Abstract
Objective
To investigate whether mutations in NPHP5 can cause Leber congenital amaurosis (LCA) without early-onset renal disease.
Methods
DNA samples from 276 individuals with non-syndromic LCA were screened for variations in the NPHP5 gene. Each had been previously screened for mutations in 8 known LCA genes without identifying a disease-causing genotype.
Results
Nine of the 276 LCA probands (3.2%) harbored 2 plausible disease-causing mutations (7 different alleles) in NPHP5. Four of these have been previously reported in patients with Senior-Loken syndrome (F141del, R461X, H506del, and R489X) and 3 are novel (A111del, E346X, and R455X). All 9 patients had severe visual loss from early childhood but none had overt renal disease in the first decade of life. Two patients were diagnosed with nephronophthisis in the second decade. Retinal imaging studies showed retained photoreceptor nuclei and retinal pigment epithelium integrity mainly in the cone-rich central retina, a phenotype with strong similarities to that of NPHP6 disease.
Conclusions
Mutations in NPHP5 can cause LCA without early-onset renal disease. Abnormalities observed in the photoreceptor outer segments (a cilial structure) may explain the severe visual loss in NPHP5-associated LCA.
Clinical Relevance
The persistence of central photoreceptor nuclei despite severe visual loss in NPHP5 disease is encouraging for future therapeutic interventions.
Many mendelian disorders have been recognized in which dysplasia, dysfunction, and/or degeneration of the retina are observed in combination with abnormalities of other organ systems. In the past decade, molecular geneticists have traced the cause of a number of these syndromes to a phylogenetically ancient organelle, the sensory cilium. Cilia-associated proteins are essential for the normal development and function of a wide array of specialized tissues including the retina, inner ear, kidney, and brain. The many manifestations of Bardet-Biedl, Usher, Joubert, and Senior-Loken syndromes result from a loss or imperfect function of a component of the cilium-centrosome complex.1,2
Senior-Loken syndrome is an autosomal recessive oculo-renal condition.3,4 The 2 major features of Senior-Loken syndrome are the cystic kidney disease known as nephronophthisis (NPHP) and an early childhood–onset retinal degeneration known as Leber congenital amaurosis (LCA).5 To date, Senior-Loken syndrome has been associated with mutations in 5 of the 10 NPHP genes.6–10 Certain genotypes of 1 of these genes (NPHP6 [OMIM 610142]) cause a nonsyndromic retinal disease known as Leber congenital amaurosis (LCA).11,12 NPHP6 is thought to form a functional complex with NPHP513 (OMIM 609237) and knockdown of either of these genes in zebrafish embryos leads to a syndromic disease with ocular and systemic manifestations. These observations led us to investigate whether mutations in NPHP5 could also cause LCA in the absence of early-onset renal disease.
With 1 exception, the retinal phenotypes associated with mutations in NPHP genes have not been described in detail. Only the nonsyndromic retinal disease caused by mutations in NPHP6 has been studied extensively.14 With the recent success in clinical trials of treatment of an inherited retinal degeneration,15–18 we felt that it was important to investigate the retinal phenotype of patients with mutations in NPHP5 for evidence that this retinal disease might be treatable.
METHODS
HUMAN SUBJECTS
The study was approved by the institutional review boards of the participating centers and adhered to the tenets set forth in the Declaration of Helsinki. Two hundred seventy-six individuals with the clinical diagnosis of nonsyndromic LCA and 276 ethnically similar control individuals were studied. The patients were chosen from a cohort of more than 1000 patients with LCA on the basis of the following criteria: (1) a clinical diagnosis of nonsyndromic LCA, (2) prior screening of the coding sequences of 8 genes known to cause the LCA phenotype (AIPL1 [OMIM 604392], CRB1 [OMIM 604210], CRX [OMIM 602225], GUCY2D [OMIM 600179], RHD12 [OMIM 608830], RPE65[OMIM 180069], RPGRIP1 [OMIM 605446], and CEP290 [OMIM 610142]) without discovery of a plausible disease-causing genotype, (3) original medical records available for detailed review, and (4) sufficient DNA remaining to allow screening and molecular confirmation.
MUTATION ANALYSES
Fifty nanograms of genomic DNA from each patient with LCA were combined into a “patient DNA pool” and 50 ng of genomic DNA from each control individual were combined into a “control DNA pool.” A series of oligonucleotide primers were designed to amplify the NPHP5 coding sequence in fragments that were 200 to 300 base pairs in length. Each primer pair was used to amplify the corresponding candidate gene segment from both the patient DNA pool and the control DNA pool. Polymerase chain reaction (PCR) amplification was confirmed by agarose gel electrophoresis with ethidium bromide visualization and the products were quantified using densitometry of the agarose gels and comparison with standard DNA samples of known concentration. All PCR products amplified from the pooled DNA from patients with LCA were combined in an equimolar fashion into 1 sample and the PCR products amplified from the pooled control subjects’ DNA were combined into a second sample. The amplified DNA segments from patients and controls were separately ligated to emulsion PCR adapters (Roche, Branford, Connecticut) as directed by the manufacturer. The ligated fragments were hybridized to sequencing beads in an amplification mixture containing DNA polymerase, deoxynucleoside triphosphates, and buffer. The mixtures were mixed with oil, emulsified, and thermocycled for 50 cycles. The DNA-coated beads were recovered from the emulsions and subjected to pyrophosphate sequencing on a Roche 454 instrument using titanium chemistry. The beads corresponding to the samples from patients with LCA were analyzed on one-half of a picotiter plate and the beads corresponding to the control individuals were analyzed on the other half of the plate during a single run of the sequencing instrument. Roche GSAmplicon software was used to analyze the standard flowgram files and detect the presence of variations from the specified reference sequences of each amplimer. Variations that were significantly more common in patients than controls were confirmed using automated DNA sequencing with dye termination chemistry on an ABI 3730 sequencer (Applied Biosystems, Carlsbad, California). Six members of 1 family—the proband, both parents, and 3 unaffected siblings—were genotyped for 16 short tandem repeat polymorphisms, as previously described.19 Five non–chromosome 3 markers (D6S1611, D1051174, D195902, D115956, and D55820) were used to confirm the biological relationships within the family while 11 chromosome 3 markers (D3S1568, D3S3664, D3S3513, D3S3620, D3S3720, D3S4497, D3S3709, D3S3576, D3S1269, D3S1267, and D3S3558) were used to investigate the possibility of uniparental isodisomy.
RETINAL CROSS-SECTIONAL IMAGING
Retinal cross-sections were obtained with a Fourier-domain OCT system (RTVue-100; Optovue Inc, Fremont, California) using recording and analysis techniques previously published.20–23 For this work, the high-definition line protocol of the Fourier domain OCT system was used to obtain 4.5-mm-long scans composed of 4091 longitudinal reflectivity profiles (LRPs) acquired at 26 000 LRPs per second. Overlapping line scans were used to cover the vertical meridian up to 9-mm eccentricity from the fovea. In addition, topographic analysis was performed by obtaining dense overlapping raster scans sampling a rectangular region of the retina (18 mm × 12 mm) centered on the fovea.
Postacquisition processing of OCT data was performed with custom programs (MatLab 6.5; MathWorks, Natick, Massachusetts). The LRPs making up the OCT scans were aligned by straightening the major retinal pigment epithelium (RPE) reflection.24 In some regions of patient retinas, the presumed RPE peak was sometimes the only signal peak deep in the retina; in other cases, it was apposed by other major peaks. In the latter case, the RPE peak was specified manually as previously described.25 In normal subjects, the signal corresponding to the RPE was assumed to be the peak nearest the sclera within the multipeaked scattering signal complex.25 Lateral sampling density of the Fourier-domain OCT scans was reduced by averaging 8 neighbors to increase the signal to noise ratio. Quantitative measurements of retinal laminae were performed after further reduction of lateral sampling density (sampling bins 0.07 mm). The outer photoreceptor nuclear layer (ONL) was defined in regions of scans showing stereotypical layering between the RPE and vitreoretinal interface. The boundaries of the ONL were determined by the minima and maxima of the LRP signal slopes and taking into account neighboring layers of higher reflectivity on LRPs; specifically, ONL boundaries were defined between the outer limiting membrane or RPE and the scleral boundary of the outer plexiform layer.
RETINAL EN FACE IMAGING
En face images of the retina were obtained with a confocal scanning laser ophthalmoscope (HRA2; Heidelberg Engineering GmbH, Heidelberg, Germany) using recording and analysis techniques previously published.14,22,23,26–29 Imaging was performed in the “high-speed” mode: 30° × 30° of the retina was sampled onto a 768 × 768 pixel image at the rate of 8.8Hz. Natural melanin fluorophores in RPE cells28 were imaged with autofluorescence (AF) using near infrared (NIR) excitation light at 790 nm and collecting emissions beyond 810 nm. This imaging mode provided an estimate of RPE health distribution since disease-related changes in RPE melanin content result in spatial variation of the NIR-AF signal intensity, appearance, or both. A sensitivity setting of 95% was invariantly used for all NIR-AF imaging. The automatic real-time averaging feature of the manufacturer’s software was used whenever possible. When the automatic real-time feature failed, image stacks were exported from the manufacturer’s software, spatially registered, and averaged. Automatic real-time–based or averaged images of the neighboring regions were digitally combined into a single mosaic image by manually specifying retinal landmark pairs.
RESULTS
DISEASE-CAUSING NPHP5 VARIANTS IN PATIENTS WITH LCA
Plausible disease-causing genotypes were identified in NPHP5 in 9 of the 276 patients with LCA (3.2%) (Table). Seven different disease alleles were observed, 4 of which have been previously observed in individuals with Senior-Loken syndrome (F141del, R461X, H506del, and R489X) and 3 of which have not been previously reported (A111del, E346X, and R455X). All of these variations would be expected to result in a truncated protein. Samples from 1 or both parents of 7 of the probands were available for assessing the phase of the observed variants. In 6 of these 7 probands, each parent contributed 1 disease allele to their affected child. In the remaining case, the affected individual was found to be homozygous for the H506del variant of NPHP5 and his mother was found to be heterozygous for this change. The child’s father was found to be homozygous for the consensus normal sequence of NPHP5. The proband, parents, and 3 unaffected siblings were then genotyped with a series of 5 short tandem repeat polymorphisms that lie on chromosomes other than chromosome 3, and these genotypes clearly confirmed the stated pedigree relationships (data not shown). Genotyping of an additional 11 short tandem repeat polymorphism markers from chromosome 3 revealed the affected child to be homozygous for a maternal allele at every locus tested, indicating maternal uniparental isodisomy (Figure 1).
Table.
Clinical and Molecular Characteristics of the 9 Patients With NPHP5 Variations
| Patient No./Sex |
Age at Visit, y |
Nucleotide Change |
Protein Change |
Nucleotide Change |
Protein Change |
Visual Acuitya | Refractionb | ERG | Renal Disease | |
|---|---|---|---|---|---|---|---|---|---|---|
| Allele 1 | Allele 1 | Allele 2 | Allele 2 | OD | OS | |||||
| 1/F | 4 | c.333delT | Ala111del1gcT | c.1381C>T | Arg461Stop | U | U | +6.75 | ND | Severe (by age 13 y) |
| 23 | 20/80 | 20/200 | NP | |||||||
| 2/M | 5 | c.1465C>T | Arg489Stop | c.1516-1517delCA | His506del2cagCA | LP | LP | +7.50 | NP | None manifestc,d |
| 3/M | 7 | c.1516-1517delCA | His506del2cagCA | c.1516-1517delCA | His506del2cagCA | 20/400 | 20/800 | +6.00 | ND | None manifestd,e |
| 4/M | 13 | c.1381C>T | Arg461Stop | c.1516-1517delCA | His506del2cagCA | 20/200 | 20/200 | +4.00 | ND | None manifestd |
| 5/M | 14 | c.1381C>T | Arg461Stop | c.1465C>T | Arg489Stop | HM | HM | NP | ND | Severe (by age 13 y) |
| 6/F | 8 | c.1363C>T | Arg455Stop | c.421-422delTT | Phe141del2cacTT | 20/200 | 20/200 | +6.00 | NP | None manifestd |
| 7/F | 3 | c.1036G>T | Glu346Stop | c.1516-1517delCA | His506del2cagCA | 20/1000 | 20/1000 | +9.00 | ND | None manifestd |
| 12 | HM | HM | +5.00 | NP | None manifestd | |||||
| 19 | LP | LP | NP | NP | Elevated creatinine level | |||||
| 8/F | 1 | c.1381C>T | Arg461Stop | c.1516-1517delCA | His506del2cagCA | 20/260 | 20/290 | +6.50 | ND | None manifest |
| 3 | LP | LP | NP | NP | ||||||
| 9/F | 14 | c.1381C>T | Arg461Stop | c.1381C>T | Arg461Stop | LP | LP | +5.25 | NP | None manifest |
Abbreviations: ERG, electroretinogram; HM, hand motions; LP, light perception; ND, not detectable; NP, not performed; U, unreliable.
Best-corrected visual acuity.
Spherical equivalent.
Ureterocele, ureteral duplication.
Normal blood urea nitrogen and creatinine levels.
Hyperaldosteronism.
Figure 1.
Chromosome 3 genotyping of patient P3 and his family. These individuals were genotyped at 11 loci distributed across chromosome 3. The mutation (H506del) lies at 121.49 centimorgans (cM) (asterisk). For all 11 markers, the patient is homozygous for a maternal marker allele, consistent with maternal uniparental isodisomy.
In addition to the 7 disease-causing NPHP5 variants, we also observed a missense variation at codon 435 (R435C) in 3 patients with LCA (1.1%) and 3 unaffected control individuals (1.1%). Although the frequency of this variant was identical in patients and controls, we noted that 2 of the 3 patients with LCA heterozygous for R435C were also heterozygous for the most common disease-causing variant in NPHP6 (IVS 26 + 1655 A>G). Because only 12% of our LCA cohort harbored this NPHP6 variant, the probability of 2 of the R435C heterozygotes harboring this variant by chance is less than 2%. However, investigation of the parents of these compound heterozygotes revealed that in 1 family, both alleles were inherited from the same unaffected parent. Thus, it is quite unlikely that these 2 alleles are acting together to cause LCA in these 2 families. Another potential modifying variant that we considered was A229T in RPGRIP1L, which was recently shown to accentuate the phenotype of NPHP6-associated disease.30 None of the 9 patients with NPHP5 mutations in this series was found to harbor this variant.
CLINICAL FINDINGS IN PATIENTS WITH NPHP5 VARIATIONS: NONSYNDROMIC LCA IS THE EARLIEST DISEASE EXPRESSION
The clinical features of the 9 individuals with NPHP5 variations are summarized in the Table. All patients had significantly reduced vision noted in the first few months of life and nystagmus. Only 1 of the 18 eyes of these 9 individuals had a visual acuity better than 20/200 and 5 of the patients had light perception or hand motions visual acuity in both eyes on their most recent visit. In both patients from whom multiple acuity measurements were available, there was progressive worsening of vision; 1 patient fell from 20/260 acuity at age 1 year to light perception at age 3 years. Three patients with visual acuity better than hand motions had visual field testing performed and all 3 exhibited only small (>20°) central islands of vision in each eye. Of the patients with recorded refractions, all had 4 to 9 diopters of hyperopia. Six of the patients were studied with electroretinography and there were no detectable responses under any stimulus condition. None of the patients exhibited signs or symptoms of renal disease in the first decade of life but 2 were diagnosed with NPHP at age 13 years. The oldest patient examined (age 23 years) had the best visual acuity (20/80 OD) but developed NPHP at age 13 years. In contrast, the second oldest patient examined (age 19 years) had the poorest vision (bare light perception) and no symptoms of renal disease. Specifically, she had no polydipsia, polyuria, or nocturia and had a normal blood urea nitrogen level of 11 mg/dL (to convert to millimoles per liter, multiply by 0.357) (reference range, 7–24 mg/dL). However, her serum creatinine level was slightly elevated (1.1 mg/dL [to convert to micromoles per liter, multiply by 88.4]; reference range, 0.6–1.0 mg/dL).
PHOTORECEPTOR AND RPE DISEASE IN NPHP5 IS SIMILAR TO THAT IN NPHP6
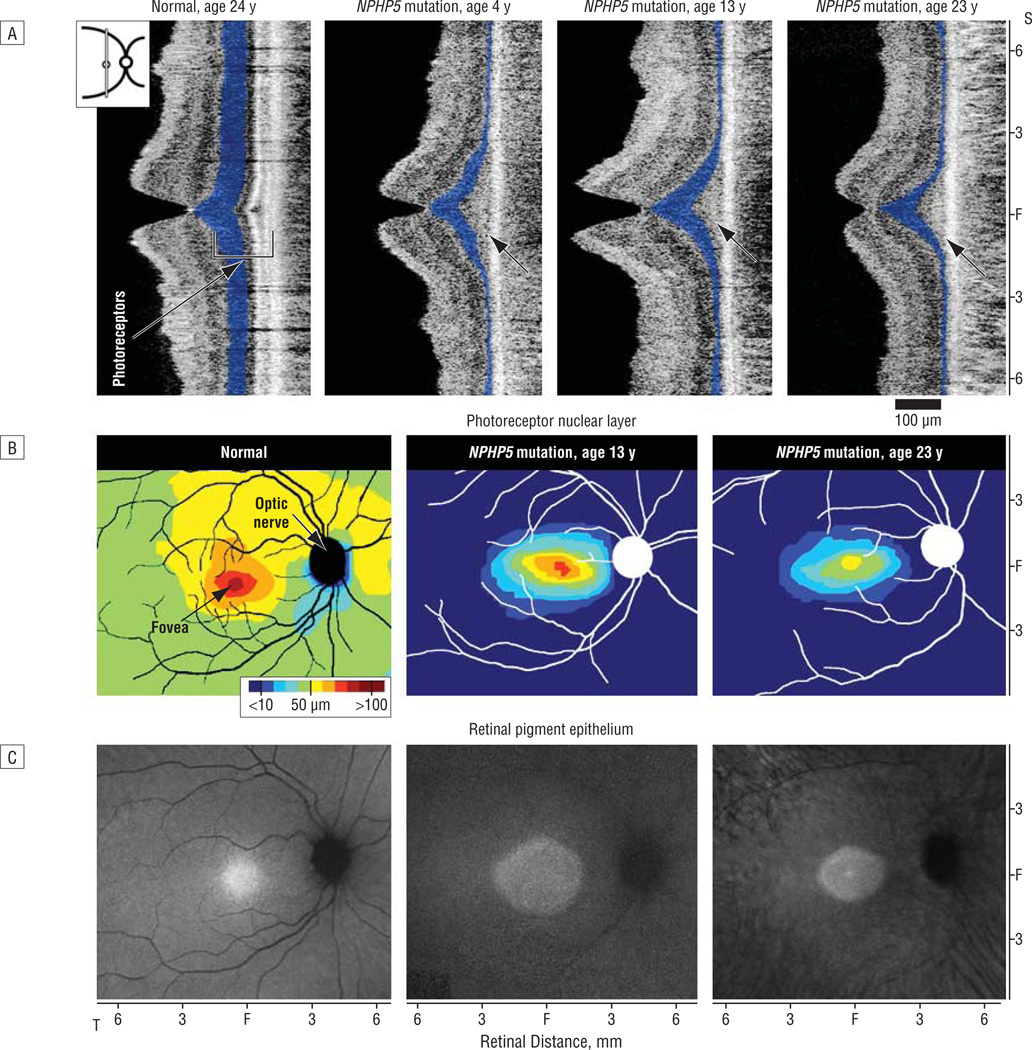
We studied the retinal photoreceptor layer and the RPE of a subset of patients with NPHP5-associated retinal disease. Cross-sectional images using OCT of the central retina of a normal subject showed a foveal depression or pit and orderly retinal layers corresponding to nuclei, synapses, and axons (Figure2A, left panel). The ONL (highlighted in blue) was thickest at the fovea and thinned with increasing distance from it. In 3 patients (aged 4, 13, and 23 years) with NPHP5 mutations, the images exhibited relative sparing of the ONL at the fovea but this was very diminished with increasing distance from the fovea (Figure 2A, right panels). A key observation was that the lamination attributed to the inner and outer segments and cilium of the photoreceptors (Figure 2A, bracketed with ONL in the normal subject and black arrows in patients) differed in the NPHP5-mutant retinas compared with normal. Whereas the laminae deep to the ONL were present in the normal retinal cross-section, a distinct laminar architecture was absent in the patients, suggesting disorganized inner and outer segment material.
Figure 2.
NPHP5-mutant retinas analyzed for photoreceptor and retinal pigment epithelium disease. A, Cross-sectional images along the vertical meridian are shown in a normal subject (left panel) and 3 patients with NPHP5 mutation of different ages (right 3 panels). The photoreceptor nuclear layer is highlighted (blue); inner and outer segment laminae and cilial structure are indicated (bracketed with the photoreceptor nuclear layer in the normal subject and unlabeled black arrows in patients). B, Topography of photoreceptor layer thickness in a normal retina (left) and 2 NPHP5-mutant retinas (right panels), displayed in pseudocolor. The location of the optic nerve head and major retinal blood vessels are overlaid (black or white) for reference. The fovea and optic nerve head are labeled in the normal subject. C, Retinal pigment epithelium integrity as measured with near infrared autofluorescence in a representative normal subject (left panel) and in the patients with NPHP5 mutation. Higher intensity represents greater melanin content. F indicates fovea; S, superior; T, temporal.
The spatial distribution of the NPHP5 retinopathy was shown in 2 of the patients (Figure 2B). Normal photoreceptor layer thickness has a central peak that declines with distance from the fovea. There is a shallower rate of decline in the superior direction that results in anisotropic contour lines extending superiorly. In the 13-year-old patient with NPHP5 mutation, there was a central ellipsoid region approximately 6 mm wide and 4 mm high centered on the fovea with retained photoreceptors; a similar region with less extent was detectable in the 23-year-old patient with NPHP5 mutation.
The topography of RPE integrity in eyes with NPHP5 mutation was defined with NIR-AF imaging (Figure 2C); the signal is considered to originate from melanin and melanolipofuscin granules in the RPE and melanin in the choroid.26,28,31 In normal human eyes, NIR-AF images show a circular central region of homogeneous appearance and higher brightness extending from the fovea. With increasing eccentricity, there is a decrease in brightness (Figure 2C). The NIR-AF topographies of eyes with NPHP5 mutation showed a central ellipsoid of higher intensity surrounded by a more peripheral region of lower intensity. In the 23-year-old patient, there was evidence of 2 zones of RPE demelanization. Surrounding the central ellipsoid was a wide homogenous annulus of lower intensity likely corresponding to partially demelanized RPE cells. Further peripheral was a heterogenous annulus likely corresponding to loss of RPE cells and appearance of choroidal structures.
COMMENT
There are numerous examples in which clinical entities that were first described in the pregenomic era fail to map crisply to the genome. Some clinical phenotypes (eg, Senior-Loken syndrome32 and LCA11) are caused by more than 1 gene while variations in some genes (eg, NPHP5 and NPHP6) cause different clinical phenotypes in different patients. This situation would be challenging enough if all clinical findings were present at the same point and the clinician needed only to look for them during a patient’s first examination. However, in many multisystem conditions, 1 or a few of the manifestations are prominent and occur early in the disease course while others are more subtle and manifest much later if at all.
The patients described by Senior et al3 and Loken et al4 in the early 1960s had profound renal disease that caused death from uremia as early as age 7 years. Knowledge of this literature might lead some clinicians to expect that if one of their patients with LCA were destined to have associated renal disease, it would be overtly manifest in the first decade of life. The patients in this report were all referred for molecular diagnosis before any sign of renal disease appeared, and 7 of them (1 now aged 19 years) are still free of overt renal disease. Similarly, Otto and colleagues10 found that end-stage renal disease can occur relatively late in life in some patients with NPHP5 mutations. These data illustrate that the frequencies of various clinical findings in patients with given genotypes will vary with the age of the patients and the degree to which they have been examined and tested. The clearest understanding of the phenotype of multisystem disorders will come from studies of large numbers of older patients. Unfortunately, for the past several decades, many children who were born blind were not carefully followed up by their ophthalmologists because of a prevailing sense that nothing could be done to help them. The advent of successful gene therapy for 1 form of LCA15–18 is starting to reverse this attitude and kindle a greater interest in the natural history of all molecular forms of this disease (https://www.carverlab.org/project3000).
Examination of the Table reveals a wide variation in clinical findings among patients with seemingly similar disease alleles. That is, both NPHP5 alleles of every patient in this series have frameshifts or nonsense mutations that might reasonably be expected to result in a complete loss of function of the protein (ie, through nonsense-mediated decay33 or protein truncation). What then could account for the variations in phenotype? There are at least 3 nonexclusive possibilities that can be considered. First, some transcripts may escape nonsense-mediated decay and be transcribed into a partially functional truncated protein or “read through” to yield a small amount of normal protein. There is evidence that the most common LCA-causing mutation in human NPHP6, a cryptic splice variant in IVS 26,12 results in some residual NPHP6 function. For example, most individuals who are homozygous for the IVS 26 variant have normal brain development as do individuals who are heterozygous for the IVS 26 change and either His406del2cAT34 or Ile1372del2gaaAT.12 In contrast, compound heterozygosity of His406del2cAT and Ile1372del2gaaAT has been reported to cause fatal maldevelopment of the brain (Meckel-Gruber syndrome).34 If partial NPHP5 function is associated with some of the mutations observed in our series, some may result in more function than others. The patients with the best and worst visual acuity in our series each harbor alleles that have not been previously observed and may account in part for their phenotypic differences. A second potential explanation for variations in the phenotypes of these patients is the effect of some environmental factor. One could imagine that a partially dysfunctional kidney might be prone to injury by infections or dietary changes that would be completely harmless to a healthier organ. If such insults were nonuniform in the population, they could account for the kinds of variation seen in the Table. A third possibility is the effect of the genetic background, that is, variations in other genes that by themselves do not alter an individual’s phenotype but that do alter the pathogenicity of a disease-causing allele.
The most likely candidates for genes that could harbor such augmenting or mitigating variations would be genes already associated with congenital retinal dysfunction. All 276 patients with LCA enrolled in this study were extensively screened for variations in other LCA-causing genes and some of them were found to harbor a single disease-causing allele in a known LCA gene. For example, 21 of the 276 patients harbor the most common LCA-causing allele of NPHP6 (IVS 26 + 1655 G>A) but none of these 21 individuals were among the 9 patients with disease-causing NPHP5 variants. An extreme case of such “background” effects occurs when heterozygous alleles of 2 different genes—neither of which is capable of causing disease on its own—cause disease when present in the compound heterozygous state (ie, digenic inheritance). The R435C variant of NPHP5 is present in approximately 1% of the general population while the IVS 26 1655G>A variant of NPHP6 is present in fewer than 0.1% of the population.11 Thus, it would seem extremely unlikely to observe 2 compound heterozygotes of these variations among only 276 patients in our LCA cohort. However, the frequency of the NPHP6 variant in the LCA cohort was actually 7.6%, not the 0.1% of the general population; thus, we might have expected to see this combination once per 1300 patients with LCA, which is not statistically different from the 2 in 276 that we did observe. The most telling observation was that the normally sighted mother of 1 of the compound heterozygous patients with LCA was herself a compound heterozygote of these 2 variants, making the pathogenicity of this genotype extremely unlikely. The final modifying variant that we considered was A229T in RPGRIP1L, which was recently shown to accentuate the phenotype of NPHP6-associated disease.30 None of the 9 patients with NPHP5 mutations in this series was found to harbor this variant.
The foregoing analysis reveals the value of access to parental genotypes when evaluating their possible pathogenicity in affected individuals. Parental genotypes are also extremely valuable for detecting de novo mutations of various types. For example, nondisjunction during meiotic cell division results in gametes with abnormal numbers of chromosomes (nullisomy or disomy). When such a gamete is joined by a normal gamete to form a zygote, either trisomy or monosomy occurs. Loss of an extra chromosome in early mitotic cell division (trisomy rescue) or gain by duplication of a monosomic chromosome (monosomy rescue) results in an individual with 2 copies of a chromosome inherited from 1 parent and none from the other (uniparental isodisomy). If a recessive disease-causing allele lies on the disomic chromosome, it can result in the inheritance of a recessive disease from only 1 parent, as it did in patient 3 (Table) in our series.
Some chromosomes are more likely to become disomic than others. Of the cases of isodisomy reported in the literature, chromosomes 14 and 15 are the most likely to be involved.35 Previous cases of isodisomic LCA observed involved a mutation in the RPE65 gene on chromosome 1.11,36 Isodisomy of chromosome 3 is very rare. We have found 2 instances of isodisomic LCA among a cohort of 260 individuals with both disease-causing alleles identified and with sufficient parental data to have revealed isodisomy if it existed. Thus, isodisomy seems to be involved in less than 1% of all cases of LCA. Despite its rarity, this inheritance pattern is important to recognize because the risk to subsequent siblings of an affected individual is near zero35 in contrast to the recurrence risk of 25% when each parent carries a disease-causing allele.
The similarity of the retinal phenotypes of patients with NPHP5- and NPHP6-associated LCA has both mechanistic and therapeutic implications. The relative preservation of photoreceptor cells in the central 4 mm of the retina in patients with disease-causing mutations in either of these genes suggests some physiologic difference between these photoreceptors and those elsewhere in the retina. The most obvious difference between the foveal and extrafoveal retina is the relative concentration of cone photoreceptors and the ratio of cone and rod photoreceptors. In vivo microscopy of the retina in our patients taken together with the normal anatomy37 suggests that the majority of the remaining photoreceptors visible by OCT are cones. We suspect that cone photoreceptors in the central 4 mm of the retina have physiologic differences from cones elsewhere in the retina in addition to their obvious anatomical differences.37 For example, the central cones could have much slower outer segment turnover than cones elsewhere and this difference could make them less dependent on the normal function of NPHP5, NPHP6, and other centrosomal proteins. Recent advances in noninvasive methods may allow testing of this hypothesis.38
Although there are certainly widespread retinal abnormalities in patients with NPHP5-associated LCA that would not be expected to respond to gene therapy, the persistence of a recognizable photoreceptor layer in the central retina of these patients is especially encouraging for future therapeutic interventions. For the subset of patients who develop end-stage renal disease, renal transplant is usually very successful and NPHP will not recur in a transplanted kidney.39 Inquiring about polydipsia, polyuria, and nocturia in juvenile and adolescent patients with LCA can help the molecular diagnostic laboratory to focus their initial efforts on the NPHP gene family. Similarly, the more common NPHP5 and NPHP6 sequence variations should be screened in all patients with molecularly uncharacterized LCA because a subset of them may harbor mutations in these genes without any evidence of renal disease. These patients should be followed up periodically by nephrologists familiar with inherited renal disease so that if kidney disease does develop, the patients can be cared for in a timely fashion.
Acknowledgments
Funding/Support: This research was supported by grants from the Grousbeck Family Foundation, the National Neuroscience Research Institute, an unrestricted grant from Research to Prevent Blindness, the Foundation Fighting Blindness, Hope for Vision, the Macula Vision Research Foundation, and the Howard Hughes Medical Institute.
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Nigg EA, Raff JW. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139(4):663–678. doi: 10.1016/j.cell.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 2.Cardenas-Rodriguez M, Badano JL. Ciliary biology: understanding the cellular and genetic basis of human ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151C(4):263–280. doi: 10.1002/ajmg.c.30227. [DOI] [PubMed] [Google Scholar]
- 3.Senior B, Friedmann AI, Braudo JL. Juvenile familial nephropathy with tapeto-retinal degeneration. Am J Ophthalmol. 1961;52:625–633. doi: 10.1016/0002-9394(61)90147-7. [DOI] [PubMed] [Google Scholar]
- 4.Loken AC, Hanssen O, Halvorsen S, Jolster NJ. Hereditary renal dysplasia and blindness. Acta Paediatr. 1961;50:177–184. doi: 10.1111/j.1651-2227.1961.tb08037.x. [DOI] [PubMed] [Google Scholar]
- 5.Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J Am Soc Nephrol. 2007;18(6):1855–1871. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- 6.Caridi G, Murer L, Bellantuono R, et al. Renal-retinal syndromes: association of retinal anomalies and recessive nephronophthisis in patients with homozygous deletion of the NPH1 locus. Am J Kidney Dis. 1998;32(6):1059–1062. doi: 10.1016/s0272-6386(98)70083-6. [DOI] [PubMed] [Google Scholar]
- 7.Otto E, Hoefele J, Ruf R, et al. A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am J Hum Genet. 2002;71(5):1161–1167. doi: 10.1086/344395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mollet G, Salomon R, Gribouval O, et al. The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat Genet. 2002;32(2):300–305. doi: 10.1038/ng996. [DOI] [PubMed] [Google Scholar]
- 9.Olbrich H, Fliegauf M, Hoefele J, et al. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat Genet. 2003;34(4):455–459. doi: 10.1038/ng1216. [DOI] [PubMed] [Google Scholar]
- 10.Otto EA, Loeys B, Khanna H, et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet. 2005;37(3):282–288. doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- 11.Stone EM. Leber congenital amaurosis—a model for efficient genetic testing of heterogeneous disorders. Am J Ophthalmol. 2007;144(6):791–811. doi: 10.1016/j.ajo.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 12.den Hollander AI, Koenekoop RK, Yzer S, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79(3):556–561. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schäfer T, Pütz M, Lienkamp S, et al. Genetic and physical interaction between the NPHP5 and NPHP6 gene products. Hum Mol Genet. 2008;17(23):3655–3662. doi: 10.1093/hmg/ddn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cideciyan AV, Aleman TS, Jacobson SG, et al. Centrosomal-ciliary gene CEP290/NPHP6 mutations result in blindness with unexpected sparing of photoreceptors and visual brain. Hum Mutat. 2007;28(11):1074–1083. doi: 10.1002/humu.20565. [DOI] [PubMed] [Google Scholar]
- 15.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 16.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105(39):15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector. Hum Gene Ther. 2008;19(10):979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fingert JH, Eliason DA, Phillips NC, Lotery AJ, Sheffield VC, Stone EM. Case of Stargardt disease caused by uniparental isodisomy. Arch Ophthalmol. 2006;124(5):744–745. doi: 10.1001/archopht.124.5.744. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson SG, Cideciyan AV, Aleman TS, et al. Photoreceptor layer topography in children with Leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci. 2008;49(10):4573–4577. doi: 10.1167/iovs.08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson SG, Aleman TS, Sumaroka A, et al. Disease boundaries in the retina of patients with Usher syndrome caused by MYO7A gene mutations. Invest Ophthalmol Vis Sci. 2009;50(4):1886–1894. doi: 10.1167/iovs.08-3122. [DOI] [PubMed] [Google Scholar]
- 22.Aleman TS, Cideciyan AV, Sumaroka A, et al. Retinal laminar architecture in human retinitis pigmentosa caused by Rhodopsin gene mutations. Invest Ophthalmol Vis Sci. 2008;49(4):1580–1590. doi: 10.1167/iovs.07-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aleman TS, Soumittra N, Cideciyan AV, et al. CERKL mutations cause an autosomal recessive cone-rod dystrophy with inner retinopathy. Invest Ophthalmol Vis Sci. 2009;50(12):5944–5954. doi: 10.1167/iovs.09-3982. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson SG, Aleman TS, Cideciyan AV, et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: prerequisite for human gene therapy success. Proc Natl Acad Sci U S A. 2005;102(17):6177–6182. doi: 10.1073/pnas.0500646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Cideciyan AV, Papastergiou GI, et al. Relation of optical coherence tomography to microanatomy in normal and rd chickens. Invest Ophthalmol Vis Sci. 1998;39(12):2405–2416. [PubMed] [Google Scholar]
- 26.Cideciyan AV, Swider M, Aleman TS, et al. Reduced-illuminance autofluorescence imaging in ABCA4-associated retinal degenerations. J Opt Soc Am A Opt Image Sci Vis. 2007;24(5):1457–1467. doi: 10.1364/josaa.24.001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrera W, Aleman TS, Cideciyan AV, et al. Retinal disease in Usher syndrome III caused by mutations in the clarin-1 gene. Invest Ophthalmol Vis Sci. 2008;49(6):2651–2660. doi: 10.1167/iovs.07-1505. [DOI] [PubMed] [Google Scholar]
- 28.Gibbs D, Cideciyan AV, Jacobson SG, Williams DS. Retinal pigment epithelium defects in humans and mice with mutations in MYO7A: imaging melanosome-specific autofluorescence. Invest Ophthalmol Vis Sci. 2009;50(9):4386–4393. doi: 10.1167/iovs.09-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobson SG, Aleman TS, Cideciyan AV, et al. Leber congenital amaurosis caused by Lebercilin (LCA5) mutation. Mol Vis. 2009;15:1098–1106. [PMC free article] [PubMed] [Google Scholar]
- 30.Khanna H, Davis EE, Murga-Zamalloa CA, et al. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet. 2009;41(6):739–745. doi: 10.1038/ng.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keilhauer CN, Delori FC. Near-infrared autofluorescence imaging of the fundus. Invest Ophthalmol Vis Sci. 2006;47(8):3556–3564. doi: 10.1167/iovs.06-0122. [DOI] [PubMed] [Google Scholar]
- 32.Harris PC. Genetic complexity in Joubert syndrome and related disorders. Kidney Int. 2007;72(12):1421–1423. doi: 10.1038/sj.ki.5002577. [DOI] [PubMed] [Google Scholar]
- 33.Stalder L, Mühlemann O. The meaning of nonsense. Trends Cell Biol. 2008;18(7):315–321. doi: 10.1016/j.tcb.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Frank V, den Hollander AI, Brüchle NO, et al. Mutations of the CEP290 gene encoding a centrosomal protein cause Meckel-Gruber syndrome. Hum Mutat. 2008;29(1):45–52. doi: 10.1002/humu.20614. [DOI] [PubMed] [Google Scholar]
- 35.Kotzot D. Maternal uniparental disomy 7 and Silver-Russell syndrome: clinical update and comparison with other subgroups. Eur J Med Genet. 2008;51(5):444–451. doi: 10.1016/j.ejmg.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Thompson DA, McHenry CL, Li Y, et al. Retinal dystrophy due to paternal isodisomy for chromosome 1 or chromosome 2, with homoallelism for mutations in RPE65 or MERTK, respectively. Am J Hum Genet. 2002;70(1):224–229. doi: 10.1086/338455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292(4):497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 38.Jonnal RS, Besecker JR, Derby JC, et al. Imaging outer segment renewal in living human cone photoreceptors. Opt Express. 2010;18(5):5257–5270. doi: 10.1364/OE.18.005257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamiwka LA, Midgley JP, Wade AW, Martz KL, Grisaru S. Outcomes of kidney transplantation in children with nephronophthisis: an analysis of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Registry. Pediatr Transplant. 2008;12(8):878–882. doi: 10.1111/j.1399-3046.2008.00942.x. [DOI] [PubMed] [Google Scholar]