This study investigated paracrine signaling effects of human Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) on keloid fibroblasts in vitro. The WJ-MSC-conditioned medium-treated keloid fibroblasts showed higher proliferation rates than their control keloid fibroblasts with no significant change in apoptosis rate or migration ability.
Keywords: Stem cells, Keloid, Scar, Skin, Fibrosis, Wharton’s jelly
Abstract
Keloid scars are abnormal benign fibroproliferative tumors with high recurrence rates and no current efficacious treatment. Accumulating evidence suggests that human umbilical cord Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) have antifibrotic properties. Paracrine signaling is considered one of the main underlying mechanisms behind the therapeutic effects of mesenchymal stem cells. However, the paracrine signaling effects of WJ-MSCs on keloids have not yet been reported. The aim of this study is to investigate paracrine signaling effects of human WJ-MSCs on keloid fibroblasts in vitro. Human umbilical cords and keloid skin samples were obtained, and WJ-MSCs and keloid fibroblasts were isolated and cultured. One-way and two-way paracrine culture systems between both cell types were investigated. Plasminogen activator inhibitor-I and transforming growth factor-β2 (TGF-β2) transcripts were upregulated in keloid fibroblasts cultured with WJ-MSC-conditioned medium (WJ-MSC-CM) and cocultured with inserts, while showing lower TGF-β3 gene expression. Interleukin (IL)-6, IL-8, TGF-β1, and TGF-β2 protein expression was also enhanced. The WJ-MSC-CM-treated keloid fibroblasts showed higher proliferation rates than their control keloid fibroblasts with no significant change in apoptosis rate or migration ability. In our culture conditions, the indirect application of WJ-MSCs on keloid fibroblasts may enhance their profibrotic phenotype.
Introduction
Scarring is the physiological response to wound healing in postnatal mammalian skin [1]. To date, there is no treatment to erase scars completely in humans, and they remain as remnants of most skin injuries. The physical and psychosocial discomfort that patients suffer varies from mild to severe [2]. Hypertrophic scars, and especially keloids, are aberrant excessive forms of pathological wounding with an excess of extracellular matrix, which appears to be mainly driven by fibroblasts [3]. Keloids are considered to be a complex polygeneic disorder whose progression is influenced by aberrant cell signaling pathways, probably as a result of both genotype and phenotype factors [4]. Keloids and hypertrophic scars represent the main clinical challenge responsible for scar-related cosmetic and functional dysfunctions and have an incidence and prevalence of 4.5%–16% and 30%–90%, respectively, in trauma, burn, and other surgical patients [5–7].
Recent reports suggest that mesenchymal stem cells (MSCs) represent a new antifibrotic treatment strategy [8–10]. They attenuate wound inflammation and reprogram resident cells to favor tissue regeneration and inhibit fibrosis [9]. They influence host cells and regulate the stem cell niche through differentiation and/or paracrine signaling mechanisms [11, 12]. Accumulating evidence suggests that paracrine signaling, the secretion of trophic or immunomodulatory factors or secretome, may represent the most pivotal underlying mechanism of MSC effects [12–14]. It has also been well documented that the MSC secretome is extremely influenced by the MSC microenvironment or stem cell niche and cell-cell communications [11].
Between the several possible sources of MSCs, umbilical cord-derived Wharton’s jelly-derived MSCs (WJ-MSCs) appear to offer the best clinical utility because of their unique beneficial characteristics [15]. WJ-MSCs represent a fetal or birth-derived adult, efficient stem cell source with many advantages, such as antifibrotic and anticancer properties [16], with no reported teratoma formation or rejection in animal models [15]. Their isolation is relatively easy and involves no relevant ethical concerns. They represent a low-cost technology and a universal cell source. Furthermore, WJ-MSC advantages also include everlasting availability (births are still happening by natural or sometimes artificial means, and women donate their umbilical cords for banking or research purposes), high number of cells (more than the umbilical cord blood and bone marrow [17] and a higher degree of stemness and self-renewal compared with bone marrow-derived MSCs [BM-MSCs] [18]). Finally, WJ-MSCs exhibit high engraftment rates with successful functional outcomes in in vivo animal models [15] and multiple potential clinical applications, including skin regeneration [18, 19].
Tumor shrinking caused by human WJ-MSCs in cancer animal models is mediated via cell-to-cell and/or noncellular contact mechanisms, such as entosis (MSC invasion-induced host cellular death). Regarding the role of WJ-MSCs in kidney fibrosis, endocrine mechanisms have been involved [20]. It has been shown that concentrated MSC-CM can modulate wound repair without MSCs being present in the wound, which has the advantage of minimal risks compared with any other cell therapy [12, 13]. BM-MSC paracrine signaling has been reported to promote skin fibrosis in normal fibroblasts in vitro, but the effect of WJ-MSC paracrine signaling has not yet been defined in normal or keloid fibroblasts.
The aim of this study is to investigate the effects of human WJ-MSC paracrine signaling on keloids.
Materials and Methods
Tissue Sources
Tissue samples were obtained with institutional review board approval and in accordance with the Declaration of Helsinki Principles at the Sunnybrook Health Sciences Centre and University of Toronto.
Cell Culture
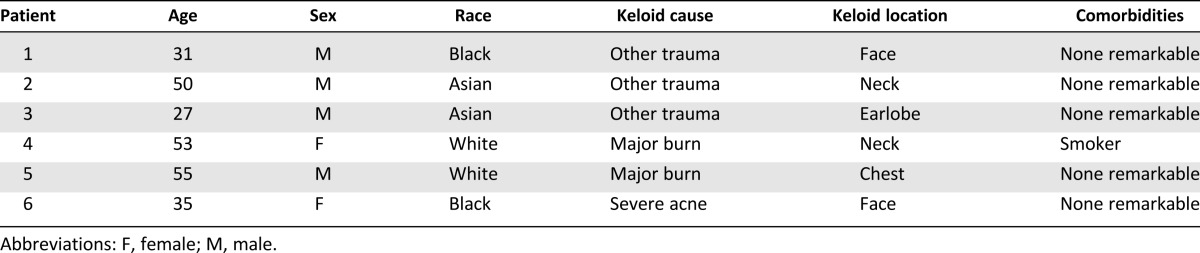
Primary human keloid fibroblasts were obtained from six skin tissue samples of patients undergoing reconstructive surgeries several years after trauma (mainly burn) at the Department of Plastic Surgery at Sunnybrook Health Sciences Centre, University of Toronto (Table 1). WJ-MSCs were isolated from umbilical cords obtained from planned cesarean sections at the Obstetrics and Gynecology Department at the same health center. All human samples were collected after signed informed consent and ethics committee approval.
Table 1.
Patients’ epidemiological data
Full-thickness keloid skin was dissected to remove any subcutaneous adipose tissue and cut in small pieces of 2–4 mm. Human skin fibroblasts were obtained from outgrowth of dermal component of explants cultured in small dishes. After trypsinization, fibroblasts were further cultured in 75-cm2 tissue culture flasks at a density of 4,500 cells per cm2 for 7 days before further passaging.
WJ-MSCs were isolated from umbilical cords according to previously described methods [21]. When fibroblasts and/or WJ-MSCs reached 70% confluence, they were trypsinized in 0.05% trypsin/0.025% EDTA vol/vol.
Characterization of Human WJ-MSCs
The cells isolated from the Wharton’s jelly of the umbilical cord were studied to confirm their MSC characteristics. They were cultured and grown in plastic plates, and flow cytometry for MSC markers (CD90+, CD73+, CD105+, CD45−, CD14−, CD34−, and HLA-DR−) [22] was performed (supplemental online Fig. 1a). Cells were differentiated into adipogenic, osteogenic, and chondrogenic lineages following previous reported protocols [21] (supplemental online Fig. 1b–1d).
Human Keloid Fibroblasts and WJ-MSC Cocultures
Two different paracrine in vitro interaction culture systems were used in this study: (a) WJ-MSC-conditioned medium (CM)-treated and untreated fibroblasts, or one-way paracrine signaling coculture, and (b) porous membrane or Transwell (Corning Enterprises, Corning, NY, http://www.corning.com) coculture using an insert well.
Experiments were done with low-passage cells (less than P5), in triplicates, and for 7 days. First, primary human fibroblasts were seeded into six-well plates (Grenier-Bio-One Cellstar, Frickenhausen, Germany, http://www.gbo.com/en), at a density of 22,000 cells per cm2. For the WJ-MSC-CM experiment or one-way coculture system, WJ-MSCs were separately seeded at the same cell density into similar plates, whereas WJ-MSC medium alone was also independently placed. When the media were refreshed, fibroblasts received WJ-MSC-CM (treatment group) or non-CM (nontreated control group). In the insert coculture method, 24 hours after seeding the fibroblasts, WJ-MSCs were seeded into 3-μm-pore inserts (at a density of 18,000 cells per cm2) and put on top of the treatment fibroblast wells. Control inserts were filled only with nonconditioned medium and were placed on top of the control fibroblast group.
RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction
A total of 10 μg of RNA from six different keloid samples, isolated with the RNeasy MicroKit (Qiagen, Valencia, CA, http://www.qiagen.com) after lysis with TRIzol (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) and quantified using a NanoDrop-2000 spectrophotometer (ThermoScientific, Waltham, MA, http://www.thermoscientific.com), was used for cDNA synthesis using a high-capacity cDNA synthesis reverse-transcription kit (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com) and thermocycler (Applied Biosystems). Amplification and analysis of cDNA fragments were carried out using the StepOnePlus RT-PCR System (Applied Biosystems). SYBR Green PCR Master Mix (Applied Biosystems) was used to relatively quantify the mRNA transcript products of the following genes of interest: TGF-β1, TGF-β2, TGF-β3, plasminogen activator inhibitor-I (PAI-1), and decorin. 18S was used as housekeeping gene. Primer sequences of the genes used above are listed in supplemental online Table 1. Relative gene expression was measured as cycle threshold (Ct) and normalized with individual housekeeping gene control Ct values. Quantitative polymerase chain reaction (qPCR) was loaded in duplicates, and Ct values from the triplicates of the same treatment group sample were averaged. The ΔδCt method was used to report qPCR results.
Protein Determination
Protein levels of human transforming growth factor (TGF)-β1, TGF-β2, and the inflammatory and profibrotic cytokines interleukin (IL)-6 and IL-8 were measured in aliquots of WJ-MSC-CM-treated and untreated human keloid fibroblasts acquired at day 7 (n = 2 keloid samples), using Luminex Multiplex technology (Millipore, Billerica, MA, http://ww.millipore.com). Protein values in the culture supernatant were measured as picograms per milliliter.
Proliferation and Viability Assays
Bromodeoxyuridine Proliferation Assay
Keloid fibroblasts and WJ-MSCs were cultured in different eight-well chamber culture slides at a cell density of 1,000 cells per cm2 for 7 days. Study design and medium refreshment were similar to the WJ-MSC-CM or one-way coculture system previously described. On day 6, cells were labeled with 50 μM bromodeoxyuridine (BrdU) per well for 24 hours. DNA incorporation of BrdU was measured to determine fibroblast proliferation.
Immunocytochemistry
After fixing and washing, cells were preincubated for 30 minutes in phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA), followed by incubation with mouse anti-human BrdU (1:200; Cell Signaling Technology, Boston, MA, http://www.cellsignal.com) overnight at 4°C. After washing with PBS, the secondary antibody was added in 1% BSA and left incubating for 1 hour at room temperature in the dark (Alexa Fluor 488 donkey anti-mouse, 1:500; Life Technologies, Grand Island, NY, http://www.lifetech.com). Images were taken using an Apotome Axiovert fluorescence imaging system (Carl Zeiss, Oberkochen, Germany, http://www.zeiss.com). Quantification was performed by counting the number of BrdU-positive nuclei in the high-power field as well as the 4′,6-diamidino-2-phenylindole (DAPI)-positive total number of nuclei.
CyQUANT Cell Proliferation Assay
The cell pellet of known number of keloid fibroblasts was frozen at −80°C for a standard curve. Both keloid fibroblasts and WJ-MSCs were cultured in separate wells at a cell density of 1,500 cells per cm2 in five 96-well culture plates, one for each time point of the proliferation study (days 0, 1, 3, 7, and 14). Medium was replaced as described, until cells reached the desired time point, when a day-labeled plate was frozen following the CyQUANT assay kit (Invitrogen) manufacturer’s instructions. Sample fluorescence was read in a spectrophotometer (Beckman Coulter, Mississauga, Canada, http://www.beckmancoulter.com).
Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling
Keloid fibroblasts were seeded into eight-chamber culture slides at a cell density of 2,000 cells per cm2 and were cultured with WJ-MSC-CM and with nonconditioned medium for 4 days. A terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) apoptosis kit (Promega, Fitchburg, WI, http://www.promega.com) was used as per the manufacturer’s instructions. Images were taken as in the BrdU assay. Cell quantification was performed by counting the number of TUNEL- and DAPI-positive nuclei, or apoptotic and alive cells, respectively.
Cell Migration Assay: Scratch Wound Assay
Keloid fibroblasts were seeded into four-well chamber culture slides (2,000 cells per cm2). A pair of scratches was performed, and 24 hours later, the cells were fixed with 4% paraformaldehyde. The staining protocol followed the same procedure as the BrdU proliferation assay previously described. Cells were incubated with phalloidin antibody conjugated to fluorescein isothiocyanate (1:30; Invitrogen) in blocking solution for 1 hour. Images were taken on an LSM META 510 confocal microscope (Zeiss). Quantification was performed using ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical Analysis
Data are graphically expressed as the mean of the target ±SEM, or 95% confidence interval. The statistical comparisons between the groups were performed using an unpaired Student’s t test or a two-way analysis of variance using GraphPad Prism software (La Jolla, CA, http://www.graphpad.com). A two-tailed p value ≤.05 was considered significant.
Results
WJ-MSC-Conditioned Media Enhanced Profibrotic Gene Expression
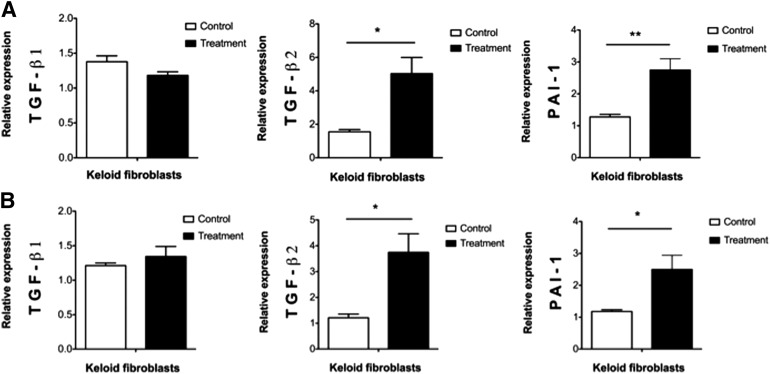
In our culture conditions, both types of WJ-MSC paracrine signaling on keloid fibroblasts, one-way (WJ-MSC-CM) and two-way (insert), enhanced mRNA expression of the profibrotic markers PAI-1 (p ≤ .01 and p ≤ .05, respectively) and TGF-β2 (p ≤ .05) (Fig. 1). Unlike the other two profibrotic transcripts, the expression level of TGF-β1 did not change significantly (p > .05) (Fig. 1).
Figure 1.
Wharton’s jelly-derived mesenchymal stem cell (WJ-MSC) paracrine signaling effect on the expression of profibrotic genes in human keloid skin fibroblast. (A): One-way paracrine signaling or WJ-MSC-conditioned medium-treated keloid skin fibroblasts versus nonconditioned media-treated keloid skin fibroblasts or control group. (B): Two-way paracrine signaling or Transwell insert system coculture. Results are from six different keloid skin samples. ∗, p ≤ .05; ∗∗, p ≤ .01. Abbreviations: PAI-1, plasminogen activator inhibitor-I; TGF, transforming growth factor.
WJ-MSC Secretome Downregulated the Expression of the Antifibrotic Gene TGF-β3 in Keloid Fibroblasts
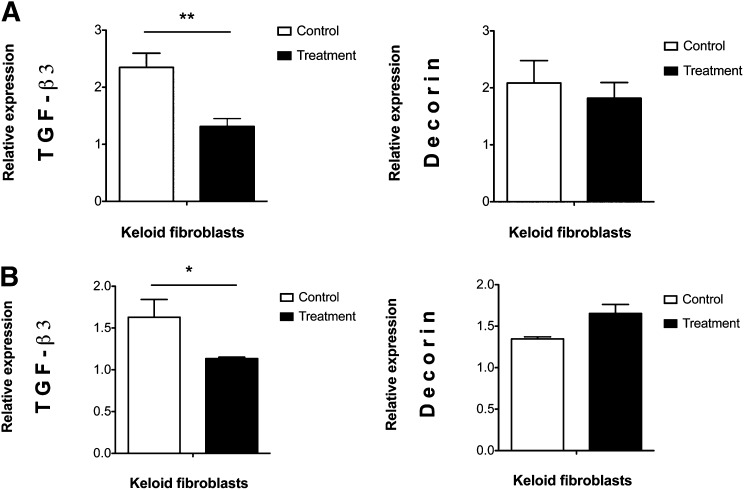
Under both of our paracrine signaling culture conditions, the transcript levels of the antifibrotic gene TGF-β3 decreased in keloid fibroblasts (p ≤ .01 in WJ-MSC-CM and p ≤ .05 in the insert system). In contrast, the expression of the antifibrotic proteoglycan decorin was not significantly modified, although it appeared downregulated in the WJ-MSC-CM-treated keloid fibroblast group (p > .05) (Fig. 2).
Figure 2.
Wharton’s jelly-derived mesenchymal stem cell (WJ-MSC) paracrine signaling effect on the expression of antifibrotic genes in human keloid skin fibroblasts. Figure shows downregulation of antifibrotic genes in keloid fibroblasts through one-way paracrine signaling WJ-MSCs (A) and two-way paracrine signaling (B). Messenger RNA levels were studied in six keloid skin samples, but four in the decorin insert system. ∗, p ≤ .05; ∗∗, p ≤ .01. Abbreviation: TGF, transforming growth factor.
WJ-MSC-CM Increased the Level of Profibrotic Proteins in Human Keloid Fibroblasts
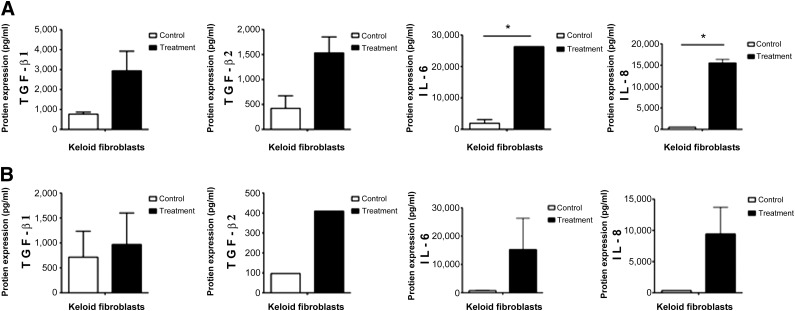
The expression of the inflammatory and profibrotic proteins IL-6 and IL-8 was significantly upregulated in keloid fibroblasts treated with WJ-MSC-CM (p ≤ .05). Unlike the one-way paracrine effect, the expression of these interleukins in the fibroblasts cocultured through an insert was not affected (p > .05). Our protein assay revealed no significant changes at the protein level for TGF-β1 and TGF-β2 in both paracrine culture systems (p > .05). Overall, our data suggested that WJ-MSC-CM upregulated the level of some profibrotic proteins (Fig. 3).
Figure 3.
Wharton’s jelly-derived mesenchymal stem cell (WJ-MSC) paracrine effects on the expression of profibrotic proteins in keloid fibroblasts. (A): Upregulation of profibrotic proteins in keloid fibroblasts through one-way paracrine signaling WJ-MSCs, or WJ-MSC-conditioned medium. (B): Upregulation of profibrotic proteins in human keloid skin fibroblasts cocultured with WJ-MSCs via a Transwell insert system. Two different keloid samples were investigated for protein studies. ∗, p ≤ .05. Abbreviations: IL, interleukin; TGF, transforming growth factor.
WJ-MSC-CM Promoted Keloid Fibroblast Proliferation
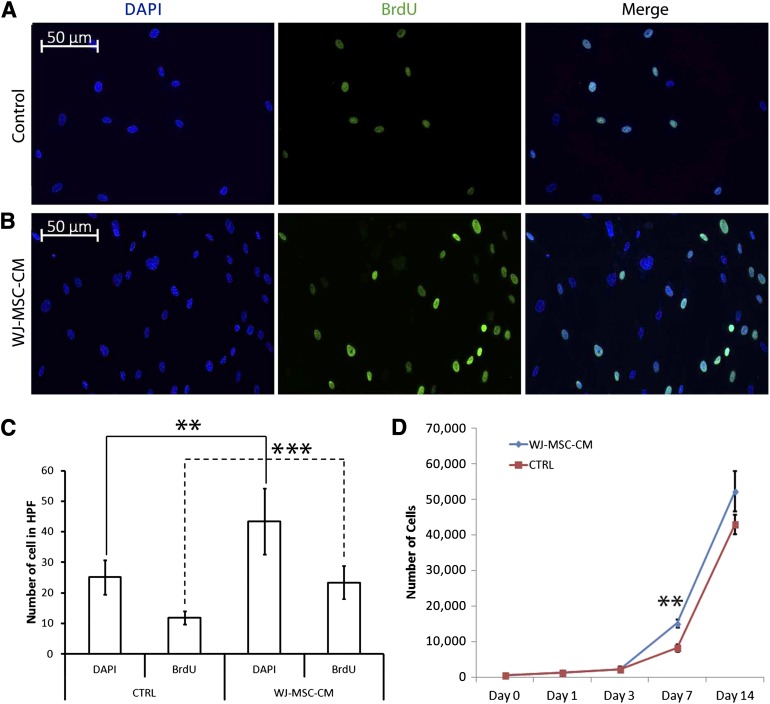
To determine the paracrine effect of WJ-MSC-CM on keloid fibroblast proliferation, cells were exposed to BrdU and were stained, as elaborated in Materials and Methods.
WJ-MSC-CM-treated human keloid fibroblasts showed a significantly higher number of viable cells as measured by DAPI staining, as well as higher number of BrdU-incorporated cells (p ≤ .001) in comparison with non-WJ-MSC-CM-treated keloid fibroblasts at 7 days after exposure (Fig. 4A–4C). We also confirmed with a DNA assay the increase in WJ-MSC-CM-treated keloid fibroblasts at day 7 ( p ≤ .01) (Fig. 4D).
Figure 4.
Kinetics growth of keloid skin fibroblasts cultured with WJ-MSC-CM. The BrdU proliferation assay (A–C) and CyQUANT assay (D) were performed in three and one keloid skin samples, respectively, after being treated or not treated with WJ-MSC-CM. ∗∗, p ≤ .01; ∗∗∗, p ≤ .001. Abbreviations: BrdU, bromodeoxyuridine; CTRL, control; DAPI, 4′,6-diamidino-2-phenylindole; HPF, high-power field; WJ-MSC-CM, Wharton’s jelly-derived mesenchymal stem cell-conditioned medium.
WJ-MSC-CM Did Not Enhance Apoptosis in Cultured Keloid Fibroblasts
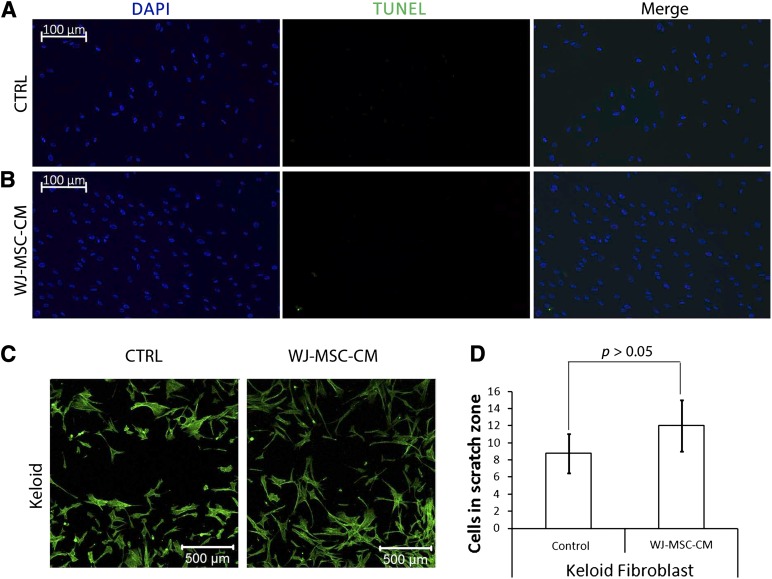
To further explore the effect of WJ-MSC-CM on keloid fibroblasts, we asked whether WJ-MSC-CM modulated the apoptosis rate of keloid fibroblasts. Under our culture conditions, the TUNEL apoptosis assay did not detect any apoptosis-related effects among human keloid skin fibroblasts following treatment with WJ-MSC-CM (Fig. 5A, 5B).
Figure 5.
Wharton’s jelly-derived mesenchymal stem cell (WJ-MSC) one-way paracrine signaling effects on human keloid skin fibroblast apoptosis and migration. A TUNEL kit was used to measure apoptosis (A, B), and a scratch wound assay (C, D) was performed to examine keloid migration, after treatment with WJ-MSC-CM or nonconditioned medium, in three different keloid samples. Abbreviations: CTRL, control; DAPI, 4′,6-diamidino-2-phenylindole; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling; WJ-MSC-CM, Wharton’s jelly-derived mesenchymal stem cell-conditioned medium.
WJ-MSC-CM Did Not Significantly Modulate Keloid Fibroblast Migration
Because keloids expand beyond the boundaries of the original injury, it is possible that the invasive localized nature of keloids is mainly the result of their migratory activity. To delineate whether WJ-MSC-CM may affect the migratory activity of keloid cells, we subjected cultured keloids to a wound scratch assay. Treatment with WJ-MSC-CM resulted in a slightly decreased keloid fibroblast wound gap compared with the nontreated control, although this difference was not statistically significant (p > .05; Fig. 5C, 5D).
Discussion
The results of this study suggest that, under our culture conditions, the effects of human WJ-MSC paracrine signaling on keloid fibroblasts may enhance their profibrotic phenotype.
The first clinical trials with MSCs hold great promise in treating several kinds of soft-tissue fibrosis [23–26], and human WJ-MSCs in particular have been reported to prevent fibrosis in liver, lung, and kidney in rats [20, 27, 28]. It has been documented that caprine WJ-MSCs diminish scarring in goats [29], but there are no described effects of human-derived WJ-MSCs on human skin fibrotic conditions, such as keloids.
Keloids remain a clinical challenge and have no efficacious treatment yet; research is now focused on their pathophysiology at the molecular level [7, 30]. PAI-1 plays a pivotal role in keloid pathogenesis [31–36]. It has been well documented that PAI-1 is overexpressed in keloid fibroblasts, in which it causes collagen accumulation [35]. This study shows that the WJ-MSC secretome upregulates PAI-1 transcript expression in keloid fibroblasts cultured under our culture conditions, suggesting that WJ-MSCs may further enhance the profibrotic keloid phenotype. PAI-1 is the most well-studied member of the serpin (serine protease inhibitor) gene family and the major physiological inhibitor of the plasminogen activator/plasmin protease system. Plasmin induces fibrinolysis and clot resolution but also participates in the destruction of extracellular matrix through the induction of matrix metalloproteinases [34, 35]. PAI-1 is considered to be necessary but not unique to develop a keloid scar [31–36]. This fact partly explains why other profibrotic markers were also examined in this study. Accordingly, PAI-1 plays many cross-talks with other fibrotic-related pathways, such as TGF-β, CTGF, HIF-1-α, and VEGF [37, 38]. Between all of them, the TGF-β superfamily plays a pivotal and complex role in the pathophysiology of multiple fibrotic conditions, including keloids [39–41]. Accumulating evidence suggests that there is a link between inflammation and fibrosis. TGF-β1, TGF-β2, PAI-1, and the proinflammatory cytokines IL-6 and IL-8, when overexpressed, are well-recognized fibrotic factors and keloid phenotype promoters [31, 42, 43]. Under our culture conditions, although we showed no differences in TGF-β1 levels in WJ-MSC-CM-treated keloid fibroblasts, PAI-1 downstream levels were upregulated, suggesting indeed that WJ-MSC-CM may contribute to enhance fibrotic characteristics proper of keloid fibroblasts. On the contrary, TGF-β3 [44, 45] and decorin [46, 47] have been reported to prevent fibrosis. According to our results regarding the profibrotic effect of WJ-MSC paracrine signaling, TGF-β3 transcript levels are downregulated when keloid fibroblasts are influenced by the WJ-MSC secretome. In contrast, it has been reported that human umbilical cord blood-derived MSCs secrete elevated levels of decorin [48], but to the authors' knowledge there are no literature reports regarding decorin secretion by WJ-MSCs in particular. Indeed, the insignificant downregulated decorin transcript levels encountered in the WJ-MSC-CM-treated keloid fibroblasts may explain why no upregulated apoptotic effects were observed, because decorin has been reported to elicit apoptosis in many cell types, including human dermal fibroblasts [49, 50]. Although the above-mentioned array of growth factors and cytokines might also elicit other pleiotropic effects depending mainly on the cellular context, and taking into account that in vitro cell studies with human samples may have particular varieties or biased cross-talks that differ with the in vivo setting, an overall profibrotic genetic response when indirectly culturing human WJ-MSCs with keloid fibroblasts occurred under our culture conditions. This finding agrees with other published reports about the role of MSCs in keloid pathogenesis [51–54] and a study by Ding et al. [55] that shows the profibrotic paracrine signaling effect of BM-MSCs in human normal skin fibroblasts. No previous studies of human WJ-MSC paracrine signaling have been reported in human skin fibroblasts, to our knowledge. MSC paracrine signaling or secretome appears to be the main mechanism underlying reported MSC effects [12–14]. Conceivably, this study focuses only on paracrine effects of WJ-MSCs on keloid fibroblasts. However, paracrine signaling and cell-cell direct MSC effects may cause different or opposing responses in fibroblasts, such as tissue repair and tissue regeneration, respectively [12], and this might partially explain why MSCs themselves have been reported to elicit immunosuppressive and antifibrotic properties [22, 25, 26, 51], in contrast to MSC-derived products, such as conditioned medium. However, the former statement appears too general and unspecific, and it is still surrounded by controversy. Mesenchyme is the embryologic layer that gives rise to many cell lineages, including fibroblasts, the heterogeneous cell type responsible for extracellular matrix deposition [56]. Fibrosis evolves from an excess of extracellular matrix synthesis or a defect on its destruction. A particular fibroblast type, the myofibroblast, has classically been considered as the main cell type responsible for skin tissue fibrosis. It has been shown that myofibroblasts in hypertrophic scars have decreased apoptotic rates, and therefore excessive scars are characterized by an overproduction of extracellular matrix [57]. Some researchers hypothesize that myofibroblasts could well be MSCs [56]. The fact that α-smooth muscle actin, the main myofibroblast marker, is also expressed in MSCs, raises this possibility. It has been suggested that MSCs from the subcutaneous fat might be responsible for the accumulation of collagen in excessive scars [56]. Although BM-MSC paracrine signaling has already been reported to enhance fibrosis [55], our study is novel because it proposes to investigate the effect of another documented more advantageous MSC source, the WJ-MSC [15]. Scarless skin regeneration in humans occurs only in the initial stages of the embryologic and fetal development, and it has been reported that the fetal fibroblast, with its special secretome, is responsible for this antifibrotic effect. The human umbilical cord starts to develop around the 5th week of gestation. Conceivably, at first thought it might well be possible that WJ-MSCs, which represent a fetal MSC source, may secrete a more scarless-like secretome than other adult MSC sources and this secretome might regulate the altered keloid niche and reverse the profibrotic phenotype of keloid fibroblasts. However, our study did not detect such antifibrotic effects but showed upregulation of many profibrotic gene markers. We therefore propose that modulation of WJ-MSC secretome might open a new avenue to manage keloids, but further research is warranted. Indeed, it still remains to be proven whether these in vitro results might be extrapolated to an in vivo setting. Second, it also remains to be examined whether direct cell-cell contact coculture of WJ-MSCs and keloid fibroblasts may elicit opposite effects and actually prevent fibrosis.
In our culture conditions, paracrine signaling of human WJ-MSCs appears to enhance the profibrotic character of keloid fibroblasts, when indirectly culturing both cell types via conditioned medium or coculturing them through a microporous membrane.
Conclusion
Among the many possible sources of MSCs, preclinical studies with WJ-MSCs have proven they emerge as a birth-derived adult or fetal cell source with many advantages, such as antifibrotic and anticancer roles with no rejection after allo-transplantation, and high engraftment rates with functional outcomes in terms of tissue repair and regeneration. However, cell therapies have inherent risks; thus, WJ-MSC clinical trials should further confirm the aforementioned reported experimental results. Accumulating evidence suggests that paracrine signaling is the main underlying mechanism behind the action of MSCs, and MSC-CM may emerge as a new therapy with less clinical risks than direct usage of MSCs. In our culture conditions, WJ-MSCs appear to promote keloid phenotype through a paracrine signaling mechanism. The direct effect of WJ-MSCs on keloids remains to be determined and may reveal new insight into the underlying process of skin fibrosis. Further research in this field may pave a new path for management of keloids.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant R01-GM087285-01, the Canada Foundation for Innovation (CFI) Leader’s Opportunity Fund (Project 25407), the Physician’s Services Incorporated Foundation–Health Research Grant Program, and the Healing Foundation/British Burn Association (BBA) and AB Wallace Memorial 2012 Award.
Author Contributions
A.I.A., S.A.-N., and P.H.B.: conception and design, administrative support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; M.A.-S. and C.B.: administrative support, collection and/or assembly of data, final approval of manuscript; E.H.: provision of study material or patients, final approval of manuscript; M.G.J.: conception and design, financial support, administrative support, provision of study material or patients collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: Recruiting developmental pathways for regeneration. Cell Mol Life Sci. 2013;70:2059–2081. doi: 10.1007/s00018-012-1152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atiyeh BS, Amm CA, El Musa KA. Improved scar quality following primary and secondary healing of cutaneous wounds. Aesthetic Plast Surg. 2003;27:411–417. doi: 10.1007/s00266-003-3049-3. [DOI] [PubMed] [Google Scholar]
- 3.Armour A, Scott PG, Tredget EE. Cellular and molecular pathology of HTS: Basis for treatment. Wound Repair Regen. 2007;15(suppl 1):S6–S17. doi: 10.1111/j.1524-475X.2007.00219.x. [DOI] [PubMed] [Google Scholar]
- 4.Shih B, Garside E, McGrouther DA, et al. Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen. 2010;18:139–153. doi: 10.1111/j.1524-475X.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence JW, Mason ST, Schomer K, et al. Epidemiology and impact of scarring after burn injury: A systematic review of the literature. J Burn Care Res. 2012;33:136–146. doi: 10.1097/BCR.0b013e3182374452. [DOI] [PubMed] [Google Scholar]
- 6.Roseborough IE, Grevious MA, Lee RC. Prevention and treatment of excessive dermal scarring. J Natl Med Assoc. 2004;96:108–116. [PMC free article] [PubMed] [Google Scholar]
- 7.Gauglitz GG, Korting HC, Pavicic T, et al. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113–125. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee MJ, Jung J, Na KH, et al. Anti-fibrotic effect of chorionic plate-derived mesenchymal stem cells isolated from human placenta in a rat model of CCl(4)-injured liver: Potential application to the treatment of hepatic diseases. J Cell Biochem. 2010;111:1453–1463. doi: 10.1002/jcb.22873. [DOI] [PubMed] [Google Scholar]
- 9.Jackson WM, Nesti LJ, Tuan RS. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res Ther. 2012;3:20. doi: 10.1186/scrt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Li T, Wei X, et al. Mesenchymal stem cell transplantation improves regional cardiac remodeling following ovine infarction. Stem Cells Transl Med. 2012;1:685–695. doi: 10.5966/sctm.2012-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Fu X. Mechanisms of action of mesenchymal stem cells in cutaneous wound repair and regeneration. Cell Tissue Res. 2012;348:371–377. doi: 10.1007/s00441-012-1393-9. [DOI] [PubMed] [Google Scholar]
- 12.Hocking AM, Gibran NS. Mesenchymal stem cells: Paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316:2213–2219. doi: 10.1016/j.yexcr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren G, Chen X, Dong F, et al. Concise review: Mesenchymal stem cells and translational medicine: Emerging issues. Stem Cells Transl Med. 2012;1:51–58. doi: 10.5966/sctm.2011-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khosrotehrani K. Mesenchymal stem cell therapy in skin: Why and what for? Exp Dermatol. 2013;22:307–310. doi: 10.1111/exd.12141. [DOI] [PubMed] [Google Scholar]
- 15.Bongso A, Fong CY. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton’s jelly of the human umbilical cord. Stem Cell Rev. 2013;9:226–240. doi: 10.1007/s12015-012-9418-z. [DOI] [PubMed] [Google Scholar]
- 16.Gauthaman K, Fong CY, Suganya CA, et al. Extra-embryonic human Wharton’s jelly stem cells do not induce tumorigenesis, unlike human embryonic stem cells. Reprod Biomed Online. 2012;24:235–246. doi: 10.1016/j.rbmo.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Kim MJ, Shin KS, Jeon JH, et al. Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: A comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011;346:53–64. doi: 10.1007/s00441-011-1249-8. [DOI] [PubMed] [Google Scholar]
- 18.Nekanti U, Rao VB, Bahirvani AG, et al. Long-term expansion and pluripotent marker array analysis of Wharton’s jelly-derived mesenchymal stem cells. Stem Cells Dev. 2010;19:117–130. doi: 10.1089/scd.2009.0177. [DOI] [PubMed] [Google Scholar]
- 19.Anzalone R, Lo Iacono M, Corrao S, et al. New emerging potentials for human Wharton’s jelly mesenchymal stem cells: Immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev. 2010;19:423–438. doi: 10.1089/scd.2009.0299. [DOI] [PubMed] [Google Scholar]
- 20.Du T, Cheng J, Zhong L, et al. The alleviation of acute and chronic kidney injury by human Wharton’s jelly-derived mesenchymal stromal cells triggered by ischemia-reperfusion injury via an endocrine mechanism. Cytotherapy. 2012;14:1215–1227. doi: 10.3109/14653249.2012.711471. [DOI] [PubMed] [Google Scholar]
- 21.Kita K, Gauglitz GG, Phan TT, et al. Isolation and characterization of mesenchymal stem cells from the sub-amniotic human umbilical cord lining membrane. Stem Cells Dev. 2010;19:491–502. doi: 10.1089/scd.2009.0192. [DOI] [PubMed] [Google Scholar]
- 22.Arno AI, Smith AH, Blit PB, et al. Stem cell therapy: A new treatment for burns? Pharmaceuticals. 2011;4:1355–1380. doi: 10.3390/ph4101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heeger CH, Jaquet K, Thiele H, et al. Percutaneous, transendocardial injection of bone marrow-derived mononuclear cells in heart failure patients following acute ST-elevation myocardial infarction: ALSTER-Stem Cell trial. EuroIntervention. 2012;8:732–742. doi: 10.4244/EIJV8I6A113. [DOI] [PubMed] [Google Scholar]
- 24.Lin H, Zhang Z, Shi M, et al. Prospective controlled trial of safety of human umbilical cord derived-mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis [in Chinese] Zhonghua Gan Zang Bing Za Zhi. 2012;20:487–491. doi: 10.3760/cma.j.issn.1007-3418.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Reinders ME, Rabelink TJ, de Fijter JW. The role of mesenchymal stromal cells in chronic transplant rejection after solid organ transplantation. Curr Opin Organ Transplant. 2013;18:44–50. doi: 10.1097/MOT.0b013e32835c2939. [DOI] [PubMed] [Google Scholar]
- 26.Voswinkel J, Francois S, Gorin NC, et al. Gastro-intestinal autoimmunity: Preclinical experiences and successful therapy of fistulizing bowel diseases and gut graft versus host disease by mesenchymal stromal cells. Immunol Res. 2013;56:241–248. doi: 10.1007/s12026-013-8397-8. [DOI] [PubMed] [Google Scholar]
- 27.Moodley Y, Atienza D, Manuelpillai U, et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;175:303–313. doi: 10.2353/ajpath.2009.080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai PC, Fu TW, Chen YM, et al. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton’s jelly in the treatment of rat liver fibrosis. Liver Transpl. 2009;15:484–495. doi: 10.1002/lt.21715. [DOI] [PubMed] [Google Scholar]
- 29.Azari OBH, Babaei H, Derakhshanfar A, et al. Effects of transplanted mesenchymal stem cells isolated from Wharton’s jelly of caprine umbilical cord on cutaneous wound healing; histopathological evaluation. Vet Res Commun. 2011;35:211–222. doi: 10.1007/s11259-011-9464-z. [DOI] [PubMed] [Google Scholar]
- 30.van der Veer WM, Bloemen MC, Ulrich MM, et al. Potential cellular and molecular causes of hypertrophic scar formation. Burns. 2009;35:15–29. doi: 10.1016/j.burns.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Tuan TL, Nichter LS. The molecular basis of keloid and hypertrophic scar formation. Mol Med Today. 1998;4:19–24. doi: 10.1016/S1357-4310(97)80541-2. [DOI] [PubMed] [Google Scholar]
- 32.Tuan TL, Zhu JY, Sun B, et al. Elevated levels of plasminogen activator inhibitor-1 may account for the altered fibrinolysis by keloid fibroblasts. J Invest Dermatol. 1996;106:1007–1011. doi: 10.1111/1523-1747.ep12338552. [DOI] [PubMed] [Google Scholar]
- 33.Tuan TL, Wu H, Huang EY, et al. Increased plasminogen activator inhibitor-1 in keloid fibroblasts may account for their elevated collagen accumulation in fibrin gel cultures. Am J Pathol. 2003;162:1579–1589. doi: 10.1016/S0002-9440(10)64292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. J Cell Physiol. 2012;227:493–507. doi: 10.1002/jcp.22783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuan TL, Hwu P, Ho W, et al. Adenoviral overexpression and small interfering RNA suppression demonstrate that plasminogen activator inhibitor-1 produces elevated collagen accumulation in normal and keloid fibroblasts. Am J Pathol. 2008;173:1311–1325. doi: 10.2353/ajpath.2008.080272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim WJ. Cellular signaling in tissue regeneration. Yonsei Med J. 2000;41:692–703. doi: 10.3349/ymj.2000.41.6.692. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q, Wu Y, Chau CH, et al. Crosstalk of hypoxia-mediated signaling pathways in upregulating plasminogen activator inhibitor-1 expression in keloid fibroblasts. J Cell Physiol. 2004;199:89–97. doi: 10.1002/jcp.10452. [DOI] [PubMed] [Google Scholar]
- 38.Steinbrech DS, Mehrara BJ, Chau D, et al. Hypoxia upregulates VEGF production in keloid fibroblasts. Ann Plast Surg. 1999;42:514–519; discussion 519–520. doi: 10.1097/00000637-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Pohlers D, Brenmoehl J, Löffler I, et al. TGF-beta and fibrosis in different organs—molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746–756. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Prud'homme GJ. Pathobiology of transforming growth factor b in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Invest. 2007;87:1077–1091. doi: 10.1038/labinvest.3700669. [DOI] [PubMed] [Google Scholar]
- 41.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mu X, Bellayr I, Walters T, et al. Mediators leading to fibrosis—how to measure and control them in tissue engineering. Oper Tech Orthop. 2010;20:110–118. doi: 10.1053/j.oto.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le AD, Zhang Q, Wu Y, et al. Elevated vascular endothelial growth factor in keloids: relevance to tissue fibrosis. Cells Tissues Organs. 2004;176:87–94. doi: 10.1159/000075030. [DOI] [PubMed] [Google Scholar]
- 44.Adzick NS, Lorenz HP. Cells, matrix, growth factors, and the surgeon: The biology of scarless fetal wound repair. Ann Surg. 1994;220:10–18. doi: 10.1097/00000658-199407000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferguson MW, O’Kane S. Scar-free healing: From embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci. 2004;359:839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meenakshi J, Vidyameenakshi S, Ananthram D, et al. Low decorin expression along with inherent activation of ERK1,2 in ear lobe keloids. Burns. 2009;35:519–526. doi: 10.1016/j.burns.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Mukhopadhyay A, Wong MY, Chan SY, et al. Syndecan-2 and decorin: Proteoglycans with a difference—implications in keloid pathogenesis. J Trauma. 2010;68:999–1008. doi: 10.1097/TA.0b013e3181c4070d. [DOI] [PubMed] [Google Scholar]
- 48.Kim JY, Kim DH, Kim JH, et al. Umbilical cord blood mesenchymal stem cells protect amyloid-β42 neurotoxicity via paracrine. World J Stem Cells. 2012;4:110–116. doi: 10.4252/wjsc.v4.i11.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honardoust D, Ding J, Varkey M, et al. Deep dermal fibroblasts refractory to migration and decorin-induced apoptosis contribute to hypertrophic scarring. J Burn Care Res. 2012;33:668–677. doi: 10.1097/BCR.0b013e31824088e3. [DOI] [PubMed] [Google Scholar]
- 50.Wu H, Wang S, Xue A, et al. Overexpression of decorin induces apoptosis and cell growth arrest in cultured rat mesangial cells in vitro. Nephrology (Carlton) 2008;13:607–615. doi: 10.1111/j.1440-1797.2008.00961.x. [DOI] [PubMed] [Google Scholar]
- 51.Akino K, Akita S, Yakabe A, et al. Human mesenchymal stem cells may be involved in keloid pathogenesis. Int J Dermatol. 2008;47:1112–1117. doi: 10.1111/j.1365-4632.2008.03380.x. [DOI] [PubMed] [Google Scholar]
- 52.Moon JH, Kwak SS, Park G, et al. Isolation and characterization of multipotent human keloid-derived mesenchymal-like stem cells. Stem Cells Dev. 2008;17:713–724. doi: 10.1089/scd.2007.0210. [DOI] [PubMed] [Google Scholar]
- 53.Iqbal SA, Sidgwick GP, Bayat A. Identification of fibrocytes from mesenchymal stem cells in keloid tissue: A potential source of abnormal fibroblasts in keloid scarring. Arch Dermatol Res. 2012;304:665–671. doi: 10.1007/s00403-012-1225-5. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Q, Yamaza T, Kelly AP, et al. Tumor-like stem cells derived from human keloid are governed by the inflammatory niche driven by IL-17/IL-6 axis. PLoS One. 2009;4:e7798. doi: 10.1371/journal.pone.0007798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding J, Ma Z, Shankowsky HA, et al. Deep dermal fibroblast profibrotic characteristics are enhanced by bone marrow-derived mesenchymal stem cells. Wound Repair Regen. 2013;21:448–455. doi: 10.1111/wrr.12046. [DOI] [PubMed] [Google Scholar]
- 56.van den Bogaerdt AJ, van der Veen VC, van Zuijlen PP, et al. Collagen cross-linking by adipose-derived mesenchymal stromal cells and scar-derived mesenchymal cells: Are mesenchymal stromal cells involved in scar formation? Wound Repair Regen. 2009;17:548–558. doi: 10.1111/j.1524-475X.2009.00501.x. [DOI] [PubMed] [Google Scholar]
- 57.Moulin V, Larochelle S, Langlois C, et al. Normal skin wound and hypertrophic scar myofibroblasts have differential responses to apoptotic inductors. J Cell Physiol. 2004;198:350–358. doi: 10.1002/jcp.10415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.