, increasing  (and decreasing
(and decreasing 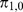 ) makes the internal retroactivity
) makes the internal retroactivity 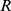 greater (since
greater (since  is proportional to
is proportional to  ), while the contribution of the Hill function remains unchanged. As a result, the response of the regulated system with retroactivity (red) becomes slower as we increase
), while the contribution of the Hill function remains unchanged. As a result, the response of the regulated system with retroactivity (red) becomes slower as we increase  (and decrease
(and decrease 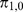 ). This is illustrated in Figure 4 with different
). This is illustrated in Figure 4 with different  pairs. Note that
pairs. Note that 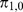 can be decreased, for example, by decreasing the ribosome binding site (RBS) strength, whereas
can be decreased, for example, by decreasing the ribosome binding site (RBS) strength, whereas 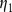 can be increased by increasing the gene copy number.
can be increased by increasing the gene copy number.
Figure 4. Negative autoregulation can make the temporal response slower.

Time response at a steady state fixed at 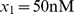 . The red and blue plots denote the cases with and without negative autoregulation, respectively, whereas the green plot represents the case of negative autoregulation neglecting retroactivity (
. The red and blue plots denote the cases with and without negative autoregulation, respectively, whereas the green plot represents the case of negative autoregulation neglecting retroactivity (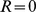 in (8)). Simulation parameters are
in (8)). Simulation parameters are  ,
,  , together with
, together with  ,
,  ,
,  for A, B and C, respectively. To carry out a meaningful comparison between the unregulated and regulated systems, we compare the response time of systems with the same steady state. To do so, we pick the same value of
for A, B and C, respectively. To carry out a meaningful comparison between the unregulated and regulated systems, we compare the response time of systems with the same steady state. To do so, we pick the same value of 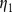 in the case of the regulated systems (
in the case of the regulated systems (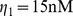 ,
,  ,
, 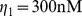 for A, B and C, respectively), but a different one for for the unregulated system (
for A, B and C, respectively), but a different one for for the unregulated system ( ,
,  ,
,  for A, B and C, respectively), such that the steady states match (see Methods for parameter ranges). Decreasing
for A, B and C, respectively), such that the steady states match (see Methods for parameter ranges). Decreasing 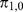 (lower production rate constant) while increasing
(lower production rate constant) while increasing  (higher DNA copy number) results in slower response, as internal retroactivity increases.
(higher DNA copy number) results in slower response, as internal retroactivity increases.
Combinatorial regulation
As a second example, we consider a single gene co-regulated by two TFs (Figure 5A). This topology appears in recurrent network motifs, such as the feedforward-loop, the bi-fan and the dense overlapping regulon [5]. Here, we show that a perturbation introduced in one of the parents (blue in Figure 5A) can affect the concentration of the other parent (red node in Figure 5A), even in the absence of a regulatory path between the two.
Figure 5. Nodes can become coupled via common downstream targets.

(A) Node 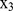 has two parents:
has two parents:  and
and 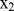 , without a regulatory path between them. (B) Perturbation
, without a regulatory path between them. (B) Perturbation  applied to
applied to  . (C) In the case of competitive binding, increasing the concentration of free
. (C) In the case of competitive binding, increasing the concentration of free  yields more of
yields more of 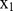 bound to the promoter of
bound to the promoter of 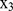 , forcing some of the molecules of
, forcing some of the molecules of 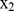 to unbind, thus increasing the free concentration
to unbind, thus increasing the free concentration 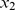 . Consequently,
. Consequently, 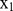 acts as if it were an activator of
acts as if it were an activator of 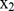 . (D) By contrast, in the case of cooperative binding, when the binding of
. (D) By contrast, in the case of cooperative binding, when the binding of  must precede that of
must precede that of 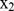 , pulses in
, pulses in 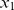 yield pulses of the opposite sign in
yield pulses of the opposite sign in 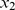 . Consequently,
. Consequently,  acts as if it were a repressor of
acts as if it were a repressor of 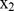 . Simulation parameters are:
. Simulation parameters are: 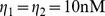 ,
,  ,
, 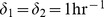 ,
,  ,
, 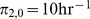 ,
, 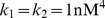 , and both
, and both  and
and 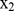 bind as tetramers.
bind as tetramers.
Referring to (5)–(6), note that  is the only node with parents (
is the only node with parents ( ), so that
), so that  . Using (6) with
. Using (6) with
where the entries of  are given in Figure 3 (depending on the binding type at
are given in Figure 3 (depending on the binding type at 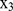 ) together with
) together with 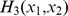 , the dynamics in (5) take the form
, the dynamics in (5) take the form
 |
This expression implies that unless 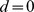 , a perturbation
, a perturbation 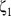 (Figure 5B) in
(Figure 5B) in  yields a subsequent perturbation in
yields a subsequent perturbation in 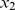 . In the case of independent binding, we have
. In the case of independent binding, we have 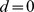 , and as a result, no perturbation is observed in
, and as a result, no perturbation is observed in  . In the case of competitive binding, instead, we have
. In the case of competitive binding, instead, we have 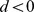 , so that perturbations
, so that perturbations  in
in  yield perturbations of the same sign in
yield perturbations of the same sign in 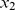 , that is,
, that is,  acts as if it were an activator of
acts as if it were an activator of 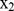 (Figure 5C). In the case of cooperative binding, instead, we have
(Figure 5C). In the case of cooperative binding, instead, we have 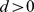 . As a result, perturbations in
. As a result, perturbations in 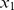 yield perturbations in
yield perturbations in  of opposite sign (Figure 5D), which implies that
of opposite sign (Figure 5D), which implies that 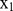 behaves as if it were a repressor of
behaves as if it were a repressor of  . As
. As  is proportional to
is proportional to 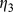 (Figure 3), higher DNA copy number for
(Figure 3), higher DNA copy number for  yields greater pulses in
yields greater pulses in  subsequent to an equal perturbation in
subsequent to an equal perturbation in  . Interestingly, if we view
. Interestingly, if we view 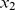 as the output of the module, the module has the adaptation property with respect to its input
as the output of the module, the module has the adaptation property with respect to its input 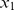 (or
(or 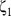 ). That is, retroactivity enables to respond to sudden changes in input stimuli, while adapting to constant stimulus values.
). That is, retroactivity enables to respond to sudden changes in input stimuli, while adapting to constant stimulus values.
Activator-repressor clock
One common clock design is based on two TFs, one of which is an activator and the other is a repressor [18], [31], [32]. Here, we illustrate the effect of internal retroactivity on the functioning of the clock design of [18] depicted in Figure 6A. In particular, 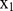 activates the production of both TFs, whereas
activates the production of both TFs, whereas  represses the production of
represses the production of 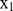 through competitive binding. Consequently, the network topology is captured by the binary matrices
through competitive binding. Consequently, the network topology is captured by the binary matrices 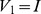 and
and 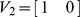 , whereas
, whereas  and
and  can be constructed by considering
can be constructed by considering 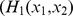 ,
,  and
and  ,
,  , respectively, in Figure 3. Here, we write
, respectively, in Figure 3. Here, we write  , while the entries of
, while the entries of 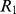 are denoted by
are denoted by 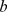 ,
,  ,
, 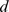 and
and  , as in (7). Then, we obtain that (5) takes the form
, as in (7). Then, we obtain that (5) takes the form
 |
(9) |
It was previously shown [33] that the principle for the clock to oscillate is that the activator dynamics are sufficiently faster than the repressor dynamics (so that the unique equilibrium point is unstable). Equation (9) shows that the activator and repressor dynamics are slowed down asymmetrically (diagonal terms in 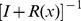 ), and that they become coupled (off-diagonal terms in
), and that they become coupled (off-diagonal terms in 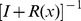 ,
,  ), because of internal retroactivity. In particular, in the case when
), because of internal retroactivity. In particular, in the case when 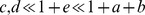 , the activator would slow down compared to the repressor. Based on the principle of functioning of the clock, we should expect that this could stabilize the equilibrium point, quenching the oscillations as a consequence. In fact, oscillations disappear even if the circuit is assembled on DNA with a single copy (
, the activator would slow down compared to the repressor. Based on the principle of functioning of the clock, we should expect that this could stabilize the equilibrium point, quenching the oscillations as a consequence. In fact, oscillations disappear even if the circuit is assembled on DNA with a single copy ( ), as it can be observed in Figure 6D. Therefore, accounting for internal retroactivity is particularly important in synthetic biology during the design process when circuit parameters and parts are chosen for obtaining the desired behavior. An effective way to restore the limit cycle in the clock, yielding sustained oscillations, is to render the repressor dynamics slower with respect to the activator dynamics. This can be obtained by adding extra DNA binding sites for the repressor [19], as shown in Figure 6B. In fact, in this case, we have
), as it can be observed in Figure 6D. Therefore, accounting for internal retroactivity is particularly important in synthetic biology during the design process when circuit parameters and parts are chosen for obtaining the desired behavior. An effective way to restore the limit cycle in the clock, yielding sustained oscillations, is to render the repressor dynamics slower with respect to the activator dynamics. This can be obtained by adding extra DNA binding sites for the repressor [19], as shown in Figure 6B. In fact, in this case, we have  given in Figure 3, which, due to (5), will yield the following change in (9): instead of
given in Figure 3, which, due to (5), will yield the following change in (9): instead of  , we will have
, we will have  , rendering the dynamics of the repressor slower with respect to the activator dynamics. As a result, the equilibrium point becomes unstable, restoring the limit cycle, verified by simulation in Figure 6E. Further studies on specific systems have investigated the effects of TF/promoter binding on the dynamics of loop oscillators, such as the repressilator [34].
, rendering the dynamics of the repressor slower with respect to the activator dynamics. As a result, the equilibrium point becomes unstable, restoring the limit cycle, verified by simulation in Figure 6E. Further studies on specific systems have investigated the effects of TF/promoter binding on the dynamics of loop oscillators, such as the repressilator [34].
Figure 6. Neglecting internal retroactivity could falsely predict that the activator-repressor clock will display sustained oscillations.

(A) The module consists of the activator protein  (dimer) and the repressor protein
(dimer) and the repressor protein  (tetramer), with dissociation constants
(tetramer), with dissociation constants 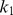 and
and 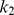 , respectively. (B) An extra node
, respectively. (B) An extra node  is introduced as a target for the repressor. (C) Without accounting for internal retroactivity, the module in A exhibits sustained oscillations. (D) When internal retroactivity is included for the module in A, however, the equilibrium point is stabilized and the limit cycle disappears. (E) Oscillations can be restored by applying a load on the repressor (module in B) with concentration
is introduced as a target for the repressor. (C) Without accounting for internal retroactivity, the module in A exhibits sustained oscillations. (D) When internal retroactivity is included for the module in A, however, the equilibrium point is stabilized and the limit cycle disappears. (E) Oscillations can be restored by applying a load on the repressor (module in B) with concentration  , so that the repressor dynamics are slowed down. Simulation parameters:
, so that the repressor dynamics are slowed down. Simulation parameters:  ,
,  ,
,  ,
,  ,
, 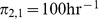 ,
,  ,
, 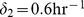 ,
, 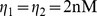 ,
, 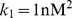 ,
, 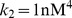 and
and 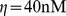 .
.
Effect of Intermodular Connections
After investigating how retroactivity due to intramodular connections affect a single module's dynamics, we next determine how the dynamics of a module change when the module is inserted into its context. To this end, we first extend the model in (5) to the case in which the module has external TFs as inputs. Hence, let 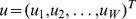 denote the concentration vector of TFs external to the module. With this, we obtain that the dynamics
denote the concentration vector of TFs external to the module. With this, we obtain that the dynamics
| (10) |
well approximate the dynamics of  in (2) with
in (2) with
 |
(11) |
where  is the set of nodes having parents from outside the module (external TFs), and the binary matrix
is the set of nodes having parents from outside the module (external TFs), and the binary matrix  has as many columns as the number of inputs of the module, and as many rows as the number of parents of
has as many columns as the number of inputs of the module, and as many rows as the number of parents of 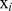 , such that its
, such that its  element is 1 if the
element is 1 if the 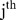 parent of
parent of 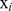 is
is  , otherwise the entry is zero. That is, an entry in the following matrix
, otherwise the entry is zero. That is, an entry in the following matrix
 |
is 1 if the species indexing the corresponding row and column are the same, otherwise the entry is zero, yielding 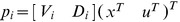 . Furthermore, note that in the presence of input
. Furthermore, note that in the presence of input  , both
, both  and
and  given in (6) depend on
given in (6) depend on  and
and  , as some of the parents of internal TFs are external TFs. For the derivation of this result, see Theorem 2 in Supporting Text S2.
, as some of the parents of internal TFs are external TFs. For the derivation of this result, see Theorem 2 in Supporting Text S2.
Before stating the main result of this section, we first provide the interpretation of  . Recall that
. Recall that 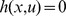 implies that the total concentrations of internal TFs are constant. In this case, (10) reduces to
implies that the total concentrations of internal TFs are constant. In this case, (10) reduces to  , where
, where  is the concentration vector of free internal TFs. This means that the concentrations of free internal TFs can still be changed subsequent to changes in the external TFs (input), despite the fact that the total concentration (free and bound) of internal TFs remains unaffected. Therefore,
is the concentration vector of free internal TFs. This means that the concentrations of free internal TFs can still be changed subsequent to changes in the external TFs (input), despite the fact that the total concentration (free and bound) of internal TFs remains unaffected. Therefore, 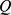 captures the phenomenon by which external TFs force internal TFs to bind/unbind, for instance, by competing for the same binding sites. Having
captures the phenomenon by which external TFs force internal TFs to bind/unbind, for instance, by competing for the same binding sites. Having 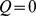 means that external TFs do not affect the binding/unbinding of internal TFs, which is the case, for example, when all bindings are independent. Thus, we call
means that external TFs do not affect the binding/unbinding of internal TFs, which is the case, for example, when all bindings are independent. Thus, we call  the external retroactivity of the module.
the external retroactivity of the module.
The main result of this section describes how the context of a module affects the module's dynamics due to retroactivity. Specifically, we consider the module of interest and we represent the rest of the network, the module's context, as a different module. As previously, we use the overbar to denote that a quantity belongs to the context. With this, we obtain that the dynamics
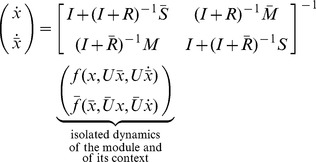 |
(12) |
well approximate the dynamics of  and
and 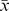 in (4) in the module connected to the context with
in (4) in the module connected to the context with
 |
(13) |
where  and
and  denote the number of nodes in the module and in the context, respectively. Furthermore, the binary matrix
denote the number of nodes in the module and in the context, respectively. Furthermore, the binary matrix  has as many rows as the number of inputs of the module, and as many columns as the number of nodes in the context, such that its
has as many rows as the number of inputs of the module, and as many columns as the number of nodes in the context, such that its  element is 1 if the
element is 1 if the 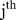 input of the module is the
input of the module is the  internal TF of the context (
internal TF of the context ( ), otherwise the entry is zero. That is, an entry in the following matrix
), otherwise the entry is zero. That is, an entry in the following matrix
 |
is 1 if the species indexing the corresponding row and column are the same, otherwise the entry is zero, yielding  . The quantities corresponding to the context, that is,
. The quantities corresponding to the context, that is, 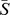 ,
,  and
and  are defined similarly with the only difference that variables with and without overbar have to be swapped (for instance,
are defined similarly with the only difference that variables with and without overbar have to be swapped (for instance,  and
and 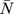 have to be swapped in (13)). For the derivation of this result, see Theorem 3 in Supporting Text S2.
have to be swapped in (13)). For the derivation of this result, see Theorem 3 in Supporting Text S2.
We next provide the interpretation of the scaling and mixing retroactivity. The reduced order model (12) describes how retroactivity between the module and the context affects each other's dynamics. Note that zero matrices  ,
,  ,
, 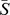 and
and  lead to no alteration in the dynamics upon interconnection. To further deepen the implications of these matrices and their physical meaning, note that when
lead to no alteration in the dynamics upon interconnection. To further deepen the implications of these matrices and their physical meaning, note that when  , the dynamics of the module after interconnection become
, the dynamics of the module after interconnection become
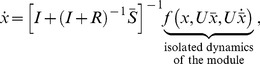 |
(14) |
that is, 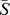 determines how the isolated dynamics of the module get “scaled” upon interconnection. Therefore, we call
determines how the isolated dynamics of the module get “scaled” upon interconnection. Therefore, we call  the scaling retroactivity of the context, accounting for the loading that the context applies on the module as some of the TFs of the module are taken up by promoter complexes in its context (we obtain
the scaling retroactivity of the context, accounting for the loading that the context applies on the module as some of the TFs of the module are taken up by promoter complexes in its context (we obtain 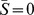 if nodes in the context do not have parents in the module, that is, if
if nodes in the context do not have parents in the module, that is, if 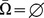 ). Since the dynamics of the context enter into the module's dynamics through
). Since the dynamics of the context enter into the module's dynamics through  , we call
, we call 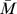 the mixing retroactivity of the context, referring to the “mixing” of the dynamics of the module and that of its context. The mixing retroactivity
the mixing retroactivity of the context, referring to the “mixing” of the dynamics of the module and that of its context. The mixing retroactivity  establishes how internal TFs force external TFs to bind/unbind, so that
establishes how internal TFs force external TFs to bind/unbind, so that  can be ensured if the binding of parents from the module is independent from that of the parents from the context. This holds if nodes in the context are not allowed to have parents in both the module and in the context (
can be ensured if the binding of parents from the module is independent from that of the parents from the context. This holds if nodes in the context are not allowed to have parents in both the module and in the context ( ). When
). When  , a perturbation applied in the context can result in a response in the upstream module, even without TFs in the context regulating TFs in the module, leading to a counter-intuitive transmission of signals from downstream (context) to upstream (module).
, a perturbation applied in the context can result in a response in the upstream module, even without TFs in the context regulating TFs in the module, leading to a counter-intuitive transmission of signals from downstream (context) to upstream (module).
With this, we can explain the simulation results in Figure 2D by analyzing  and
and 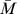 for the system in Figure 2C. Let
for the system in Figure 2C. Let  and let
and let  be defined as in (7), where
be defined as in (7), where  ,
,  ,
,  ,
,  and
and  are given in Figure 3. Then, we have
are given in Figure 3. Then, we have  by (13) and
by (13) and  and
and 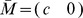 by (6). Hence, expression (12) yields
by (6). Hence, expression (12) yields
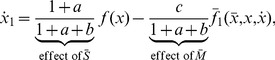 |
(15) |
describing the dynamics of  upon interconnection with its context, where
upon interconnection with its context, where  and
and 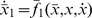 describe the dynamics of
describe the dynamics of 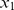 and
and  , respectively, when the module and the context are not connected to each other. If
, respectively, when the module and the context are not connected to each other. If  , then (15) reduces to
, then (15) reduces to
that is, the context rescales the dynamics of the module. The smaller  , that is, the greater the scaling retroactivity
, that is, the greater the scaling retroactivity 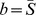 , the greater the effect of this scaling. Note that since the scaling factor is smaller than 1 (unless the scaling retroactivity is zero, i.e.,
, the greater the effect of this scaling. Note that since the scaling factor is smaller than 1 (unless the scaling retroactivity is zero, i.e., 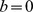 ), the effect of the scaling retroactivity of the context in this case is to make the temporal dynamics of the module slower.
), the effect of the scaling retroactivity of the context in this case is to make the temporal dynamics of the module slower.
Once  , in addition to this sclaing effect, the dynamics of the context appear in the dynamics of the module (Figure 2D). Referring to (15), we can quantify the effect of the context on the module, considering the ratio
, in addition to this sclaing effect, the dynamics of the context appear in the dynamics of the module (Figure 2D). Referring to (15), we can quantify the effect of the context on the module, considering the ratio 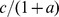 . The greater
. The greater  , that is, the greater
, that is, the greater 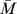 , the stronger the contribution of the context compared to that of the module to the dynamics of the module upon interconnection. Here, both
, the stronger the contribution of the context compared to that of the module to the dynamics of the module upon interconnection. Here, both  and
and  increase, for instance, with the copy number of
increase, for instance, with the copy number of  (Figure 3).
(Figure 3).
Connecting the module to its context such that  and
and  are competing for the same binding sites is less desirable than employing independent binding, as the dynamics of the context (downstream system) can suppress the dynamics of the module (upstream system). Dismantling the dynamics of the module will “misinform” other downstream systems in the network that are regulated by
are competing for the same binding sites is less desirable than employing independent binding, as the dynamics of the context (downstream system) can suppress the dynamics of the module (upstream system). Dismantling the dynamics of the module will “misinform” other downstream systems in the network that are regulated by  . From a design perspective, multi-module systems should be designed and analyzed such that the modules have zero mixing retroactivity. This can be achieved, for instance, by avoiding non-independent binding at the interface nodes (at
. From a design perspective, multi-module systems should be designed and analyzed such that the modules have zero mixing retroactivity. This can be achieved, for instance, by avoiding non-independent binding at the interface nodes (at  in Figure 2B). However, since completely independent binding can be hard to realize in the case of combinatorial regulation, nodes integrating signals from different modules should not be placed into the input layer (nodes having parents from other modules), yielding
in Figure 2B). However, since completely independent binding can be hard to realize in the case of combinatorial regulation, nodes integrating signals from different modules should not be placed into the input layer (nodes having parents from other modules), yielding  . This can be achieved by introducing an extra input node in the downstream module (see Supporting Figure S1).
. This can be achieved by introducing an extra input node in the downstream module (see Supporting Figure S1).
Next, we quantify the difference between the isolated and connected module behavior. In particular, we provide a metric of the change in the dynamics of a module upon interconnection with its context, dependent on  and
and  and under the assumption that
and under the assumption that  (obtained, for instance, by avoiding mixing parents from the module and the context). The isolated dynamics of the module can be well approximated by the reduced order model
(obtained, for instance, by avoiding mixing parents from the module and the context). The isolated dynamics of the module can be well approximated by the reduced order model  in (10), and let
in (10), and let 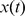 denote its solution. Once we connect the module to its context, its dynamics change according to (14), which we write as
denote its solution. Once we connect the module to its context, its dynamics change according to (14), which we write as  and let
and let  denote the corresponding solution. Using the sub-multiplicative property of the induced 2-norm, we have that the percentage change of the dynamics upon interconnection can be bounded from above as follows:
denote the corresponding solution. Using the sub-multiplicative property of the induced 2-norm, we have that the percentage change of the dynamics upon interconnection can be bounded from above as follows:
| (16) |
Furthermore, with 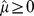 independent of
independent of  and
and  , such that
, such that 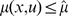 , we obtain that
, we obtain that
that is,  provides an upper bound on the percentage change in the dynamics of the module, and on the difference in the trajectories of the module upon interconnection with its context. Furthermore, by using the properties of the induced 2-norm, we obtain that we can pick
provides an upper bound on the percentage change in the dynamics of the module, and on the difference in the trajectories of the module upon interconnection with its context. Furthermore, by using the properties of the induced 2-norm, we obtain that we can pick
provided that  for all
for all  and
and  , where
, where 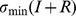 and
and 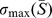 denote the smallest singular value of
denote the smallest singular value of  and largest singular value of
and largest singular value of  , respectively. For the mathematical derivations, see Supporting Text S2. This suggests that the module becomes more robust to interconnection by increasing
, respectively. For the mathematical derivations, see Supporting Text S2. This suggests that the module becomes more robust to interconnection by increasing 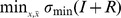 or by decreasing
or by decreasing  .
.
Such a metric can be used both in the analysis and in the design of complex gene transcription networks as follows. Given any network and a desired module size 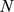 (number of nodes within the module), we can identify the module that has the least value of
(number of nodes within the module), we can identify the module that has the least value of 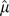 , that is, the module with the greatest guaranteed robustness to interconnection. Furthermore, we can also evaluate existing partitionings based on other measures (e.g., edge betweenness [35], its extension to directed graphs with nonuniform weights [36], round trip distance [25] or retroactivity [37]) with respect to robustness to interconnection. From a design point of view, one can design multi-module systems such that internal, scaling and mixing retroactivities allow for low values of
, that is, the module with the greatest guaranteed robustness to interconnection. Furthermore, we can also evaluate existing partitionings based on other measures (e.g., edge betweenness [35], its extension to directed graphs with nonuniform weights [36], round trip distance [25] or retroactivity [37]) with respect to robustness to interconnection. From a design point of view, one can design multi-module systems such that internal, scaling and mixing retroactivities allow for low values of  , leading to modules that behave almost the same when connected or isolated.
, leading to modules that behave almost the same when connected or isolated.
Practical Implications of Intermodular Connections
We next illustrate the effect of intermodular connections on the dynamics of interconnected modules, considering both a synthetic genetic module that is being employed in a number of applications and a natural recurring network motif.
Toggle switch
Here, we consider the toggle switch of [38], a bistable system that can be permanently switched between two steady states upon presentation of a transient input perturbation. This module has been proposed for synthetic biology applications in biosensing (see, for example, [34], [39]). In this paper, we consider the toggle switch inserted into the context of the synthetic circuit for controlling tissue homeostasis as proposed in [24], and investigate how the context of the toggle affects its switching characteristics. Figure 7A illustrates the toggle switch in isolation, whereas Figure 7B shows the configuration when connected to the context [24]. Note that all nodes, both in the toggle switch and in its context, have a single parent. Therefore,  ,
,  ,
,  ,
, 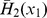 ,
,  , and similarly,
, and similarly, 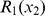 ,
,  ,
,  ,
, 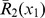 ,
, 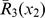 are given in Figure 3.
are given in Figure 3.
Figure 7. Effects of the context on the switching characteristics of the toggle switch.
(A) The toggle switch in isolation. (B) The toggle switch connected to its context [40]. (C) A narrow pulse in  (input perturbation in
(input perturbation in  , depicted in green) causes the isolated toggle to switch between the two stable equilibria. (D) When connected to the context, the same pulse is insufficient to yield a switch. (E) With a wider pulse, the switching is restored (however, dynamics are slower compared to the isolated case). Simulation parameters: both
, depicted in green) causes the isolated toggle to switch between the two stable equilibria. (D) When connected to the context, the same pulse is insufficient to yield a switch. (E) With a wider pulse, the switching is restored (however, dynamics are slower compared to the isolated case). Simulation parameters: both 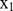 and
and  bind as dimers,
bind as dimers,  ,
,  ,
, 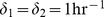 ,
,  ,
,  , and the height of the input perturbation pulse is
, and the height of the input perturbation pulse is 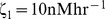 .
.
We first consider the model of the toggle switch when not connected to its context (Figure 7A). Since the toggle switch has no input, its isolated dynamics are described by (5), where 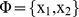 ,
,  and
and  yield
yield
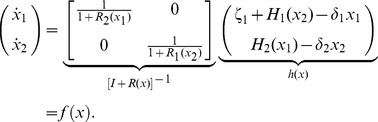 |
Next, we consider the toggle switch connected to its context (Figure 7B). As nodes in the toggle switch have no parents from outside it, we have  and
and  by (13). Nodes in the context have no parents in the context, leading to
by (13). Nodes in the context have no parents in the context, leading to 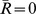 from (6), and to
from (6), and to  , referring to (11). With this, the isolated dynamics of the context are given by
, referring to (11). With this, the isolated dynamics of the context are given by
 |
according to (10). The fact that nodes in the context do not have mixed parents from the toggle switch and from the context results in 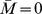 from (13). With
from (13). With 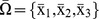 ,
,  ,
,  , and
, and  we obtain
we obtain
As a result, with 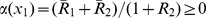 and
and 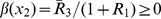 , the dynamics of the toggle switch once connected to the context (Figure 7B) are given by
, the dynamics of the toggle switch once connected to the context (Figure 7B) are given by
 |
according to (12), so that the dynamics of  and
and 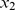 are unaffected if
are unaffected if 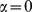 and
and 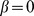 , respectively. When
, respectively. When  , both the
, both the  and
and 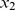 dynamics become slower upon interconnection, so that the response to an input stimulation will also be slower. As a consequence, upon removal of the stimulation, the displacement in the toggle state may not be sufficient to trigger a switch. This is illustrated in Figure 7C–D. In order to recover the switch, a wider pulse is required (Figure 7E) to compensate for the slow-down due to the context (also, note that the switching dynamics are slower than in the isolated case). As a result, even if the toggle had been characterized in isolation, it would fail to function as expected when inserted into its context. Note that we have
dynamics become slower upon interconnection, so that the response to an input stimulation will also be slower. As a consequence, upon removal of the stimulation, the displacement in the toggle state may not be sufficient to trigger a switch. This is illustrated in Figure 7C–D. In order to recover the switch, a wider pulse is required (Figure 7E) to compensate for the slow-down due to the context (also, note that the switching dynamics are slower than in the isolated case). As a result, even if the toggle had been characterized in isolation, it would fail to function as expected when inserted into its context. Note that we have  , where
, where  represents the amount of load on
represents the amount of load on  imposed by the context compared to that by the module, and
imposed by the context compared to that by the module, and  can be interpreted similarly. The greater
can be interpreted similarly. The greater  (or
(or  ), the slower the dynamics of
), the slower the dynamics of 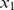 (of
(of  ) become upon interconnection with the context. Greater
) become upon interconnection with the context. Greater  and
and  yield greater
yield greater  , suggesting decreased robustness to interconnection, verified by the simulation results.
, suggesting decreased robustness to interconnection, verified by the simulation results.
Single-input motif
As a second example, we focus on a recurrent motif in gene transcription networks, called the single-input motif [5]. The single-input motif is defined by a set of operons (context) controlled by a single TF (module), which is usually autoregulated (Figure 8A). It is found in a number of instances, including the temporal program controlling protein assembly in the flagella biosynthesis [40]. Here, we show that the dynamic performance (speed) of the module and its robustness to interconnection with its context are not independent, and that this trade-off can be analyzed by focusing on the interplay between the internal retroactivity  of the module and the scaling retroactivity
of the module and the scaling retroactivity 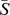 of the context.
of the context.
Figure 8. Internal retroactivity makes a module more robust to interconnection at the expense of speed.

(A) The module consists of a single negatively autoregulated node, whereas the context comprises 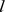 nodes repressed by the TF in the module. (B) The internal retroactivity
nodes repressed by the TF in the module. (B) The internal retroactivity 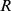 of the module increases with the DNA copy number
of the module increases with the DNA copy number  of
of 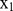 . As a result, the module becomes slower as
. As a result, the module becomes slower as  increases. (C) The percentage increase in the response time of the module decreases with
increases. (C) The percentage increase in the response time of the module decreases with  , that is, internal retroactivity
, that is, internal retroactivity  increases the robustness to interconnection. Simulation parameters:
increases the robustness to interconnection. Simulation parameters:  ,
, 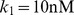 and
and  is changed such that
is changed such that  at the steady state. The context contains
at the steady state. The context contains  nodes each with DNA concentration
nodes each with DNA concentration  for
for 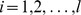 (low load:
(low load: 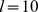 ; medium load:
; medium load:  ; high load:
; high load: 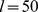 ). The response time is calculated as the time required to reach
). The response time is calculated as the time required to reach 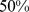 of the steady state value.
of the steady state value.
The isolated dynamics of the module are given in (8), which we write here as  . Furthermore, we have
. Furthermore, we have 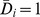 for
for  and
and  , so that
, so that  by (13), where
by (13), where  is the number of nodes in the context and
is the number of nodes in the context and  is given in Figure 3 (single parent). Consequently, upon interconnection, the dynamics of the module change according to (14) as
is given in Figure 3 (single parent). Consequently, upon interconnection, the dynamics of the module change according to (14) as
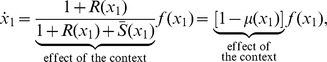 |
where  and equals the expression in (16). The smaller
and equals the expression in (16). The smaller  , the more robust the module to interconnection. Note that
, the more robust the module to interconnection. Note that  is proportional to
is proportional to  , therefore, while increasing
, therefore, while increasing  makes the module slower (Figure 8B), it also makes it more robust to interconnection (Figure 8C). It was previously shown that negative autoregulation increases robustness to perturbations [41]. Here, we have further shown that increasing the internal retroactivity
makes the module slower (Figure 8B), it also makes it more robust to interconnection (Figure 8C). It was previously shown that negative autoregulation increases robustness to perturbations [41]. Here, we have further shown that increasing the internal retroactivity 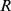 of the module provides an additional mechanism to increase robustness to interconnection, at the price of slower response. For a fixed steady state (the product of
of the module provides an additional mechanism to increase robustness to interconnection, at the price of slower response. For a fixed steady state (the product of 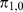 and
and  is held constant), smaller
is held constant), smaller  yields greater
yields greater  , that is, increased
, that is, increased 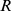 and, in turn, smaller
and, in turn, smaller 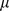 . From a design perspective, if speed is a priority, one should choose a strong RBS with a low copy number plasmid, or alternatively, a promoter with high dissociation constant
. From a design perspective, if speed is a priority, one should choose a strong RBS with a low copy number plasmid, or alternatively, a promoter with high dissociation constant 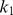 . By contrast, if robustness to interconnection is central, a weak RBS with a high copy number plasmid (or with low
. By contrast, if robustness to interconnection is central, a weak RBS with a high copy number plasmid (or with low  ) is a better choice. If both speed and robustness to interconnection are desired, other design approaches may be required, such as the incorporation of insulator devices, as proposed in other works [42].
) is a better choice. If both speed and robustness to interconnection are desired, other design approaches may be required, such as the incorporation of insulator devices, as proposed in other works [42].
Remark
The above presented trade-off between robustness to interconnection and dynamic performance can be observed also in electrical systems. To illustrate this, consider the electrical circuit in Figure 9A consisting of the series interconnection of a voltage source 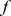 , a resistor
, a resistor 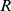 and a capacitor
and a capacitor  , in which the output voltage is
, in which the output voltage is 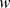 . The speed of the circuit can be characterized by its time constant
. The speed of the circuit can be characterized by its time constant  : the greater
: the greater  , the slower the response. Upon interconnection with its context, represented by the capacitor
, the slower the response. Upon interconnection with its context, represented by the capacitor  , the time constant of the system changes to
, the time constant of the system changes to  , while the steady state remains the same. Note that the percentage change in
, while the steady state remains the same. Note that the percentage change in  decreases with
decreases with  , making the module more robust to interconnection, at the expense of slower response when isolated.
, making the module more robust to interconnection, at the expense of slower response when isolated.
Figure 9. Analogy with electrical systems.

(A) The module consists of the series interconnection of a voltage source  , a resistor
, a resistor  and a capacitor
and a capacitor  . The speed of the module can be characterized by the time constant
. The speed of the module can be characterized by the time constant 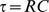 , which increases upon interconnection with the context. The greater
, which increases upon interconnection with the context. The greater  , the slower the module in isolation, but the smaller the percentage change in its speed upon interconnection. (B) According to the fundamental theorem by Thevenin [48], any linear electrical network can be equivalently represented by a series interconnection of a voltage source and an impedance. As a result, a generic module consists of the series interconnection of a voltage source
, the slower the module in isolation, but the smaller the percentage change in its speed upon interconnection. (B) According to the fundamental theorem by Thevenin [48], any linear electrical network can be equivalently represented by a series interconnection of a voltage source and an impedance. As a result, a generic module consists of the series interconnection of a voltage source  and an impedance
and an impedance  , and similarly, any context can be represented with the series interconnection of
, and similarly, any context can be represented with the series interconnection of  and
and  .
.
To further generalize the analogy between electrical systems and gene transcription networks [43], consider the electrical circuits in Figure 9B. When the module is not connected to its context, we have  and
and  , which changes to
, which changes to
 |
upon interconnection. This relationship is conceptually analogous to (12). That is, the module is robust to interconnection with its context if  is small compared to
is small compared to  , whereas the genetic module is robust to interconnection with its context if
, whereas the genetic module is robust to interconnection with its context if  and
and  are “small” compared to
are “small” compared to  . Therefore,
. Therefore,  is conceptually analogous to
is conceptually analogous to 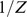 (output admittance), whereas
(output admittance), whereas  and
and  play a role similar to
play a role similar to  (input admittance).
(input admittance).
Discussion
In this paper, we have focused on retroactivity, one source of context-dependence, and demonstrated that the internal, scaling, and mixing retroactivity provide missing knowledge that captures loading effects due to intramodular and intermodular connections. The internal retroactivity quantifies the effect of intramodular load, applied by nodes within a module onto each other because of binding to promoter sites within the module. Given a module of interest, the effects of intramodular loading on the module's dynamics are captured by equations (5)–(6), in which one needs to replace the specific expressions of the Hill functions 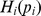 and node retroactivities
and node retroactivities 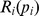 provided in Figure 3, and the binary matrices
provided in Figure 3, and the binary matrices  encoding the network topology. The scaling retroactivity accounts for the intermodular loading that the context applies on a module, due to having some TFs of the module bound to promoter sites belonging to the context. The mixing retroactivity couples the dynamics of the module and that of the context upon interconnection, and it is non-zero when TFs from different modules bind non-independently at promoter sites. The effects of intermodular loading are captured by equations (10)–(13). To obtain this description, it is sufficient to consider the Hill functions
encoding the network topology. The scaling retroactivity accounts for the intermodular loading that the context applies on a module, due to having some TFs of the module bound to promoter sites belonging to the context. The mixing retroactivity couples the dynamics of the module and that of the context upon interconnection, and it is non-zero when TFs from different modules bind non-independently at promoter sites. The effects of intermodular loading are captured by equations (10)–(13). To obtain this description, it is sufficient to consider the Hill functions  and node retroactivities
and node retroactivities  provided in Figure 3, together with the binary matrices
provided in Figure 3, together with the binary matrices  ,
,  and
and  representing the network topology. In general, the effects of the retroactivity matrices tend to increase with increased DNA copy number and/or decreased dissociation constants.
representing the network topology. In general, the effects of the retroactivity matrices tend to increase with increased DNA copy number and/or decreased dissociation constants.
We have illustrated that accounting for retroactivity reveals surprising dynamical properties of modules and, at the same time, can aid design. For example, negative autoregulation, depending on the gene copy number and production rates, can slow down the response of a system instead of speeding it up. A gene can respond to a perturbation applied to a different gene even in the absence of a regulatory path between the two genes. We have shown that this can occur when a group of TFs co-regulate common targets and these common targets are found in abundance. This type of motif, referred to as the dense overlapping regulon, is highly frequent in natural regulatory networks [1]. As a result, system identification techniques based on perturbation analysis [23] could erroneously identify non-existent regulatory linkages if retroactivity is not accounted for in the corresponding models. An activator-repressor clock on low copy DNA plasmid displays sustained oscillations when internal retroactivity is neglected, while oscillations are quenched once internal retroactivity is accounted for. However, by carefully adjusting the module's internal retroactivity through the addition of DNA load for the repressor, we can restore oscillations. A genetic toggle switch that can be flipped by a transient external stimulation requires a substantially longer stimulation to be flipped once it is connected to just few downstream targets. These facts are relevant, in particular, when designing synthetic biology circuits and multi-module systems.
Similar to synthetic systems, natural systems are subject to retroactivity. For example, clocks responsible for circadian rhythms have a large number of downstream targets [44], [45], which, in turn, may apply substantial load. This load can affect the amplitude and frequency of oscillations of the clock as well as the stability of the corresponding limit cycle. This suggests that natural systems may have evolved to use retroactivity in advantageous ways such as using it to properly tune the dynamic behavior of a module without changing the module's components. This hypothesis is further supported by the fact that there are a large number of TF binding sites on the chromosome that do not have a regulatory function [46], [47]. These sites have an impact on the temporal response of TFs, and could therefore be exploited by nature to further control the dynamics of gene regulation. More generally, retroactivity provides means for information to travel from downstream targets to upstream regulators, therefore establishing indirect connections. In highly interconnected topologies, this information transfer can result in previously unknown ways of realizing sophisticated functions. One such example that we have provided is the adaptation function that topologies such as the dense overlapping regulon can realize by virtue of having nodes co-regulate multiple downstream targets.
Based on the three retroactivity matrices, we provided a metric of robustness to interconnection, quantifying the percent change between the dynamics of a module in isolation and once connected to other modules. This metric is an explicit function of measurable parameters and becomes smaller when a module's internal retroactivity is large compared to the scaling retroactivity of the modules it connects to. This interplay may help uncover trade-offs in natural systems, providing a new angle for understanding natural principles of network organization. From an engineering perspective, we have provided quantitative design tools that can be employed in synthetic biology to appropriately match the internal and scaling retroactivity of connected circuits to preserve the circuits' behavior upon interconnection with different contexts. Our metric of robustness to interconnection further allows to evaluate the extent of modularity of a dynamical module, possibly enabling the discovery of previously unknown core processes. Our metric could be employed by currently available methods for partitioning networks into modules. Specifically, to evaluate connectivity, these methods rely on several metrics, for instance, edge betweenness [35], its extension to directed graphs with nonuniform weights [36], round trip distance [25] or retroactivity [37]. The metric of robustness to interconnection that we have introduced can enhance these methods by providing a way to evaluate modules on the basis of their functional robustness to interconnection in addition to distinguishing them at the connectivity level.
The framework that we have proposed carries substantial conceptual analogies with the electrical circuit theory established by Thevenin [48], which has been used for more than a hundred years to analyze and to design electrical networks. Within this theory, each circuit has an equivalent input and output impedance (conceptually analogous to the scaling/mixing and internal retroactivity, respectively), and an equivalent energy source (playing a role similar to the isolated module dynamics). This theory has been instrumental for answering key questions in the analysis and design of electrical networks including, for example, how the output of a circuit changes after it is interconnected in a network; how to design circuits to maximize the power transfer upon connection (impedance matching); and how to design circuits whose input/output response is unaffected by loads. We believe that the framework proposed in this paper can be used in a similar way for the analysis and design of gene regulatory networks.
Although our framework can be applied to a general gene transcription network, there are a number of aspects that it does not currently capture. These include post-translational protein modifications, such as phosphorylation, which are present in many regulatory networks and may potentially affect retroactivity. Including these will require to extend our framework to mixed gene transcription and signaling network models, leading to systems with multiple time scales. Furthermore, the transcription and translation processes use shared resources such as RNA polymerase and ribosomes, which may create couplings among unconnected circuits [17]. The dynamics of shared resources has not been included in our modeling framework and will be the focus of our future work.
Methods
Detailed Description of the System Model
The production of TF  is regulated by its parents
is regulated by its parents 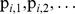 : they bind to the promoter of
: they bind to the promoter of 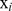 , and form complexes
, and form complexes 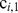 ,
,  with the promoter. Each of these complexes, in turn, produce
with the promoter. Each of these complexes, in turn, produce 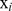 with a different rate, where we use a one-step production process encapsulating both transcription and translation [3]. As a result, the reactions we consider for node
with a different rate, where we use a one-step production process encapsulating both transcription and translation [3]. As a result, the reactions we consider for node 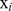 are
are
| (17) |
modeling the following physical phenomena. We denote by 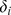 protein decay, whereas
protein decay, whereas 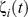 represents the production rate that may be due to external inputs or perturbations (inducer, noise or disturbance). The second reversible reaction in (17) describes the binding of parent
represents the production rate that may be due to external inputs or perturbations (inducer, noise or disturbance). The second reversible reaction in (17) describes the binding of parent 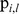 with multimerization factor
with multimerization factor 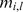 to promoter complex
to promoter complex  forming complex
forming complex  , where
, where 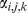 and
and  are the association and dissociation rate constants, respectively. Furthermore, each promoter complex
are the association and dissociation rate constants, respectively. Furthermore, each promoter complex  will contribute to the production of
will contribute to the production of 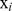 through the production rate constant
through the production rate constant  , modeled by the third reaction in (17). This production rate constant is a lumped parameter that incorporates features such as the RBS strength and the promoter strength. Finally, we assume that the total concentration of the promoter, denoted by
, modeled by the third reaction in (17). This production rate constant is a lumped parameter that incorporates features such as the RBS strength and the promoter strength. Finally, we assume that the total concentration of the promoter, denoted by  , for each transcription component is conserved, so that
, for each transcription component is conserved, so that  , where
, where 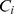 is the number of possible complexes formed with the promoter of
is the number of possible complexes formed with the promoter of  . This concentration is proportional to the concentration of copies of the promoter, which can be controlled, for example, by changing plasmid copy numbers in synthetic systems.
. This concentration is proportional to the concentration of copies of the promoter, which can be controlled, for example, by changing plasmid copy numbers in synthetic systems.
The reaction flux vector  contains all the reactions in the system, that is, binding/unbinding and protein production/decay. Given that binding/unbinding reactions occur on a much faster time-scale than protein production/decay [1], we partition
contains all the reactions in the system, that is, binding/unbinding and protein production/decay. Given that binding/unbinding reactions occur on a much faster time-scale than protein production/decay [1], we partition  into
into  and
and  , where
, where 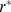 contains the slow processes, whereas
contains the slow processes, whereas  is composed of the fast reactions, that is,
is composed of the fast reactions, that is,
 |
(18) |
Biochemical Parameters
Since the production of a typical protein takes approximately 5 minutes [1], and a few dozen mRNAs can be transcribed from the same gene simultaneously by [49], and similarly, a few dozen proteins can be translated from the same mRNA at the same time by [50], the effective production rate of protein from a gene can be as high as  . This value can be arbitrarily decreased, for instance, by decreasing the RBS strength in synthetic circuits. The cell volume of Escherichia coli is typically between
. This value can be arbitrarily decreased, for instance, by decreasing the RBS strength in synthetic circuits. The cell volume of Escherichia coli is typically between  by [51], so that 1 molecule/cell corresponds to approximately
by [51], so that 1 molecule/cell corresponds to approximately  concentration. By [52], a typical value of the dissociation constant of bacterial promoters is
concentration. By [52], a typical value of the dissociation constant of bacterial promoters is  , whereas [22] suggests
, whereas [22] suggests 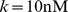 , and experimentally obtained values are provided in [53]. One of the most widely used high copy number vectors is the pUC plasmid [54], which can have hundreds of copies per cell [55]. A frequently used medium copy number plasmid is p15A with a few dozen copies per cell [56], whereas pSC101 is regarded as a low copy number plasmid with only a few copies per cell [56]. Finally, since the lifetime of a protein is on the order of a cell-cycle [1], we have
, and experimentally obtained values are provided in [53]. One of the most widely used high copy number vectors is the pUC plasmid [54], which can have hundreds of copies per cell [55]. A frequently used medium copy number plasmid is p15A with a few dozen copies per cell [56], whereas pSC101 is regarded as a low copy number plasmid with only a few copies per cell [56]. Finally, since the lifetime of a protein is on the order of a cell-cycle [1], we have  [50]. The typical range of macroscopic parameters in Escherichia coli is summarized in Table 1.
[50]. The typical range of macroscopic parameters in Escherichia coli is summarized in Table 1.
Table 1. Typical range of macroscopic parameters in Escherichia coli.
If we had not neglected mRNA dynamics, there would be three different time scales in the system. Binding and unbinding reactions occur on the time scale of seconds (or even subseconds) [1], representing the fastest time scale. The intermediate time scale is that of mRNA dynamics, as the average lifetime of mRNA is on the time scale of minutes [57], [58], [59]. Finally, the dynamics of proteins evolve on the slowest time scale (hours). As we are interested in describing the dynamics of the system on the time scale of gene expression, the concentration of promoter complexes and mRNA transcripts can be both approximated with their quasi-steady state values, leading to the models we have proposed in the paper. However, we would like to point out that including mRNA dynamics would not change anything substantial in the results and it would simply add  more ODEs to the ODE model of an
more ODEs to the ODE model of an  -node module without any effects on the retroactivity matrices (shown in [8] considering a specific example).
-node module without any effects on the retroactivity matrices (shown in [8] considering a specific example).
Definition of  and
and 
First, note that  in (2) has a block diagonal structure, yielding
in (2) has a block diagonal structure, yielding
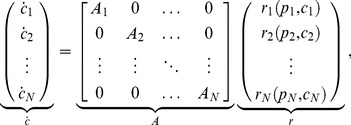 |
(19) |
where 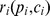 denotes the reaction flux vector corresponding to reversible binding reactions with the promoter of
denotes the reaction flux vector corresponding to reversible binding reactions with the promoter of  . Let
. Let  denote the vector of concentrations of complexes with the promoter of
denote the vector of concentrations of complexes with the promoter of 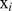 at the quasi-steady state, obtained by setting
at the quasi-steady state, obtained by setting  .
.
We first define  as follows:
as follows:
 |
(20) |
where  is the
is the 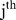 entry in
entry in 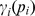 and
and 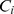 is the number of complexes with the promoter of
is the number of complexes with the promoter of 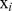 .
.
Next, define the matrix 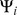 as follows: it has as many columns as the number of complexes formed with the promoter of
as follows: it has as many columns as the number of complexes formed with the promoter of  , and as many rows as the number of parents of
, and as many rows as the number of parents of  :
:
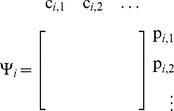 |
such that its  element is
element is  if the
if the  parent of
parent of  is bound as an
is bound as an  -multimer in
-multimer in 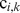 (
( if the
if the  parent is not bound). Finally, for nodes having parents, define the retroactivity
parent is not bound). Finally, for nodes having parents, define the retroactivity
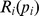 of node
of node
 as
as
| (21) |
For the most common binding types, 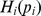 and
and  are given in Figure 3. For details on their derivation, see Supporting Text S3.
are given in Figure 3. For details on their derivation, see Supporting Text S3.
Supporting Information
Mixing retroactivity can be avoided by introducing an extra node. Rectangles represent promoter regions, arrows denote coding regions. Promoters are regulated by TFs expressed from coding regions of the same color. (A) The production of  is regulated by two TFs:
is regulated by two TFs:  from the module, and
from the module, and  from the context. If the binding of
from the context. If the binding of  is not completely independent from that of
is not completely independent from that of 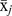 , the mixing retroactivity
, the mixing retroactivity 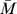 of the context is non-zero. As a result, the dynamics of the context can suppress that of the module by (12). (B) One possible solution to obtain zero mixing retroactivity
of the context is non-zero. As a result, the dynamics of the context can suppress that of the module by (12). (B) One possible solution to obtain zero mixing retroactivity  is to introduce an extra input node
is to introduce an extra input node 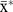 in the context, so that parents from the module and from the context are not mixed. In particular, replace the promoter of
in the context, so that parents from the module and from the context are not mixed. In particular, replace the promoter of  with one that is regulated by
with one that is regulated by 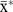 . As a result, parents from the module and from the context are not mixed anymore, yielding
. As a result, parents from the module and from the context are not mixed anymore, yielding  , in the meantime,
, in the meantime,  still integrates the signal coming from
still integrates the signal coming from  (through
(through 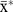 ) and from
) and from  .
.
(EPS)
ODE model of the system in Figure 2 together with the parameter values used for simulation.
(PDF)
Appendix containing the Theorems and Propositions together with the corresponding proofs. Subsections include the following: (1) Isolated module without input; (2) Isolated module with input; (3) Interconnection of modules; and (4) Bounding the difference between the trajectories of an isolated and a connected module.
(PDF)
Funding Statement
Grant #FA9550-12-1-0129. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Alon U (2007) Network motifs: theory and experimental approaches. Nature Reviews Genetics 8: 450–461. [DOI] [PubMed] [Google Scholar]
- 2.Kirschner MW, Gerhart JC (2006) The plausibility of life: Resolving Darwin's dilemma. Yale University Press. [Google Scholar]
- 3. Milo R, Shen-Orr SS, Kashtan N, Chlovskii DB, Alon U (2002) Network motifs: simple building blocks of complex networks. Science 298: 824–827. [DOI] [PubMed] [Google Scholar]
- 4. Ravasz E, Somera AL, Mongru DA, Oltvai ZN, Barabasi AL (2002) Hierarchical organization of modularity in metabolic networks. Science 297: 1551–1555. [DOI] [PubMed] [Google Scholar]
- 5. Shen-Orr SS, Milo R, Mangan S, Alon U (2002) Network motifs in the transcriptional regulation network of Escherichia coli. Nature Genetics 31: 64–68. [DOI] [PubMed] [Google Scholar]
- 6. Lauffenburger DA (2000) Cell signaling pathways as control modules: complexity for simplicity? PNAS 97: 5031–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guido NJ, Wang X, Adalsteinsson D, McMillen D, Hasty J, et al. (2006) A bottom-up approach to gene regulation. Nature 439: 856–860. [DOI] [PubMed] [Google Scholar]
- 8. Purnick PEM, Weiss R (2009) The second wave of synthetic biology: from modules to systems. Nature Reviews Molecular Cell Biology 10: 410–422. [DOI] [PubMed] [Google Scholar]
- 9. Cardinale S, Arkin AP (2012) Contextualizing context for synthetic biology – identifying causes of failure of synthetic biological systems. Biotechnology Journal 7: 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bentley W, Mirjalili N, Andersen D, Davis R, Kompal D (1990) Plasmid-encoded protein: the principal factor in the “metabolic burden” associated with recombinant bacteria. Biotechnology and Bioengineering 35: 668–681. [DOI] [PubMed] [Google Scholar]
- 11. Scott M, Gunderson C, Mateescu E, Zhang Z, Hwa T (2010) Interdependence of cell growth and gene expression: origins and consequences. Science 330: 1099–1102. [DOI] [PubMed] [Google Scholar]
- 12. Cookson N, Mather W, Danino T, Mondragon-Palomino O, Williams R, et al. (2011) Queueing up for enzymatic processing: correlated signaling through coupled degradation. Molecular Systems Biology 7: 561 doi:10.1038/msb.2011.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saez-Rodriguez J, Kremling A, Gilles ED (2005) Dissecting the puzzle of life: modularization of signal transduction networks. Computers and Chemical Engineering 29: 619–629. [Google Scholar]
- 14. Del Vecchio D, Ninfa AJ, Sontag ED (2008) Modular cell biology: retroactivity and insulation. Nature/EMBO Molecular Systems Biology 4: 161 doi:10.1038/msb4100204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slusarczyk AL, Lin A, Weiss R (2012) Foundations for the design and implementation of synthetic genetic circuits. Nature Reviews Genetics 13: 406–420. [DOI] [PubMed] [Google Scholar]
- 16. Jayanthi S, Nilgiriwala KS, Del Vecchio D (2013) Retroactivity controls the temporal dynamics of gene transcription. ACS Synthetic Biology 2: 431–441. [DOI] [PubMed] [Google Scholar]
- 17. Franco E, Friedrichs E, Kim J, Jungmann R, Murray R, et al. (2011) Timing molecular motion and production with a synthetic transcriptional clock. PNAS 108: E787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Atkinson MR, Savageau MA, Myers JT, Ninfa AJ (2003) Development of genetic circuitry exhibiting toggle switch or oscillatory behavior in Escherichia coli. Cell 113: 597–607. [DOI] [PubMed] [Google Scholar]
- 19. Jayanthi S, Del Vecchio D (2012) Tuning genetic clocks employing DNA binding sites. PLoS One 7: e41019 doi:10.1371/journal.pone.0041019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang P, Ventura AC, Sontag ED, Merajver SD, Ninfa AJ, et al. (2011) Load-induced modulation of signal transduction networks. Science Signaling 4: ra67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim Y, Paroush Z, Nairz K, Hafen E, Jimnez G, et al. (2011) Substrate-dependent control of MAPK phosphorylation in vivo. Molecular Systems Biology 7: 467 doi:10.1038/msb.2010.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenfeld N, Elowitz MB, Alon U (2002) Negative autoregulation speeds the response times of transcription networks. Journal of Molecular Biology 323: 785–793. [DOI] [PubMed] [Google Scholar]
- 23. Chou IC, Voit EO (2009) Recent developments in parameter estimation and structure identification of biochemical and genomic systems. Mathematical Biosciences 219: 57–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller M, Hafner M, Sontag E, Davidsohn N, Subramanian S, et al. (2012) Modular design of artificial tissue homeostasis: robust control through synthetic cellular heterogeneity. PLoS Computational Biology 8: e1002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sridharan GV, Hassoun S, Lee K (2011) Identification of biochemical network modules based on shortest retroactive distances. PLoS Computational Biology 7: e1002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson J, Chang YC, Papachristodoulou A (2011) Model decomposition and reduction tools for large-scale networks in systems biology. Automatica 47: 1165–1174. [Google Scholar]
- 27. Fell DA (1992) Metabolic control analysis: a survey of its theoretical and experimental development. Biochemical Journal 286: 313–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinrich R, Schuster S (1999) The regulation of cellular systems. Chapman & Hall. [Google Scholar]
- 29.Alon U (2006) An introduction to systems biology – Design principles of biological circuits. Chapman and Hall. [Google Scholar]
- 30.Klipp E, Liebermeister W, Wierling C, Kowald A, Lehrach H, et al.. (2009) Systems biology: A textbook. John Wiley & Sons.
- 31. Stricker J, Cookson S, Bennett MR, Mather WH, Tsimring LS, et al. (2008) A fast, robust and tunable synthetic gene oscillator. Nature 456: 516–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Danino T, Mondragon-Palomino O, Tsimring L, Hasty J (2010) A synchronized quorum of genetic clocks. Nature 463: 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Vecchio D (2007) Design and analysis of an activator-repressor clock in E. coli. Proc of American Control Conference : 1589–1594.
- 34. Bennett MR, Volfson D, Tsimring L, Hasty J (2007) Transient dynamics of genetic regulatory networks. Biophysical Journal 392: 3501–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Girvan M, Newman MEJ (2002) Community structure in social and biological networks. PNAS 99: 7821–7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoon J, Blumer A, Lee K (2006) An algorithm for modularity analysis of directed and weighted biological networks based on edge-betweenness centrality. Bioinformatics 22: 3106–3108. [DOI] [PubMed] [Google Scholar]
- 37. Saez-Rodriguez J, Gayer S, Ginkel M, Gilles ED (2008) Automatic decomposition of kinetic models of signaling networks minimizing the retroactivity among modules. Bioinformatics 24: 213–219. [DOI] [PubMed] [Google Scholar]
- 38. Gardner TS, Cantor CR, Collins JJ (2000) Construction of a genetic toggle switch in Escherichia coli. Nature 403: 339–342. [DOI] [PubMed] [Google Scholar]
- 39. Kobayashi H, Kaern M, Araki M, Chung K, Gardner TS, et al. (2004) Programmable cells: Interfacing natural and engineered gene networks. PNAS 101: 8414–8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kalir S, McClure J, Pabbaraju K, Southward C, Ronen M, et al. (2001) Ordering genes in a agella pathway by analysis of expression kinetics from living bacteria. Science 292: 2080–2083. [DOI] [PubMed] [Google Scholar]
- 41. Becskei A, Serrano L (2000) Engineering stability in gene networks by autoregulation. Nature 405: 590–593. [DOI] [PubMed] [Google Scholar]
- 42. Jayanthi S, Del Vecchio D (2011) Retroactivity attenuation in bio-molecular systems based on timescale separation. IEEE Transactions on Automation and Control 56: 748–761. [Google Scholar]
- 43. Kyung KH, Sauro HM (2010) Fan-out in gene regulatory networks. Journal of Biological Engineering 4: 16 doi:10.1186/1754-1611-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu Y, Tsinoremas NF, Johnson CH, Lebedeva NV, Golden SS, et al. (1995) Circadian orchestration of gene expression in cyanobacteria. Genes & Development 9: 1469–1478. [DOI] [PubMed] [Google Scholar]
- 45. Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, et al. (2005) Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nature Reviews Genetics 6: 544–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Robison K, McGuire A, Church G (1998) A comprehensive library of DNA-binding site matrices for 55 proteins applied to the complete Escherichia coli K-12 genome. Journal of Molecular Biology 284: 241–254. [DOI] [PubMed] [Google Scholar]
- 47. Burger A, Walczak AM, Wolynes PG (2010) Abduction and asylum in the lives of transcription factors. PNAS 107: 4016–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thevenin LC (1883) Extension de la loi d'ohm aux circuits electromoteurs complexes [extension of ohm's law to complex electromotive circuits]. Annales Telegraphiques 10: 222–224. [Google Scholar]
- 49. Bremer H, Dennis P, Ehrenberg M (2003) Free RNA polymerase and modeling global transcription in Escherichia coli. Biochimie 85: 597–609. [DOI] [PubMed] [Google Scholar]
- 50.Bremer H, Dennis P (1996) Modulation of chemical composition and other parameters of the cell by growth rate in Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, 1553–1569 pp. [Google Scholar]
- 51. Klumpp S, Hwa T (2008) Growth-rate-dependent partitioning of RNA polymerases in bacteria. PNAS 105: 20245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sanchez A, Garcia HG, Jones D, Phillips R, Kondev J (2011) Effect of promoter architecture on the cell-to-cell variability in gene expression. PLoS Computational Biology 7: e1001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Peerez AG, Angarica VE, Collado-Vides J, Vasconcelos ATR (2009) From sequence to dynamics: the effects of transcription factor and polymerase concentration changes on activated and repressed promoters. BMC Molecular Biology 10: 92 doi:10.1186/1471-2199-10-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vieira J, Messing J (1982) The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19: 259–268. [DOI] [PubMed] [Google Scholar]
- 55.Casali N, Preston A (2003) E. Coli Plasmid Vectors: Methods and Applications. Springer. [Google Scholar]
- 56. Lutz R, Bujard H (1997) Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Research 25: 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mohanty B, Kushner S (1999) Analysis of the function of Escherichia coli poly(A) polymerase I in RNA metabolism. Molecular Microbiology 34: 1094–1108. [DOI] [PubMed] [Google Scholar]
- 58. Selinger DW, Saxena RM, Cheung KJ, Church GM, Rosenow C (2003) Global RNA half-life analysis in Escherichia coli reveals positional patterns of transcript degradation. Genome Research 13: 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bernstein JA, Khodursky AB, Lin PH, Lin-Chao S, Cohen SN (2002) Global analysis of mRNA decay and abundance in Escherichia coli at single-gene resolution using two-color uorescent DNA microarrays. PNAS 99: 9697–9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mixing retroactivity can be avoided by introducing an extra node. Rectangles represent promoter regions, arrows denote coding regions. Promoters are regulated by TFs expressed from coding regions of the same color. (A) The production of  is regulated by two TFs:
is regulated by two TFs:  from the module, and
from the module, and  from the context. If the binding of
from the context. If the binding of  is not completely independent from that of
is not completely independent from that of 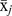 , the mixing retroactivity
, the mixing retroactivity 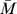 of the context is non-zero. As a result, the dynamics of the context can suppress that of the module by (12). (B) One possible solution to obtain zero mixing retroactivity
of the context is non-zero. As a result, the dynamics of the context can suppress that of the module by (12). (B) One possible solution to obtain zero mixing retroactivity  is to introduce an extra input node
is to introduce an extra input node 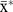 in the context, so that parents from the module and from the context are not mixed. In particular, replace the promoter of
in the context, so that parents from the module and from the context are not mixed. In particular, replace the promoter of  with one that is regulated by
with one that is regulated by 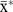 . As a result, parents from the module and from the context are not mixed anymore, yielding
. As a result, parents from the module and from the context are not mixed anymore, yielding  , in the meantime,
, in the meantime,  still integrates the signal coming from
still integrates the signal coming from  (through
(through 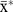 ) and from
) and from  .
.
(EPS)
ODE model of the system in Figure 2 together with the parameter values used for simulation.
(PDF)
Appendix containing the Theorems and Propositions together with the corresponding proofs. Subsections include the following: (1) Isolated module without input; (2) Isolated module with input; (3) Interconnection of modules; and (4) Bounding the difference between the trajectories of an isolated and a connected module.
(PDF)