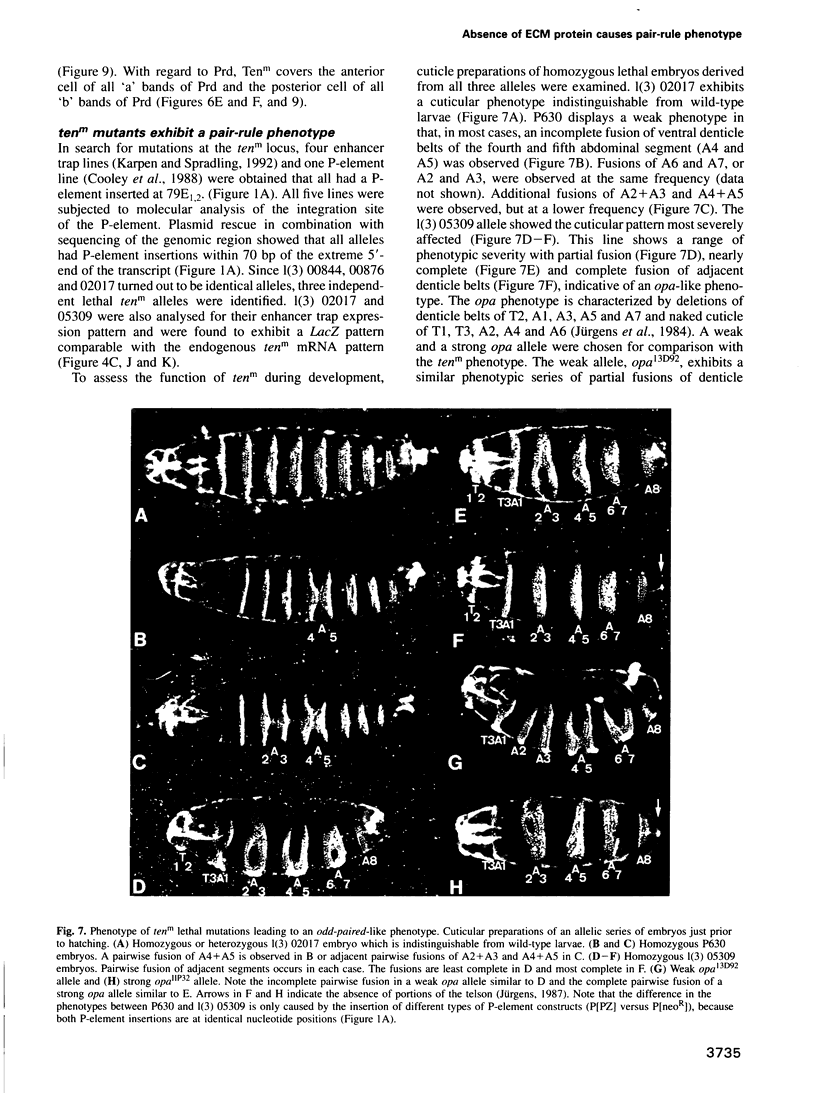
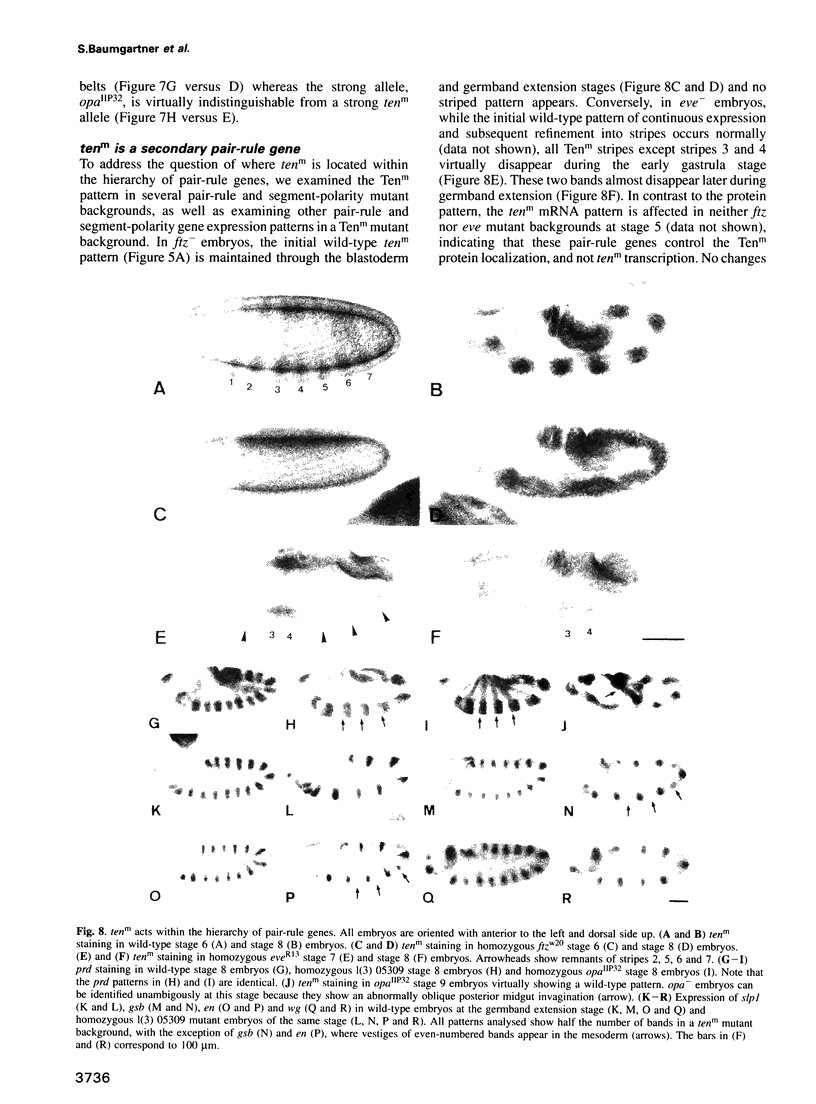
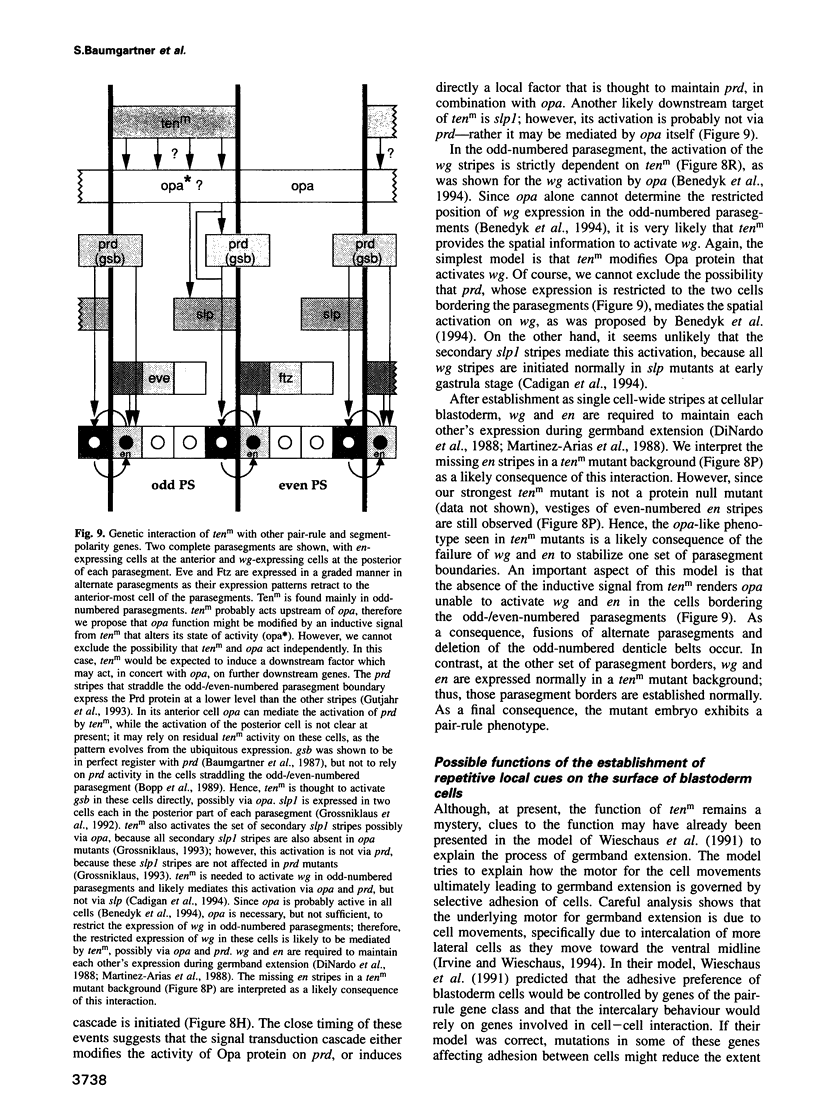
Abstract
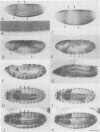
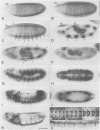
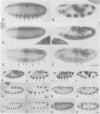
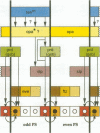
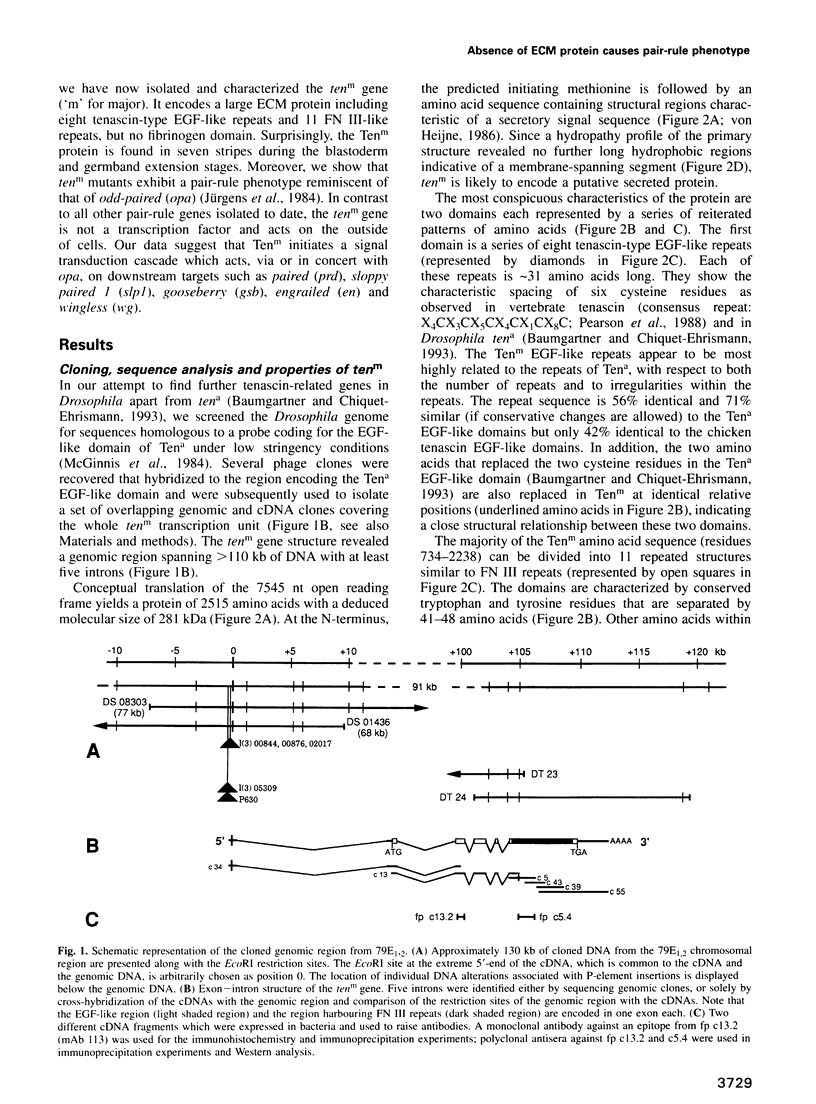
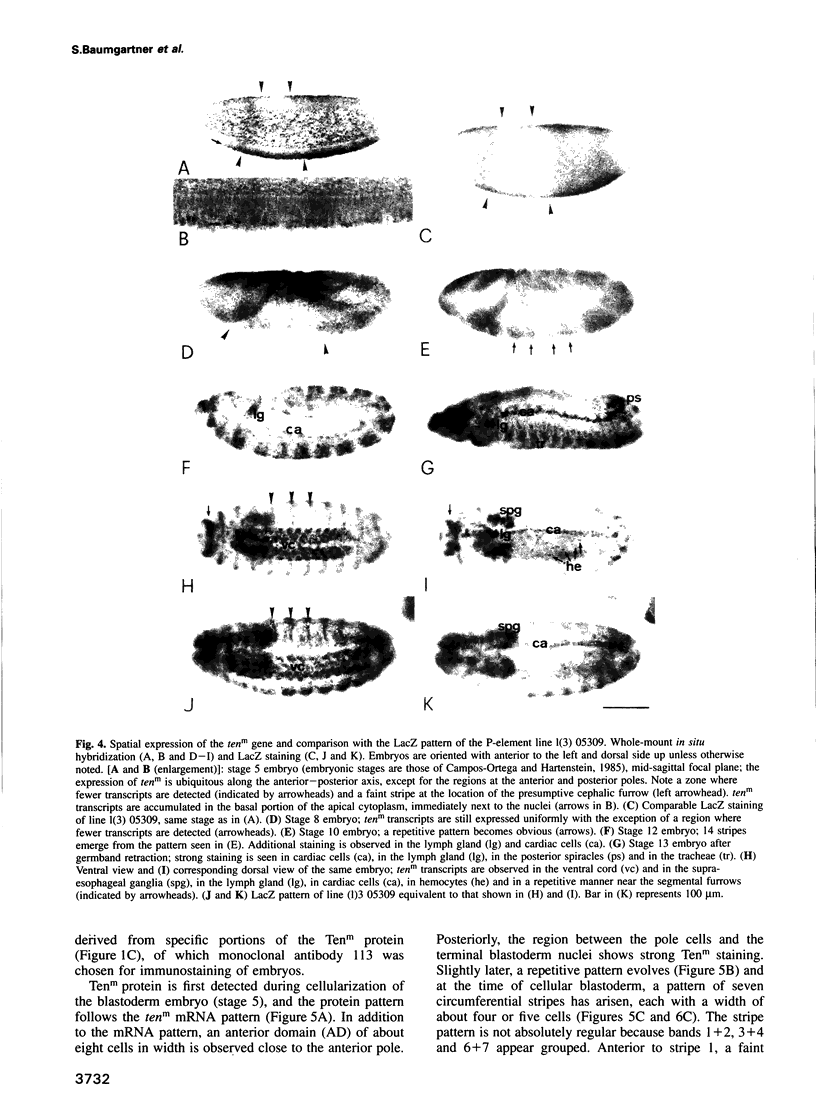
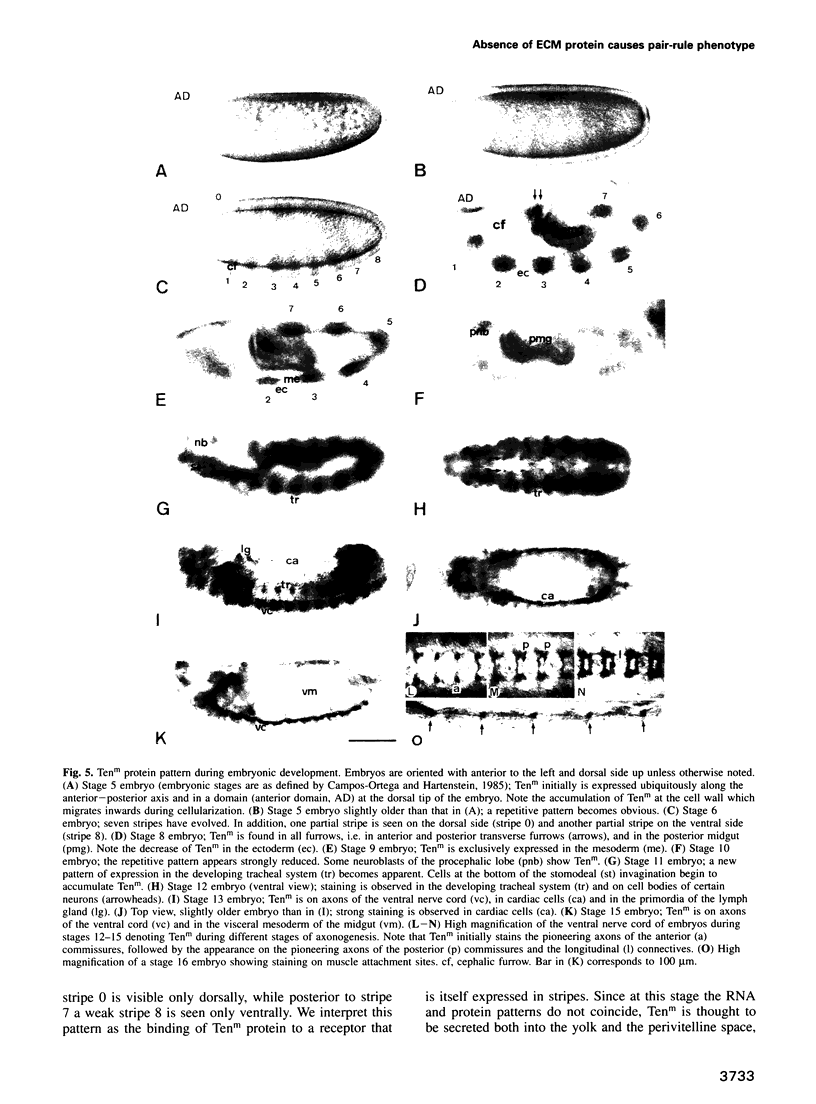
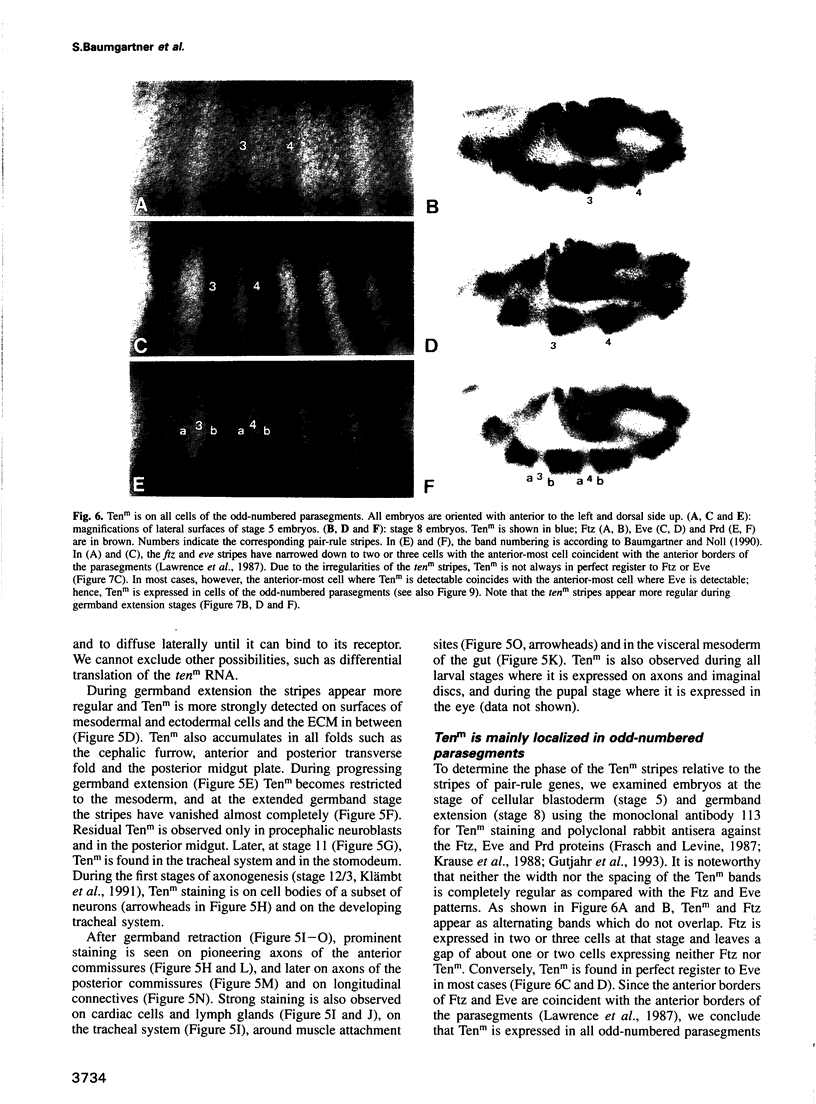
We describe the molecular characterization of the Drosophila gene tenm, a large transcription unit spanning > 110 kb of DNA. tenm encodes a large extracellular protein of 2515 amino acids related to the extracellular matrix molecule tenascin. The Tenm protein is found in seven stripes during the blastoderm stage, and each stripe overlaps with the even-skipped stripes. tenm mutants show a phenotype resembling that of odd-paired (opa), a member of the pair-rule class of segmentation genes. Thus, Tenm is the first example of a pair-rule gene product acting from outside the cell. While the Tenm protein is under the control of fushi tarazu and even-skipped, but not of opa, at least two pair-rule genes, paired (prd) and sloppy paired (slp), and all segment-polarity genes analysed to date are under the control of tenm. Our data suggest that Tenm initiates a signal transduction cascade which acts, via or in concert with opa, on downstream targets such as prd, slp, gooseberry, engrailed and wingless, leading to an opa-like phenotype.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aukhil I., Joshi P., Yan Y., Erickson H. P. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J Biol Chem. 1993 Feb 5;268(4):2542–2553. [PubMed] [Google Scholar]
- Baumgartner S., Bopp D., Burri M., Noll M. Structure of two genes at the gooseberry locus related to the paired gene and their spatial expression during Drosophila embryogenesis. Genes Dev. 1987 Dec;1(10):1247–1267. doi: 10.1101/gad.1.10.1247. [DOI] [PubMed] [Google Scholar]
- Baumgartner S., Chiquet-Ehrismann R. Tena, a Drosophila gene related to tenascin, shows selective transcript localization. Mech Dev. 1993 Mar;40(3):165–176. doi: 10.1016/0925-4773(93)90074-8. [DOI] [PubMed] [Google Scholar]
- Baumgartner S., Noll M. Network of interactions among pair-rule genes regulating paired expression during primordial segmentation of Drosophila. Mech Dev. 1990 Dec;33(1):1–18. doi: 10.1016/0925-4773(90)90130-e. [DOI] [PubMed] [Google Scholar]
- Benedyk M. J., Mullen J. R., DiNardo S. odd-paired: a zinc finger pair-rule protein required for the timely activation of engrailed and wingless in Drosophila embryos. Genes Dev. 1994 Jan;8(1):105–117. doi: 10.1101/gad.8.1.105. [DOI] [PubMed] [Google Scholar]
- Bopp D., Jamet E., Baumgartner S., Burri M., Noll M. Isolation of two tissue-specific Drosophila paired box genes, Pox meso and Pox neuro. EMBO J. 1989 Nov;8(11):3447–3457. doi: 10.1002/j.1460-2075.1989.tb08509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P., Doolittle R. F. Proposed acquisition of an animal protein domain by bacteria. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8990–8994. doi: 10.1073/pnas.89.19.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow J., Tee M. K., Gitelman S. E., Mellon S. H., Miller W. L. Tenascin-X: a novel extracellular matrix protein encoded by the human XB gene overlapping P450c21B. J Cell Biol. 1993 Jul;122(1):265–278. doi: 10.1083/jcb.122.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. H., Kafatos F. C. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988 Sep 20;203(2):425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- Cadigan K. M., Grossniklaus U., Gehring W. J. Localized expression of sloppy paired protein maintains the polarity of Drosophila parasegments. Genes Dev. 1994 Apr 15;8(8):899–913. doi: 10.1101/gad.8.8.899. [DOI] [PubMed] [Google Scholar]
- Cardin A. D., Weintraub H. J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989 Jan-Feb;9(1):21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. What distinguishes tenascin from fibronectin? FASEB J. 1990 Jun;4(9):2598–2604. doi: 10.1096/fasebj.4.9.1693347. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cooley L., Kelley R., Spradling A. Insertional mutagenesis of the Drosophila genome with single P elements. Science. 1988 Mar 4;239(4844):1121–1128. doi: 10.1126/science.2830671. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., O'Farrell P. H. Establishment and refinement of segmental pattern in the Drosophila embryo: spatial control of engrailed expression by pair-rule genes. Genes Dev. 1987 Dec;1(10):1212–1225. doi: 10.1101/gad.1.10.1212. [DOI] [PubMed] [Google Scholar]
- DiNardo S., Sher E., Heemskerk-Jongens J., Kassis J. A., O'Farrell P. H. Two-tiered regulation of spatially patterned engrailed gene expression during Drosophila embryogenesis. Nature. 1988 Apr 14;332(6165):604–609. doi: 10.1038/332604a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P., Bourdon M. A. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Erickson H. P. Tenascin-C, tenascin-R and tenascin-X: a family of talented proteins in search of functions. Curr Opin Cell Biol. 1993 Oct;5(5):869–876. doi: 10.1016/0955-0674(93)90037-q. [DOI] [PubMed] [Google Scholar]
- Frasch M., Levine M. Complementary patterns of even-skipped and fushi tarazu expression involve their differential regulation by a common set of segmentation genes in Drosophila. Genes Dev. 1987 Nov;1(9):981–995. doi: 10.1101/gad.1.9.981. [DOI] [PubMed] [Google Scholar]
- Gitelman S. E., Bristow J., Miller W. L. Mechanism and consequences of the duplication of the human C4/P450c21/gene X locus. Mol Cell Biol. 1992 May;12(5):2124–2134. doi: 10.1128/mcb.12.5.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus U., Pearson R. K., Gehring W. J. The Drosophila sloppy paired locus encodes two proteins involved in segmentation that show homology to mammalian transcription factors. Genes Dev. 1992 Jun;6(6):1030–1051. doi: 10.1101/gad.6.6.1030. [DOI] [PubMed] [Google Scholar]
- Gutjahr T., Frei E., Noll M. Complex regulation of early paired expression: initial activation by gap genes and pattern modulation by pair-rule genes. Development. 1993 Feb;117(2):609–623. doi: 10.1242/dev.117.2.609. [DOI] [PubMed] [Google Scholar]
- Irvine K. D., Wieschaus E. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development. 1994 Apr;120(4):827–841. doi: 10.1242/dev.120.4.827. [DOI] [PubMed] [Google Scholar]
- Karpen G. H., Spradling A. C. Analysis of subtelomeric heterochromatin in the Drosophila minichromosome Dp1187 by single P element insertional mutagenesis. Genetics. 1992 Nov;132(3):737–753. doi: 10.1093/genetics/132.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klämbt C., Jacobs J. R., Goodman C. S. The midline of the Drosophila central nervous system: a model for the genetic analysis of cell fate, cell migration, and growth cone guidance. Cell. 1991 Feb 22;64(4):801–815. doi: 10.1016/0092-8674(91)90509-w. [DOI] [PubMed] [Google Scholar]
- Krause H. M., Klemenz R., Gehring W. J. Expression, modification, and localization of the fushi tarazu protein in Drosophila embryos. Genes Dev. 1988 Aug;2(8):1021–1036. doi: 10.1101/gad.2.8.1021. [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Johnston P., Macdonald P., Struhl G. Borders of parasegments in Drosophila embryos are delimited by the fushi tarazu and even-skipped genes. 1987 Jul 30-Aug 5Nature. 328(6129):440–442. doi: 10.1038/328440a0. [DOI] [PubMed] [Google Scholar]
- Li X., Gutjahr T., Noll M. Separable regulatory elements mediate the establishment and maintenance of cell states by the Drosophila segment-polarity gene gooseberry. EMBO J. 1993 Apr;12(4):1427–1436. doi: 10.1002/j.1460-2075.1993.tb05786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martizez Arias A., Baker N. E., Ingham P. W. Role of segment polarity genes in the definition and maintenance of cell states in the Drosophila embryo. Development. 1988 May;103(1):157–170. doi: 10.1242/dev.103.1.157. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Ishihara N., Ando A., Inoko H., Ikemura T. Extracellular matrix protein tenascin-like gene found in human MHC class III region. Immunogenetics. 1992;36(6):400–403. doi: 10.1007/BF00218048. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Levine M. S., Hafen E., Kuroiwa A., Gehring W. J. A conserved DNA sequence in homoeotic genes of the Drosophila Antennapedia and bithorax complexes. 1984 Mar 29-Apr 4Nature. 308(5958):428–433. doi: 10.1038/308428a0. [DOI] [PubMed] [Google Scholar]
- Mozer B., Marlor R., Parkhurst S., Corces V. Characterization and developmental expression of a Drosophila ras oncogene. Mol Cell Biol. 1985 Apr;5(4):885–889. doi: 10.1128/mcb.5.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nörenberg U., Wille H., Wolff J. M., Frank R., Rathjen F. G. The chicken neural extracellular matrix molecule restrictin: similarity with EGF-, fibronectin type III-, and fibrinogen-like motifs. Neuron. 1992 May;8(5):849–863. doi: 10.1016/0896-6273(92)90199-n. [DOI] [PubMed] [Google Scholar]
- PRESCOTT D. M., BENDER M. A. Synthesis of RNA and protein during mitosis in mammalian tissue culture cells. Exp Cell Res. 1962 Mar;26:260–268. doi: 10.1016/0014-4827(62)90176-3. [DOI] [PubMed] [Google Scholar]
- Pearson C. A., Pearson D., Shibahara S., Hofsteenge J., Chiquet-Ehrismann R. Tenascin: cDNA cloning and induction by TGF-beta. EMBO J. 1988 Oct;7(10):2977–2982. doi: 10.1002/j.1460-2075.1988.tb03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen F. G., Wolff J. M., Chiquet-Ehrismann R. Restrictin: a chick neural extracellular matrix protein involved in cell attachment co-purifies with the cell recognition molecule F11. Development. 1991 Sep;113(1):151–164. doi: 10.1242/dev.113.1.151. [DOI] [PubMed] [Google Scholar]
- Robertson H. M., Preston C. R., Phillis R. W., Johnson-Schlitz D. M., Benz W. K., Engels W. R. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 1988 Mar;118(3):461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y., Yagi T., Ikawa Y., Sakakura T., Aizawa S. Mice develop normally without tenascin. Genes Dev. 1992 Oct;6(10):1821–1831. doi: 10.1101/gad.6.10.1821. [DOI] [PubMed] [Google Scholar]
- Shermoen A. W., O'Farrell P. H. Progression of the cell cycle through mitosis leads to abortion of nascent transcripts. Cell. 1991 Oct 18;67(2):303–310. doi: 10.1016/0092-8674(91)90182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring J., Beck K., Chiquet-Ehrismann R. Two contrary functions of tenascin: dissection of the active sites by recombinant tenascin fragments. Cell. 1989 Oct 20;59(2):325–334. doi: 10.1016/0092-8674(89)90294-8. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Wilson R., Ainscough R., Anderson K., Baynes C., Berks M., Bonfield J., Burton J., Connell M., Copsey T., Cooper J. 2.2 Mb of contiguous nucleotide sequence from chromosome III of C. elegans. Nature. 1994 Mar 3;368(6466):32–38. doi: 10.1038/368032a0. [DOI] [PubMed] [Google Scholar]
- Wingender E., Bercz G., Blöcker H., Frank R., Mayer H. Expression of human parathyroid hormone in Escherichia coli. J Biol Chem. 1989 Mar 15;264(8):4367–4373. [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]