Abstract
Introduction: Severe sepsis is a leading cause of non-coronary death in hospitals across the United States. Early identification and risk stratification in the emergency department (ED) is difficult because there is limited ability to predict escalation of care. In this study we evaluated if a sustained shock index (SI) elevation in the ED was a predictor of short-term cardiovascular collapse, defined as vasopressor dependence within 72 hours of initial presentation.
Methods: Retrospective dual-centered cross-sectional study using patients identified in the Yale-New Haven Hospital Emergency Medicine sepsis registry.
Results: We included 295 patients in the study with 47.5% (n=140) having a sustained SI elevation in the ED. Among patients with a sustained SI elevation, 38.6% (54 of 140) required vasopressors within 72 hours of ED admission contrasted to 11.6% (18 of 155) without a sustained SI elevation (p=0.0001; multivariate modeling OR 4.42 with 95% confidence intervals 2.28–8.55) . In the SI elevation group the mean number of organ failures was 4.0 ± 2.1 contrasted to 3.2 ± 1.6 in the non-SI elevation group (p=0.0001).
Conclusion: ED patients with severe sepsis and a sustained SI elevation appear to have higher rates of short-term vasopressor use, and a greater number of organ failures contrasted to patients without a sustained SI elevation. An elevated SI may be a useful modality to identify patients with severe sepsis at risk for disease escalation and cardiovascular collapse.
INTRODUCTION
Severe sepsis and septic shock are the 10th leading cause of death in the United States (U.S.) with mortality rates ranging from 28–50%.1,2 Over the past several decades the incidence of each has progressively increased3,4 with roughly two thirds of patients initially presenting to the emergency department (ED).2,5 Of the 2.3 million annual visits to U.S. EDs, severe sepsis represents about 1–3% of all people presenting with an infectious disease related illness6,7 and accounts for 1 in 10 admissions to the intensive care unit,8 culminating in healthcare costs around 16.7 billion dollars per year.2
Currently for emergency physicians (EP) or other healthcare providers responsible for initial management, there are limited modalities to risk-stratify patients at risk for short-term cardiovascular collapse and escalation of disease (i.e. vasopressor dependence). The shock index (SI, heart rate divided by systolic blood pressure)9 is a simple formula useful for detecting changes in cardiovascular performance before the onset of systemic hypotension.10–17 A SI elevation greater than 0.8 has a reported 95% sensitivity for predicting shock.10 It is an easily accessible, non-invasive, and non-costly risk stratification tool that may enhance current EP methods for differentiating severe sepsis patients at risk for imminent cardiovascular collapse. While several studies have evaluated the initial SI value upon presentation to the ED,9,11,16 no studies to our knowledge have evaluated the influence of a sustained SI elevation in any clinical environment.
The objective of this preliminary study is to evaluate the role of a sustained SI elevation as a predictor of short-term cardiovascular collapse, defined as vasopressor dependence within 72 hours of ED initial presentation. We hypothesize that severe sepsis patients with a sustained SI elevation greater than 0.8 are at greater risk for short-term vasopressor use, and, that a sustained SI elevation is one instrument that may help to risk stratify patients with severe sepsis at risk for progression to shock.
METHODS
Study Setting and Design
The study was performed at a dual-site teaching hospital ED with nearly 100,000 patient visits annually. It was a retrospective cross-sectional study using patients identified prospectively in the Yale-New Haven Hospital Emergency Medicine sepsis registry. The study was approved by the Yale Human Investigation Committee for the review of medical records by study personnel. The registry is comprised of a patient list created between July 1, 2005, and July 31, 2007. In a systematic and standardized fashion, we prospectively and consecutively identified sepsis registry patients during pre-defined time periods at 2 EDs as a quality improvement initiative tracking sepsis outcomes (i.e., short term mortality) and quality measures (i.e.–lactate measurement, time to antibiotics, implementation of EGDT) for ED patients in the Yale Health System. Over the 2-year time period there were 189,867 cumulative visits at both sites (155,757 patient visits in the Adult Section of the Yale-New Haven Hospital ED; 34,110 visits at the Shoreline Medical Center ED). We screened 5,228 patients, generating a list of 359 septic patients in the registry over the cited time period.
Study Population and Measurements
Inclusion criteria for the study were as follows: at least 18 years of age, fulfillment of at least 2 of the 4 systemic inflammatory response syndrome (SIRS) criteria18, a documented clinical source of infection, and fulfillment of at least one type of organ dysfunction (i.e.–severe sepsis) at the time of presentation to the ED.
We defined end-organ dysfunction19–21 as having at least one of the following: transient hypotension defined as at least one documented systolic blood pressure reading below 90 mmHg in the ED; lactate level greater than 2.0 mmol/mL; unexplained acidosis defined as either an arterial blood gas pH below 7.35 or serum bicarbonate below 21 mg/dl; documented change in mental status from baseline; a serum platelet count less than 150,000/mm3 with no history of prior thrombocytopenia; a total bilirubin elevation greater than 1.2 mg/dl in the absence of underlying chronic liver disease; an elevation of serum coagulation factors in the absence of chronic liver disease or anticoagulant medications (PT >12sec, PTT>45 sec, INR>1.8); evidence of acute kidney injury defined as a serum creatinine increase above 0.5 mg/dl from baseline or greater than 1.2 mg/dl if no baseline was available; documented hypoxemia with at least one oxygen saturation less than 90%, or an elevated serum troponin above 0.04 mg/dl. We calculated cumulative organ dysfunction scores (i.e., Physiology and Chronic Health Evaluation II [APACHE II], Mortality in Emergency Department Sepsis [MEDS] score) from physiologic parameters and laboratory results acquired in the ED.
We excluded patients from the study if they were discharged to home or to another facility from the ED, if they arrived at the hospital in extremis (defined as having an initial systolic blood pressure less than 80 mmHg and being administered a vasopressor medication within 15 minutes of arrival to the ED), or if they had a pre-existing advance directive for the implementation of comfort care measures prior to ED presentation.
We calculated the SI for each set of vital signs that was documented until admission from the ED or the initiation of a vasopressor in the ED. The percentage of SI elevation for each patient was determined by taking the total number of SI values greater than 0.8 and dividing this number by the total number of vital signs taken. We then used this calculation to estimate the total percentage of time that each patient maintained a SI elevation in the ED. The sustained SI elevation group was defined as having a SI greater than 0.8 for at least 80% of the ED vital sign measurements. The non-sustained SI elevation group was defined as having a SI greater than 0.8 for less than 80% of the vital sign measurements in the ED. As an alternative analysis, we further sub-divided patients based on the total percentage of time each patient had an elevated SI. We also looked at the initial SI for all study patients and compared it to our outcomes of short-term vasopressor use and hospital mortality.
Study Protocol and Measurements
Data were extracted in a standardized and systematic fashion22 from medical records by two medical students (MW, SB) under the supervision of a faculty investigator (CW) with internal procedures to ensure extraction accuracy >90%. Medical students used a customized data collection form and glossary of terms to extract pre-defined demographical and clinical data points. Both electronic and paper records were used for abstraction. We obtained electronic records using MD Link™, Sunrise Clinical Manager™ and Lynx Medical Systems.™ All hard-copy medical charts were reviewed in the Yale-New Haven Hospital office of medical records. Collected data were transcribed from data collection forms into a customized Microsoft Excel database created by the faculty investigator. Patient subjects were randomly assigned study ID numbers to protect personal health information according to guidelines established by our Human Investigation Committee. We conducted weekly meetings to review extracted data and to ensure internal consistency in data extraction. To demonstrate internal accuracy in data extraction, the two medical students collected 551 overlapping data points with 95% agreement.
Data Analysis
Investigators performed statistical analysis in consultation with a statistical consultant from the Yale Department of Emergency Medicine. In performing a 2-tailed post-hoc power calculation (using a Type I error rate of 5%, the total sample size of 295–140 in the sustained SI group; 155 in the group without a sustained SI elevation) we calculated that our study has 100% statistical power, and that it has the appropriate sample size to detect a 12% difference between each group (80% power threshold). Continuous data were reported as the mean and standard deviation. We performed a comparison of means using an unpaired t-test. Fisher's exact test was used to compare groups with categorical variables, including the rates of vasopressor use among patients with a sustained SI elevation contrasted to those without a sustained SI elevation. Statistical significance was indicated by a p-value (or alpha error) <0.05. To perform statistical analyses', investigators initially used Graph Pad Quick Calcs, GraphPad Software, (San Diego California USA, www.graphpad.com). Multivariate modeling was performed by a Department of Emergency Medicine faculty member and statistical expert who adjusted for potential confounding variables using SAS software (Cary, NC).
RESULTS
Of the 359 patients identified in the Yale sepsis registry, 82.2% (n=295) met study inclusion criteria. Of the 64 patients excluded from the study, no patients were excluded for age, 7.8% (n=5) were excluded for being in extremis at presentation, 12.5% (n=8) were excluded for having pre-existing comfort measures prior to ED presentation, 23.4% (n=15) were discharged to home or to a facility from the ED, and 57.8% (n=37) were excluded for having fewer than 2 SIRS criteria in the ED or no evidence of end organ dysfunction.
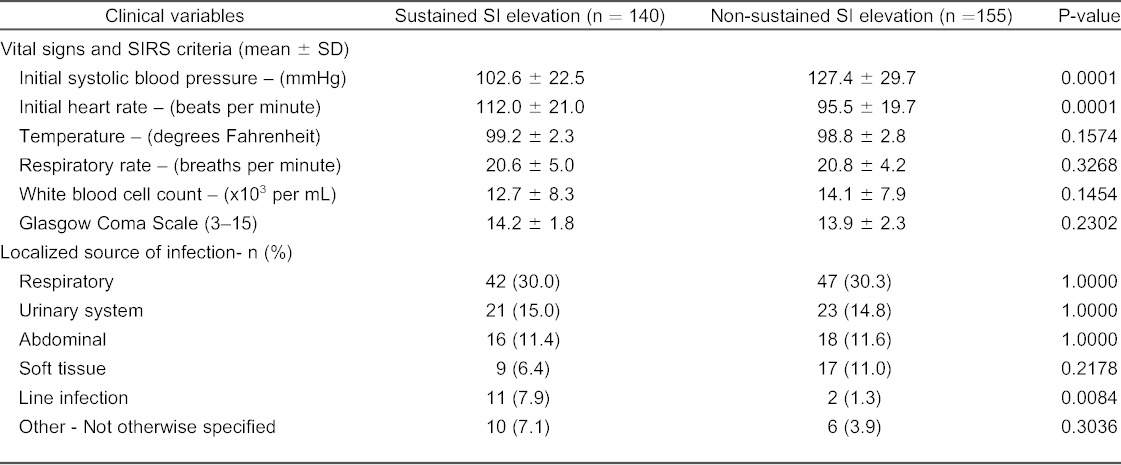
In our cumulative sample, 47.4% (n=140) patients had a sustained SI elevation. Forty-eight percent (142 of 295) were female and the mean age at presentation for all patients was 62.5 ±18.5 years (Table 1). Of the 16 co-morbid conditions reviewed, patients with a sustained SI were less likely to have a history of coronary artery disease (19.3 versus 30.3%, p=0.0319) and hypertension (40.7 versus 61.9 %, p=0.0003). Patients in the sustained SI elevation group had a lower initial systolic blood pressure (102.6 ± 22.5 versus 127.4 ± 29.7 mmHg, p<0.0001), and higher initial heart rate (112 ± 21.0 versus 96 ± 19.7 beats per minute, p<0.0001, Table 2).
Table 1.
Baseline characteristics comparing patients with and without a sustained shock index (SI) elevation in the emergency department.

Table 2.
Vital signs, systemic inflammatory response syndrome (SIRS) criteria, and localized source of infection in patients with a sustained shock index (SI) elevation and patients without a sustained SI elevation. Results are either reported as the mean ± SD, or the absolute number (%) of patients with a specific source of infection.
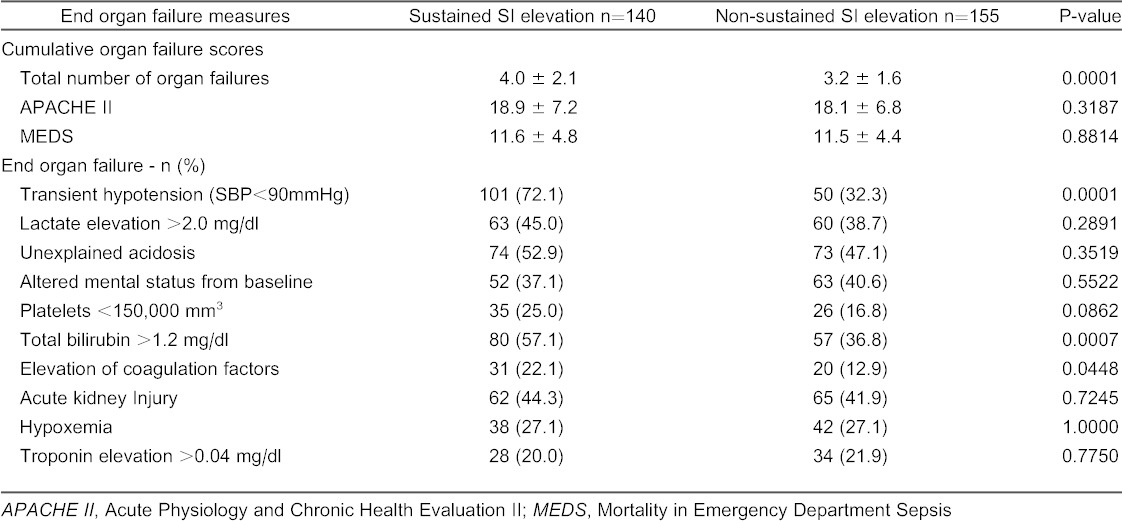
The mean number of organ dysfunctions at initial presentation was greater in patients with a sustained elevated SI (4.0 ± 2.1 versus 3.2 ± 1.6, p=0.0001) compared to those without a sustained SI (Table 3). In contrast, there was no difference in the mean APACHE II (18.8 ± 7.2 versus 18.1 ± 6.8, p=0.3187) or MEDS score (11.6 ± 4.8 versus 11.5 ± 4.4, p=0.88 between patients with a sustained elevated SI compared to those without.
Table 3.
End-organ dysfunction in patients with a sustained shock index (SI) elevation versus a non-sustained SI elevation.
Initial laboratory data showed no difference between groups except that there were higher initial lactate levels (2.8 ± 2.6 versus 2.3 ± 1.7 mmol/dl, p=0.0426) in the sustained SI elevation group. Patients with a sustained SI elevation were more likely to receive blood products in the ED, and they also received a greater amount of crystalloid fluid volume resuscitation (3.6 ± 2.4 versus 2.7 ± 3.9 liters, p=0.0267). 42.1% (n=59) with a sustained SI elevation underwent central line placement in the ED compared to 27.1% (n=42) without a sustained SI elevation (p<0.005).
There was no difference in the mean ED length of stay between the sustained SI elevation group and the non-sustained SI elevation group respectively: the time in the ED was 6:27 hours ± 3:48 hours versus 7:07 hours ± 4:18 hours (p=0.287). In our total sample, 24.4% (72 of 295) received a vasopressor agent within 72 hours of initial presentation. Of the 140 patients with a sustained SI elevation, 38.6% (n=54) were placed on vasopressors within 72 hours of presentation, compared to 11.6% (n=18) of the 155 patients who did not have a sustained SI elevation (p=0.0001). The proportion of patients placed on vasopressors appeared to be directly related to the total percentage of time patients had a shock index elevation (Figure). Multivariate modeling with correction for potential confounding variables resulted in an OR of 4.42 for vasopressor use within 72 hours among patients with a sustained SI elevation (95% CI 2.28 to 8.55).
Figure.

The rate of vasopressor use within 72 hours in relation to the total percentage of emergency department vital sign measurements with an elevated shock index.(*P<0.05 when the 80–100% group is compared to all other groups).
In the initial ED presentation, 83.0% (n=245) had an early systolic blood pressure greater than 100 mmHg defined as having at least one of the first 3 systolic blood pressure measurements greater than 100 mmHg. In this subgroup, there was also a significant difference in rates of 72-hour vasopressor use between patients with a sustained SI elevation (n=98) compared to those without a sustained SI elevation (n=147)–30.6% versus 8.2% respectively, (p<0.0001).
In further analyses', the isolated initial shock index elevation did not correlate with rates of vasopressor use, inhospital mortality, or ICU admission. Overall, for our total sample the in-hospital 28-day mortality rate was 15.6% (46 of 295). Patients with a sustained elevated SI had a 19.3% (n=27) mortality, compared to 12.3% (n=19) in patients who did not have an elevated SI (p=0.1093). Regardless of SI elevation, all patients placed on vasopressors within 72 hours had higher 28-day mortality rates compared to those who did not receive vasopressors (41.7% versus 7.2%, p=0.0001).
DISCUSSION
The SI was first described in the 1960s as the ratio of heart rate to systolic blood pressure.23 While it was originally designed to identify apparently stable yet critically ill trauma patients, the SI has since been shown to be a simple, non-invasive risk stratification tool useful for detecting changes in cardiovascular performance before the onset of systemic hypotension and cardiorespiratory collapse.11 Since its original description, the SI has been evaluated for this purpose in patients with cardiogenic shock,12 sepsis,13 ectopic pregnancy,14 gastro-intestinal hemorrhage,24 and pulmonary embolism.15
In a precursor study,13 Rady et al. looked at patients with apparently stable vital signs and divided them into 2 groups based on whether the patient had a SI elevation. The study associated an elevated SI with higher admission rates to hospital floor beds and to ICUs, as well as poorer outcomes. Authors concluded that when used alone, an elevated SI was more sensitive than using heart rate or systolic blood pressure alone to predict the severity of illness, and had a higher specificity.
What is considered an abnormal SI elevation? The reported range in the literature is between 0.8 to 1.0.10,13,25 One study from Mexico showed an improvement in sensitivity to 95% when using a SI of 0.8, although their population evaluated surgical patients and did not focus specifically on sepsis.10 Given the variable definition of an elevated SI, there is no established cut-off for an elevated SI above normal (0.5–0.7) that has been routinely applied to critical care literature.
Several areas of focus differentiate our study from others. This is the first study to our knowledge evaluating the impact of a sustained SI elevation. All prior studies evaluating the SI have evaluated single, isolated, initial values rather than taking into account the trajectory of change once fluid resuscitation, antibiotics, and other therapies are instituted. Many have suggested that the SI is potentially a macro-endpoint to resuscitation—but to our knowledge this has also never been studied. We believe there is great merit for the EP using the simple, non-costly, and non-invasive SI measurements to risk stratify patients who are at risk for potential cardiovascular collapse. Also, the sustained SI elevation is something that could be incorporated into future scoring systems aimed at differentiating patients at risk for decompensation versus those that aren't. Furthermore, given the pressure to reduce healthcare costs—a non-costly risk stratification tool for determining which patients truly need the costly resources of an ICU admission is needed.
What further differentiates our research is that while prior studies evaluating the SI have used hospital admission and mortality rates as primary endpoints, we used the endpoint of short-term vasopressor dependence to represent escalation of disease because, for initial providers, progression to vasopressor dependence is a more relevant outcome measure contrasted to overall 28-day mortality. Furthermore, given that the SI is a hemodynamic variable, we believed that short-term vasopressor use was a good marker of hemodynamic decompensation. Although it is still unclear whether the SI is a useful tool when used alone to aid EPs in treatment decisions and triage13,25, it may prove useful in combination with predictors of illness severity and other information routinely available to practitioners.
In this study we identified that a sustained SI elevation in the ED was indeed associated with higher rates of short-term vasopressor use. We also observed a potential relationship between the total percentage of time that patients maintained a SI elevation in the ED and vasopressor use, suggesting that the longer a patient maintains an elevated SI, the more likely they are to require vasopressors within 72 hours.
Similar to the Jones et al study25 we did not find discerning value predictive of outcome when looking only at the initial SI value. There was no difference in vasopressor use or mortality between patients with an initial SI elevation contrasted to those without. Thus, from our data we surmise that a sustained SI elevation may be a more useful measure for risk stratification rather than a single initial SI elevation.
We also found that patients with a sustained SI elevation had a higher mean number of organ failures than those without a sustained elevated SI (4.0 versus 3.1, p=0.0001), although there was no difference in APACHE II and MEDS score. This observation does suggest, however, that a sustained SI elevation may serve to identify patients with a potentially greater number of organ failures in the ED, and, could prove to be valuable in clinical settings where laboratory turn-around times for results may approach 60 to 90 minutes.
Looking at other initial vital signs as a predictor of vasopressor use, we found that the initial systolic blood pressure of patients who were placed on vasopressors was lower than those who were not placed on vasopressors (101 versus 120 mmHg). There was also a difference in systolic blood pressure between patients who had a sustained SI elevation and those who did not (102 v. 127 mmHg). While common sense suggests that patients with lower systolic blood pressures would have higher rates of vasopressor use, we identified several points underscoring the potential value of also looking at the SI as a predictor of disease progression. First, nearly 60 percent of the patients with a sustained SI had an initial SBP>100 mmHg. This finding suggests that a proportion of patients with normal blood pressures and an elevated SI may be at risk for hemodynamic decompensation. Second, the non-sustained SI group had a proportion of patients who were hypertensive in the ED, thus skewing the blood pressure comparison between groups (systolic blood pressure >170 mmHg, n=14, range 170 to 224 mmHg). Third, when we performed a sub-group analysis on patients with an early systolic blood pressure above 100 mmHg (n=245), we still found a significant difference in vasopressor use between patients with a sustained SI elevation and those who did not have a sustained SI (30.6% versus 8.2%, p<0.0001). Thus, our data suggest that the SI is potentially a valuable and non-costly marker to enhance existing methods to risk stratify septic patients.
LIMITATIONS
General limitations of this study were retrospective data extraction and a relatively small sample size. Additionally, patient selection came from a registry that was not all-inclusive but did implement procedures (i.e., prospective and consecutive enrollment during predefined time periods) to reduce inclusion or selection bias. There was temporal variability, and variability in the total number of vital sign measurements performed in the ED by the initial providers –the mean number of ED vital sign measurements in our total patient population was 8.06 ± 4.38, but in a separate analysis this did not depend upon normal or abnormal values. The retrospective data extraction was limited by many factors inherent to the process, including possible errors in the medical record. The 95% accuracy among the 2 data extractors confirmed overall accuracy.
CONCLUSIONS
ED patients with severe sepsis and a sustained SI elevation appear to have higher rates of short-term vasopressor use contrasted to patients without a sustained SI elevation. A sustained SI elevation may be a promising simple, cost-efficient, and non-invasive measurement to help risk stratify patients who present to the ED with severe sepsis, and may complement other predictors of disease progression. A sustained SI elevation may be a useful modality to identify patients with severe sepsis at risk for disease progression.
Footnotes
Supervising Section Editor: Joel M. Schofer, MD
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
- 1.Hoyert DL, Arias E, Smith BL et al. National Vital Statistics Reports [serial online], 21 September 2001. [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Friedman G, Silva E, Vincent JL. Has the mortality of septic shock changed with time? Crit Care Med. 1998;26:2078–2086. doi: 10.1097/00003246-199812000-00045. [DOI] [PubMed] [Google Scholar]
- 4.Martin GS, Mannino DM, Eaton S et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 5.McCaig LF, Burt CW. National hospital ambulatory medical care survey: 2003 emergency department survey. Adv Data. 2005:1–38. [PubMed] [Google Scholar]
- 6.Ho BC, Bellomo R, McGain F et al. The incidence and outcome of septic shock patients in the absence of early-goal directed therapy. Crit Care. 2006;10:R80. doi: 10.1186/cc4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Shapiro N, Angus D et al. National estimates of severe sepsis in the United States emergency departments. Crit Care Med. 2007;35:1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 8.Angus DC, Pereira CA, Silva E. Epidemiology of severe sepsis around the world. Endocr Metab Immune Disord Drug Targets. 2006;6:207–212. doi: 10.2174/187153006777442332. [DOI] [PubMed] [Google Scholar]
- 9.Rady MY. The role of central venous oximetry, lactic acid concentration and shock index in the evaluation of clinical shock: a review. Resuscitation. 1992;24:55–60. doi: 10.1016/0300-9572(92)90173-a. [DOI] [PubMed] [Google Scholar]
- 10.Catellanos J, Martin L, Pineda ML, Revista Sensitivity and specificity of the shock index in the diagnosis of shock from intraperitoneal hemorrhage in patients with abdominal contusion. Revista Cubana De Medicina Intensiva y Emergencias. 2005;5:1–10. [Google Scholar]
- 11.Rady MY. The role of central venous oximetry, lactic acid concentration and shock index in the evaluation of clinical shock: a review. Resuscitation. 1992;24:55–60. doi: 10.1016/0300-9572(92)90173-a. [DOI] [PubMed] [Google Scholar]
- 12.Oestern HJ, Trentz O, Hempelmann G et al. Cardiorespiratory and metabolic patterns in multiple trauma patients. Resuscitation. 1979;7:169–183. doi: 10.1016/0300-9572(79)90024-8. [DOI] [PubMed] [Google Scholar]
- 13.Rady MY, Rivers EP, Nowak RM. Resuscitation of the critically ill in the ED: responses of blood pressure, heart rate, shock index, central venous oxygen saturation. Am J Emerg Med. 1996;14:218–225. doi: 10.1016/s0735-6757(96)90136-9. [DOI] [PubMed] [Google Scholar]
- 14.Birkhahn RH, Gaeta TJ, Bel R et al. Shock index in the first trimester of pregnancy and its relationship to ruptured ectopic pregnancy. Acad Emerg Med. 2002;9:115–119. doi: 10.1111/j.1553-2712.2002.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 15.Kline JA, Nelson RD, Jackson RE et al. Criteria for the safe use of D-dimer testing in emergency department patients with suspected pulmonary embolism: a multicenter US study. Ann Emerg Med. 2002;39:144–152. doi: 10.1067/mem.2002.121398. [DOI] [PubMed] [Google Scholar]
- 16.Rady MY, Smithline HA, Blake H et al. A comparison of the shock index and conventional vital signs to identify acute, critical illness in the emergency department. Ann Emerg Med. 1994;24:685–690. doi: 10.1016/s0196-0644(94)70279-9. [DOI] [PubMed] [Google Scholar]
- 17.Rady MY. Triage and resuscitation of critically ill patients in the emergency department: current concepts and practice. Eur J Emerg Med. 1994;1:175–189. [PubMed] [Google Scholar]
- 18.Levy M et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conf. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 19.Marshall JC, Cook DJ, Christou NV et al. Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira FL, Bota DP, Bross A et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2002;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 21.Mehta NJ, Khan IA, Gupta V et al. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol. 2004;95:13–17. doi: 10.1016/j.ijcard.2003.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert EH, Lowenstein SR, Koziol-McLain J et al. Chart reviews in emergency medicine research: where are the methods? Ann Emerg Med. 1996;27:305–308. doi: 10.1016/s0196-0644(96)70264-0. [DOI] [PubMed] [Google Scholar]
- 23.Allgower M, Burri C. Shock Index. Ger Med Mon. 1968;12:14–19. [PubMed] [Google Scholar]
- 24.Nakasone Y, Ikeda O, Yamashita Y et al. Shock index correlates with extravasation on angiographs of gastrointestional hemorrhage: a logistics regression analysis. Cardiovasc Intervent Radiol. 2007;30:861–865. doi: 10.1007/s00270-007-9131-5. [DOI] [PubMed] [Google Scholar]
- 25.Jones A, Aborn L, Kline J. Severity of Emergency Department Hypotension predicts Adverse Hospital Outcome. Shock. 2004;22:410–414. doi: 10.1097/01.shk.0000142186.95718.82. [DOI] [PubMed] [Google Scholar]


