In this study of the effect of human Müller glia-derived photoreceptors in the restoration of rod photoreceptor function, cells migrated and integrated into the degenerate rat retina and led to significant improvement in rod photoreceptor function. These observations suggest that human Müller glia with stem cell characteristics are a potential cell source for the development of cell-replacement therapies to treat human photoreceptor degeneration and may also offer potential for the development of autologous transplantation.
Keywords: Repair and regeneration, Müller glia, Stem cells, Retina, Photoreceptors, Transplantation
Abstract
Müller glia possess stem cell characteristics that have been recognized to be responsible for the regeneration of injured retina in fish and amphibians. Although these cells are present in the adult human eye, they are not known to regenerate human retina in vivo. Human Müller glia with stem cell characteristics (hMSCs) can acquire phenotypic and genotypic characteristics of rod photoreceptors in vitro, suggesting that they may have potential for use in transplantation strategies to treat human photoreceptor degenerations. Much work has been undertaken in rodents using various sources of allogeneic stem cells to restore photoreceptor function, but the effect of human Müller glia-derived photoreceptors in the restoration of rod photoreceptor function has not been investigated. This study aimed to differentiate hMSCs into photoreceptor cells by stimulation with growth and differentiation factors in vitro to upregulate gene and protein expression of CRX, NR2E3, and rhodopsin and various phototransduction markers associated with rod photoreceptor development and function and to examine the effect of subretinal transplantation of these cells into the P23H rat, a model of primary photoreceptor degeneration. Following transplantation, hMSC-derived photoreceptor cells migrated and integrated into the outer nuclear layer of the degenerated retinas and led to significant improvement in rod photoreceptor function as shown by an increase in a-wave amplitude and slope using scotopic flash electroretinography. These observations suggest that hMSCs can be regarded as a cell source for development of cell-replacement therapies to treat human photoreceptor degenerations and may also offer potential for the development of autologous transplantation.
Introduction
The unique capacity of fish and amphibians to regenerate retina throughout life has been well established [1], with Müller glia arising from a common multipotent retinal progenitor [2] constituting the source of new neurons for the regenerative process observed in these species. Although retinal regeneration has not been observed in higher mammals, Müller glia with stem cell characteristics have been identified in lower mammals [3, 4] and also within the adult human retina [5, 6]. In recent years, transplantation studies to restore photoreceptor function have been successful when performed in rodent models using allogeneic retinal cells sourced from various stages of development [7–9] as well as photoreceptors derived from both human embryonic [10] and induced pluripotent [11] stem cells. Stem cells derived from the adult human retina offer a novel opportunity to develop cell-based therapies for human application that may circumvent many concerns regarding the use of embryonic and pluripotent cells [12, 13] while offering the potential for autologous therapies.
Proliferation and differentiation of Müller glia toward rod photoreceptors in response to injury, growth factors, or modulation of the Notch pathway in lower mammals [3, 14–18] suggest that the neurogenic capacity of Müller glia to facilitate photoreceptor regeneration seen in fish and amphibians [19] may be conserved to some extent within the mature retina of mammalian species in vivo. It was initially reported that the ontogenetic stage of transplanted rod precursors was crucial to successful integration and functionality in the mouse eye [8]. However, recent reports have shown that mature rods are also able to successfully integrate within the host retina, suggesting that the ontogenetic stage of the donor cell may be less critical than previously thought [7].
Previous studies have shown that human Müller glia have the ability to differentiate in vitro toward rod photoreceptors [20], but the capacity of these cells to integrate within the host retina following transplantation and to induce any functional improvement of rod function has yet to be investigated. In this study, we developed an in vitro protocol to differentiate human Müller glia with stem cell characteristics (hMSCs) toward rod photoreceptors and examined the functional outcomes of subretinal transplantation of these differentiated cells into a rodent model of primary rod photoreceptor degeneration. The outcome of transplantation was examined by immunohistochemical analysis of the transplanted retina ex vivo in order to assess cellular migration and integration as well by investigation of changes in rod photoreceptor function in vivo using scotopic flash electroretinography (ERG).
Materials and Methods
Isolation and Culture of hMSCs
Isolation and culture of hMSCs were performed, as previously reported [6]. Briefly, hMSCs were isolated from the neural retina of donor human eyes using trypsin/EDTA at 37°C. The resultant cell suspension was centrifuged and the cells placed in culture on fibronectin-coated flasks in Dulbecco’s modified Eagle’s medium, 10% fetal calf serum, and epidermal growth factor (40 ng/ml). Colonies were formed at 3–4 weeks, and confluent monolayers were obtained after another 1–2 weeks. After this period of time, cells that expressed nestin and CRALBP continued to grow indefinitely and exhibited stem cell characteristics [6]. Using modifications of published protocols [21–23], optimization of photoreceptor differentiation was achieved by culturing hMSCs for 5 days in flasks coated with basement membrane protein (bMP; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) in the presence of 20 ng/ml recombinant human basic fibroblast growth factor (FGF2; Peprotech, Rocky Hill, NJ, http://www.peprotech.com), 20 μM taurine (Sigma-Aldrich), 5 μM retinoic acid (Sigma-Aldrich), and 100 ng/ml insulin-like growth factor type 1 (IGF-1; Peprotech).
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction Analysis
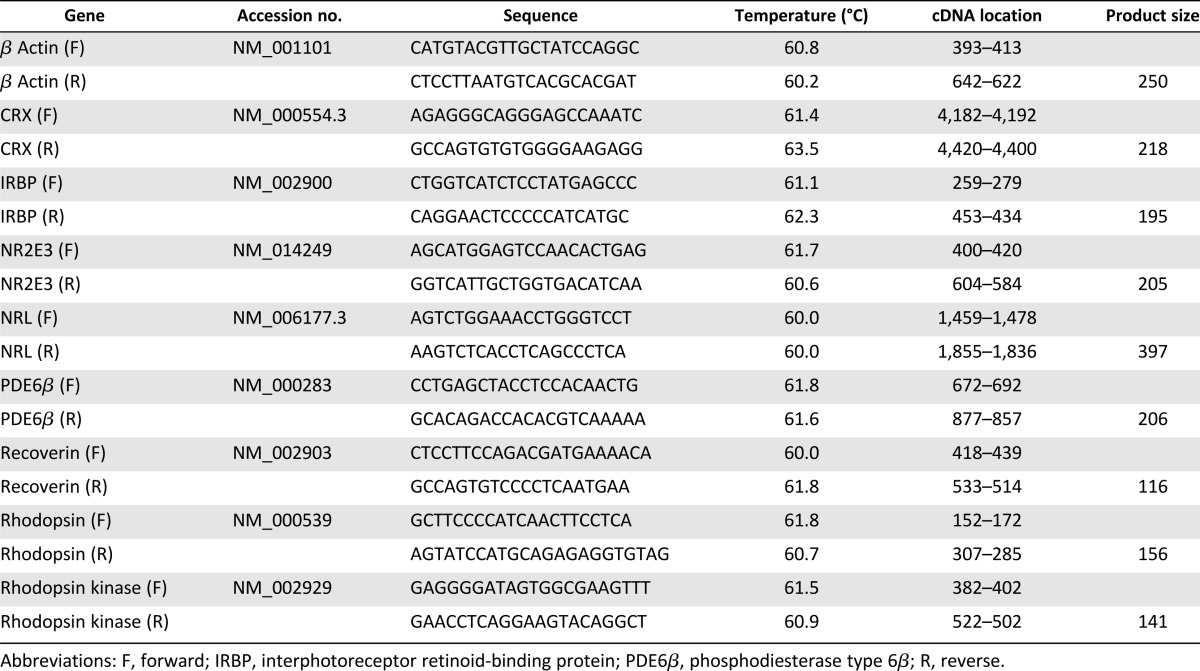
Total cellular RNA was isolated from cell pellets using the RNeasy system according to the manufacturer’s instructions (Qiagen, Hilden, Germany, http://www.qiagen.com). cDNA was generated by reverse transcription of 500 ng of total in 20-μl reactions containing 5 mM MgCl2, 1 mM dNTP, 1 U of RNAse inhibitor, 0.75 U of avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI, http://www.promega.com), and 25 ng/μl oligo(dT)-15 primers (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) in 10 mM Tris/HCl buffer containing 50 mM KCl for 40 minutes at 42°C and 5 minutes at 95°C in a thermal cycler (Mastercycler Pro; Eppendorf, Hamburg, Germany, http://www.eppendorf.com). Polymerase chain reaction (PCR) amplification was performed using primers derived from the GenBank database (sequences shown in Table 1). Amplification was performed in a 50-μl volume by addition of 1.5 mM MgCl2, 0.2 mM dNTP, 2U Taq DNA polymerase (Promega), 0.5 μM primers in 50 mM KCl, and 10 mM Tris/HCl, pH 8.0. The mixture was incubated at 94°C for 5 minutes, followed by 34 cycles of 94°C for 1 minute, 58°C for 1 minute, and 72°C for 1 minute, and 1 cycle of 72°C for 5 minutes. Kinetic analysis of amplified products was applied to all samples for each gene to ensure that signals derived only from the exponential phase of amplification. PCR products were analyzed by agarose gel electrophoresis (1%) containing 25 ng/ml ethidium bromide.
Table 1.
Primers
Confocal Microscopy
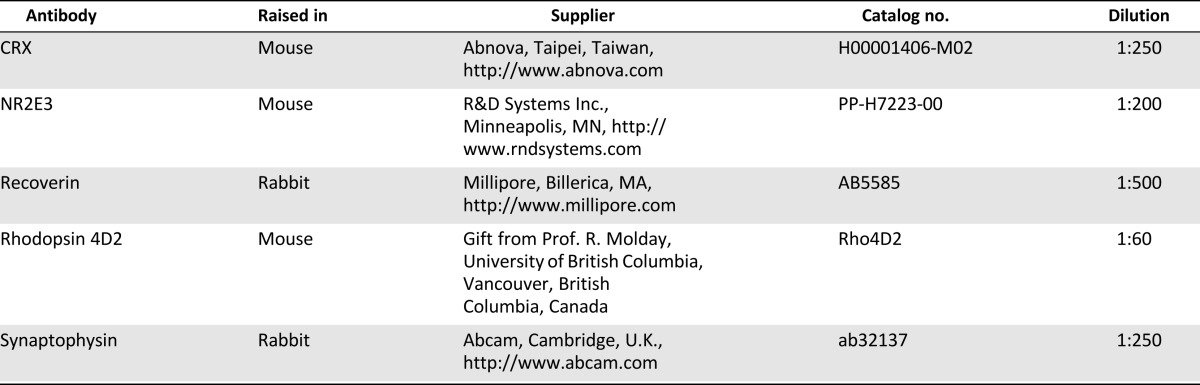
For immunocytochemistry, cells were cultured on bMP-coated (10 μg/ml) Lab-Tek glass chamber slides (Nunc, Rochester, NY, http://www.nuncbrand.com). After fixation with 4% paraformaldehyde in phosphate-buffered saline (PBS), cells or cryosections of rodent eyes were blocked for 1 hour in 0.3% Triton-X in Tris-buffered saline and incubated overnight at 4°C with primary antibodies diluted in the blocking solution. Details of the antibodies used are shown in Table 2. Mouse IgG isotypes matching test antibodies were used as negative controls. After incubation with primary antibodies, specimens were washed in Tris-buffered saline, followed by a 2-hour incubation with Alexa-conjugated secondary antibodies (Invitrogen). Slides were washed and counterstained with 2 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) for 1 minute and mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com). Fluorescent images were recorded using a Zeiss LSM 510 confocal microscope operating in multitrack mode for fluorescein isothiocyanate, DAPI, and Cy5 fluorochromes.
Table 2.
Primary antibodies
Transplantation Studies
The use of animals in this study was in accordance with the U.K. Home Office regulations for the care and use of laboratory animals and the Animals (Scientific Procedures) Act (1986). Animals were kept under a 12-hour/12-hour light-dark cycle (light cycle mean illumination: 30 candelas [cd]/m2) and had ad libitum access to food and water. In order to trace the transplanted cells, hMSCs were transfected with a retroviral vector containing enhanced green fluorescent protein (GFP; Clontech, Palo Alto, CA, http://www.clontech.com) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Transgenic P23H (line 1) rats were produced by Xenogen Biosciences (Cranbury, NJ, http://www.taconic.com) and developed and supplied with the support of the National Eye Institute by Dr. Matthew LaVail, University of California San Francisco. These animals have a proline-to-histidine substitution at codon 23 of the rhodopsin gene, a mutation commonly associated with human autosomal dominant retinitis pigmentosa [24]. Heterozygous P23H-1 rats were generated by mating homozygous P23H (line 1) with wild-type Sprague-Dawley rats. Heterozygotes display a rapid primary photoreceptor degeneration with a 50% loss of outer nuclear layer thickness observed at 3 weeks of age [25]. This particular line were chosen because of the development of a significant degree of retinal degeneration by 7 weeks of age, allowing for the determination of any structural and functional rescue by the various experimental treatments. The presence of the transgene in all experimental studies was confirmed by reverse transcription PCR using RNA isolated from the liver at the end of the experiment.
To avoid severe xenorejection, all animals were tolerized at birth by intraperitoneal injection of 105 hMSCs, using established protocols [26, 27]. Although animals that later received human Tenon’s fibroblasts were not specifically tolerized with this cell type, based on our previous work, neonatal desensitization to human epitopes together with additional immunosuppression produced a satisfactory reduction of the host immune response to xenotransplantation. Animals used in the experiments were anesthetized for surgery and immunosuppressed with oral cyclosporine A (20 mg/l), prednisolone (5 mg/l), and azathioprine (25 mg/l) in the drinking water for 2 days prior to transplantation and for the duration of the experiment, as previously described [6, 28]. Subretinal injections were performed into one eye under direct vision using an operating microscope following pupil dilation with tropicamide 1% eye drops, with the unoperated fellow eye serving as an internal control. Following application of 5% povidone iodine drops, the dorsolateral conjunctiva was opened out with a single cut followed by blunt dissection, and a 30-gauge metal needle attached to a glass Hamilton syringe containing the materials to be injected was inserted into the subretinal space. A total volume of 2 μl was delivered into the subretinal space comprising 1 μl of the cell suspension containing 4 × 104 cells per microliter of either photoreceptor differentiated cells (as determined by the expression of the photoreceptor markers CRX, NR2E3, recoverin, and rhodopsin), undifferentiated hMSCs or human Tenon’s fibroblasts (control) combined with 0.5 μl (40 μg) triamcinolone acetonide (washed and reconstituted in sterile water to 80 mg/ml) (Kenalog; Bristol-Myers Squibb, New York, NY, http://www.bms.com), and 0.5 μl chondroitinase (0.04 U/μl) (Chondroitinase ABC Protease Free; AMS Biotechnology, Abingdon, U.K., http://www.amsbio.com), as previously described [27, 28]. A combination of topical steroid and antibiotic eye drops (Maxitrol; Alcon, Hünenberg, Switzerland, http://www.alcon.com) was applied to the eye at the end of the procedure. Under terminal anesthesia and following intracardiac perfusion with 4% paraformaldehyde in PBS, eyes were enucleated, washed in PBS, and cryoprotected in 30% sucrose. OCT-embedded eyes were sectioned at a thickness of 15–20 μm, and the fate of the transplanted cells was investigated by costaining with antibodies to neural retinal markers, as described above, and confocal microscopy.
ERG
Scotopic ERG was performed in all animals at 4 weeks following transplantation. Animals were dark adapted overnight and prepared for ERG under dim red light illumination. The animals were anesthetized, followed by administration of proxymetacaine drops as a topical anesthetic and tropicamide drops in order to achieve pupillary dilation.
ERG was performed using the Diagnosys system and Espion software (Diagnosys, Lowell, MA, http://www.diagnosysllc.com). Once anesthetized the animal was placed on a heated table beneath the Ganzfeld stimulator, which was located inside a Faraday cage. A subcutaneous ground electrode was inserted near to the base of the tail, and a subcutaneous reference electrode was inserted on the nasal bridge between the eyes. Gold wire loop electrodes were placed onto each cornea and lubricated with hypromellose eye drops. Animals were allowed to dark adapt for a further 10 minutes prior to commencing the stimulation protocol.
Simultaneous bilateral recordings were taken using scotopic ERG protocols based on the International Society for Clinical Electrophysiology of Vision standards [29]. Responses were elicited with either short duration LED flashes or a xenon strobe delivered from within the Ganzfeld dome with interstimulus intervals of 5–60 seconds, depending on the stimulus intensity. The flash intensities ranged from 6 × 10−6 to 722 cd⋅s⋅m−2 and increased sequentially in 0.25- to 0.5-log steps. Two to five responses were averaged for each step, depending on the stimulus intensity. ERG responses were filtered using low-pass (0.15 Hz) and high-pass (100 Hz) filters and collected with the Espion software for subsequent analysis. The a-wave amplitude was measured from the prestimulus baseline to the peak of the first trough, and the b-wave was measured from the prestimulus baseline or a-wave trough to the maximum positive value. The slope of the leading edge of the a-wave was measured by examining the first 4 ms of the a-wave downslope with the gradient for each trace being calculated directly from the measuring tools within the software package. Both of these parameters are established and well-accepted measures of rod photoreceptor function [30–33].
Statistical Analysis
Gene expression results were analyzed using the paired two-tailed Student t test. ERG data were analyzed using one-way analysis of variance with Bonferroni post-test correction and paired two-tailed Student’s t test where appropriate. Statistical analyses and creation of graphs were carried out using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA, http://www.graphpad.com).
Results
Acquisition of Rod Photoreceptor Precursor Phenotype by hMSCs In Vitro
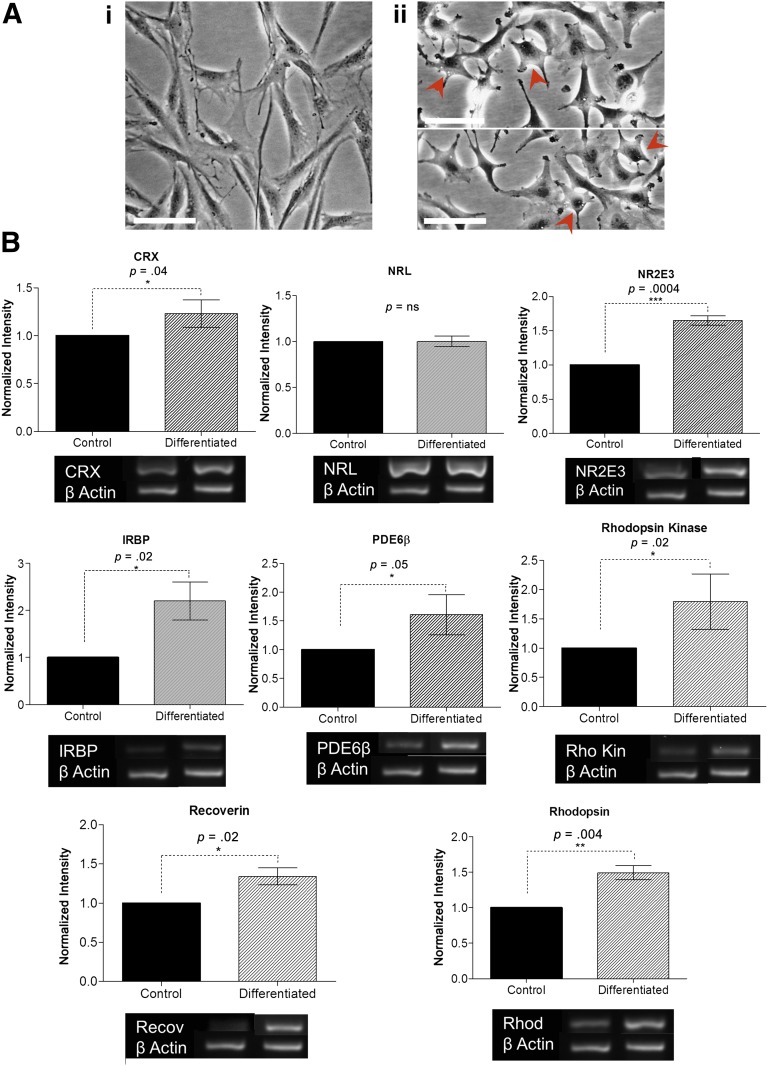
In order to optimize the differentiation of hMSCs toward a photoreceptor fate in vitro, we evaluated factors previously studied in the development of photoreceptor differentiation protocols for pluripotent stem cells [21–23]. Cells cultured on bMP in the presence of FGF2, taurine, retinoic acid, and IGF-1 for 5 days exhibited marked changes in cellular morphology consistent with previous reports of photoreceptors in culture. These cells exhibited shorter and condensed cell bodies, phase bright nuclei, prominent nuclear chromatin, a reduced cytoplasm-nucleus ratio, and short elongations resembling small neurite-like processes (Fig. 1A). Qualitative analysis of cell cultures when compared with control conditions demonstrated a greater proportion of cells exhibiting this characteristic morphology [34–38].
Figure 1.
In vitro differentiation with recombinant human basic fibroblast growth factor, taurine, retinoic acid, and insulin-like growth factor type 1 on basement membrane protein induces morphological changes and upregulates rod photoreceptor gene expression in human Müller glia with stem cell characteristics (hMSCs). (Ai): hMSCs cultured in the absence of growth or differentiation factors exhibit a typical elongated glial morphology. (Aii): hMSCs cultured for 5 days in the presence of fibroblast growth factor, taurine, retinoic acid, and insulin-like growth factor type 1 exhibited a marked change in cellular morphology, as seen with phase microscopy, with condensed cell bodies, phase bright nuclei, prominent nuclear chromatin, and several short neurite-like processes, similar to previous published reports of photoreceptors in culture [34–38] (red arrowheads). Scale bars = 100 μm. (B): Analysis of reverse transcription-polymerase chain reaction band densities showed that differentiated cells exhibited a significant increase in mRNA expression of CRX (30%), Nr2e3 (60%), rhodopsin (50%), recoverin (40%), rhodopsin kinase (80%), PDE6β (60%), and IRBP (110%) when compared with untreated cells cultured in the absence of extracellular matrix (n = 5; error bars represent the SEM). Abbreviations: IRBP, interphotoreceptor retinoid-binding protein; ns, not significant; PDE6β, phosphodiesterase type 6β Recov, recoverin; Rhod, rhodopsin.
To demonstrate further evidence of photoreceptor differentiation, we studied the gene expression of differentiated cells at the same time point. This demonstrated significant upregulation of many of the genes associated with rod photoreceptor development [39]. These included increased expression of CRX (30%, p = .04), NR2E3 (60%, p = .002), rhodopsin (50%, p = .004), and recoverin (40%, p = .02) when compared with undifferentiated cells (Fig. 1B). Interestingly, no increase in the constitutively high expression of NRL was observed following in vitro differentiation. These changes in gene expression were consistent across three different hMSC lines studied. There was also a significant upregulation of mRNA transcripts encoding for components of the phototransduction cascade including rhodopsin kinase (80%, p = .02), phosphodiesterase type 6β (0%, p = .04), and interphotoreceptor retinoid-binding protein (110%, p = .02) (Fig. 1B).
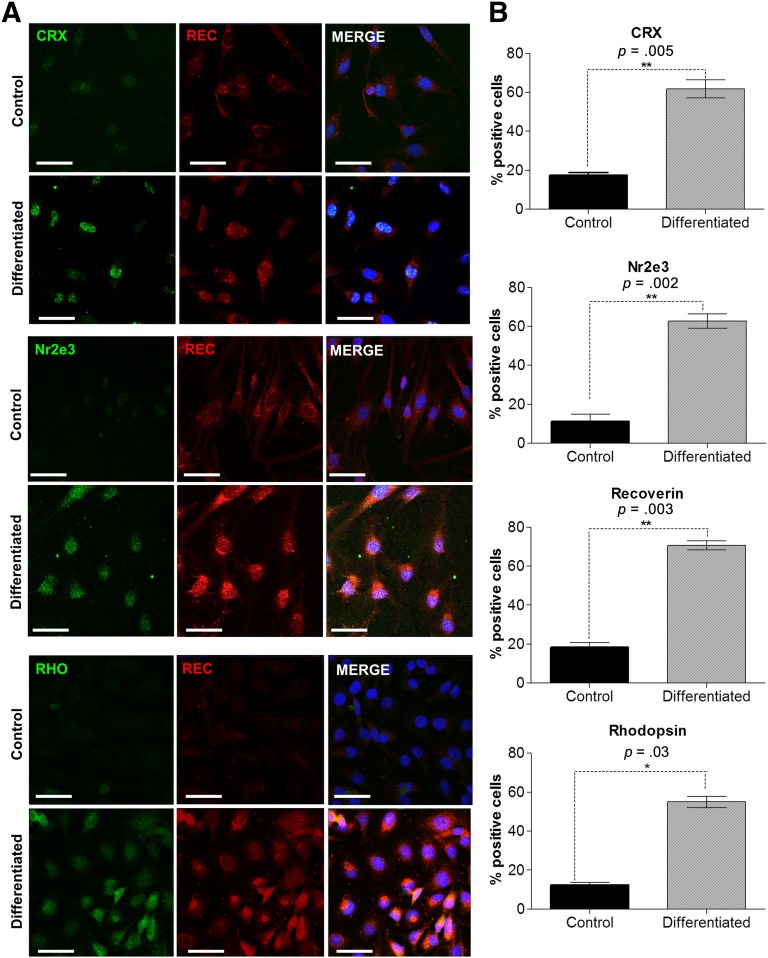
In order to confirm increased protein expression of these markers, we performed immunocytochemistry of differentiated cells in culture. This confirmed an increase in the expression of CRX (p = .005), NR2E3 (p = .002), recoverin (p = .003), and rhodopsin (p = .03) when compared with undifferentiated hMSCs. The images presented illustrate areas showing larger numbers of positive cells for each marker (Fig. 2A). Quantitative analysis of the expression of these markers showed a significant increase in the percentage of cells expressing rod photoreceptor markers in cultures of differentiated cells when compared with untreated controls (Fig. 2B).
Figure 2.
In vitro differentiation of human Müller glia with stem cell characteristics with fibroblast growth factor, taurine, retinoic acid, and insulin-like growth factor type 1 upregulates the expression of rod photoreceptor markers. (A): Differentiation of human Müller glia with stem cell characteristics was performed on basement membrane protein-coated glass slides in the presence of fibroblast growth factor, taurine, retinoic acid, and insulin-like growth factor type 1 and on uncoated plastic chamberwell slides as a control. Differentiating conditions led to an observed increase in staining for Nr2e3, CRX, rhodopsin, and recoverin. The images shown illustrate areas showing larger numbers of positive cells for each marker. Scale bars = 100 μm. (B): The histograms show the increase in the percentage of cells within the population staining for each marker. A significant increase in expression of CRX (p = .005), rhodopsin (p = .03), NR2E3 (p = .005), and recoverin (p = .003) was seen in differentiated cells when compared with control cells. These results are the average of four different fields in three separate experiments. Abbreviations: REC, recoverin; RHO, rhodopsin.
Integration of Transplanted Photoreceptor Precursors Into the Degenerate Host Retina
To investigate whether hMSC-derived photoreceptor precursors were able to integrate within the degenerate retina following subretinal transplantation, studies were performed in rats heterozygous for the P23H mutation. This particular strain exhibits a rapid primary photoreceptor degeneration [25] that is evident by 3 weeks of age, the time point at which animals underwent transplantation. hMSCs used for transplantation were transfected with a lentiviral vector expressing human recombinant GFP in order to facilitate localization after surgery. In order to minimize the risk of xenograft rejection, animals were immune tolerized at birth with an intraperitoneal injection of undifferentiated GFP-labeled hMSCs [26]. Further modulation of the host immune response was achieved by administration of oral immunosuppression (cyclosporine A, prednisolone, azathioprine) from 2 days prior to transplantation until the end of the experiment.
GFP-labeled hMSCs were differentiated toward rod photoreceptor precursors, as described above. Also as previously described by our group [28], survival and migration of transplanted cells were promoted by breakdown of the local extracellular matrix with chondroitinase ABC and by the suppression of microglial activity with triamcinolone [27].
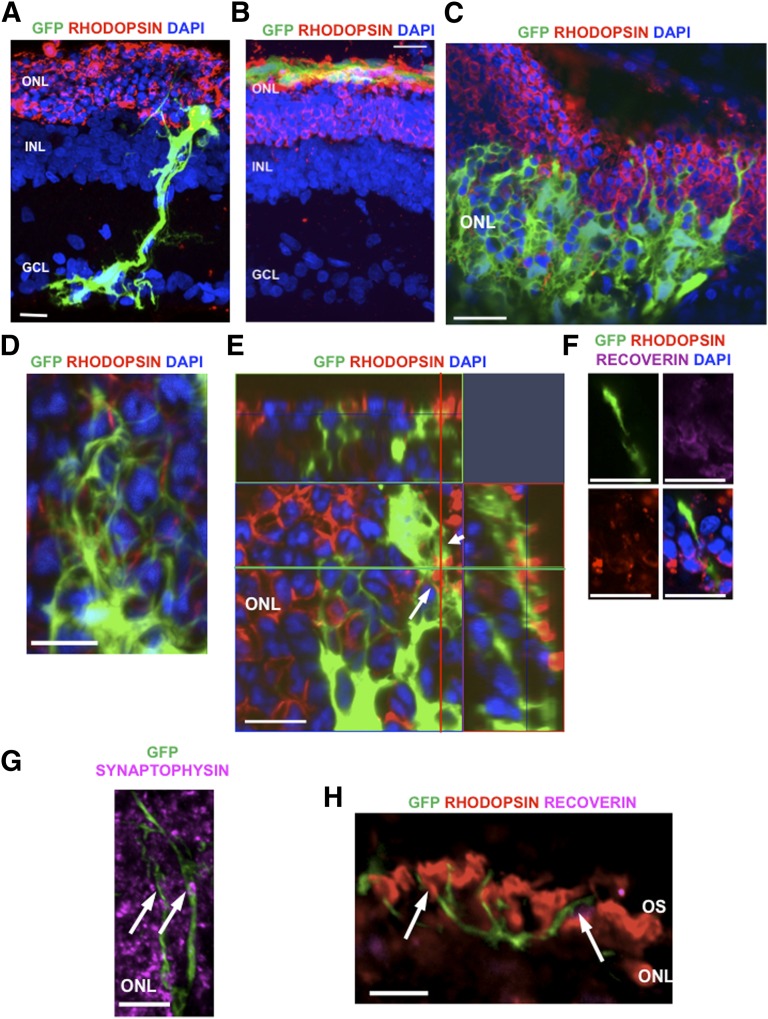
Following scotopic ERG at 4 weeks following transplantation, animals were sacrificed for histological analysis. Undifferentiated hMSCs migrated longitudinally across the host retina without localization to a specific retinal lamina (Fig. 3A). Differentiated hMSCs were seen to migrate and integrate within the host outer nuclear layer (Fig. 3B, 3C), although their migration was restricted to the site of injection. Transplanted cells appeared to have integrated well, as judged by their interwoven cellular projections around the host photoreceptors (Fig. 3D). Cells that appeared to be well integrated within the outer nuclear layer were seen to express rhodopsin, as determined by analysis of the orthogonal view of the z-stack plane (Fig. 3E), whereas others were observed migrating toward the host outer segments with spherule-like projections remaining in the outer nuclear layer (Fig. 3F).
Figure 3.
Migration of human Müller glia with stem cell characteristics-derived photoreceptor cells into the retina of P23H-1 transgenic rats. (A): Undifferentiated human Müller glia with stem cell characteristics migrated longitudinally across the host retina without localization to a specific retinal lamina adopting a typical Müller glial morphology. (B): Low-magnification confocal microscopy image of the whole retina showing localization of differentiated donor GFP+ cells at the outer retina following subretinal transplantation. (C): At 3 weeks after transplantation, differentiated donor GFP+ cells had migrated into the ONL of the host retina. (D): Differentiated donor GFP+ cells appeared to be well integrated into the ONL, as judged by their interwoven cellular projections observed around the host cells. (E): An example of differentiated donor GFP+ cells demonstrating colocalization with rhodopsin (arrow) within the ONL of the host retina. (F): Differentiated donor GFP+ cells were seen to localize within the host outer nuclear layer extending toward the location of the remaining host outer segments with a spherule-like projection remaining in the outer nuclear layer. (G): Synaptic connectivity between the differentiated donor GFP+ cells and host ONL was suggested by colocalization of synaptophysin (purple) with GFP+ cells (arrows). (H): Although classic outer segments were not seen in the differentiated donor GFP+ cells, neural-like projections of GFP+ cells were observed surrounding the host OS (arrows). Scale bars = 50 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; GCL, ganglion cell layer; GFP, green fluorescent protein; INL, inner nuclear layer; ONL, outer nuclear layer; OS, outer segments.
Differentiated hMSCs expressed synaptophysin in situ suggestive of synaptic connectivity with the host retina (Fig. 3G). Although classic rod outer segment morphology was not observed with transplanted cells, several neural-like projections of GFP+ cells were observed in the outer segment zone, in close association with the residual outer segments of the host retina (Fig. 3H).
Transplantation of Müller Glia-Derived Photoreceptor Cells Improves Rod Photoreceptor Function in P23H Rats
Scotopic ERG was performed at 4 weeks after transplantation prior to sacrifice using a standardized protocol conforming to the International Society for Clinical Electrophysiology of Vision guidelines [29]. Sixteen rats were engrafted unilaterally with either differentiated cells or undifferentiated cells. In addition, six animals received human Tenon’s fibroblasts, and three received injections of sterile tissue culture media as experimental controls. ERG recordings from 25 unoperated eyes were also examined.
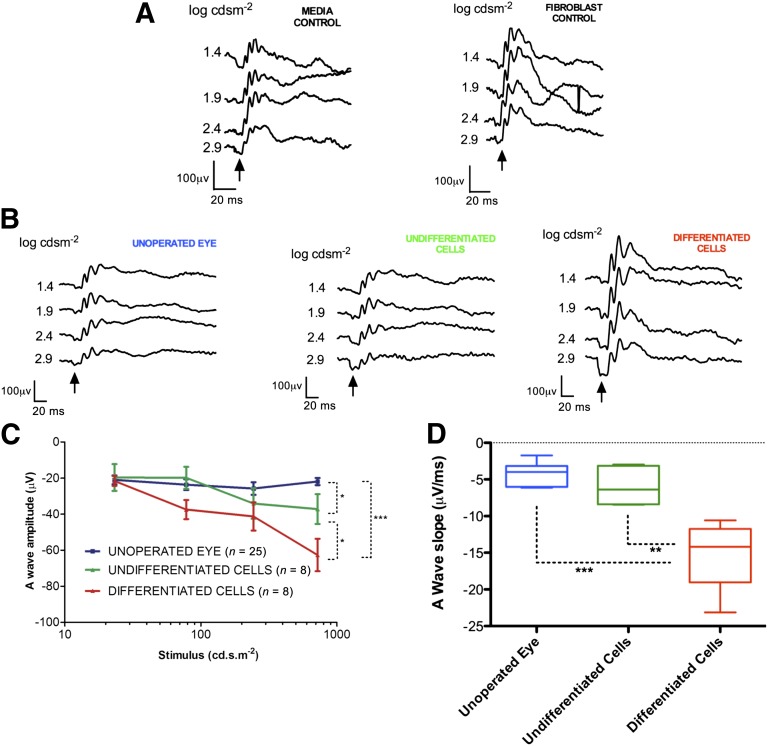
Evaluation of the scotopic a-wave was used to study rod photoreceptor function. Qualitative evaluation of the ERG waveforms from each group demonstrated the presence of a-waves in all experimental groups at the higher light intensities used in the protocol (Fig. 4A, 4B). Eyes transplanted with differentiated cells demonstrated greater a-wave amplitudes than the other experimental groups, and these amplitudes were more apparent at the higher stimulus intensities (>2.4-log cd⋅s⋅m−2). There was no significant effect of sham injections on a-wave amplitudes observed at the higher stimulus intensities (Fig. 4A), with quantitative analysis demonstrating that these were very similar to those observed in the unoperated eyes (supplemental online Fig. 1A).
Figure 4.
Scotopic flash electroretinography performed 3 weeks following subretinal transplantation in the P23H-1 transgenic rat. (A): Representative waveforms in controls that were transplanted with sham injections of tissue culture media alone or human Tenon’s fibroblasts, with the a-wave indicated by the arrow. (B): Representative waveforms from unoperated eyes and those transplanted with undifferentiated human Müller glia with stem cell characteristics. The a-wave (arrows) in the eyes transplanted with the differentiated cells showed significantly greater amplitude than the other experimental groups at the higher intensity flash stimuli. (C): Eyes transplanted with differentiated cells showed significantly greater a-wave amplitude than those transplanted with undifferentiated cells (p < .01) and unoperated eyes (p < .001). A difference in a-wave amplitudes was also observed between unoperated eyes and those transplanted with undifferentiated Müller stem cells (p < .03) (error bars represent the SEM). (D): Analysis of slope of the leading edge of the a-wave through quantification of the gradient of the first 4 ms of the leading edge of the waveform, a more specific measure of rod photoreceptor function before other light-induced currents commence, showed a significantly greater a-wave slope in eyes transplanted with differentiated cells when compared with unoperated eyes (p < .0001) or eyes transplanted with undifferentiated cells (p < .001).
Statistical comparison of the a-wave amplitudes over a range of stimulus intensities confirmed the greater a-wave amplitude observed in eyes that had received the differentiated cells compared with the other experimental groups (Fig. 4C; supplemental online Fig. 1A). Transplantation of undifferentiated cells was associated with an increase in a-wave amplitude at the highest stimulus intensity (p < .03) when compared with unoperated eyes. However, the a-wave amplitude in eyes that received differentiated cells was significantly greater than that observed in both eyes with undifferentiated cells (p < .01) and unoperated controls (p < .001) (Fig. 4C).
Analysis of the leading edge of the a-wave was performed in order to further isolate the response of rod photoreceptors [30] by measuring the change in electrical potential (measured in voltage) over the first 4 ms of the a-wave downslope. Measurement of this parameter showed no significant difference between unoperated eyes and those transplanted with undifferentiated cells. However, a highly significant increase in a-wave slope was observed in eyes that had received differentiated cells when compared with both undifferentiated cells (p < .001) and unoperated eyes (p < .0001) (Fig. 4D). In order to take into account the possible neurotrophic response attributable to the transplantation of Müller glia, the data were analyzed by subtracting the effect of transplantation of undifferentiated cells from that observed with the differentiated cell population. This adjusted a-wave slope for the eyes transplanted with differentiated cells remained significantly greater than that observed in unoperated eyes (p = .002) (supplemental online Fig. 1B).
Discussion
The differentiation of retinal progenitors into photoreceptors and the roles of specific transcription factors have been well characterized during mammalian retinogenesis [39]. It has been increasingly recognized that Müller glia play an important role in neurogenesis within the mammalian retina [40, 41] and that they are a source of new photoreceptors in rodent models of photoreceptor loss [16–18]. However, the pathway taken across the developmental landscape [42] by human Müller glia in their differentiation toward a photoreceptor fate is currently unknown. Although complex protocols have been reported regarding the derivation of rod photoreceptors from human embryonic [21] and induced pluripotent stem cells [11] in vitro, it has yet to be established whether the same conditions and combinations of growth factors apply to the in vitro differentiation of hMSCs obtained from the adult human retina.
The present experiments demonstrate phenotypic changes in the hMSC population studied, including morphological changes as well as altered gene and protein expression in vitro that occurred following treatment with differentiation protocols used by other laboratories for embryonic and induced pluripotent stem cell differentiation [21–23].
Culture of hMSCs on bMP in the presence of FGF2, taurine, retinoic acid, and IGF-1 induced morphological changes, producing striking similarities to previous reports of cultured photoreceptors. Rod photoreceptors in vitro characteristically possess a small rounded cell body without an apparent outer segment [37, 38, 43, 44] but with a prominent nucleolus [36]. The presence of short filopodial and longer neuritic processes have also been described as arising from cultured photoreceptors [35] and may be enhanced in the presence of components of bMP such as S-laminin [34].
In addition, in the present study we observed increased gene expression of the transcription factors CRX, NR2E3, and rhodopsin, which are associated with normal rod photoreceptor development. It is noteworthy that upregulation of NRL was not observed, which may indicate a constitutive expression of this gene by hMSC, possibly resistant to modifications by exogenous factors. Importantly, increased gene expression of several components of the rod phototransduction cascade, such as rhodopsin kinase, phosphodiesterase type 6β, and interphotoreceptor retinoid-binding protein, was observed in differentiated cultures, thus providing supportive evidence for differentiation toward a rod phenotype.
Although NRL expression has been suggested to be critical for rodent photoreceptor commitment [8], we hypothesize that the constitutive expression of NRL in hMSCs may represent a particular state of photoreceptor competence or that their differentiation toward rod photoreceptors may occur via a pathway independent of NRL; alternatively, differences in the expression of this factor may exist across retinal progenitors in different mammalian species. Because of their increased rhodopsin expression and upregulated expression of markers of the phototransduction cascade, the differentiated cells produced by our current protocol may be regarded as ontogenetically more advanced than murine NRL+ precursors grafted by MacLaren et al. [8] and as similar to the mature photoreceptors that were transplanted by Gust and Reh [7]. The differentiation pathway and ontogenetic stage of the hMSC-derived photoreceptor precursors may be further elucidated by studies of gene expression arrays, which would enable the identification of the molecular targets of the differentiation protocol developed in this study.
Our experiments clearly showed that subretinal transplantation of hMSC-derived photoreceptor precursors leads to the migration and integration of grafted cells into the outer nuclear layer of the heterozygous P23H-1 rat. Importantly, our finding that the transplanted cells expressed photoreceptors and synaptic markers in situ is significant if the grafted photoreceptor precursors are to integrate into the host retinal circuitry and lead to the functional replacement of photoreceptors.
The heterogeneous nature of the differentiated cultures was emphasized by the presence of some cells adopting a Müller glia-like morphology spanning the whole thickness of the retina. The present study demonstrated that using the photoreceptor differentiation protocol described, approximately 60% of hMSCs were induced to express photoreceptor markers, possibly underlying the observed heterogeneity of cell localization following transplantation. Enrichment of the population of photoreceptor precursors for transplantation by fluorescence activated cell sorting prior to transplantation may have rendered the transplant more efficient. However, the markers of photoreceptor differentiation studied, which represent intracellular proteins and nuclear transcription factors, would have been unsuitable for cell sorting. The design of future protocols to enrich differentiated cell populations for transplantation may be enhanced by characterization of surface markers, such as the CD73 antigen that has been identified in the mouse and marmoset [45], that might aid in the isolation of photoreceptor cell populations.
Extensive repopulation of the outer nuclear layer was not seen, with transplanted cells tending to migrate and integrate into areas of retina close to the site of transplantation. This may be related to the number of transplanted cells and the inherent anatomical difficulty in dispersing the graft throughout the whole retina with a single bolus injection, despite the enzymatic promotion of cell migration using chondroitinase ABC to degrade the extracellular matrix [28]. Transplantation of greater cell numbers and the use of cellular scaffolds in larger animal models may facilitate widespread dispersion of grafted cells throughout the retina.
No evidence of classic outer segment formation was observed in vivo, which is consistent with other reports of xenogeneic transplantation of photoreceptor precursors [11, 20]. Arbored segments were observed emerging from transplanted cells within the outer nuclear layer extending into the outer segment zone and were associated with the expression of markers of synaptic connectivity. Although the characteristic bipolar morphology of rod photoreceptors (inner and outer segment) was not observed in vivo, the presence of multiple arbored segments may be indicative of the formation of primordial outer segments. However, the absence of outer segments at the time point examined does not necessarily exclude their formation, and it may be possible to examine the retinal pigment epithelium (RPE) to look for evidence of phagocytosed outer segments derived from transplanted cells. Growth factors from the RPE have also been shown to play an important role in photoreceptor morphogenesis late in development [46], and further maturation of transplanted cells may occur over time in vivo as a response to cytokines such as pigment epithelium-derived growth factor. In addition, in vitro transplantation onto retinal explants with or without RPE may help elucidate the role of the RPE in photoreceptor morphogenesis from hMSC-derived precursors.
Scotopic ERG studies provide an objective and specific measure of photoreceptor function in vivo. Having presented evidence for the in vitro generation of an enriched population of photoreceptor precursors from hMSCs and demonstrated that they are able to migrate and integrate within the appropriate lamina and express markers of photoreceptors within the degenerate rodent retina, it was important to look for evidence of any improvement of rod photoreceptor function in these animals. The heterozygous P23H-1 transgenic rat displays primary rod photoreceptor degeneration due to a mutation within the rhodopsin gene in the presence of a healthy RPE [25]. These features make it a useful model in which to explore photoreceptor precursor transplantation in the absence of pathological RPE. Transplanted animals showed a significant increase in rod photoreceptor function 4 weeks after subretinal delivery of hMSC-derived photoreceptor precursors. This was demonstrated by an increase in the a-wave amplitude, which is largely dependent on rod function under scotopic conditions. The observed enhancement of rod photoreceptor performance is likely to be related to both functional replacement of transplanted photoreceptor precursors and a neurotrophic effect from differentiated or undifferentiated Müller glia. Undifferentiated Müller glia, which are present because of the heterogeneous nature of the enriched culture used for transplantation, are known to secrete pigment epithelium-derived factor [47] and have been shown to have protective effects on photoreceptors [46], brain-derived neurotrophic factor [48, 49], and nerve growth factor [50]. In order to further isolate the effect on rod function, analysis of the slope of the leading edge of the a-wave [30] was performed. This measure of pure rod photoreceptor function in scotopic conditions did not demonstrate any significant difference between eyes receiving undifferentiated cells and unoperated control eyes, as observed using the more crude measure of a-wave amplitude. However, a highly significant improvement in rod photoreceptor function was observed in eyes transplanted with differentiated cells in comparison with undifferentiated cells. Further subtraction analysis to take into account any neurotrophic effects of undifferentiated Müller glia confirmed the significant increase in rod function as being attributable to the effect of transplanted photoreceptor precursors.
Conclusion
This study has demonstrated that hMSCs isolated from the normal adult human retina may be cultured in the laboratory to generate a source of rod photoreceptor precursors suitable for transplantation. Such cells may also offer the potential of developing autologous therapies for human application. On transplantation into the subretinal space of a rodent model of primary photoreceptor degeneration, these cells migrated and integrated into the retina and caused significant improvement in photoreceptor function in vivo. hMSCs may therefore be regarded as an alternative source of cells for the development of future therapeutic strategies to treat photoreceptor disease.
Supplementary Material
Acknowledgments
This work was supported by a Clinical Research Training Fellowship awarded by the Medical Research Council and the Royal College of Surgeons of Edinburgh (Grant G0701341 to H.J.), the Medical Research Council (Grant G0900002 to G.A.L.), the NIHR Biomedical Research Centre for Ophthalmology at Moorfields Eye Hospital (G.A.L., M.F.J.), and Fight for Sight (through a donation from Tony Bickford). We thank Prof. Graham Holder from Moorfields Eye Hospital for advice on analysis of electrophysiology results.
Author Contributions
H.J.: conception and design, experimental work, collection and assembly of data, data analysis and interpretation, manuscript writing, financial support, final approval of manuscript; M.F.J., K.E., P.B.C.: experimental work, collection and assembly of data, final approval of manuscript; S.B.: data analysis and interpretation, final approval of manuscript; J.W.: experimental work, final approval of manuscript; P.T.K.: financial support, discussions and advice on the work; G.A.L.: conception and design, data analysis and interpretation, manuscript writing, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
G.A.L. and P.T.K. have intellectual property rights as patent holders.
References
- 1.Meyer RL. Evidence from thymidine labeling for continuing growth of retina and tectum in juvenile goldfish. Exp Neurol. 1978;59:99–111. doi: 10.1016/0014-4886(78)90204-2. [DOI] [PubMed] [Google Scholar]
- 2.Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- 3.Das AV, Mallya KB, Zhao X, et al. Neural stem cell properties of Müller glia in the mammalian retina: Regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283–302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 4.Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia B, Singhal S, Lawrence JM, et al. Distribution of Müller stem cells within the neural retina: Evidence for the existence of a ciliary margin-like zone in the adult human eye. Exp Eye Res. 2009;89:373–382. doi: 10.1016/j.exer.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence JM, Singhal S, Bhatia B, et al. MIO-M1 cells and similar muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25:2033–2043. doi: 10.1634/stemcells.2006-0724. [DOI] [PubMed] [Google Scholar]
- 7.Gust J, Reh TA. Adult donor rod photoreceptors integrate into the mature mouse retina. Invest Ophthalmol Vis Sci. 2011;52:5266–5272. doi: 10.1167/iovs.10-6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 9.Pearson RA, Barber AC, Rizzi M, et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485:99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamba DA, McUsic A, Hirata RK, et al. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hentze H, Graichen R, Colman A. Cell therapy and the safety of embryonic stem cell-derived grafts. Trends Biotechnol. 2007;25:24–32. doi: 10.1016/j.tibtech.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Jalving M, Schepers H. Induced pluripotent stem cells: Will they be safe? Curr Opin Mol Ther. 2009;11:383–393. [PubMed] [Google Scholar]
- 14.Del Debbio CB, Balasubramanian S, Parameswaran S, et al. Notch and Wnt signaling mediated rod photoreceptor regeneration by Müller cells in adult mammalian retina. PLoS One. 2010;5:e12425. doi: 10.1371/journal.pone.0012425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karl MO, Hayes S, Nelson BR, et al. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci USA. 2008;105:19508–19513. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ooto S, Akagi T, Kageyama R, et al. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci USA. 2004;101:13654–13659. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan J, Zheng H, Chen ZL, et al. Preferential regeneration of photoreceptor from Müller glia after retinal degeneration in adult rat. Vision Res. 2008;48:223–234. doi: 10.1016/j.visres.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Wan J, Zheng H, Xiao HL, et al. Sonic hedgehog promotes stem-cell potential of Müller glia in the mammalian retina. Biochem Biophys Res Commun. 2007;363:347–354. doi: 10.1016/j.bbrc.2007.08.178. [DOI] [PubMed] [Google Scholar]
- 19.Bernardos RL, Barthel LK, Meyers JR, et al. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannelli SG, Demontis GC, Pertile G, et al. Adult human Müller glia cells are a highly efficient source of rod photoreceptors. Stem Cells. 2011;29:344–356. doi: 10.1002/stem.579. [DOI] [PubMed] [Google Scholar]
- 21.Lamba DA, Karl MO, Ware CB, et al. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine EM, Fuhrmann S, Reh TA. Soluble factors and the development of rod photoreceptors. Cell Mol Life Sci. 2000;57:224–234. doi: 10.1007/PL00000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- 24.Sohocki MM, Daiger SP, Bowne SJ, et al. Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum Mutat. 2001;17:42–51. doi: 10.1002/1098-1004(2001)17:1<42::AID-HUMU5>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinberg RH, Flannery JG, Naash M, et al. Transgenic rat models of inherited retinal degeneration caused by mutant opsin genes. Invest Ophthalmol Vis Sci. 1996;37:S698. [Google Scholar]
- 26.Kelly CM, Precious SV, Scherf C, et al. Neonatal desensitization allows long-term survival of neural xenotransplants without immunosuppression. Nat Methods. 2009;6:271–273. doi: 10.1038/nmeth.1308. [DOI] [PubMed] [Google Scholar]
- 27.Singhal S, Lawrence JM, Salt TE, et al. Triamcinolone attenuates macrophage/microglia accumulation associated with NMDA-induced RGC death and facilitates survival of Müller stem cell grafts. Exp Eye Res. 2010;90:308–315. doi: 10.1016/j.exer.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Singhal S, Lawrence JM, Bhatia B, et al. Chondroitin sulfate proteoglycans and microglia prevent migration and integration of grafted Müller stem cells into degenerating retina. Stem Cells. 2008;26:1074–1082. doi: 10.1634/stemcells.2007-0898. [DOI] [PubMed] [Google Scholar]
- 29.Marmor MF, Fulton AB, Holder GE, et al. ISCEV Standard for full-field clinical electroretinography (2008 update) Doc Ophthalmol. 2009;118:69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- 30.Baron WS. Electroretinogram a-wave slope as a measure of photoreceptor activity. J Opt Soc Am. 1982;72:296–297. [Google Scholar]
- 31.Brown KT. The eclectroretinogram: Its components and their origins. Vision Res. 1968;8:633–677. doi: 10.1016/0042-6989(68)90041-2. [DOI] [PubMed] [Google Scholar]
- 32.Granit R. The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. J Physiol. 1933;77:207–239. doi: 10.1113/jphysiol.1933.sp002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hood DC, Birch DG. The A-wave of the human electroretinogram and rod receptor function. Invest Ophthalmol Vis Sci. 1990;31:2070–2081. [PubMed] [Google Scholar]
- 34.Hunter DD, Murphy MD, Olsson CV, et al. S-laminin expression in adult and developing retinae: A potential cue for photoreceptor morphogenesis. Neuron. 1992;8:399–413. doi: 10.1016/0896-6273(92)90269-j. [DOI] [PubMed] [Google Scholar]
- 35.Mandell JW, MacLeish PR, Townes-Anderson E. Process outgrowth and synaptic varicosity formation by adult photoreceptors in vitro. J Neurosci. 1993;13:3533–3548. doi: 10.1523/JNEUROSCI.13-08-03533.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messing A, Kim SU. Long-term culture of adult mammalian central nervous system neurons. Exp Neurol. 1979;65:293–300. doi: 10.1016/0014-4886(79)90099-2. [DOI] [PubMed] [Google Scholar]
- 37.Townes-Anderson E, MacLeish PR, Raviola E. Rod cells dissociated from mature salamander retina: Ultrastructure and uptake of horseradish peroxidase. J Cell Biol. 1985;100:175–188. doi: 10.1083/jcb.100.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zayas-Santiago A, Kang Derwent JJ. Preservation of intact adult rat photoreceptors in vitro: Study of dissociation techniques and the effect of light. Mol Vis. 2009;15:1–9. [PMC free article] [PubMed] [Google Scholar]
- 39.Swaroop A, Kim D, Forrest D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat Rev Neurosci. 2010;11:563–576. doi: 10.1038/nrn2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karl MO, Reh TA. Studying the generation of regenerated retinal neuron from Müller glia in the mouse eye. Methods Mol Biol. 2012;884:213–227. doi: 10.1007/978-1-61779-848-1_15. [DOI] [PubMed] [Google Scholar]
- 41.Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013;61:651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- 42.Enver T, Pera M, Peterson C, et al. Stem cell states, fates, and the rules of attraction. Cell Stem Cell. 2009;4:387–397. doi: 10.1016/j.stem.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Gaudin C, Forster V, Sahel J, et al. Survival and regeneration of adult human and other mammalian photoreceptors in culture. Invest Ophthalmol Vis Sci. 1996;37:2258–2268. [PubMed] [Google Scholar]
- 44.Hicks D, Forster V, Dreyfus H, et al. Survival and regeneration of adult human photoreceptors in vitro. Brain Res. 1994;643:302–305. doi: 10.1016/0006-8993(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 45.Koso H, Minami C, Tabata Y, et al. CD73, a novel cell surface antigen that characterizes retinal photoreceptor precursor cells. Invest Ophthalmol Vis Sci. 2009;50:5411–5418. doi: 10.1167/iovs.08-3246. [DOI] [PubMed] [Google Scholar]
- 46.Jablonski MM, Tombran-Tink J, Mrazek DA, et al. Pigment epithelium-derived factor supports normal development of photoreceptor neurons and opsin expression after retinal pigment epithelium removal. J Neurosci. 2000;20:7149–7157. doi: 10.1523/JNEUROSCI.20-19-07149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eichler W, Yafai Y, Keller T, et al. PEDF derived from glial Müller cells: A possible regulator of retinal angiogenesis. Exp Cell Res. 2004;299:68–78. doi: 10.1016/j.yexcr.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 48.Sato T, Fujikado T, Lee TS, et al. Direct effect of electrical stimulation on induction of brain-derived neurotrophic factor from cultured retinal Müller cells. Invest Ophthalmol Vis Sci. 2008;49:4641–4646. doi: 10.1167/iovs.08-2049. [DOI] [PubMed] [Google Scholar]
- 49.Seki M, Tanaka T, Sakai Y, et al. Müller Cells as a source of brain-derived neurotrophic factor in the retina: Noradrenaline upregulates brain-derived neurotrophic factor levels in cultured rat Müller cells. Neurochem Res. 2005;30:1163–1170. doi: 10.1007/s11064-005-7936-7. [DOI] [PubMed] [Google Scholar]
- 50.de Melo Reis RA, Cabral-da-Silva M, de Mello FG, et al. Müller glia factors induce survival and neuritogenesis of peripheral and central neurons. Brain Res. 2008;1205:1–11. doi: 10.1016/j.brainres.2008.02.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.