Abstract
Alzheimer’s disease is one of the most devastating neurodegenerative disorders, and this disease is characterized by severe memory impairment and decline of cognition. Hippocampal neurons are vulnerable to injury induced by Alzheimer’s disease. Physical exercise is known to promote cell survival and functional recovery after brain injuries. In the present study, we investigated the effects of treadmill exercise on short-term memory in relation with neurogenesis in the rats with amyloid β25–35 (Aβ25–35)-induced Alzheimer’s disease. The rat model of Alzheimer’s disease was induced by the intracerebroventricular (ICV) injection of Aβ25–35, using a stereotaxic instrument. The rats in the exercise group were forced to run on a treadmill for 30 min once daily for 4 consecutive weeks, starting 2 days after Aβ25–35 injection. Presently, short-term memory was deteriorated and apical dendritic length in the hippocampus was shortened in the hippocampus by Aβ25–35 injection. In contrast, treadmill exercise alleviated memory impairment and increased apical dendritic length in the Aβ25–35-injected rats. Neurogenesis and brain-derived neurotorphic factor (BDNF) and tyrosine kinase B (trkB) in the hippocampal dentate gyrus were decreased by Aβ25–35 injection. Treadmill exercise increased neurogenesis and expressions of BDNF and trkB expressions. The present study shows that treadmill exercise may provide therapeutic value for the alleviating symptoms of Alzheimer’s disease.
Keywords: Alzheimer’s disease, Treadmill exercise, Short-term memory, Neurogenesis, Apical dendritic length
INTRODUCTION
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder with progressive cognitive dysfunction and characterized by presence of senile plaques in the brain (Savla and Palme, 2005). β-Amyloid peptide (Aβ) is the major component of senile plaques, and Aβ is considered as a causal factor in the development and progress of AD (Alkam et al., 2007). AD in animal models causes long-term and progressive deficits in cognitive function (Veerendra Kumar and Gupta, 2003), and these symptoms are similar to the sporadic type of AD (Tanzi and Bertram, 2005). Intracerebroventricular (ICV) injection of Aβ causes prolonged impairment of brain glucose and energy metabolism by desensitization of neuronal insulin receptors (Lannert and Hoyer, 1998). ICV injection of Aβ has been used for the animal model of AD (Selkoe, 2008; Shi et al., 2010).
The neurons in the hippocampus are especially vulnerable to the AD (Alkam et al., 2007). Hippocampal synaptic function is important in learning ability and memory function (Eichenbaum, 2004). Alterations of hippocampal structures probably account for early symptoms of the AD (Braak et al., 2006). Hippocampus is the brain area that cell proliferation continues throughout life in the adult mammals including humans (Eriksson et al., 1998; Lee et al., 2013).
Activity of the apical dendrite maintains persistence of neural states. Dendritic morphology in the hippocampus was evaluated by Juárez-Méndez et al. (2006). Golgi-impregnated pyramidal neurons in the hippocampus are readily identified by their characteristic triangular soma shape, apical dendritic extension toward the pial surface, and numerous dendritic spines (Brocca, 2013).
Physical exercise is currently advocated as a behavioral intervention to ameliorate neurological impairments (Kim et al., 2013; Seo et al., 2013). Exercise enhances neurogenesis and increases dendritic spine density in the hippocampus (Redila and Christie, 2006; Stranahan et al., 2007; van Praag et al., 2007).
In this study, we evaluated the effect of treadmill exercise on short-term memory in relation with cell proliferation using AD rats induced by ICV injection of Aβ25–35. Step-through avoidance task for short-term memory, 5-bromo-2′-deoxyridine (BrdU) immunohistochemistry for neurogenesis, apical dendritic analysis for apical dendritic length, and western blot for brain-derived neurotrophic factor (BDNF) and tyrosine kinase B (TrkB) were conducted.
MATERIALS AND METHODS
Experimental animals and treatments
The experimental procedures were performed in accordance with the animal care guidelines of the National Institute of Health and the Korean Academy of Medical Sciences. Male Sprague-Dawley rats, weighing 220±10 g (7 weeks old), were used in this experiment. Each animal was housed under controlled temperature (20 ±2°C) and lighting (07:00 h–19:00 h) conditions with food and water made available ad libitum. The animals were randomly divided into 4 groups (n=10 in each group): the sham-operation group, the sham-operation and treadmill exercise group, the Aβ25–35-injection group, and the Aβ25–35-injection and treadmill exercise group.
ICV administration of Aβ25–35
The animals were anesthetized with Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France) and placed in a stereotaxic frame. Burr holes were drilled in the skull on both the sides over the lateral ventricles using the following coordinates: 0.8 mm posterior to bregma, 1.5 mm lateral to sagittal suture, 3.6 mm beneath the surface of brain. Through a hole drilled in the skull, a 26-gauge needle was inserted manually into each lateral ventricle. The lesioned groups received a bilateral ICV injection of Aβ25–35 (1.5 mg/kg, 5 μL in saline), according to the previously described method (Jee et al., 2008). The animals in the sham-operation group underwent the same surgical procedures, but same volume of saline was injected instead of Aβ25–35.
Treadmill exercise protocol
The rats in the exercise groups were made to run on the treadmill 30 min once a day, five times a week during 4 weeks, starting 2 days after Aβ25–35 injection. The workload of the exercise consisted of running at a speed of 3 meters/min for the first 5 min, 5 meters/min for the next 5 min, and then 8 meters/min for the last 20 min, with 0% grade of inclination. The animals in the sham-operation group and in the Aβ25–35-injection group were left for the same duration without running. BrdU (50 mg/kg; Sigma Chemical Co., St. Louis, MO, USA) was given intraperitoneally to all animals 1 h before the treadmill running, once a day for 3 consecutive days stating one day after surgery.
Step-through avoidance task
Short-term memory was evaluated by a step-through avoidance task after the last exercise session, according to the previously described method (Kim et al., 2013). The apparatus is a dark/light shuttle box consisted of two compartments. A guillotine door separated the two compartments. The dark compartment had a stainless steel shock grid floor. During the acquisition trial, each rat was placed in the light chamber. After 60 sec of habituation period, the guillotine door was opened, and the initial latency of animals to enter the dark chamber was recorded. Immediately after the rat had entered the dark chamber, the guillotine door was closed and an electric foot shock (75 V, 0.2 mA, 50 Hz) was delivered to the floor grids with a stimulator for 3 sec. Five seconds later, the rat was removed from the dark chamber and returned to its home cage. After 24 h, the time of retention in dark chamber (latency) was measured in the same way as in the acquisition trial, but foot shock was not delivered. The latency was recorded to a maximum of 600 sec.
Tissue preparation
The animals were sacrificed immediately after determination of latency in the step-through avoidance task. The animals were anesthetized using Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories), transcardially perfused with 50 mM phosphate-buffered saline (PBS), and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100 mM phosphate buffer (PB, pH 7.4). The brains were dissected and postfixed in the same fixative overnight and transferred into a 30% sucrose solution for cryoprotection. Coronal sections of 40 μm thickness were made with a freezing microtome (Leica, Nussloch, Germany).
BrdU immunohistochemistry
For the detection of newly generated cells in the dentate gyrus, BrdU-specific immunohistochemistry was performed, according to a previously described method (Kim et al., 2013; Seo et al., 2013). The sections were first permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min. They were then pretreated in 50% formamide-2×standard saline citrate (SSC) at 65°C for 2 h, denaturated in 2 N HCl at 37°C for 30 min, and rinsed twice in 100 mM sodium borate (pH 8.5). Thereafter, the sections were incubated overnight at 4°C with BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany). The sections were then washed three times with PBS and incubated for 1 h with a biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). The sections were then incubated with avidin-peroxidase complex (1:100; Vector Laboratories) for 1 h. For visualization, the sections were incubated in 50 mM Tris-HCl (pH 7.6) containing 0.02% diaminobenzidine tetrahydrochloride (DAB), 40 mg/mL nickel chloride, and 0.03% hydrogen peroxide for 5 min.
After BrdU-specific staining, differentiation of BrdU-positive cells was determined on the same section using a mouse anti-neuronal nucleic (NeuN) antibody (1:300; Chemicon International, Temecula, CA, USA). The sections were washed three times with PBS and incubated for l h with a biotinylated anti-mouse secondary antibody. For staining, the sections were incubated in a reaction mixture consisting of 0.02% DAB and 0.03% hydrogen peroxide for 5 min. The sections were finally mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and coverslips were mounted with Permount® (Fisher Scientific, New Jersey, NJ, USA).
DCX immunohistochemistry
DCX immunohistochemistry was performed, according to a previously described method (Arisi and Garcia-Cairasco, 2007). The sections were washed with 0.1 M PBS (pH 7.4), then immersed in a 3% hydrogen peroxide and 20% methanol in PBS solution, washed in PBS and incubated for 48 h at 4°C with the α-doublecortin antibody (1 μg/mL; Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted in a blocking solution of 3% normal donkey serum in PBS. The slices were washed in PBS and in 0.05% Tween 20 in PBS for 15 min before incubation with donkey α-goat biotinylated antibody for 1 h at room temperature (Chemicon) diluted in the blocking solution. The slices were washed in PBS before incubation with ABC Elite kit (Vector Laboratories) for 1 h, washed again in PBS, and incubated with DAB with 0.03% hydrogen peroxide for 5 min. The sections were mounted onto gelatin-coated slides, air-dried overnight at room temperature, and coverslips were mounted using Permount® (Fisher Scientific).
Dendritic morphological analysis
For the morphological analysis of the DCX-positive dendritic trees, the cells were choosen for the reconstruction, and the length for each dendritic branch was measured according to the following method (Arisi and Garcia-Cairasco, 2007).
Western blot analysis for BDNF and TrkB
The hippocampal tissues were collected, and then were immediately frozen at −70°C. The hippocampal tissues were homogenized on ice, and lysed in a lysis buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM PMSF, 1 mM EGTA, 1.5 mM MgCl2 · 6H2O, 1 mM sodium orthovanadate, and 100 mM sodium flouride. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Protein (30 μg) was separated on SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane. Mouse actin antibody (1:500; Santa Cruz Biotechnology), rabbit BDNF antibody (1:1,000; Santa Cruz Biotechnology) and rabbit TrkB antibody (1:1,000; Santa Cruz Biotechology) were used as the primary antibodies. Horseradish peroxidase-conjugated anti-rabbit antibody for BDNF (1:2,000; Vector Laboratories) and TrkB (1:3,000; Vector Laboratories) were used as the secondary antibodies.
Experiments were performed in normal laboratory conditions and at room temperature, except for the transferred membranes. Transferred membranes were performed at 4°C with the cold pack and pre-chilled buffer. Band detection was performed using the enhanced chemiluminescence (ECL) detection kit (Santa Cruz Biotechnology).
Data analysis
For confirming the expressions of BDNF and TrkB, the detected bands were calculated densitometrically using Molecular Analyst™, version 1.4.1 (Bio-Rad). The number of BrdU-positive cells in the dentate gyrus were counted hemilaterally under a light microscope (Olympus, Tokyo, Japan), and expressed as the numbers of cells per mm2 in the dentate gyrus. The area of the dentate gyrus was measured by Image-Pro® Plus image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA). Statistical analysis was performed using one-way ANOVA followed by Duncan’s post-hoc test, and the results are expressed as the mean±standard error of the mean (SEM). Significances were accepted at P<0.05.
RESULTS
Effect of treadmill exercise on short-term memory in the step-through avoidance task
The result of step-through avoidance task is presented in Fig. 1. The latency was 577.00±15.39 sec in the sham-operation group, 600.00±0.00 sec in the sham-operation and treadmill exercise group, 284.26±30.11 sec in the Aβ25–35-injection group, and 415.66±31.92 sec in the Aβ25–35-injection and treadmill exercise group. The present results show that ICV injection of Aβ25–35 deteriorated short-term memory and that treadmill exercise alleviated Aβ25–35-induced memory impairment.
Fig. 1.
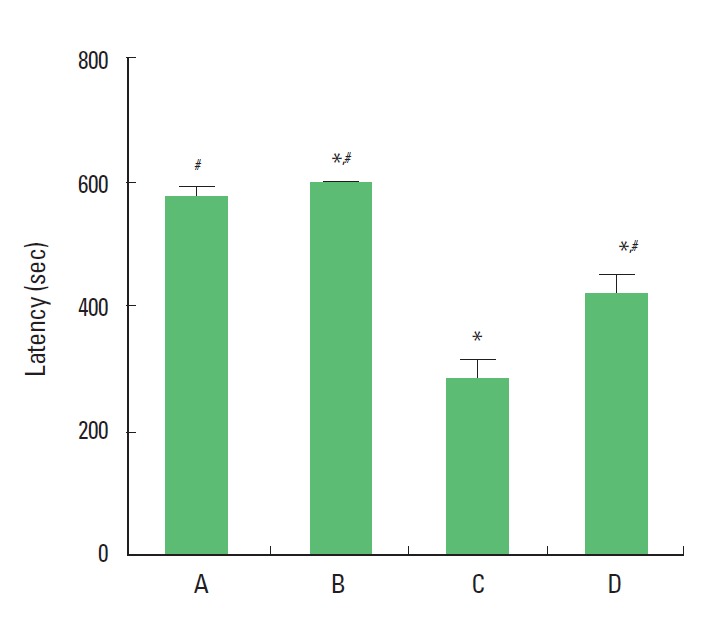
Effect of treadmill exercise on latency in the step-through avoidance task. (A) Sham-operation group, (B) sham-operation and treadmill exercise group, (C) Aβ25–35-injection group, and (D) Aβ25–35-injection and treadmill exercise group. *Represents P< 0.05 compared to the sham-operation group. #Represents P< 0.05 compared to the Aβ25–35-injection group.
Effect of treadmill exercise on neurogenesis in the hippocampal dentate gyrus
Photomicrographs of BrdU-positive cells in the dentate gyrus are presented in Fig. 2. The number of BrdU-positive cells was 72.88±4.91/mm2 in the sham-operation group, 114.09±5.86/mm2 in the sham-operation and treadmill exercise group, 33.08± 3.36/mm2 in the Aβ25–35-injection group, and 60.65±4.19/mm2 in the Aβ25–35-injection and treadmill exercise group. The present results show that ICV injection of Aβ25–35 decreased neurogenesis in the dentate gyrus and that treadmill exercise increased neurogenesis in the rats with ICV injection of Aβ25–35.
Fig. 2.
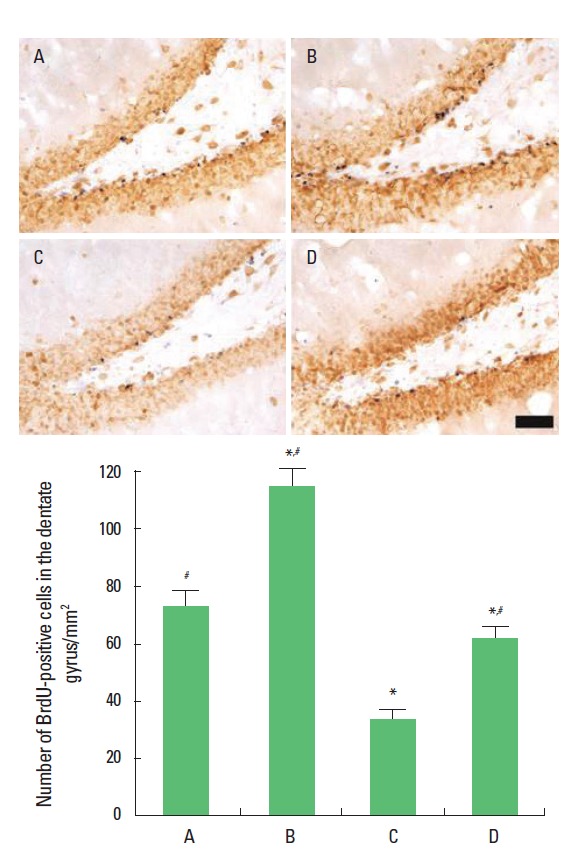
Effect of treadmill exercise on neurogenesis in the hippocampal dentate gyrus. Upper: Photomicrographs of 5-bromo-2′-deoxyridine (BrdU)-positive cells in the dentate gyrus. The scale bar represents 50 μm. Lower: The number of BrdU-positive cells in the hippocampus. (A) Sham-operation group, (B) sham-operation and treadmill exercise group, (C) Aβ25–35-injection group, and (D) Aβ25–35-injection and treadmill exercise group. *Represents P< 0.05 compared to the sham-operation group. #Represents P< 0.05 compared to the Aβ25–35-injection group.
Effect of treadmill exercise on apical dendritic length in the hippocampal dentate gyrus
Photomicrographs of apical dendritic cells in the dentate gyrus are presented in Fig. 3. The length of apical dendrite was 132.75 ±3.59 μm in the sham-operation group, 139.26±3.96 μm in the sham-operation and treadmill exercise group, 101.47±3.17 μm in the Aβ25–35-injection group, and 117.40±2.86 μm in the Aβ25–35-injection and treadmill exercise group. The present results show that ICV injection of Aβ25–35 shortened apical dendritic length in the dentate gyrus and that treadmill exercise increased apical dendritic length in the rats with ICV injection of Aβ25–35.
Fig. 3.
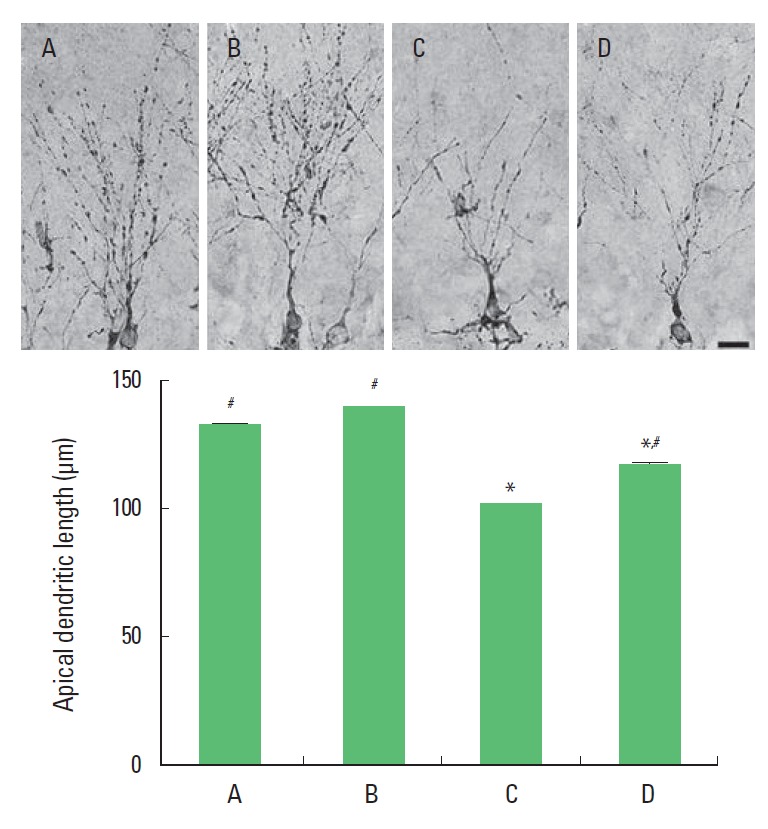
Effect of treadmill exercise on apical dendritic length in the hippocampal dentate gyrus. Upper: Photomicrographs of apical dendritic cells in the dentate gyrus. The scale bar represents 50 μm. Lower: The length of apical dendritic cells in the hippocampus. (A) Sham-operation group, (B) sham-operation and treadmill exercise group, (C) Aβ25–35-injection group, and (D) Aβ25–35-injection and treadmill exercise group. *Represents P< 0.05 compared to the sham-operation group. #Represents P< 0.05 compared to the Aβ25–35-injection group.
Effect of treadmill exercise on expressions of BDNF and TrkB in the hippocampus
The BDNF (14 kDa) and TrkB (95–145 kDa) protein expression are presented Fig. 4. When the level of BDNF in the sham-operation was set at 1.00, the level of BDNF was 1.34±0.10 in the sham-operation and treadmill exercise group, 0.59±0.05 in the Aβ25–35-injection group, and 0.83±0.08 in the Aβ25–35-injection and treadmill exercise group. The present results show that ICV injection of Aβ25–35 decreased BDNF expression in the hippocampus and that treadmill exercise increased BDNF expression in the rats with ICV injection of Aβ25–35.
Fig. 4.
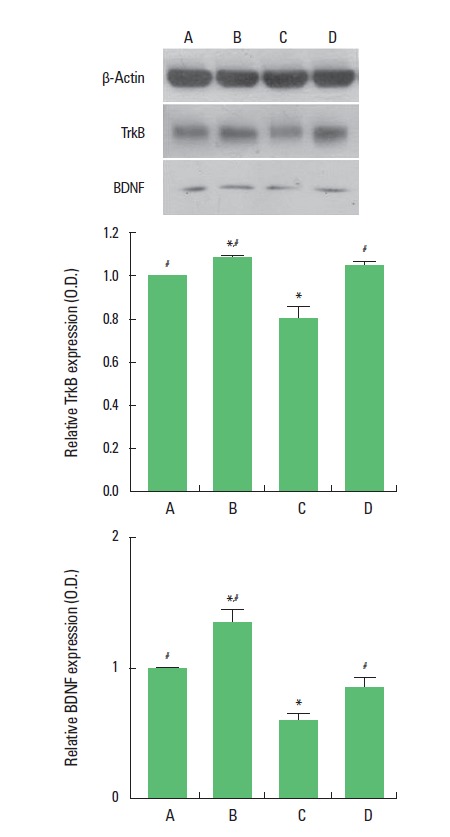
Effect of treadmill exercise on brain-derived neurotrophic factor (BDNF) and tyrosine kinase B (trkB) expressions. (A) Sham-operation group, (B) sham-operation and treadmill exercise group, (C) Aβ25–35-injection group, and (D) Aβ25–35-injection and treadmill exercise group. *Represents P< 0.05 compared to the sham-operation group. #Represents P< 0.05 compared to the Aβ25–35-injection group.
When the level of TrkB in the control group was set at 1.00, the level of TrkB was 1.08±0.01 in the sham-operation and treadmill exercise group, 0.80±0.15 in the Aβ25–35-injection group, and 1.04±0.24 in the Aβ25–35-injection and treadmill exercise group. The present results show that ICV injection of Aβ25–35 decreased TrkB expression in the hippocampus and that treadmill exercise increased TrkB expression in the rats with ICV injection of Aβ25–35.
DISCUSSION
Subtle accumulation of Aβ is correlated with cognitive deficits in AD (Chauhan and Sandoval, 2007). Increased proteolytic degradation of APP, the precursor for Aβ, is considered as the primary cause of AD process (Selkoe, 2008). These effects reduce brain oxidative metabolism, inhibit insulin receptor function, and induce progressive deficits in learning ability and memory capability, and deteriorate cerebral energy balance (Hoyer and Lannert, 1999; Chauhan and Sandoval, 2007; Verri et al., 2012).
The present results showed that the latency in the step-through avoidance task was shortened by ICV injection of Aβ25–35. This indicates that ICV injection of Aβ25–35 impaired short-term memory. Intrastriatal injection of the cholinergic neurotoxin AF64A and ICV injection of Aβ25–35 into the caudate nucleus reduced memory retention in the passive avoidance test (Ishrat et al., 2006). Memory impairment induced by ICV injection of Aβ25–35 in rats is associated with impaired brain glucose and energy metabolism, oxidative stress, and disturbed cholinergic neurotransmission (Verri et al., 2012). We evaluated the effect of treadmill exercise on the memory impairment caused by ICV injection of Aβ25–35. In the present study, treadmill exercise alleviated Aβ25–35-induced memory impairment. These observations are consistent with other studies, which demonstrated that memory was impaired by ICV injection of Aβ25–35 in the rats (Alkam et al., 2007; Shi et al., 2010).
DCX is a brain-specific microtubule-associated protein and it is present in migrating neuroblasts and young neurons (Francis et al., 1999). DCX is expressed in the mitotic and early postmitotic neurons, and DCX plays a role in the neuronal growing processes (Friocourt et al., 2003; Kim et al., 2013). Numerous changes in dendritic architecture have been observed, including loss of dendritic spines in transgenic mice overexpressing APP and in the brains of AD (Moolman et al., 2004). Synaptic loss is correlated with the degree of dementia in humans, and similar synaptic loss occurs in the amyloid deposited transgenic mice (Masliah, 1995). Treadmill exercise enhanced dendritic spine density in granule cells of the dentate gyrus (Stranahan et al., 2007). In the oresent study, ICV injection of Aβ25–35 shortened apical dendritic length in the dentate gyrus. The present results showed that treadmill exercise increased apical dendritic length in the Aβ25–35-induced AD rats.
Enhancing neurogenesis and increasing BDNF by treadmill exercise are well documented (Chapleau et al., 2008; Cho et al., 2013; Lee et al., 2013). BDNF is known to increase dendritic spine density of CA1 pyramidal neurons (Chapleau et al., 2008; Scharfman et al., 2005). Clinical evidences have shown that exercise decreases risk for developing cognitive impairment and dementia, particularly in the Alzheimer type (Berchtold et al., 2002; Sonkusare et al., 2005; Jee et al., 2008). In the present study, neurogenesis and expressions of BDNF and trkB in the hippocampus were suppressed by ICV injection of Aβ25–35. Treadmill exercise enhanced neurogenesis and expressions of BDNF and trkB in the hippocampus.
Based on the present results, treadmill exercise might overcome the Aβ25–35-induced memory deficits by enhancing neurogenesis. The present study revealed the possibility that treadmill exercise may be used as the useful strategy for alleviating memory dysfunction induced by neurodegenerative diseases including AD.
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2010-327-G00099).
Footnotes
CONFLICT OF INEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Alkam T, Nitta A, Mizoguchi H, Itoh A, Nabeshima T. A natural scavenger of peroxynitrites, rosmarinic acid, protects against impairment of memory induced by Aβ25–35. Behav Brain Res. 2007;180:139–145. doi: 10.1016/j.bbr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Arisi GM, Garcia-Cairasco N. Doublecortin-positive newly born granule cells of hippocampus have abnormal apical dendritic morphology in the pilocarpine model of temporal lobe epilepsy. Brain Res. 2007;1165:126–134. doi: 10.1016/j.brainres.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Cotman CW. Hippocampal brain-derived neurotrophic factor gene regulation by exercise and the medial septum. J Neurosci Res. 2002;68:511–521. doi: 10.1002/jnr.10256. [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocca ME, Pietranera L, Beauquis J, De Nicola AF. Estradiol increases dendritic length and spine density in CA1 neurons of the hippocampus of spontaneously hypertensive rats: A Golgi impregnation study. Exp Neurol. 2013;247:158–164. doi: 10.1016/j.expneurol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Chapleau CA, Carlo ME, Larimore JL, Pozzo-Miller L. The actions of BDNF on dendritic spine density and morphology in organotypic slice cultures depend on the presence of serum in culture media. J Neurosci Methods. 2008;169:182–190. doi: 10.1016/j.jneumeth.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan NB, Sandoval J. Amelioration of early cognitive deficits by aged garlic extract in Alzheimer’s transgenic mice. Phytother Res. 2007;21:629–640. doi: 10.1002/ptr.2122. [DOI] [PubMed] [Google Scholar]
- Cho HS, Shin MS, Song W, Jun TW, Lim BV, Kim YP, Kim CJ. Treadmill exercise alleviates short-term memory impairment in 6-hydroxydopamine-induced Parkinson’s rats. J Exerc Rehabil. 2013;9:354–361. doi: 10.12965/jer.130048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;4:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjök-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;11:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- Friocourt G, Koulakoff A, Chafey P, Boucher D, Fauchereau F, Chelly J, Francis F. Doublecortin functions at the extremities of growing neuronal processes. Cereb Cortex. 2003;13:620–626. doi: 10.1093/cercor/13.6.620. [DOI] [PubMed] [Google Scholar]
- Hoyer S, Lannert H. Inhibition of the neuronal insulin receptor causes Alzheimer-like disturbances in oxidative/energy brain metabolism and in behavior in adult rats. Ann N Y Acad Sci. 1999;893:301–303. doi: 10.1111/j.1749-6632.1999.tb07842.x. [DOI] [PubMed] [Google Scholar]
- Ishrat T, Khan MB, Hoda MN, Yousuf S, Ahmad M, Ansari MA, Ahmad AS, Islam F. Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav Brain Res. 2006;171:9–16. doi: 10.1016/j.bbr.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Jee YS, Ko IG, Sung YH, Lee JW, Kim YS, Kim SE, Kim BK, Seo JH, Shin MS, Lee HH, Cho HJ, Kim CJ. Effects of treadmill exercise on memory and c-Fos expression in the hippocampus of the rats with intracerebroventricular injection of streptozotocin. Neurosci Lett. 2008;443:188–192. doi: 10.1016/j.neulet.2008.07.078. [DOI] [PubMed] [Google Scholar]
- Juárez-Méndez S, Carretero R, Martínez-Tellez R, Silva-Gómez AB, Flores G. Neonatal caffeine administration causes a permanent increase in the dendritic length of prefrontal cortical neurons of rats. Synapse. 2006;60:450–455. doi: 10.1002/syn.20318. [DOI] [PubMed] [Google Scholar]
- Kim SE, Ko IG, Park CY, Shin MS, Kim CJ, Jee YS. Treadmill and wheel exercise alleviate lipopolysaccharide-induced short-term memory impairment by enhancing neuronal maturation in rats. Mol Med Rep. 2013;7:31–316. doi: 10.3892/mmr.2012.1160. [DOI] [PubMed] [Google Scholar]
- Lannert H, Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci. 1998;112:1199–1208. doi: 10.1037//0735-7044.112.5.1199. [DOI] [PubMed] [Google Scholar]
- Lee SW, Kim YS, Jun TW, Seo JH, Kim K, Shin MS, Kim CJ. The impact of duration of one bout treadmill exercise on cell proliferation and central fatigue in rats. J Exerc Rehabil. 2013;9:463–469. doi: 10.12965/jer.130069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E. Mechanisms of synaptic dysfunction in Alzheimer’s disease. Histol Histopathol. 1995;10:509–519. [PubMed] [Google Scholar]
- Moolman DL, Vitolo OV, Vonsattel JP, Shelanski ML. Dendrite and dendritic spine alterations in Alzheimer models. J Neurocytol. 2004;33:377–387. doi: 10.1023/B:NEUR.0000044197.83514.64. [DOI] [PubMed] [Google Scholar]
- Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Savla GN, Palmer BW. Neuropsychology in Alzheimer’s disease and other dementia research. Curr Opin Psychiatry. 2005;18:621–627. doi: 10.1097/01.yco.0000184413.36706.8e. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid β-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo TB, Cho HS, Shin MS, Kim CJ, Ji ES, Baek SS. Treadmill exercise improves behavioral outcomes and spatial learning memory through up-regulation of reelin signaling pathway in autistic rats. J Exerc Rehabil. 2013;9:220–229. doi: 10.12965/jer.130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YQ, Huang TW, Chen LM, Pan XD, Zhang J, Zhu YG, Chen XC. Ginsenoside Rg1 attenuates amyloid-β content, regulates PKA/CREB activity, and improves cognitive performance in SAMP8 mice. J Alzheimer’s Dis. 2010;19:977–989. doi: 10.3233/JAD-2010-1296. [DOI] [PubMed] [Google Scholar]
- Sonkusare S, Srinivasan K, Kaul C, Ramarao P. Effect of donepezil and lercanidipine on memory impairment induced by intracerebroventricular streptozotocin in rats. Life Sci. 2005;77:1–14. doi: 10.1016/j.lfs.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, Yip E, Afanador M, Schroeter H, Hammerstone J, Gage FH. Plant-derived flavanol (−)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci. 2007;27:5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerendra Kumar MH, Gupta YK. Effect of centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer’s disease in rats. Clin Exp Pharmacol Physiol. 2003;30:336–342. doi: 10.1046/j.1440-1681.2003.03842.x. [DOI] [PubMed] [Google Scholar]
- Verri M, Pastoris O, Dossena M, Aquilani R, Guerriero F, Cuzzoni G, Venturini L, Ricevuti G, Bongiorno AI. Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer’s disease. Int J Immunopathol Pharmacol. 2012;25:345–353. doi: 10.1177/039463201202500204. [DOI] [PubMed] [Google Scholar]


