This study demonstrates the feasibility of using arthritic tissue resected during hip replacement as a source of autologous stem cells. Periosteum tissue that is resected with the femoral neck in replacing the hip represents an unprecedented and to date unstudied source of stem cells from osteoarthritis and rheumatoid arthritis patients.
Keywords: Stem cell, Periosteum, Arthritis, Rheumatoid arthritis, Osteoarthritis, Differentiation, Clinical translation, Regenerative medicine
Abstract
The overarching aim of this study is to assess the feasibility of using periosteal tissue from the femoral neck of arthritic hip joints, usually discarded in the normal course of hip replacement surgery, as an autologous source of stem cells. In addition, the study aims to characterize intrinsic differences between periosteum-derived cell (PDC) populations, isolated via either enzymatic digestion or a migration assay, including their proliferative capacity, surface marker expression, and multipotency, relative to commercially available human bone marrow-derived stromal cells (BMSCs) cultured under identical conditions. Commercial BMSCs and PDCs were characterized in vitro, using a growth assay, flow cytometry, as well as assay of Oil Red O, alizarin red, and Safranin O/Fast Green staining after respective culture in adipo-, osteo-, and chondrogenic media. Based on these outcome measures, PDCs exhibited proliferation rate, morphology, surface receptor expression, and multipotency similar to those of BMSCs. No significant correlation was observed between outcome measures and donor age or diagnosis (osteoarthritis [OA] and rheumatoid arthritis [RA], respectively), a profound finding given recent rheumatological studies indicating that OA and RA share not only common biomarkers and molecular mechanisms but also common pathophysiology, ultimately resulting in the need for joint replacement. Furthermore, PDCs isolated via enzymatic digestion and migration assay showed subtle differences in surface marker expression but otherwise no significant differences in proliferation or multipotency; the observed differences in surface marker expression may indicate potential effects of isolation method on the population of cells isolated and/or the behavior of the respective isolated cell populations. This study demonstrates, for the first time to our knowledge, the feasibility of using arthritic tissue resected during hip replacement as a source of autologous stem cells. In sum, periosteum tissue that is resected with the femoral neck in replacing the hip represents an unprecedented and, to date, unstudied source of stem cells from OA and RA patients. Follow-up studies will determine the degree to which this new, autologous source of stem cells can be banked for future use.
Introduction
The net aging of the population, and associated need for disease treatments and augmented healing strategies, provides great impetus to develop novel stem cell therapies, implants, and associated regenerative medicine solutions [1]. In turn, these advances call for a safe and reliable method to acquire and bank stem cells [2]. Nonembryonic stem cells from sources including perinatal tissues [3], bone marrow [4], adipose tissue [5], and dental pulp [6] have been shown to be possible candidates for cell banking and cryopreservation. However, today’s aging adults were born a generation too early to benefit from banking of perinatal tissues. Furthermore, periosteum-derived cells (PDCs) from long bone diaphyses have garnered increased interest of late, showing great potential as a source of adult multipotent stem cells [1, 7]. Although not as easily accessible as adipose tissue or dental pulp for cell banking, previously published studies have demonstrated PDCs’ in vivo capacity for bone and cartilage formation [8–10]. However, there have been conflicting reports regarding the relative advantages of using PDCs versus multipotent cells derived from other tissues for regenerative medicine purposes [11–13]. Furthermore, in the absence of trauma or tumor resection, access to periosteal tissue of long bone diaphyses, the tissue source of most previous studies, is limited.
In hip replacement surgery, the femoral head and part of the neck are resected to accommodate the neck of the implant. Typically this tissue is discarded after pathologic examination, yet this discarded tissue may provide an unprecedented and untapped source of autologous progenitor cells for aging adults who were born a generation too early to benefit from banking of, for example, perinatal tissues. Interestingly, in contrast to the bone marrow niche and its resident stem cell population, which have been characterized as a function of age and diseases states, including cardiac disease and osteoarthritis [14], to our knowledge, not a single study has been published on periosteum-derived stem cells from arthritic patients. Given the increasing population of elderly in the United States (12.9% of the current population, 20% of the population in 2030), the number of people affected by arthritis and the associated number of total hip arthroplasties are expected to increase significantly [15]. Hence, the aim of this study is to compare periosteum-derived cell properties from arthritis patients with those of bone marrow-derived stem cells to determine the feasibility of using the patient’s own tissue, removed during routine joint replacement, to potentially heal and/or repair failing organs and to treat diseases.
In addition, although historically characterized as different diseases with distinct etiologies, recent advances in the field demonstrate that osteoarthritis (OA) and rheumatoid arthritis (RA) share similar biomarkers [16] and common molecular mechanisms [17]. Interestingly, although little is known regarding the effect of chronic inflammation on the regenerative potential of mesenchymal stem cells, a recent study of muscle stem cells showed no differences in regenerative potential between satellite cells of OA and RA patients, although mean telomere length was shorter in the RA than in the OA group [18]. Furthermore, chemokines common to the synovial fluid of OA and RA patients may play a role in progenitor cell recruitment to microfractured areas of subchondral bone [19]. Finally, no differences in osteogenic differentiation capacity have been reported for human-derived bone marrow stem cells from OA and RA patients [20]. Ultimately, the pathophysiologic effects of OA and RA lead to loss of joint structure and function over time, necessitating joint replacement.
According to the U.S. Centers for Disease Control and Prevention, approximately 327,000 total hip replacements are performed in the U.S. each year [15], and the resected tissue is discarded as medical waste. Whereas human femoral neck periosteum has been shown to be less cellular than mid-diaphyseal periosteum [21], no significant correlation has been shown between age and cellularity of mid-diaphyseal periosteum in aging patients from 68 to 99 years of age [22]. Aging individuals would benefit greatly from access to autologous tissue serving as a niche for stem cells, as the incidence of virtually every adult onset disease increases with age. Furthermore, banking and use of a patient’s own tissues or cells prevents risks associated with use of donor tissue, including the risks of immune system rejection and disease transmission. A critical first step in translation of the concept is to assess the feasibility of using periosteal tissue, discarded in the normal course of hip replacement surgery, as an autologous source of adult stem cells.
Furthermore, the effect of cell isolation protocol on cell behavior, including expression of surface markers and differentiation capacity, is poorly understood in general [23–25] and is of particular interest for cells derived from the periosteum [1]. The periosteum is a bilayered tissue with an elastic and tough (attributable to elastin and collagen, respectively) outer fibrous layer and an inner cambium layer [15, 25]. Its anisotropic composition and structure confer to the tissue unique, environmentally responsive (“smart”) properties, including direction-dependent permeability and anisotropic elastic modulus [26, 27]. The purpose of cell isolation is to liberate the cells confined within the tissue, a goal that is typically achieved by enzymatic digestion or cellular migration. Given the composite architecture and resulting direction-dependent properties of periosteum, one might expect the isolation method to exert profound influence not only on the population of cells isolated but also on the behavior of the respective isolated cell populations.
Furthermore, in a previously tested in vivo ovine study, cells isolated enzymatically from mid-diaphyseal periosteum (harvested from bone removed to create a critical-sized defect) were seeded on collagen and incubated overnight before they were tucked into a periosteum substitute membrane designed for directional delivery of stem cells and biologics/pharmaceuticals [8]. The delivery of PDCs in this manner hastens defect healing. Although this approach shows promise for translation to human patients and has been shown to be effective using endogenous periosteum in limited numbers of patients [28, 29], issues of PDC accessibility and, in particular, feasibility of isolating PDCs from joint replacement patients have yet to be addressed. These considerations provided the impetus for our current study.
Hence, the objective of this first feasibility study is to determine whether PDCs can be isolated from discarded femoral explants from OA and RA patients undergoing total hip arthroplasty. Furthermore, the study aims to determine how different isolation protocols affect PDC behavior (surface marker expression, proliferation, and multipotency). In addition, the study aims to characterize the populations of PDCs, isolated through either enzymatic digestion or migration, and their relative capacity to differentiate down multiple lineages; direct comparison with commercially available human marrow-derived stromal cells cultured under identical conditions will enable the placement of the PDC data in context of the current state of the field.
Materials and Methods
Periosteum Harvest and Cell Isolation
Proximal femoral head/neck explants (n = 4) were acquired from human patients following hip replacement surgery (total hip arthroplasty), within 8 hours of surgery (IRB 12-335, Institutional Review Board of the Cleveland Clinic Foundation, ethical approval in compliance with the Helsinki Declaration) and immediately after routine examination and diagnosis by pathology. All samples were assigned anonymized numbers prior to transfer to the Experimental Mechanobiology Laboratory at Case Western Reserve University.
The periosteum from the femoral neck was detached from the underlying bone using a periosteal elevator. The tissue was then finely minced using a scalpel blade. Half of the minced tissue was used to isolate enzymatically digested periosteum-derived cells (dPDCs), and the remaining tissue was used to isolate migrated periosteum-derived cells (mPDCs).
In order to isolate dPDCs, the minced tissue was suspended in 3 mg/ml collagenase II (Gibco, Grand Island, NY, http://www.invitrogen.com) solution in α-minimal essential medium (α-MEM) with GlutaMAX (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) with 1% antibiotic-antimycotic (Invitrogen) overnight in a 37°C incubator. Any undigested tissue was filtered from the cells using a 100-μm filter, and the isolated cells were cultured in standard culture media.
In order to isolate mPDCs, the minced tissue was directly plated into tissue culture flasks in α-MEM with GlutaMAX supplemented with 10% fetal bovine serum (FBS) (Invitrogen), 1% antibiotic-antimycotic overnight, and cultured in standard culture media. The cells were left to egress from the tissue for 1 week.
Validated bone marrow-derived human mesenchymal stem cells (hMSCs) were purchased from four independent vendors (Lonza, Walkersville, MD, http://www.lonza.com; PromoCell, Heidelberg, Germany, http://www.promocell.com; ScienCell, Carlsbad, CA, http://www.sciencellonline.com; and Cell Applications, San Diego, CA, http://www.cellapplications.com) as standards for comparison.
Cell Culture and Cryopreservation
All cells were cultured in standard culture medium, α-MEM with GlutaMAX supplemented with 10% FBS and 1% penicillin-streptomycin (Invitrogen). The medium was replaced every 2-3 days. Cells were maintained in a humidified incubator at 37°C with 5% CO2. Cells were cultured in tissue culture-treated plastic flasks until 80% confluent. Cells were detached using 0.25% trypsin-EDTA (Invitrogen) and counted using a hemocytometer.
For cryopreservation, cells were resuspended at 1 × 106 cells per milliliter in α-MEM with GlutaMAX with 40% FBS, 10% dimethyl sulfoxide (Fisher Scientific International, Hampton, NH, http://www.fisherscientific.com), and 1% penicillin-streptomycin. The suspension was aliquoted into 1-ml cryovials and placed in a Mr. Frosty freezing container at −80°C overnight to control the freezing rate. Cryovials were then transferred for long-term storage in liquid nitrogen. All cells were expanded for one passage and cryopreserved for up to 1 month prior to these studies to synchronize donors. All experiments were performed on passage 4 (P4) mPDCs and dPDCs and on bone marrow-derived stromal cells (BMSCs) passaged twice after cryopreserved acquisition.
Cell Proliferation Assay
Each cell type was seeded at 5,000 cells per cm2 (n = 6) in 96-well plates (1,600 cells per well) and cultured. Plates were drained of all medium and frozen at −80°C every 3 days for 21 days. DNA was quantified using a CyQUANT assay (Invitrogen) using a fluorescent microplate reader and normalized for each cell type using measurements for day 0.
Cell Population Analysis by Flow Cytometry
Cells were stained for CD73 (Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com), CD90 (Becton Dickinson), and CD105 (eBioscience Inc., San Diego, CA, http://www.ebioscience.com), which have been established as positive surface antigens in mesenchymal stem cells (MSCs) [28]. CD34 (Becton Dickinson), which is expressed in leukocytes, and CD45 (Becton Dickinson), which is expressed in T and B lymphocytes, have been established as negative surface antigens in MSCs [22]. Myosin heavy chain (MyHC) (R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com) is expressed in skeletal muscle cells, and it was also used to exclude muscle contamination. Then, 1 × 106 cells of each cell type were detached and stained with CD73, CD90, CD105, CD34, and CD45 antibodies in bovine serum albumin stain buffer (Becton Dickinson). Cells were fixed and permeabilized using the BD Cytofix/Cytoperm Kit (Becton Dickinson) and then stained with MyHC. Compensation beads (Becton Dickinson) were stained in the same manner. The stained cells were then analyzed using a LSRII flow cytometer (Becton Dickinson) at the Cytometry and Imaging Microscopy Core Facility of the Case Comprehensive Cancer Center, and analysis was performed using WinList (Verity Software House, Topsham, ME, http://www.vsh.com)
Culture in Adipogenic Medium and Assessment of Oil Red O Staining
Each cell type was seeded at 8,000 cells per cm2 (n = 6) in 12-well plates. The cells were cultured in standard medium for 48 hours until the cells reached confluence. The cells were then cultured in adipogenic induction medium comprising high-glucose DMEM (Invitrogen), 10% FBS, 40 IU/ml penicillin-streptomycin (pen/strep), 1μM dexamethasone (Sigma-Aldrich), 0.2 mM indomethacin (Sigma-Aldrich), 0.1 mg/ml insulin (Sigma-Aldrich), and 1 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich) for 5 days. Then the cells were cultured in adipogenic maintenance medium comprising high-glucose DMEM, 10% FBS, 40 IU/ml pen/strep, and 0.1 mg/ml insulin for 2 days. The cells were then cultured in the adipogenic maintenance medium for an additional 2 days, and then assessed for Oil Red O staining. Control cells were cultured identically using standard culture medium.
To assess Oil Red O staining, cells were rinsed in phosphate-buffered saline (PBS) (Invitrogen) and fixed using cold, neutral buffered 4% formaldehyde solution (Fisher Scientific) for 2 minutes at −20°C. Cells were rinsed with cold 50% ethanol solution (Fisher Scientific) and then incubated in 0.2% Oil Red O solution (Sigma-Aldrich) for 20 minutes. Cells were washed once with 50% ethanol solution then thoroughly rinsed with distilled water three times. To quantify staining, Oil Red O was extracted using 100% isopropanol (Fisher Scientific), and then absorbance was measured at a wavelength of 520 nm in a microplate reader.
Culture in Osteogenic Medium and Assessment of Alizarin Red Staining
Each cell type was seeded at 4,000 cells per cm2 (n = 6) in 12-well plates. PDCs and BMSCs were cultured in hMSC Osteogenic Differentiation BulletKit (Lonza) for 21 days; medium was changed every 3–4 days. After fixation using cold, neutral buffered 4% formaldehyde solution, cells were stained with alizarin red, and staining was assessed quantitatively using an Osteogenesis Quantification Kit (Millipore, Billerica, MA, http://www.millipore.com). Staining of cells cultured in inductive medium was compared with that of cells cultured identically using standard culture medium.
Culture in Chondrogenic Medium and Histological Assessment of Safranin O/Fast Green Staining
mPDCs and dPDCs were seeded at 200,000 cells per well (n = 3) in a V-bottomed 96-well plate and centrifuged for 5 minutes at 400g to from cell pellets. The pellets were incubated undisturbed for 24 hours and then cultured in chondrogenic medium comprising high glucose DMEM, 100 IU/ml pen/strep, 10 μM dexamethasone (Sigma-Aldrich), 1 mM sodium pyruvate (Sigma-Aldrich), 10 μg/ml l-ascorbic acid (Sigma-Aldrich), 1× insulin-transferrin-selenium (Invitrogen), and 10 μg/ml TGF-β (R&D Systems). Medium was changed every 2–3 days for 4 weeks, and cells were then assessed for differentiation. Diameters of the pellets were measured before and after culture in chondrogenic and control media. After culture, the pellets were fixed for 2 hours in cold, neutral buffered 4% formaldehyde solution. Pellets were placed in histology cassettes and stored in 70% ethanol. They were embedded in paraffin and sectioned, and then stained for Safranin O and counterstained with Fast Green at the Case Medical Center Hard Tissue Histology Core.
Statistical Analysis
Statistical analysis was performed using JMP (SAS Institute, Inc., Cary, NC, http://www.sas.com). Error bars in figures indicate 95% confidence intervals. Nonparametric multiple comparisons were performed using Wilcoxon tests. Significance was defined as p < .05.
Results
Cell Collection
Femoral neck periosteum was obtained from the male and female joint replacement patients diagnosed with osteoarthritis or rheumatoid arthritis, aged 30–72 years (Table 1). Post hoc inquiries to bone marrow hMSC vendors showed that cells were derived from prenatal (22 weeks of gestation) to middle-aged (45 years) and aged (56 and 72 years) donors (Table 1) of undeclared health status.
Table 1.
Cell source information

Cell Characterization
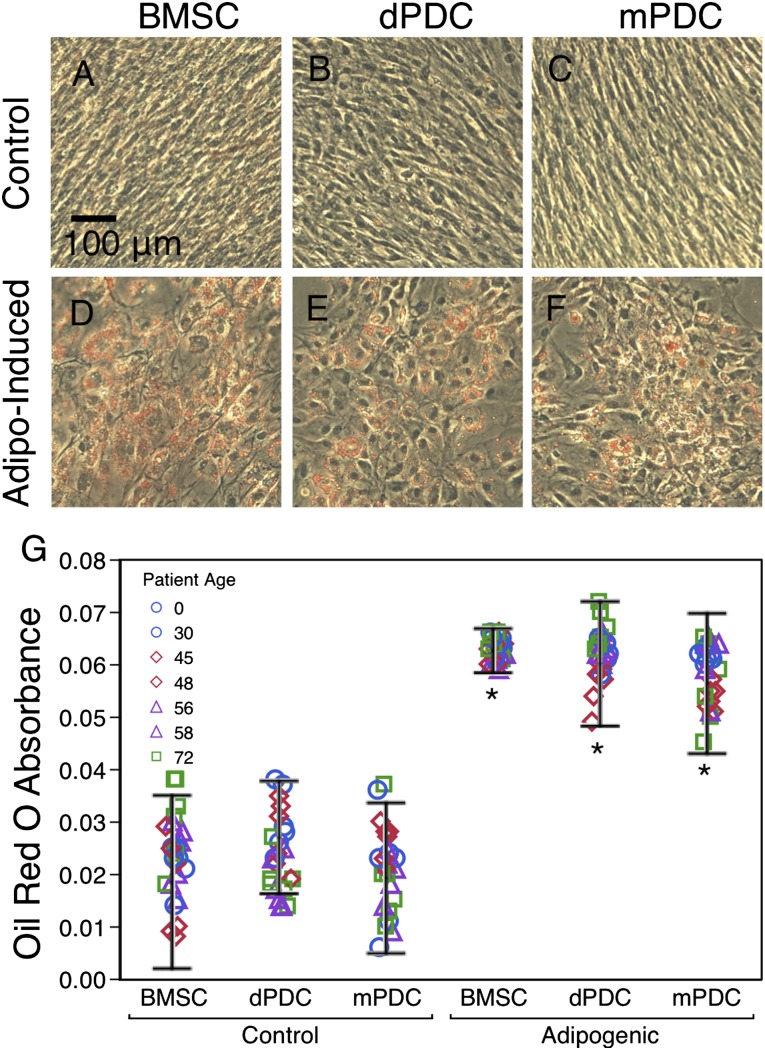
Using the protocols for culture and expansion described above, both mPDCs and dPDCs were successfully acquired from periosteum of each arthroplasty patient. Both mPDCs and dPDCs were fibroblastic in shape and were of similar size and morphologically indistinguishable from BMSCs (Fig. 1A–1C). Proliferation characteristics of mPDCs, dPDCs, and BMSCs were similar, with all presenting similar growth rates from day 0 to day 6 and showing no significant differences in maximum achieved density (Fig. 1D). BMSCs showed higher variability (larger standard deviation) in growth characteristics than the PDCs.
Figure 1.
Cell morphology and growth curves. (A–C): Representative phase contrast (×10) microscopic images of BMSCs (A), dPDCs (B), and mPDCs (C). Cells exhibited similar morphologies and sizes. Scale bar = 100 μm. (D): Growth curves of cells were quantified for 15 days. All cell types showed similar growth rates (slope) (n = 4). Error bars indicate 95% confidence intervals. Abbreviations: BMSC, bone marrow-derived stromal cell; dPDC, enzymatically digested periosteum-derived cells; mPDC, migrated periosteum-derived cell.
Flow cytometry analysis showed that dPDCs exhibited a significantly greater percentage of cells positive for CD73, and mPDCs exhibited a significantly greater percentage of cells positive for CD105, compared with the BMSCs, but all cell groups exhibited at least 95% cells positive for CD73, CD90, and CD105 (Table 2). BMSCs showed a greater percentage of cells positive for CD34 than dPDCs and mPDCs, and BMSCs showed a greater percentage of cells positive for MyHC than mPDCs. There were no significant correlations between surface marker expression and donor age. A significant correlation was observed between mPDCs and CD90 expression, which showed an R2 value of .85 (supplemental online Table 1).
Table 2.
Flow cytometry results

Assessment of Oil Red O Staining After Culture in Adipogenic Medium
Each cell type showed a significant increase in Oil Red O absorbance after treatment with adipogenic medium, but there were no significant differences between the cell types. Each cell type (BMSCs, dPDCs, and mPDCs) from each arthroplasty donor (ages 30, 48, 58, and 72) showed morphological changes typical of adipogenic differentiation (Fig. 2A–2F), as well as significantly higher levels of Oil Red O staining compared with the corresponding undifferentiated control (Fig. 2G). Finally, when data were pooled per cell type, there were no significant correlations between the amount of Oil Red O absorbance and age, where the highest R2 value was .515 (supplemental online Table 1).
Figure 2.
Oil Red O staining after culture in adipogenic medium. (A–F): Control BMSCs (A), dPDCs (B), and mPDCs (C) and induced BMSCs (D), dPDCs (E), and mPDCs (F) stained with Oil Red O. Scale bar = 100 μm. (G): Oil Red O absorbance for control and induced cells. Six replicates of each donor are included to show consistency and distribution. Asterisks (∗) indicate difference from the control (p < .05) (n = 4). Error bars indicate 95% confidence intervals. Abbreviations: BMSC, bone marrow-derived stromal cell; dPDC, enzymatically digested periosteum-derived cells; mPDC, migrated periosteum-derived cell.
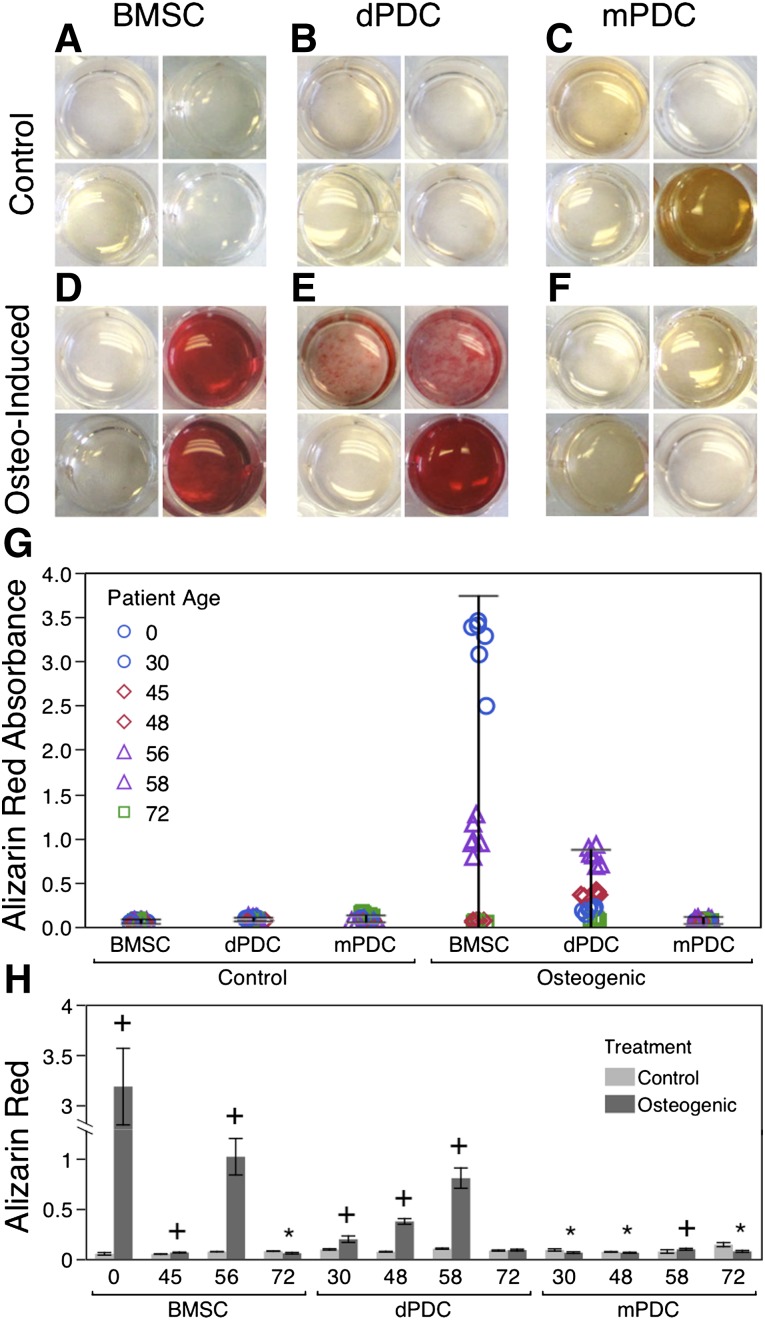
Assessment of Alizarin Red Staining After Culture in Osteogenic Medium
Neither BMSCs nor PDCs showed a statistically significant increase in alizarin red absorbance after culture in osteogenic medium. Furthermore, no differences in alizarin red absorbance were observed between the cell types (Fig. 3G). In general, high variability was seen both between cell types and between donors (Fig. 3A–3F) but not within cell cohorts from each donor (Fig. 3H). BMSCs showed higher variability in alizarin red absorbance between donors compared with the dPDCs and mPDCs, and dPDCs showed higher variability between donors than comparable mPDC cohorts from the same donors. Three out of the four BMSC donors (ages 22 weeks of gestation, 45, and 56), three of the four dPDC donors (ages 30, 48, and 58), and one of the four mPDC donors (age 58) showed a significant increase in alizarin red absorbance after culture in osteogenic medium compared with the control (Fig. 3H). Three of four mPDC donors (ages 30, 48, and 72) showed a significant decrease in alizarin red absorbance after culture in osteogenic medium compared with the control. BMSCs from the 22-week-old donor (fetal) exhibited the highest amount of mineralization (alizarin red absorbance), significantly greater than that of all other cell types. There were no significant correlations between the amount of alizarin red absorbance and age, where the highest R2 value was .515 (supplemental online Table 1).
Figure 3.
Alizarin red staining after culture in osteogenic medium. (A–F): Control BMSCs (A), dPDCs (B), and mPDCs (C) and induced BMSCs (D), dPDCs (E), and mPDCs (F), stained with alizarin red. Each well represents a single donor: top left, age 30; top right, age 48; bottom left, age 58; and bottom right, age 72. (G): Alizarin red absorbance for control and induced cells. Six replicates of each donor are included to show consistency and distribution (n = 4). Error bars indicate 95% confidence intervals. (H): Alizarin red absorbance for different donors. Plus sign (+) indicates increase from control, and asterisk (∗) indicates decrease from control (p < .05) (n = 6). Error bars indicate 95% confidence intervals. Abbreviations: BMSC, bone marrow-derived stromal cell; dPDC, enzymatically digested periosteum-derived cells; mPDC, migrated periosteum-derived cell.
Assessment of Pellet Size and Safranin O/Fast Green Staining After Culture in Chondrogenic Medium
Treatment of pellet-cultured BMSCs, dPDCs, and mPDCs with chondrogenic medium resulted in significantly larger pellets compared with those cultured in control medium (Fig. 4G). Although no statistically significant differences were observed in the size of pellets, and no obvious qualitative differences in cellularity were observed between the three groups cultured in control medium, stark differences in baseline staining of BMCs and PDCs were noted (Fig. 4A–4C). Specifically, whereas BMSC pellets exhibited faint background (matrix) staining and dark blue nuclear staining, dPDC and mPDC pellets exhibited deep maroon staining, darkening around the edges of the pellet. Pellets from all groups cultured in chondrogenic medium (Fig. 4D–4F) showed a lower density of darkly stained nuclei compared with the corresponding control pellets; these observations, in combination with the larger pellet size, provide evidence for the production of extracellular matrix (ECM) in the pellets cultured in chondrogenic medium. Furthermore, BMSC pellets cultured in chondrogenic medium showed typical pink staining for glycosaminoglycans (GAGs) (Fig. 4D) and morphological features, including enlarged lacunae around cells, typical of hyaline cartilage (supplemental online Fig. 1). In contrast, mPDC pellets cultured in chondrogenic medium resembled less mature fibrocartilage (Fig. 4F; supplemental online Fig. 1) with staining similar to that of the baseline control. dPDC pellets cultured in chondrogenic medium exhibited the least obvious morphological changes over baseline controls (Fig. 4E; supplemental online Fig. 1), perhaps indicative of even less mature fibrocartilage than the dPDC pellets. There were no significant correlations between pellet size and age; the greatest correlation (R2 = .613) was observed with mPDC chondrogenic pellet size. (supplemental online Table 1).
Figure 4.
Safranin O/Fast Green staining after culture in chondrogenic medium. (A–F): Pellets of control (A–C) and induced (D-F) BMSCs, dPDCs, and mPDCs, stained for GAG production with Safranin O/Fast Green. Scale bars = 250 and 50 μm. (G): Cross-sectional pellet area of control and induced cells. Three replicates of each donor are included to show consistency and distribution. Asterisks (∗) indicate difference from the control (p < .05) (n = 3). Error bars indicate 95% confidence intervals. Abbreviations: BMSC, bone marrow-derived stromal cell; Chondro, chondrogenic medium; dPDC, enzymatically digested periosteum-derived cells; mPDC, migrated periosteum-derived cell.
Further Statistical Analysis
No differences in data were attributable to the disease state of patients from which the periosteum was derived; specifically, when data were pooled, no significant correlations were found between “healthy” (as defined by not being treated for joint replacement) and arthritic donors, or between OA and RA donors (supplemental online Table 2). Furthermore, a power analysis was carried out based on the data from this feasibility study to determine the number of subjects needed per group to observe significant differences. First, the effect size was calculated for the outcome variable quantifying osteogenesis (Fig. 3), where data from the fetal sample were excluded from the analysis, as experimental (arthritic) and control samples would be age matched in future studies. Effect was calculated as the difference of the means divided by the difference of their standard deviations, resulting in an effect size of 0.2. For a two-sample (nonarthritic versus arthritic) t test, estimating the sample size necessary to obtain a power of 0.25 (low for biomedical studies) with significance defined as a p < .05 and an effect size of 0.2, at least 84 individuals would be needed per group of 168 patients total, for parametric testing of the data [30]. For nonparametric testing, such as that carried out in the current feasibility study, it is generally recommended to increase sample size by 15%, which would increase sample size to 97 individuals per group, or 194 total [30]. Furthermore, for the purposes of biomedical studies, a power of at least 0.8 is considered a “standard for adequacy” [31], which would result in an even higher required sample size of 393 individuals per group or 786 individuals total to show significant differences between groups.
Discussion
This study demonstrates, for the first time to our knowledge, the feasibility of isolating cells from periosteum of the femoral neck of arthritic patients. Based on the results of this study, the periosteum-derived cells exhibit remarkable similarities in proliferation rate, morphology, and surface receptor expression to commercially available bone marrow stromal cells cultured under identical in vitro conditions. Of note, both the cells from the periosteum of the femoral neck, as well as bone marrow stromal cells from commercial vendors, display marked donor-to-donor differences in multipotency as measured by Oil Red O, alizarin red, and Safranin O/Fast Green staining when cultured in respective inductive media for adipo-, osteo-, and chondrogenesis. Interestingly, the observation of no significant correlation in multipotency between OA and RA patient-derived is of particular significance, as recent rheumatological studies indicate that OA and RA share not only common biomarkers and molecular mechanisms but also common pathophysiology, resulting in the need for joint replacement. Furthermore, no significant differences in multipotency are associated with donor age or disease state per se (arthritic versus “healthy”). In sum, the use of periosteum tissue that is discarded with the femoral neck in replacing the hip is highly novel, as it represents an unprecedented and to date unstudied source of stem cells from OA and RA patients.
Although studies have demonstrated the isolation of stem cells from periosteum of long bone diaphyses, this source of cells is relatively inaccessible except in cases of trauma or tumor resection [8]. The novelty of the current study is that stem cells were isolated from femoral neck periosteal tissue resected from arthritis patients during the normal course of hip replacement surgery. Not only is this tissue highly accessible, it is usually discarded. Taken together with recent studies indicating the regenerative potential of periosteum-derived cells and periosteum transplants in vivo [7, 8, 29, 32], the use of femoral neck periosteum as a source of stem cells from arthritic tissue removed during joint replacement has profound translational implications and has never been shown before.
In general, cells from the periosteum show no significant differences in alizarin red and Oil Red O staining compared with marrow stromal cells. Although BMSCs and PDCs show similar, significant increases in pellet size when cultured in chondrogenic medium, Safranin O/Fast Green stained BMSC pellet morphology resembles more mature hyaline cartilage (supplemental online Fig. 1), whereas mPDC pellets resemble less mature fibrocartilage (supplemental online Fig. 1), and dPDC pellets exhibit the least morphological change over baseline controls. Interestingly, cell cohorts derived from the same periosteal tissue using different cell isolation protocols show intrinsic differences in their multipotency. Given the striking similarity in differentiation behavior of PDCs and BMSCs under the in vitro conditions of the current study, it is interesting to note the particular ways in which they did vary. Notably, PDCs expressed MSC surface markers, including CD73, CD90, and CD105, more consistently (all three markers at greater than 99%) and at significantly higher levels (CD90 and CD105) than BMSCs. Although high levels of these surface antigens indicate the isolation of an MSC-like population of cells, they can persist in the phenotype even after the loss of multipotency [33]. As a result, the expression of such MSC typical surface markers can assist in the identification of potential multipotent cell populations, but the performance of in vitro tests in inductive media and in vivo experimentation are necessary to confirm multipotency and respective target tissue genesis.
In addition to relative comparison of multipotency between cell types, effects of intraindividual variability may profoundly affect the implications of this study, for example, for clinical implementation within a naturally diverse patient population. Although all cell types demonstrated similar potentials (small intraindividual differences) when cultured in adipogenic medium, intraindividual differences in alizarin red staining were unexpectedly large for all cell types when cultured in osteogenic medium. Follow-up studies for specific markers of osteogenic differentiation—for example, at the level of protein transcription (reverse transcription polymerase chain reaction [rtPCR]) or secretion (immunohistochemistry) [34]—will allow for precise comparison of intrinsic differentiation capacities between mPDCs, dPDCs and BMSCs. Furthermore, mPDCs and dPDCs demonstrated similar fibrous ECM formation in chondrogenic medium; although small intraindividual variability in pellet size was observed, follow-up studies using, for example, rtPCR and/or immunohistochemistry may shed light on mechanisms of chondrogenesis and nascent tissue maturation, similar to use of rtPCR to elucidate intramembranous and endochondral osteogenesis mechanisms [34].
Intraindividual variability in osteogenic capacity has been reported previously for both BMSCs and PDCs [14, 35]. The intraindividual variability in alizarin red staining observed in the current study could be attributed to the range of individual ages, disease states, or inherent differences in cell acquisition and/or maintenance. Given the power analysis, carried out in context of the current study and indicating the need for samples from more than 800 patients, a potentially more effective approach for follow-up studies would be to prescreen cells derived from periosteum of femoral necks for their relative differentiation capacity. Cells showing adequate multipotency could be cryopreserved as in the current study and then passaged to test the same hypotheses on a larger cohort of individuals to elucidate how best to harness the cells’ regenerative capacity in a translational context.
Interestingly, this study shows that dPDCs and mPDCs show intrinsic differences in staining characteristics after culture in adipo-, osteo-, and chondrogenic induction media. Although no statistically significant differences were found between cell types, differences in staining characteristics of dPDCs and mPDCs were observed, most strikingly in the osteogenic and chondrogenic inductive media; this was not expected. These differences are of particular importance, since the mPDCs and dPDCs from each donor were isolated from the same small section of tissue. On the one hand, the enzymatic digestion of PDCs is a faster method of acquiring cells but does use biochemical means, which may have unknown effects on cell behavior. On the other hand, isolation of cells using the migration protocol takes longer and appears to select for a different population of cells [1, 36]. Taken as a whole, the data from the current study strongly suggest either that the isolation method of migration or enzymatic digestion selects for different subpopulations of the total native PDC population or that isolation methods per se affect the differentiation capacities of the acquired cells. The implications of this observation are important, as the method of cell acquisition may play a larger role in determining stem cell population and function than previously assumed.
Furthermore, the results of this study indicate that commercially available BMSCs do not ensure consistent multipotency with typical differentiation protocols. Despite the fact that most commercially available BMSCs are tested for positive and negative surface markers and in vitro differentiation capabilities upon sample acquisition, the loss of these capabilities due to storage, continuous expansion, or altered culture conditions is not well understood. Additionally, most companies strongly recommend the use of their proprietary culture media and in vitro adipogenic, osteogenic, and chondrogenic kits to optimize differentiation results. For the sake of consistency, the current study used the same standardized culture conditions (as described in Materials and Methods) for all cell types (although tests using some proprietary media as a cross-control did not yield results different from those reported here; data not shown). Furthermore, primary cells that were cryopreserved and then passaged at most four times were used to maximize the clinical relevance of the work. Although colony forming capacity and/or clonal analysis would be interesting to study in the future, we aimed to use the cells as they would likely be used in the clinic, for example, with resection and cryopreservation of either the isolated cells or the tissue as a whole and later expansion for regenerative medicine therapies using autologous, patient-banked cells.
Ultimately, considering clinical relevance, the sensitivity of BMSCs and PDCs to different culture media and protocols is secondary to the in vivo tissue-building capacity of these cells. Furthermore, although in vitro results are strongly indicative of multipotency, in vitro culture is insufficient for accurately mimicking a true in vivo environment. Additional tests of in vivo tissue and colony forming capacities, as well as passagability of these cells, should be conducted using the most promising groups from these in vitro experiments. These tests would likely be carried out using human cells in SCID animal models, or in human patients after follow-up testing of differentiation capacity in prescreened, larger cohorts.
Finally the femoral neck PDC in vitro culture results are not consistent with previously published diaphyseal PDC results, which have demonstrated both in vitro and in vivo adipogenic, osteogenic, chondrogenic, and myogenic differentiation capabilities in single-cell lineages [7]. However, most published studies on long bone human PDCs have used diaphyseal periosteum tissue from diaphyseal regions of the tibia or femur of healthy patients. Since these regions are not routinely accessed during, for example, joint replacement surgery, they do not make ideal tissue harvesting sites compared with the proximal or distal femur or the proximal tibia. PDCs have exhibited distinctive characteristics depending on the site of the harvest [36–38]. Therefore, further investigation is necessary to determine whether there are significant differences between femoral neck periosteum and periosteum of the femoral diaphysis.
Additional factors that may contribute to the observed discrepancies in PDC multipotency are the medical conditions of the donors, in particular in relation to diseases with involvement of proinflammatory factors. Total hip arthroplasty is most commonly performed on patients suffering from bone or joint disorders; therefore, it is essential to properly understand how stem cell populations are affected by these disorders as well. Previously published studies report that BMSCs of patients with osteoarthritis show mixed results in their differentiation capacities compared with healthy patients [39–41], and BMSCs, synovial tissue-derived stem cells, and adipose tissue-derived stem cells of patients diagnosed with rheumatoid arthritis also show mixed results in their cell reserve, renewal, and differentiation capacities compared with those of healthy patients [42–44]. Given the results of the current study, no correlations could be made between OA and RA disease state and differentiation capacity; these results are particularly intriguing given a new paradigm in rheumatology where OA and RA are considered diseases with common biomarkers and molecular mechanisms and with a pathophysiology leading ultimately to joint replacement. Specific effects of OA or RA on PDCs are unknown, and it is unclear whether such diseases equally affect multipotent cells residing in different tissues. Additional follow-up studies will be important to identify factors allowing for best isolation practices for autologous stem cell source in patients with disease, and these studies will help solidify the rationale for using arthritic tissues resected during the normal course of hip replacement as a source for autologous stem cells.
Additional studies examining the effects of cryopreservation temperature and duration as well as cell viability and multipotency will be required if PDCs are to be considered for long-term cell banking purposes. Furthermore, although the cells used for this study did not exceed P5, PDCs have been shown to retain differentiation capacity and not become senescent for many population doublings [45]; these characteristics could indicate an advantage of the periosteum over other tissue sources for industrial or commercial tissue engineering applications.
Conclusion
PDCs isolated from the femoral neck of donors with osteoarthritis or rheumatoid arthritis exhibit many defining characteristics similar to BMSCs. mPDCs, dPDCs, and BMSCs demonstrate similar multipotency as measured by staining assays for adipo-, osteo-, and chondrogenesis under identical in vitro culture conditions. Furthermore, PDCs and BMSCs show no significant difference in alizarin red staining when cultured under identical in vitro osteogenic conditions. PDCs from the femoral neck of joint replacement patients may exhibit the capacity to form fibrocartilage in vitro, whereas BMSCs show hyaline cartilage forming capacity (supplemental online Fig. 1). Moreover, cell isolation protocol may exert a previously unappreciated influence on the subpopulation or differentiation capacity of cells originating from the same tissue. In addition, the intraindividual variability in PDCs is comparable to that seen in commercially acquired BMSCs. Taken together, although the femoral neck periosteum offers a feasible source for isolation and a possible source for banking of autologous multipotent cells with similar potential as BMSCs in vitro, further studies are needed to determine the regenerative capacity of these cells in a more physiological context, such as in vivo. Looking toward the future, prescreening and larger scale studies allowing for inter- and intraindividual comparisons of proliferation and differentiation capacities between BMSCs, PDCs, and potentially other multipotent adult cells derived from specific tissues (e.g., adipose tissue) will provide standards for implementation of autologous multipotent cells and thus a foundation for clinical translation.
Supplementary Material
Acknowledgments
We acknowledge the expert advice of Prof. Stefan Milz of the Institute for Anatomy at Ludwig Maximilians University in Munich, Drs. Thomas Bauer and Ang Liu from the Orthopaedic Pathology Section of the Cleveland Clinic Pathology Department, the assistance of Teresa Pizzuto from the Hard Tissue Histology Core, and Favia Merritt of Case Western Reserve University Medical School. This research was supported by the Training Program in Musculoskeletal Research, NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 2T32AR007505 (H.C.), National Science Foundation Grant CMMI-0826435 (M.L.K.T. and H.C.), an Alexander von Humboldt Foundation Fellowship Award for Senior Researchers (M.L.K.T.), and the Cleveland Clinic Department of Orthopaedic Surgery. This research was also supported by the Cytometry & Imaging Microscopy Core Facility of the Case Comprehensive Cancer Center (Grant P30 CA43703), Hard Tissue Histology Core Case Medical Center, Cleveland Clinic Tissue Procurement Center, Cleveland Clinic Orthopaedic Pathology, and the University of New South Wales. Dr. Michael Sramkoski from the Cytometry & Imaging Microscopy Core Facility at Case Western Reserve University helped us immensely with the antibody selection and sample analysis.
Author Contributions
H.C.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; D.D.: conception and design, final approval of manuscript; U.R.K: conception and design, financial support, provision of study material or patients, final approval of manuscript; M.L.K.T.: conception and design, financial support, data analysis and interpretation, manuscript writing, final revision and approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Chang H, Knothe Tate ML. Concise review: The periosteum: Tapping into a reservoir of clinically useful progenitor cells. Stem Cells Translational Medicine. 2012;1:480–491. doi: 10.5966/sctm.2011-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thirumala S, Goebel WS, Woods EJ. Clinical grade adult stem cell banking. Organogenesis. 2009;5:143–154. doi: 10.4161/org.5.3.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badowski MS, Harris DT. Collection, processing, and banking of umbilical cord blood stem cells for transplantation and regenerative medicine. Methods Mol Biol. 2012;879:279–290. doi: 10.1007/978-1-61779-815-3_16. [DOI] [PubMed] [Google Scholar]
- 4.Ginis I, Grinblat B, Shirvan MH. Evaluation of bone marrow-derived mesenchymal stem cells after cryopreservation and hypothermic storage in clinically safe medium. Tissue Eng Part C Methods. 2012;18:453–463. doi: 10.1089/ten.TEC.2011.0395. [DOI] [PubMed] [Google Scholar]
- 5.Dhanasekaran M, Indumathi S, Poojitha R, et al. Plasticity and banking potential of cultured adipose tissue derived mesenchymal stem cells. Cell Tissue Bank. 2013;14:303–315. doi: 10.1007/s10561-012-9311-7. [DOI] [PubMed] [Google Scholar]
- 6.Kerkis I, Caplan AI. Stem cells in dental pulp of deciduous teeth. Tissue Eng Part B Rev. 2012;18:129–138. doi: 10.1089/ten.teb.2011.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bari C, Dell’Accio F, Vanlauwe J, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54:1209–1221. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]
- 8.Knothe Tate ML, Chang H, Moore SR, et al. Surgical membranes as directional delivery devices to generate tissue: Testing in an ovine critical sized defect model. PLoS ONE. 2011;6:e28702. doi: 10.1371/journal.pone.0028702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breitbart AS, Grande DA, Kessler R, et al. Tissue engineered bone repair of calvarial defects using cultured periosteal cells. Plast Reconstr Surg. 1998;101:567–574; discussion 575–576. doi: 10.1097/00006534-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Wakitani S, Goto T, Pineda SJ, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi Y, Sekiya I, Yagishita K, et al. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 12.Ng AM, Saim AB, Tan KK, et al. Comparison of bioengineered human bone construct from four sources of osteogenic cells. J Orthop Sci. 2005;10:192–199. doi: 10.1007/s00776-004-0884-2. [DOI] [PubMed] [Google Scholar]
- 13.Stockmann P, Park J, von Wilmowsky C, et al. Guided bone regeneration in pig calvarial bone defects using autologous mesenchymal stem/progenitor cells - a comparison of different tissue sources. J Craniomaxillofac Surg. 2012;40:310–320. doi: 10.1016/j.jcms.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Siddappa R, Licht R, van Blitterswijk C, et al. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res. 2007;25:1029–1041. doi: 10.1002/jor.20402. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. FastStats: Inpatient surgery. Available at http://www.cdc.gov/nchs/fastats/insurg.htm Accessed March 25, 2013
- 16.Malemud CJ, Schulte ME. Is there a final common pathway for arthritis? Future Rheumatol. 2008;3:253–268. [Google Scholar]
- 17.Xue F, Zhang C, He Z, et al. Analysis of critical molecules and signaling pathways in osteoarthritis and rheumatoid arthritis. Mol Med Rep. 2013;7:603–607. doi: 10.3892/mmr.2012.1224. [DOI] [PubMed] [Google Scholar]
- 18.Duijnisveld BJ, Bigot A, Beenakker KG, et al. Regenerative potential of human muscle stem cells in chronic inflammation. Arthritis Res Ther. 2011;13:R207. doi: 10.1186/ar3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endres M, Andreas K, Kalwitz G, et al. Chemokine profile of synovial fluid from normal, osteoarthritis and rheumatoid arthritis patients: CCL25, CXCL10 and XCL1 recruit human subchondral mesenchymal progenitor cells. Osteoarthritis Cartilage. 2010;18:1458–1466. doi: 10.1016/j.joca.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto D, Kuroda S, Kizawa T, et al. Equivalent osteoblastic differentiation function of human mesenchymal stem cells from rheumatoid arthritis in comparison with osteoarthritis. Rheumatology (Oxford) 2009;48:643–649. doi: 10.1093/rheumatology/kep044. [DOI] [PubMed] [Google Scholar]
- 21.Allen MR, Burr DB. Human femoral neck has less cellular periosteum, and more mineralized periosteum, than femoral diaphyseal bone. Bone. 2005;36:311–316. doi: 10.1016/j.bone.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Moore SR, Milz S, Knothe Tate ML. Relation of human periosteal thickness, cellularity to age and loading history. J Anat. 2013 doi: 10.1111/joa.12133. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karamzadeh R, Eslaminejad MB, Aflatoonian R. Isolation, characterization and comparative differentiation of human dental pulp stem cells derived from permanent teeth by using two different methods. J Vis Exp. 2012;69:e4372. doi: 10.3791/4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Futami I, Ishijima M, Kaneko H, et al. Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PLoS ONE. 2012;7:e45517. doi: 10.1371/journal.pone.0045517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reich CM, Raabe O, Wenisch S, et al. Isolation, culture and chondrogenic differentiation of canine adipose tissue- and bone marrow-derived mesenchymal stem cells—a comparative study. Vet Res Commun. 2012;36:139–148. doi: 10.1007/s11259-012-9523-0. [DOI] [PubMed] [Google Scholar]
- 26.McBride SH, Evans SF, Knothe Tate ML. Anisotropic mechanical properties of ovine femoral periosteum and the effects of cryopreservation. J Biomech. 2011;44:1954–1959. doi: 10.1016/j.jbiomech.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans SF, Parent JB, Lasko CE, et al. Periosteum, bone’s “smart” bounding membrane, exhibits direction-dependent permeability. J Bone Miner Res. 2013;28:608–617. doi: 10.1002/jbmr.1777. [DOI] [PubMed] [Google Scholar]
- 28.Sauser B. Faster healing for severe fractures. MIT Technology Review 2010. Available at http://www.technologyreview.com/printer_friendly_article.aspx?id=24711 Accessed March 15, 2011.
- 29.Knothe UR, Springfield DS. A novel surgical procedure for bridging of massive bone defects. World J Surg Oncol. 2005;3:7. doi: 10.1186/1477-7819-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenny DA. Statistics for the Social and Behavioral Sciences. Boston, MA: Little, Brown; 1987. The two-group design. pp. 203–223. [Google Scholar]
- 31.Ellis PD. The Essential Guide to Effect Sizes: An Introduction to Statistical Power, Meta-Analysis and the Interpretation of Research Results. Cambridge, U.K.: Cambridge University Press; 2010. [Google Scholar]
- 32.Knothe UR, Dolejs S, Miller RM, et al. Effects of mechanical loading patterns, bone graft, and proximity to periosteum on bone defect healing. J Biomech. 2010;43:2728–2737. doi: 10.1016/j.jbiomech.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 33.Coipeau P, Rosset P, Langonne A, et al. Impaired differentiation potential of human trabecular bone mesenchymal stromal cells from elderly patients. Cytotherapy. 2009;11:584–594. doi: 10.1080/14653240903079385. [DOI] [PubMed] [Google Scholar]
- 34.Knothe Tate ML, Falls TD, McBride SH, et al. Mechanical modulation of osteochondroprogenitor cell fate. Int J Biochem Cell Biol. 2008;40:2720–2738. doi: 10.1016/j.biocel.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonardi E, Devescovi V, Perut F, et al. Isolation, characterisation and osteogenic potential of human bone marrow stromal cells derived from the medullary cavity of the femur. Chir Organi Mov. 2008;92:97–103. doi: 10.1007/s12306-008-0057-0. [DOI] [PubMed] [Google Scholar]
- 36.Hutmacher DW, Sittinger M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003;9(suppl 1):S45–S64. doi: 10.1089/10763270360696978. [DOI] [PubMed] [Google Scholar]
- 37.Gallay SH, Miura Y, Commisso CN, et al. Relationship of donor site to chondrogenic potential of periosteum in vitro. J Orthop Res. 1994;12:515–525. doi: 10.1002/jor.1100120408. [DOI] [PubMed] [Google Scholar]
- 38.Bilkay U, Tokat C, Helvaci E, et al. Osteogenic capacities of tibial and cranial periosteum: a biochemical and histologic study. J Craniofac Surg. 2008;19:453–458. doi: 10.1097/SCS.0b013e318052fe3d. [DOI] [PubMed] [Google Scholar]
- 39.Jones E, English A, Churchman SM, et al. Large-scale extraction and characterization of CD271+ multipotential stromal cells from trabecular bone in health and osteoarthritis: Implications for bone regeneration strategies based on uncultured or minimally cultured multipotential stromal cells. Arthritis Rheum. 2010;62:1944–1954. doi: 10.1002/art.27451. [DOI] [PubMed] [Google Scholar]
- 40.Scharstuhl A, Schewe B, Benz K, et al. Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells. 2007;25:3244–3251. doi: 10.1634/stemcells.2007-0300. [DOI] [PubMed] [Google Scholar]
- 41.Murphy JM, Dixon K, Beck S, et al. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 42.Jones E, Churchman SM, English A, et al. Mesenchymal stem cells in rheumatoid synovium: Enumeration and functional assessment in relation to synovial inflammation level. Ann Rheum Dis. 2010;69:450–457. doi: 10.1136/ard.2008.106435. [DOI] [PubMed] [Google Scholar]
- 43.Skalska U, Kontny E, Prochorec-Sobieszek M, et al. Intra-articular adipose-derived mesenchymal stem cells from rheumatoid arthritis patients maintain the function of chondrogenic differentiation. Rheumatology (Oxford) 2012;51:1757–1764. doi: 10.1093/rheumatology/kes129. [DOI] [PubMed] [Google Scholar]
- 44.Papadaki HA, Kritikos HD, Gemetzi C, et al. Bone marrow progenitor cell reserve and function and stromal cell function are defective in rheumatoid arthritis: Evidence for a tumor necrosis factor alpha-mediated effect. Blood. 2002;99:1610–1619. doi: 10.1182/blood.v99.5.1610. [DOI] [PubMed] [Google Scholar]
- 45.De Bari C, Dell’Accio F, Luyten FP. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001;44:85–95. doi: 10.1002/1529-0131(200101)44:1<85::AID-ANR12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.