Current surgical therapy involving partial or complete meniscectomy relieves pain in the short-term but often leads to osteoarthritis in the long-term. Results show that intra-articular injection of human meniscus stem/progenitor cells enhanced meniscus regeneration through the stromal cell-derived factor-1/CXCR4 axis. This is a new strategy of intra-articular injection of human meniscus stem/progenitor cells for meniscus regeneration.
Keywords: Meniscus injury, Osteoarthritis, Meniscus-derived stem/progenitor cells, Cell transplantation, SDF-1/CXCR4 axis, Regeneration
Abstract
Meniscus injury is frequently encountered in clinical practice. Current surgical therapy involving partial or complete meniscectomy relieves pain in the short-term but often leads to osteoarthritis (OA) in the long-term. In this study, we report a new strategy of articular cartilage protection by intra-articular injection of novel human meniscus stem/progenitor cells (hMeSPCs). We found that hMeSPCs displayed both mesenchymal stem cell characteristics and high expression levels of collagen II. In the rat meniscus injury model, hMeSPC transplantation not only led to more neo-tissue formation and better-defined shape but also resulted in more rounded cells and matured extracellular matrix. Stromal cell-derived factor-1 (SDF-1) enhanced the migration of hMeSPCs, whereas AMD3100 abolished the chemotactic effects of SDF-1 on hMeSPCs, both in vitro and in vivo. In an experimental OA model, transplantation of hMeSPCs effectively protected articular cartilage, as evidenced by reduced expression of OA markers such as collagen I, collagen X, and hypoxia-inducible factor 2α but increased expression of collagen II. Our study demonstrated for the first time that intra-articular injection of hMeSPCs enhanced meniscus regeneration through the SDF-1/CXCR4 axis. Our study highlights a new strategy of intra-articular injection of hMeSPCs for meniscus regeneration.
Introduction
Tearing of the meniscus frequently occurs during athletic injuries to the knee [1]. As meniscus possesses poor self-regenerative capacity upon injury [2], meniscectomy is currently the most common treatment modality [3]. However, meniscectomy is associated with complications that may ultimately lead to osteoarthritis (OA) [4–8]. Hence, it is imperative to repair meniscal tears rather than perform surgical excision [9–12].
Over the last two decades, tissue-engineering strategies have emerged to repair injured meniscus [13, 14]. To date, various cell types have been examined in meniscus tissue engineering such as bone marrow mesenchymal stem cells (B-MSCs), synovium MSCs (S-MSCs), meniscal fibrochondrocytes, and chondrocytes [15–19]. However, these cell sources are associated with donor site morbidity. Moreover, the outcomes are unsatisfactory with these cell types because of poor proliferative capacity and ossification [20]. Therefore, a new source of seed cells is needed to promote the regeneration of injured meniscus. A recent study showed that multipotent stem cells could be present in the meniscus [21, 22]. This finding raises the possibility of using meniscus-derived stem/progenitor cells for meniscal regeneration in vivo. However, the effect of human meniscus-derived stem/progenitor cells (hMeSPCs) on meniscus regeneration and OA prevention in vivo and the mechanisms involved in the migration of injected hMeSPCs have not yet been reported.
Systemically transplanted mesenchymal stem cells (MSCs) have been previously shown to migrate to injured tissues and contribute to tissue regeneration [23]. The migration of MSCs is mediated by stromal cell-derived factor-1 (SDF-1) that is upregulated at injury sites and its cognate receptor CXCR4 expressed on the migratory cells [24–29]. However, it is still unknown whether the SDF-1/CXCR4 chemokine axis will facilitate the migration of intra-articular injected MeSPCs to promote meniscus repair in vivo. In this study, we demonstrated that intra-articular injection of hMeSPCs enhances meniscus regeneration through SDF-1/CXCR4-mediated homing, underscoring its potential use for meniscus repair in the clinic.
Materials and Methods
Monoclonal Selection of hMeSPCs
Human meniscus tissue was digested with collagenase (3 mg/ml) for 6 hours. The cells from the digested tissue were cultured in low-glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with penicillin-streptomycin and 20% (vol/vol) fetal bovine serum. After passage 0, they were seeded at a very low density to form colonies on 6-cm dishes (300 cells in one dish). The putative cells isolated from human meniscus were designated as hMeSPCs [30]. All hMeSPCs in this study were of polyclonal origin. hMeSPCs between passages 1 and 3 were used for all experiments.
Flow Cytometry
Cells (5 × 105) were incubated with 1 μg phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated mouse antibodies specific to human cell surface markers, including CD34 (FITC), CD90 (FITC), CD44 (PE), CD45 (FITC), and CD166 (FITC) for 1 hour at 4°C. The nonconjugated mouse-specific antibody to human CD105 was incubated with 1 × 106 cells for 1 hour at 4°C. After washing, the cells were incubated with FITC-conjugated rabbit anti-mouse immunoglobulin G for 45 minutes on ice and analyzed using a Coulter Epics XL flow cytometer.
Evaluation of Multipotent Differentiation Potential of hMeSPCs
The multipotent differentiation potential of hMeSPCs toward the adipogenic, osteogenic, and chondrogenic lineages was evaluated in vitro according to established protocols [30]. Positive induction of adipogenesis, osteogenesis, and chondrogenesis was confirmed by Oil Red O staining, alkaline phosphatase staining, and safranin O staining, respectively.
Colony Formation Assay and Expression Analysis of Meniscus-Related Genes by MeSPCs, S-MSCs, and B-MSCs
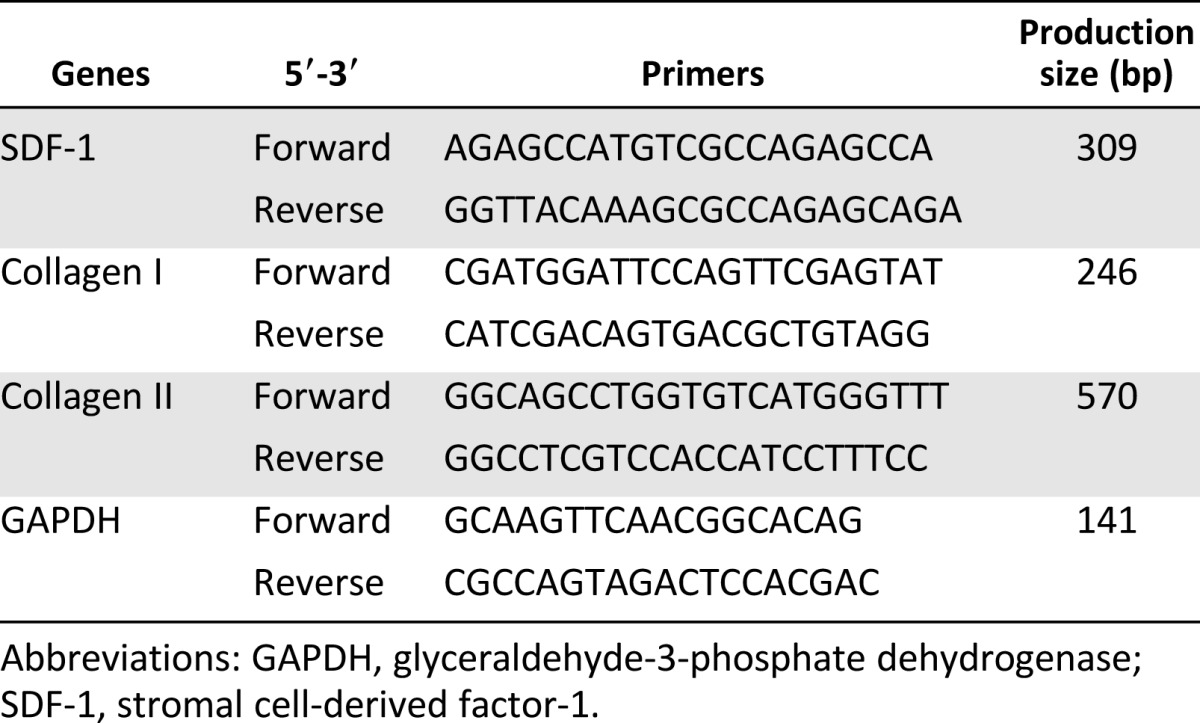
Human MeSPCs, S-MSCs, and B-MSCs were harvested from total knee arthroplasty of donors with osteoarthritis, as described previously [21, 30, 31]. Twelve days after initial seeding, some of the dishes were stained with 1% (wt/vol) crystal violet solution (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) in methanol, and the number of colonies (with diameter >2 mm) was counted. Total cellular RNA was isolated by lysis in TRIzol (Invitrogen, Carlsbad, CA, http://www.invitrogen.com), followed by a one-step phenol chloroform-isoamyl alcohol extraction, as described by the manufacturer’s protocol. Polymerase chain reaction (PCR) analysis of collagen I and collagen II expression was performed, as described previously [30], and the results are presented as target gene expression normalized to glyceraldehyde-3-phosphate dehydrogenase. The primer sequences used for PCR analysis in this study are listed in Table 1.
Table 1.
List of primer sequences used for real-time polymerase chain reaction
In Vitro Chemotaxis Assay
To determine whether recombinant human SDF-1α (rhSDF-1α) can regulate the migration of hMeSPCs, an in vitro chemotaxis assay was performed using a Transwell system (Costar 3422; Corning Inc., Corning, NY, http://www.corning.com), as described previously [27, 30]. Briefly, 1 × 10 5 cells suspended in 100 µL serum-free DMEM plus 0.5% (wt/vol) bovine serum albumin were placed within the upper chamber. To induce chemotaxis, 60 µL rhSDF-1α (200 ng/ml) in serum-free DMEM was added to the lower chamber (−/+). Additionally, to observe chemokinesis, rhSDF-1α was also added to the upper chambers (+/+). To evaluate whether rhSDF-1 promoted the migration of hMeSPCs through the SDF-1/CXCR4 axis, hMeSPCs were pretreated with AMD3100 (10 μg/ml; CXCR4-specific antagonist) for 2 hours at 37°C in some experimental groups (AMD3100 −/+). Twenty-four hours later, the upper surface of the filters was scraped free of hMeSPCs and debris. hMeSPCs that had migrated through the filter were fixed in 4% (v/v) paraformaldehyde and subjected to 4′,6-diamidino-2-phenylindole (DAPI) staining (Beyotime Institute of Biotechnology, Jiangsu, China). The total number of cells on the lower surface of the membrane was counted under light microscopy in five randomly selected fields (×400). The data were expressed as mean number of cells per high-power field (cells per HPF) ± SD, and were subsequently analyzed for statistically significant differences between experimental groups.
Expression of SDF-1 After Meniscectomy
The injured meniscus tissues were harvested from the meniscectomy groups, as well as from the sham groups (n = 3 in each time point) at 1, 2, and 3 weeks postsurgery. The mRNA expression levels of SDF-1 within injured meniscus were then analyzed, as previously described [30]. The primer sequences used in this study are listed in Table 1.
In Vivo Chemotaxis and Loss-of-Function Assay
One week after meniscectomy, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)-stained hMeSPCs (6 × 106 cells in 50 µL phosphate-buffered saline [PBS], pretreated with 10 μg/ml AMD3100 for 2 hours at 37°C) were injected into the right knee. As control, the left knee was injected with normal hMeSPCs in PBS alone. Four weeks after operation, the Kodak in vivo FX small animal imaging system was used to evaluate the migration of injected hMeSPCs within the meniscus defect. This experiment was repeated three times.
Meniscectomy and hMeSPC Injection
Six female rats weighing 200–220 g were used in this study. The rats were treated with cyclophosphamide (150 mg/kg) 24 hours before the meniscectomy. The anterior half of medial meniscus was removed at the level of the medial collateral to create a defect [18]. hMeSPCs (6 × 106 in 50 μl PBS) were injected into the right knee at 1 week and 2 weeks after meniscectomy, whereas the same volume of PBS was injected into the left knee as control.
After euthanasia, three meniscuses of rats from each experimental group were subjected to histological evaluation at the 4-week and 12-week time points. Treatment of animals was in accordance with standard guidelines approved by the Zhejiang University Ethics Committee.
Cell Labeling and Detection
The hMeSPCs used for in situ repair of meniscus were prestained with DiI/6-carboxyfluorescein diacetate (CFDA). To evaluate the survival of implanted hMeSPCs in the meniscus defect, a noninvasive Kodak in vivo FX small animal imaging system was used to analyze the samples at 4 and 12 weeks postmeniscectomy [32]. The positively stained cells within histological sections of the hMeSPC-treated group were observed under fluorescence microscopy, whereas DAPI was used to stain the cell nuclei.
Histology
Hematoxylin and eosin and safranin O staining were performed, as described previously [33]. Macroscopically, regeneration of the injured meniscus was evaluated by area assay, and the degeneration of femoral and tibial articular cartilage was evaluated directly after ink staining [34]. Histological scoring was performed, as described previously [33]. Briefly, four sections from each sample were graded blindly by three observers. Histology evaluation was performed using the International Cartilage Repair Society visual histological assessment scale, including surface, matrix, cell distribution, and depth.
Transmission Electron Microscopy
At 4 and 12 weeks postsurgery, tissue specimens from the hMeSPC-treated and control groups were fixed according to standard procedures for transmission electron microscopy (TEM) to assess the cell morphology of the regenerated meniscus [30].
Immunostaining
A series of 8-µm-thick sections were used for immunohistochemical staining. Rabbit anti-Col1 (Anbo Biotechnology Co., San Francisco, CA, http://www.anbobio.com), mouse anti-Col2 (Calbiochem, San Diego, CA, http://www.emdbiosciences.com), rabbit anti-Col10 (Abcam, Cambridge, U.K. , http://www.abcam.com), and rabbit anti-Hif-2α (Abcam), together with goat anti-mouse (Beyotime) or goat anti-rabbit (Beyotime) secondary antibodies, were used to detect the expression of these proteins within the degenerated articular cartilage [35].
Statistical Analysis
All quantitative data sets are expressed as mean ± SD. Student’s t test was performed to assess whether there were statistically significant differences in the results of different data sets, with a value of p < .05 being considered significantly different.
Results
Characterization of hMeSPCs
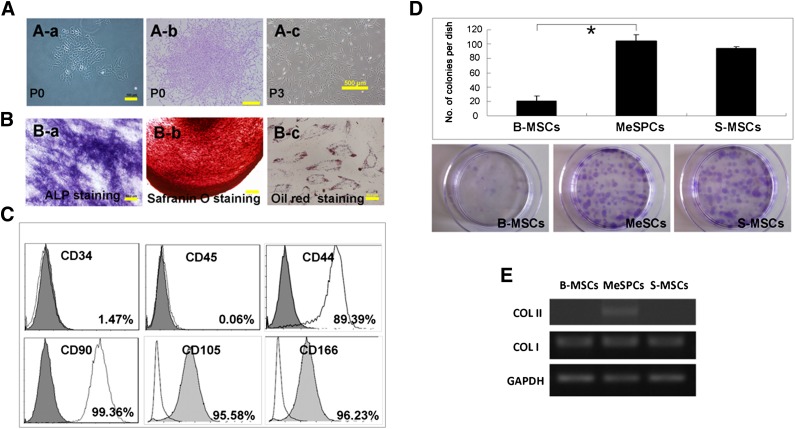
A subpopulation of meniscus cells attached and formed colonies 10–12 days after initial seeding (Fig. 1A). The colonies were heterogeneous in morphology at P0, possibly reflecting differences in cell origin from the meniscus tissue. A multipotent homogeneous population of MSC-like cells became apparent after further culture (Fig. 1B). These results confirmed the clonogenicity and multipotency of meniscus-derived cells in vitro, as previously reported [21]. Flow cytometry results showed that the cells expressed high levels of MSC markers, including CD44 (89.39%), CD90 (99.36%), CD105 (95.58%), and CD166 (96.23%), but not the hematopoietic markers CD34 (1.47%) and CD45 (0.06%) (Fig. 1C). Together, these results suggest that hMeSPCs possess stem cell properties similar to MSCs. However, MeSPCs exhibited higher clonogenicity and displayed a higher level of collagen II expression than B-MSCs (p < .05) and S-MSCs (Fig. 1D, 1E), which is indicative of their unique features.
Figure 1.
Isolation and characterization of human MeSPCs (hMeSPCs). hMeSPCs displayed defining stem cell characteristics (clonogenicity [A], multidifferentiation potential [B], and MSC surface markers [C]). (D, E): Colony formation assay (D) and expression of meniscus-related genes (COL I and COL II) (E) by MeSPCs, S-MSCs, and B-MSCs. hMeSPCs formed colonies at a higher frequency than B-MSCs (B) (p < .05) and S-MSCs. Reverse-transcriptase polymerase chain reaction showed that hMeSPCs expressed higher levels of collagen II as compared with B-MSCs and S-MSCs. Scale bars = 50 μm (Ba, Bc), 100 μm (Aa, Bb), 500 μm (Ab, Ac). ∗, p < .05 versus control group. Abbreviations: ALP, alkaline phosphatase; B-MSCs, bone marrow-derived mesenchymal stem cells; COL I, collagen I; COL II, collagen II; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MeSPCs, meniscus-derived stem/progenitor cells; P, passage; S-MSCs, synovium-derived mesenchymal stem cells.
Migration of hMeSPCs Was Mediated by SDF-1/CXCR4 In Vitro and In Vivo
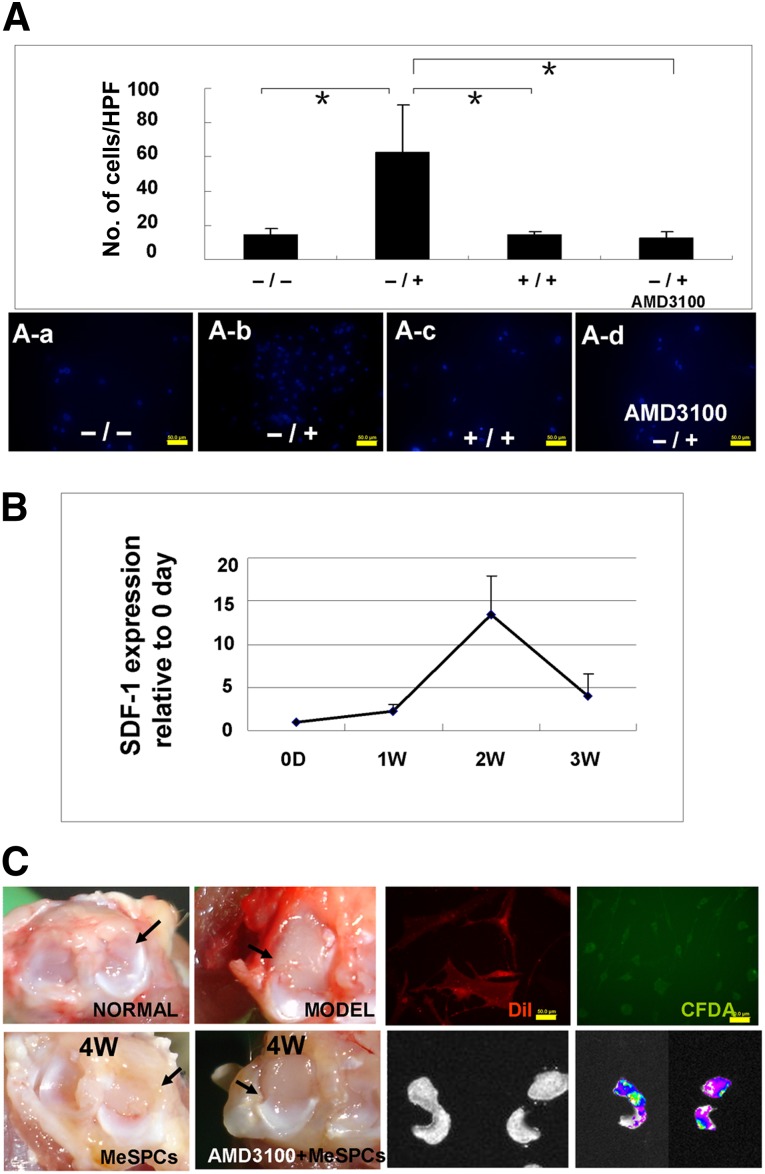
To investigate the direct influence of SDF-1 on the migration of hMeSPCs, the following in vitro and in vivo studies were performed. For the in vitro study, it was observed that SDF-1 significantly (62.79 ± 27.53 vs. 14.39 ± 3.56/HPF; p < .05; n = 3 wells per group) increased the numbers of migratory hMeSPCs on the lower surface of the membrane, as compared with the untreated control (Fig. 2Aa, 2Ab). The number of hMeSPCs that migrated in response to SDF-1 was decreased when SDF-1 was introduced into both the upper and lower chambers at the same concentrations (Fig. 2Aa–2Ac). To further investigate the effects of the SDF-1/CXCR4 chemokine axis on hMeSPC migration, AMD3100-pretreated (2 hours) hMeSPCs were placed into the upper chambers. The results showed that AMD3100 abolished the chemotactic effects of SDF-1 on hMeSPCs significantly (12.43 ± 4.08 vs. 62.79 ± 27.53/HPF; p < .01; n = 3 wells per group), suggesting that the signaling events leading to migration of hMeSPCs are transmitted through the SDF-1/CXCR4 chemokine axis (Fig. 2Aa–2Ad).
Before performing the in vivo study, we first analyzed the expression of SDF-1 in the injured meniscus at 1, 2, and 3 weeks after meniscectomy. Real-time PCR results showed that the expression of SDF-1 started to increase at 1 week after meniscectomy (Fig. 2B). Subsequently, the expression of SDF-1 was significantly increased after 2 weeks (13.42 ± 4.57 vs. 2.24 ± 0.87; p < .01) and remained elevated at 3 weeks after meniscectomy.
Figure 2.
Effects of SDF-1 on the migration of human MeSPCs (hMeSPCs). (A): Migration assay of hMeSPCs in vitro using a Transwell system. (B): Expression of SDF-1 after meniscectomy. (C): Gross morphology (left panels) and Cold electric coupling device analysis (bottom right panels) of meniscal regeneration at 4 weeks postimplantation in the MeSPC-treated group and AMD3100 + MeSPC (hMeSPCs pretreated with AMD3100)-treated group suggests that migration of hMeSPCs is mediated by the SDF-1/CXCR4 chemokine axis. hMeSPCs were labeled with DiI and CFDA before injection and traced in vivo (top right panels). Injected hMeSPCs adhered to the injured meniscus at 3 weeks after injection, whereas the adhesion of hMeSPCs was almost inhibited in the AMD3100-pretreated group (bottom panels). Black arrows show the anterior half of medial meniscus. Scale bars = 50 µm (A, C). ∗, p < .05 versus control group. Abbreviations: CFDA, 6-carboxyfluorescein diacetate; DiI, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; HPF, high power field; MeSPCs, meniscus-derived stem/progenitor cells; N, number; SDF-1, stromal cell-derived factor-1α; W, weeks.
Based on the observation that SDF-1 promoted MeSPC migration in vitro and that expression of SDF-1 was upregulated within the injured meniscus in vivo, we further investigated the effect of meniscus injury on the migration of intra-articular injected hMeSPCs in vivo through the SDF-1/CXCR4 chemokine axis. One week after meniscectomy, DiI-stained hMeSPCs were injected into the left knee. As control, the AMD3100-pretreated hMeSPCs were injected into the right knee. As shown by gross morphology and image tracking (Kodak in vivo FX small animal imaging system), injected hMeSPCs adhered to the injured meniscus at 3 weeks after injection (Fig. 2C). By contrast, in the AMD3100-pretreated group, the adhesion of hMeSPCs was almost inhibited. These results suggest that the SDF-1/CXCR4 chemokine axis mediated the migration and adhesion of injected hMeSPCs.
Intra-Articular Injection of hMeSPCs Promoted Meniscus Repair
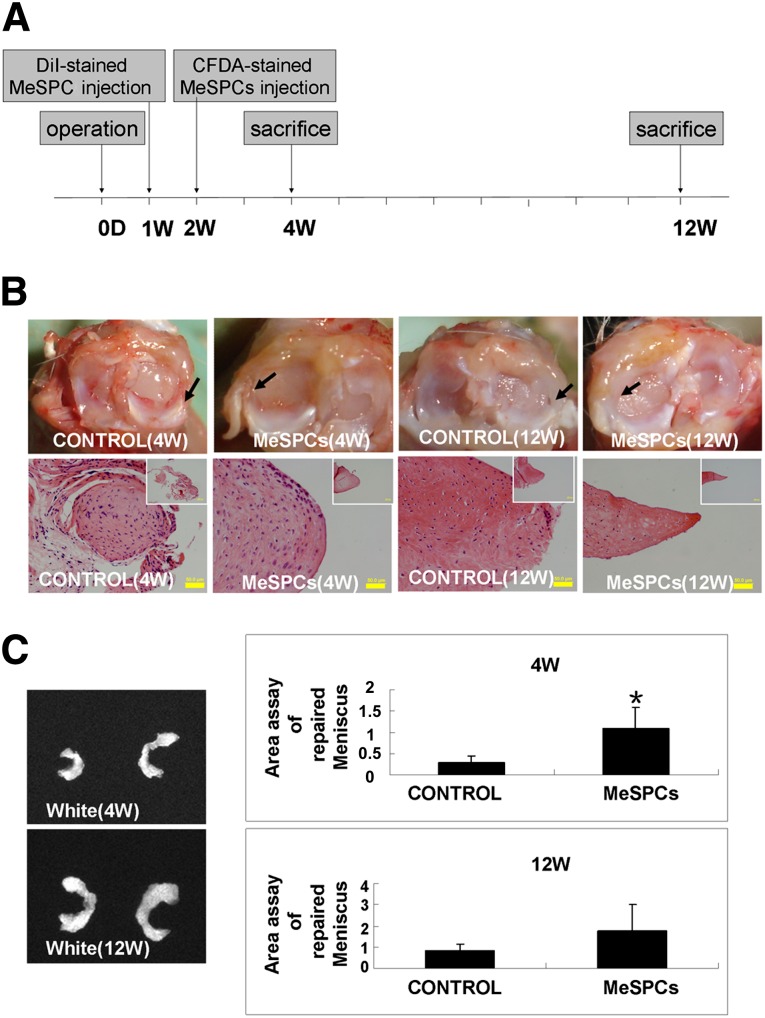
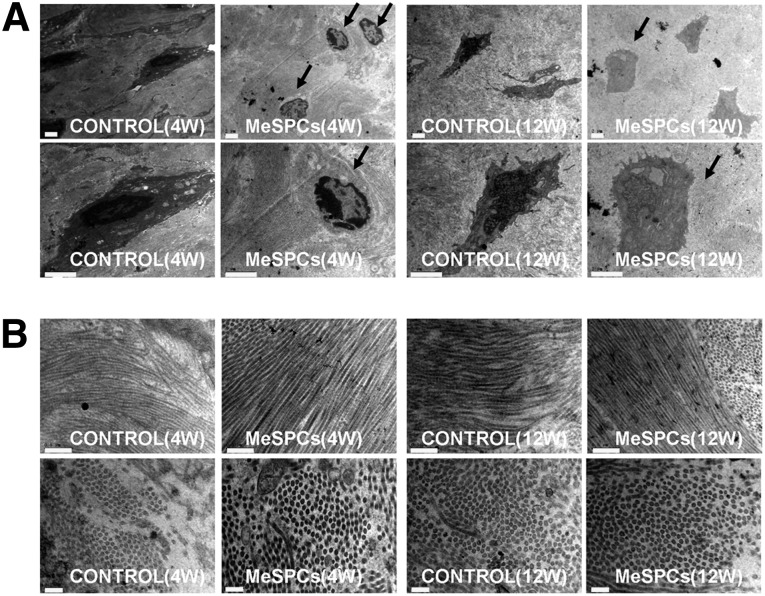
We further examined the effects of intra-articular implanted hMeSPCs on meniscus repair in vivo (Fig. 3A). Compared with the PBS control, intra-articular injection of hMeSPCs induced significantly more neo-tissue formation and extracellular matrix (ECM) deposition in the meniscal defect at 4 weeks postmeniscectomy (Fig. 3B, black arrows; Fig. 3C, 1.09 ± 0.48 vs. 0.28 ± 0.18; p < .05). At 12 weeks postmeniscectomy, there were no significant differences between the hMeSPC injection group and the control group in terms of gross morphology (Fig. 3B, black arrows; Fig. 3C, 1.78 ± 1.25 vs. 0.81 ± 0.34; p > .05). It is a major limitation of our in vivo study that rat meniscus could heal spontaneously in the control group. For future studies, larger animal models, such as sheep, dog, and pig [36], are required to demonstrate the clinical efficacy of intra-articular injection of MeSPCs for meniscus repair. TEM imaging also showed that the cells of the hMeSPC injection group were morphologically more similar to that of normal meniscus with rounded shape (Fig. 4A, black arrows), and with their collagen fibrils being more ordered and larger than that of the control group (Fig. 4B), at both the 4- and 12-week time points.
Figure 3.
The result of meniscus repair after intra-articular injection of human MeSPCs (hMeSPCs). (A): Experimental design for the in vivo treatment of meniscus injury with hMeSPCs. (B): Gross morphology and typical hematoxylin and eosin staining of regenerated meniscus in the control group and hMeSPC-treated group at 4 and 12 weeks postimplantation. Black arrows show the neo-tissue formation in the anterior half of the medial meniscus. (C): Area assays of repaired meniscus relative to surplus normal meniscus at 4 and 12 weeks postimplantation. Scale bars = 50 µm ([B], main panels), 200 µm ([B], insets). ∗, p < .05 versus control group. Abbreviations: CFDA, 6-carboxyfluorescein diacetate; D, day; DiI, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; MeSPCs, meniscus-derived stem/progenitor cells; W, weeks.
Figure 4.
The result of meniscus repair after intra-articular injection of human MeSPCs (hMeSPCs). Shown is transmission electron microscopy imaging of typical cells (A) (black arrows) and collagen fibrils (B) in the control group and hMeSPC-treated group at 4 and 12 weeks postimplantation. Scale bars = 5 µm (A), 0.5 µm ([B], top panels), and 0.2 µm ([B], bottom panels). Abbreviations: MeSPCs, meniscus-derived stem/progenitor cells; W, weeks.
To evaluate the contribution of injected hMeSPCs to meniscus regeneration, hMeSPCs were labeled with DiI and CFDA before injection and traced in vivo (top right panels of Fig. 2C). DiI and CFDA signals were detected at the injury site at 4 and 12 weeks postmeniscectomy, as shown by both the small animal fluorescent imaging system and fluorescence microscopy (supplemental online Fig. 1A, 1B), thus suggesting that injected hMeSPCs may contribute to meniscus regeneration. However, we didn’t detect the percentage of the cells administered remain at the subsequent time points. It is another limitation of our in vivo study.
Intra-Articular Injection of hMeSPCs Suppressed the Progression of OA
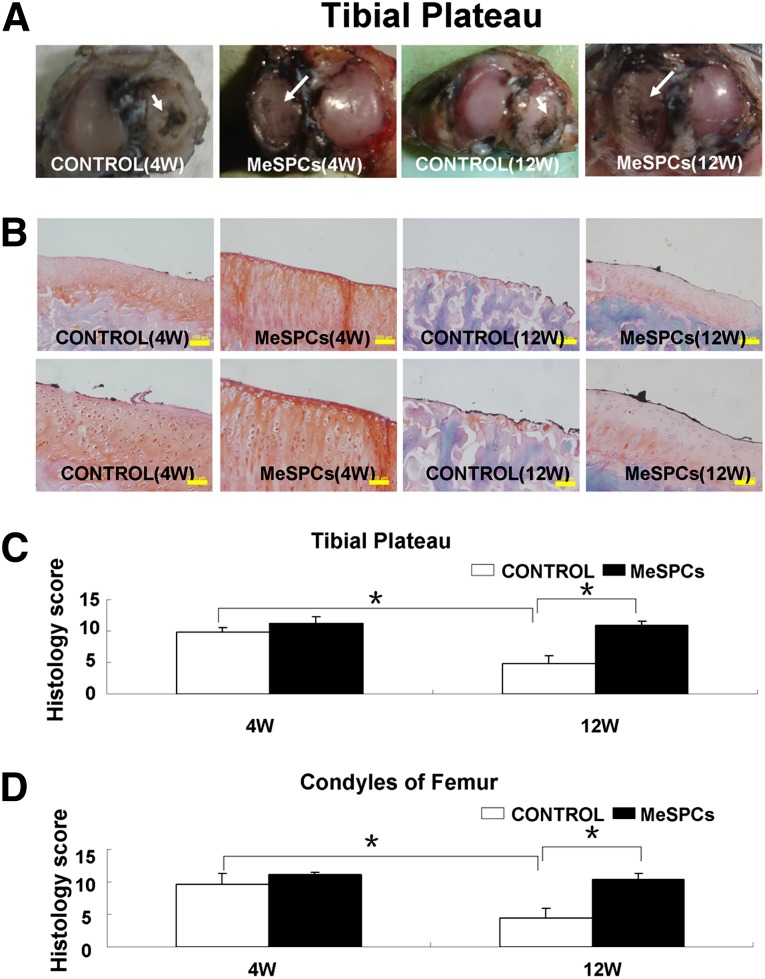
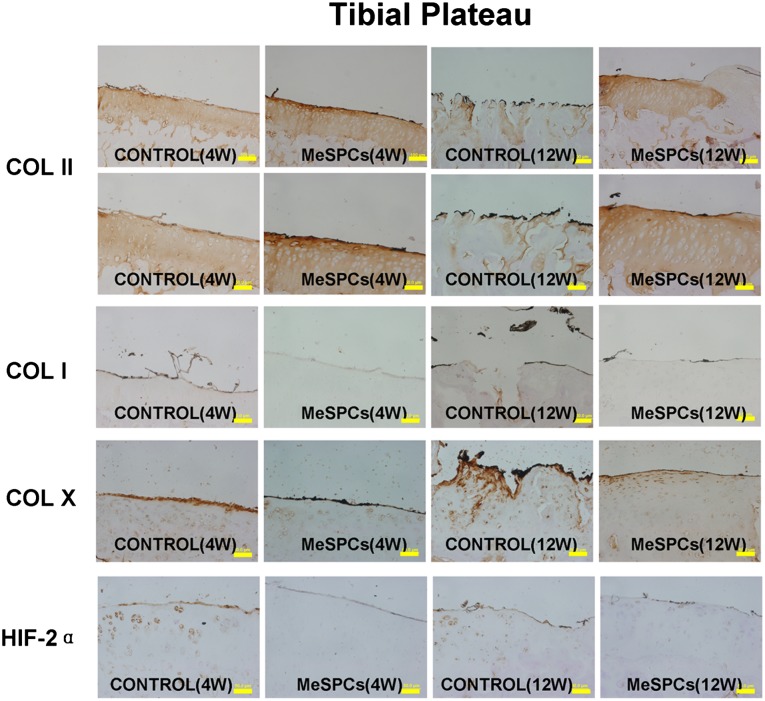
We further examined the therapeutic effect of hMeSPCs in an animal model of OA. Craters, present on the surface of the medial femoral condyle and medial tibial cartilage of the control, were undetectable in the hMeSPC-treated cartilage surface at 12 weeks postmeniscectomy (Fig. 5A; supplemental online Fig. 2A). Compared with the control, intra-articular injection of hMeSPCs reduced surface irregularities (Fig. 5B–5D, p < .05; supplemental online Fig. 2B, 2C). Immunohistochemistry analysis demonstrated that, in contrast to the control, hMeSPC treatment decreased the expression of the OA markers collagen I and X but maintained high collagen II expression, thus indicating that hMeSPCs ameliorated the degeneration of cartilage (Fig. 6; supplemental online Fig. 3).
Figure 5.
The suppression of experimental osteoarthritis after intra-articular injection of human MeSPCs (hMeSPCs). Shown are the gross morphologies of the cartilage surface of tibia (A) in the control group and hMeSPC-treated group at 4 and 12 weeks postimplantation. The cartilage was stained with india ink. White arrows show the surface of the medial tibial cartilage. (B–D): The typical hematoxylin and eosin staining (B) and histological evaluation of the cartilage of tibia (C) and femur (D) in the control group and hMeSPC-treated group at 4 and 12 weeks postimplantation. Scale bars = 50 µm ([B], bottom panels) and 100 µm ([B], top panels). ∗, p < .05 versus control group. Abbreviations: MeSPCs, meniscus-derived stem/progenitor cells; W, weeks.
Figure 6.
The suppression of experimental osteoarthritis after intra-articular injection of human MeSPCs (hMeSPCs). At 4 and 12 weeks postinjection, immunohistochemistry of the cartilage of tibia showed that the osteoarthritis process was suppressed within the hMeSPC-treated group with high COL II expression and low expression levels of COL I, COL X, and HIF-2α. Scale bars = 50 µm (COL II bottom row, COL I, COL X, HIF-2α), 100 µm (COL II top row). Abbreviations: COL I, collagen I; COL II, collagen II; COL X, collagen X; HIF-2α, hypoxia-inducible factor 2α; MeSPCs, meniscus-derived stem/progenitor cells; W, weeks.
Discussion
This study was performed to evaluate our hypothesis that intra-articular injected hMeSPCs can enhance the regeneration of injured meniscus through induced cell homing via the SDF-1/CXCR4 chemokine axis. The results positively confirmed that: (a) a unique cell population (hMeSPCs) with the characteristics of both mesenchymal stem cells and cartilage phenotype was successfully isolated from human meniscus and characterized; (b) the SDF-1/CXCR4 chemokine axis can mediate the migration of hMeSPCs in vitro, as well as promote the trafficking of hMeSPCs to the meniscus injury site in vivo; (c) intra-articular injection of hMeSPCs enhanced the regeneration of meniscus injury in vivo within a rat model, resulting in more physiological structure of the ECM; (d) intra-articular injection of hMeSPCs also suppressed the process of osteoarthritis in vivo through the inhibition of hypoxia-inducible factor 2α (HIF-2α). Collectively, these results demonstrated that intra-articular injection of hMeSPCs has great potential for clinical applications in meniscus regeneration and OA prevention in the future.
hMeSPCs Can be an Ideal Seed Cell for Meniscus Regeneration In Vivo
Tissue engineering is a multidisciplinary field comprising an intersection of biology, engineering, and clinical applications, the ultimate objective of which is to grow and maintain living tissues both in situ and ex vivo. Most researchers agree that no tissue-engineered construct can be derived without cells. Recently, some studies suggest that multipotent stem cells are present in the meniscus [21, 22]. Our previous study found that stem cells can be isolated from rabbit meniscus and that these cells possess immunosuppressive properties. Intra-articular injection of meniscus derived stem cells promoted meniscus regeneration and maintained joint space width. These data thus suggest that meniscus-derived MSCs have great potential for the regeneration of meniscus injuries. Nevertheless, to date, no report has yet mentioned the injection of human meniscus-derived MSCs for regeneration of injured meniscus. In our study, we found that 35% of cells derived from human meniscus formed circular colonies, which is higher than the proportion of putative adult stem cells derived from other human tissues, such as bone marrow and synovium. We also found that hMeSPCs displayed higher expression of collagen II compared with the putative stem cells derived from synovium and bone marrow. Hence, we selected hMeSPCs as seed cells for our in vivo study. The in vivo study demonstrated that the hMeSPC-transplanted group yielded better histological staining results. We observed that the hMeSPC-transplanted groups exhibited higher density of ECM deposition with round meniscal cells within it, thus indicating the key role of hMeSPCs in the process of meniscus healing. Our findings suggested a new strategy of articular cartilage protection through meniscus regeneration induced by intra-articular injection of hMeSPCs for patients undergoing meniscectomy. Meniscus regeneration is feasible using this tissue-engineering technique.
Injected hMeSPCs Enhance the Regeneration of Injured Meniscus Through the SDF-1/CXCR4 Chemokine Axis
A number of studies have previously demonstrated that intra-articular injection of cells is an effective treatment strategy for regenerating injured meniscus. For example, Murphy et al. [37, 38] found that local delivery of adult MSCs to injured knee joints could stimulate the regeneration of meniscal tissue. Sekiya and colleagues [18] reported that the injected stem cells differentiated directly into meniscal cells within the meniscal defect, but the mechanisms by which these cells adhered to the injury sites are not well understood. SDF-1, a major chemokine of the bone marrow, has been reported to be constitutively expressed in a wide range of tissues, including brain, kidney, liver, heart, spleen, and lung [39]. The upregulated expression of SDF-1 in the meniscus after meniscectomy in vivo and the migration of hMeSPCs in response to SDF-1 were observed in our in vitro study, indicating that SDF-1 might be involved in the preferential migration and homing of intra-articular injected hMeSPCs. Our previous study demonstrated that specific interaction between chemokines and their receptors mediates the migratory process of fibroblast-like cells, including MSCs [30]. In this study, the CXCR4-selective antagonist AMD3100 blocked the migratory effect induced by SDF-1 in vitro. With the in vivo study, we observed that AMD3100-treated hMeSPCs did not adhere to the injured meniscus defect, even under high expression of SDF-1. These results further suggest that the SDF-1/CXCR4 chemokine axis is involved in the homing of injected hMeSPCs to injured meniscus in the knee. However, more evidence is needed to explore the cell therapy for human injured meniscus. In further studies, SDF-1-releasing scaffold or CXCR4-modified stem cells may be used in the tissue engineering of meniscus to enhance the regeneration of injured meniscus.
Intra-Articular Injected hMeSPCs Suppress the Process of OA In Vivo
In our study, we found that intra-articular injection of hMeSPCs can delay or reduce the progression of OA induced by meniscectomy, including the degree of cartilage degeneration, osteophyte formation, and subchondral sclerosis. Some researchers have hypothesized that the neo-meniscal tissue in the treated groups is associated with protection against degenerative changes induced by OA after meniscal injury [4, 37]. It is thought that MSCs may have a direct role in cartilage protection through direct remodeling of the articular cartilage or by acting to preserve subchondral or trabecular bone based on the relationship between early bone remodeling and OA development [40, 41]. However, Sekiya et al. [42] found that only a small proportion of the cells adhered to the cartilage defect after injection, which is similar to our previous results [22]. These results suggest that other mechanisms may be inferred. Many studies have shown that the destruction of articular cartilage is induced by the upregulation of catabolic factors, such as matrix metalloproteinases (MMP1, MMP3, MMP9, MMP12, and MMP13) and aggrecanase-1 [43–45]. HIF-2α is a major catabolic transcription factor associated with the osteoarthritic process and is known to directly induce the expression of these catabolic factors. Chun and colleagues also demonstrated that gene knockout of HIF-2α in mice could suppress cartilage destruction, thus suggesting that HIF-2α plays a leading role in causing osteoarthritis [46]. In our study, we observed that HIF-2α expression was inhibited in our hMeSPC-treated group. Therefore, this suggests that hMeSPCs may impede OA progression by inhibiting HIF-2α expression.
Conclusion
Our study demonstrated for the first time that intra-articular injection of hMeSPCs promoted meniscus regeneration through SDF-1/CXCR4-mediated migration and ameliorated the progression of OA. Our study highlights the potential of using intra-articular injection of hMeSPCs for the treatment of meniscus injuries in the clinic.
Supplementary Material
Acknowledgments
We thank Wang Li for transmission electron microscopy imaging. This work was supported by National Natural Science Foundation of China Grants (81125014, 81071461, 81202396, 81271970, J1103603, 31000436), National Key Scientific Program (2012CB966600), National High Technology Research and Development Program of China (2012AA020503), Zhejiang Province Grants (Z2100086, LY12H06006), The Ministry of education program for New Century Excellent Talents foundation project-08-0487, Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents, and Medical Science and Technology Project of Zhejiang Province (201341741).
Author Contributions
W.S.: conception and design, collection of data, data analysis and interpretation, manuscript writing; J.C.: collection and assembly of data, data analysis and interpretation, manuscript writing; T.Z., L.C., W.Z., and Z.F.: collection and assembly of data; B.C.H.: manuscript writing, final approval of manuscript; Z.Y. and X.C.: administrative support, data analysis and interpretation; J.J.: manuscript writing, data analysis and interpretation; W.C.: conception and design, provision of study material, data analysis and interpretation, final approval of manuscript; H.-W.O.: conception and design, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Majewski M, Susanne H, Klaus S. Epidemiology of athletic knee injuries: A 10-year study. Knee. 2006;13:184–188. doi: 10.1016/j.knee.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Hennerbichler A, Moutos FT, Hennerbichler D, et al. Repair response of the inner and outer regions of the porcine meniscus in vitro. Am J Sports Med. 2007;35:754–762. doi: 10.1177/0363546506296416. [DOI] [PubMed] [Google Scholar]
- 3.van Tienen TG, Hannink G, Buma P. Meniscus replacement using synthetic materials. Clin Sports Med. 2009;28:143–156. doi: 10.1016/j.csm.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Roos H, Laurén M, Adalberth T, et al. Knee osteoarthritis after meniscectomy: Prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41:687–693. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Hunter DJ, Zhang YQ, Niu JB, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 6.Lotz MK, Kraus VB. New developments in osteoarthritis: Posttraumatic osteoarthritis: Pathogenesis and pharmacological treatment options. Arthritis Res Ther. 2010;12:211. doi: 10.1186/ar3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badlani JT, Borrero C, Golla S, et al. The effects of meniscus injury on the development of knee osteoarthritis: Data from the osteoarthritis initiative. Am J Sports Med. 2013;41:1238–1244. doi: 10.1177/0363546513490276. [DOI] [PubMed] [Google Scholar]
- 8.Arno S, Hadley S, Campbell KA, et al. The effect of arthroscopic partial medial meniscectomy on tibiofemoral stability. Am J Sports Med. 2013;41:73–79. doi: 10.1177/0363546512464482. [DOI] [PubMed] [Google Scholar]
- 9.Sonoda M, Harwood FL, Amiel ME, et al. The effects of hyaluronan on tissue healing after meniscus injury and repair in a rabbit model. Am J Sports Med. 2000;28:90–97. doi: 10.1177/03635465000280012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tienen TG, Heijkants RG, de Groot JH, et al. Replacement of the knee meniscus by a porous polymer implant: A study in dogs. Am J Sports Med. 2006;34:64–71. doi: 10.1177/0363546505280905. [DOI] [PubMed] [Google Scholar]
- 11.Cook JL, Tomlinson JL, Kreeger JM, et al. Induction of meniscal regeneration in dogs using a novel biomaterial. Am J Sports Med. 1999;27:658–665. doi: 10.1177/03635465990270051901. [DOI] [PubMed] [Google Scholar]
- 12.Weinand C, Peretti GM, Adams SB, Jr, et al. An allogenic cell-based implant for meniscal lesions. Am J Sports Med. 2006;34:1779–1789. doi: 10.1177/0363546506290666. [DOI] [PubMed] [Google Scholar]
- 13.Sweigart MA, Athanasiou KA. Toward tissue engineering of the knee meniscus. Tissue Eng. 2001;7:111–129. doi: 10.1089/107632701300062697. [DOI] [PubMed] [Google Scholar]
- 14.Buma P, Ramrattan NN, van Tienen TG, et al. Tissue engineering of the meniscus. Biomaterials. 2004;25:1523–1532. doi: 10.1016/s0142-9612(03)00499-x. [DOI] [PubMed] [Google Scholar]
- 15.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 16.Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10:223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker BM, Nathan AS, Huffman GR, et al. Tissue engineering with meniscus cells derived from surgical debris. Osteoarthritis Cartilage. 2009;17:336–345. doi: 10.1016/j.joca.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horie M, Sekiya I, Muneta T, et al. Intra-articular injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells. 2009;27:878–887. doi: 10.1634/stemcells.2008-0616. [DOI] [PubMed] [Google Scholar]
- 19.Sato M, Uchida K, Nakajima H, et al. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14:R31. doi: 10.1186/ar3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris MT, Butler DL, Boivin GP, et al. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998–1003. doi: 10.1016/j.orthres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Segawa Y, Muneta T, Makino H, et al. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27:435–441. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- 22.Shen W, Chen J, Zhu T, et al. Osteoarthritis prevention through meniscal regeneration induced by intra-articular injection of meniscus stem cells. Stem Cells Dev. 2013;22:2071–2082. doi: 10.1089/scd.2012.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 24.Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 25.Hoehn M, Küstermann E, Blunk J, et al. Monitoring of implanted stem cell migration in vivo: A highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci USA. 2002;99:16267–16272. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 27.Ji JF, He BP, Dheen ST, et al. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415–427. doi: 10.1634/stemcells.22-3-415. [DOI] [PubMed] [Google Scholar]
- 28.Tögel F, Isaac J, Hu Z, et al. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 29.Ratajczak MZ, Zuba-Surma E, Kucia M, et al. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 30.Shen W, Chen X, Chen J, et al. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials. 2010;31:7239–7249. doi: 10.1016/j.biomaterials.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 31.Zou XH, Cai HX, Yin Z, et al. A novel strategy incorporated the power of mesenchymal stem cells to allografts for segmental bone tissue engineering. Cell Transplant. 2009;18:433–441. doi: 10.3727/096368909788809839. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Song XH, Yin Z, et al. Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells. 2009;27:1276–1287. doi: 10.1002/stem.61. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, Chen LK, Zhu DC, et al. The inductive effect of bone morphogenetic protein-4 on chondral-lineage differentiation and in situ cartilage repair. Tissue Eng Part A. 2010;16:1621–1632. doi: 10.1089/ten.TEA.2009.0681. [DOI] [PubMed] [Google Scholar]
- 34.Visco DM, Hill MA, Widmer WR, et al. Experimental osteoarthritis in dogs: A comparison of the Pond-Nuki and medial arthrotomy methods. Osteoarthritis Cartilage. 1996;4:9–22. doi: 10.1016/s1063-4584(96)80003-3. [DOI] [PubMed] [Google Scholar]
- 35.Fan H, Liu H, Toh SL, et al. Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials. 2009;30:4967–4977. doi: 10.1016/j.biomaterials.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 36.Peretti GM, Gill TJ, Xu JW, et al. Cell-based therapy for meniscal repair: A large animal study. Am J Sports Med. 2004;32:146–158. doi: 10.1177/0095399703258790. [DOI] [PubMed] [Google Scholar]
- 37.Murphy JM, Fink DJ, Hunziker EB, et al. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 38.Abdel-Hamid M, Hussein MR, Ahmad AF, et al. Enhancement of the repair of meniscal wounds in the red-white zone (middle third) by the injection of bone marrow cells in canine animal model. Int J Exp Pathol. 2005;86:117–123. doi: 10.1111/j.0959-9673.2005.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Q, Jones D, Borghesani PR, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dequeker J, Mokassa L, Aerssens J, et al. Bone density and local growth factors in generalized osteoarthritis. Microsc Res Tech. 1997;37:358–371. doi: 10.1002/(SICI)1097-0029(19970515)37:4<358::AID-JEMT10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 41.Li B, Aspden RM. Composition and mechanical properties of cancellous bone from the femoral head of patients with osteoporosis or osteoarthritis. J Bone Miner Res. 1997;12:641–651. doi: 10.1359/jbmr.1997.12.4.641. [DOI] [PubMed] [Google Scholar]
- 42.Koga H, Shimaya M, Muneta T, et al. Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Res Ther. 2008;10:R84. doi: 10.1186/ar2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yatabe T, Mochizuki S, Takizawa M, et al. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann Rheum Dis. 2009;68:1051–1058. doi: 10.1136/ard.2007.086884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato T, Konomi K, Fujii R, et al. Prostaglandin EP2 receptor signalling inhibits the expression of matrix metalloproteinase 13 in human osteoarthritic chondrocytes. Ann Rheum Dis. 2011;70:221–226. doi: 10.1136/ard.2009.118620. [DOI] [PubMed] [Google Scholar]
- 45.Hui W, Litherland GJ, Elias MS, et al. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann Rheum Dis. 2012;71:455–462. doi: 10.1136/annrheumdis-2011-200372. [DOI] [PubMed] [Google Scholar]
- 46.Yang S, Kim J, Ryu JH, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.