The objective of the study was to assess the safety and efficacy of transplanting bone marrow nucleated cells (BMNCs) to treat children with complete interruption of spinal cord (SC) continuity. The results demonstrate the safety and feasibility of BMNC transplantation in children with complete SC injury and indicate that a certain degree of neurological and quality-of-life improvement can be attained by children with chronic complete SC injury who receive multiple BMNC implantations.
Keywords: Cell therapy, Spinal cord injury, Transplantation, Cell transplantation, Tissue regeneration
Abstract
The objective of this study was to assess the safety and efficacy of transplanting bone marrow nucleated cells (BMNCs) to treat children with complete interruption of spinal cord (SC) continuity. The present study was conducted from 2005 to 2011. The inclusion criteria were a magnetic resonance imaging-confirmed complete interruption of SC continuity and no improvement in neurological status within 6 months after standard therapy. Bone marrow was isolated from the iliac ala and submitted to BMNC isolation. Subsequently, the cell suspension was administered into the SC cavity and intravenously. In total, 18 of 19 intraspinal and intravenous BMNC transplantation procedures performed caused no adverse events. One case was connected with transient bradycardia. The experimental therapy showed no late complications in the 1- to 6-year follow-up evaluation period. Neurological improvement was observed in two patients who received multiple implantations. One patient demonstrated improved superficial sensation from Th3 to Th12/L1 and a restored bladder-filling sensation. In the other case, superficial sensation was improved from C2 to C5, and the respiratory drive, the swallowing reflex, and tongue movements were restored. Spasticity and quality of life were improved in three of five patients. In addition, skin pressure ulcers healed and did not recur. Our preliminary results demonstrate the safety and feasibility of BMNC transplantation in children with complete SC injury. The results indicate that a certain degree of neurological and quality-of-life improvement can be attained by children with chronic complete SC injury who receive multiple BMNC implantations.
Introduction
Spinal cord injury (SCI) with an interruption of spinal cord (SC) continuity results in an irreversible loss of neuronal connections, leading to SC dysfunction, profound disability, significantly affected quality of life, and even death. More than 130,000 people sustain new SCIs each year worldwide [1]; most are young individuals, and 82% are young males. Progress in emergency medicine, nursing care, and long-term care for SCI patients has resulted in increased survival; however, survival decreases with age and depends on the injury level [2].
Standard management after SCI includes stabilization of the injured region and subsequent long-term conservative and rehabilitation treatment [1], although the optimal duration of the intervention is disputable [3]. Patients with SCI develop several long-term complications, such as pneumonia, atelectasis, deep venous thrombosis, pulmonary embolism, pressure ulcers, autonomic dysreflexia, renal calculi, fractures [4], spasticity (up to 70%) [5], and neuropathic pain (40%–50%) [6]. The complications are a frequent cause of morbidity and mortality and lead to an increased rate of rehospitalization [4]. Thus, conservative treatment involves the prevention of post-traumatic complications as well as analgesic treatment and rehabilitation. Nevertheless, we still lack standard treatment methods that can regenerate sensory and motor neurons, which would enable the complete or partial functional recovery of the patient.
Experimental approaches to SCI treatment using the cellular delivery of growth factors demonstrated that insulin-like growth factor 1 supports corticospinal axon survival [7]; that neurotrophin-3 causes partial functional recovery [8]; and that the placement of molecular, cellular, or “synthetic” bridges in the lesion cavity supports axonal regeneration across the lesion site [9]. Sciatic nerve conditioning of lesions result in regeneration of the injured SC [10], and stimulating cAMP signaling increased the intrinsic growth capacity of injured sensory axons [11], overcoming myelin inhibition [12].
The combination of cAMP, neurotrophin-3, and intralesional mesenchymal stem cell (MSC) administration causes better regeneration than single factors [13]. A phosphodiesterase type 4 inhibitor causes regeneration when applied together with embryonic spinal tissue implantation [14] or with Schwann cell grafts, which promote significant axon sparing and myelination [15].
Sensory and functional recovery was also demonstrated using glial scar inhibition by chondroitinase ABC [16], and neutralization of an important neurite outgrowth inhibitor, Nogo-A, stimulated axonal sprouting [17]. Neuronal and axonal regeneration, resulting in improvements in sensory and motor function, after the administration of embryonic stem cells, MSCs, neural stem/progenitor cells, olfactory ensheathing cells (OECs) or Schwann cells was nicely reviewed by Li and Lepski [18].
Bone marrow (BM) has been demonstrated to be a source of different stem and progenitor cells, including hematopoietic, angiogenic and epithelial cells [19], and MSCs [20], but BM also contains neural tissue-committed stem cells, which are mobilized into the peripheral blood during a cerebral stroke [21]. These cells produce numerous trophic growth factors [22]. The intravenous administration of CD34+ BM cells has been demonstrated to promote an environment that supports the neovascularization of the ischemic brain, enabling neuronal regeneration [23]. In addition, intracerebral transplantation of CD34+ and/or CD133+ cells improved neurological function in animal models [24]. BM MSCs administered intracerebrally [25] or via the carotid artery [26] into the ischemic brains of rats caused significant improvement in functional performance. Remyelination of SC axons following the intravenous delivery of BM cells [27, 28] and functional improvement after the intraspinal administration of BM stromal cells have also been demonstrated [29].
Nine clinical trials evaluating the safety and efficacy of using autologous BM-derived cells alone or in combination with granulocyte-macrophage colony-stimulating factor in acute [30–35], subacute [34, 35], and chronic [32, 34–38] adult SCI patients have been published since 2005, involving a total of 401 patients. The safety and feasibility of intravenous and intraspinal single implantations were demonstrated by all groups, with only minor adverse events in some patients, such as fever, increased leukocytosis, skin rash, dizziness, itching, headache, rigidity, and abdominal discomfort [30, 35, 37]. These studies provide evidence of the possibility of neurological, sensory, and functional improvement; quality of life improvement; and partial restoration of the neural paths in the SC for patients with acute, subacute, or chronic SCI. However, greater improvement was observed in patients with acute SCI, and a negative correlation between the time interval from the injury to cell therapy and the outcome was noted [32, 34]. Three groups demonstrated improvements in patients with chronic SCIs who received BM cells by intrathecal administration [32, 37, 38], whereas one study found no improvement [35]. The importance of intramedullary administration compared with only intravenous administration was demonstrated for attaining neurological improvement [34], regardless of the length of time since injury. The cell dose seemed to influence the appearance and degree of the improvement [33]. Single implantations of BM MSCs mainly produced slight improvements in patients with acute [39], subacute, or chronic SCI [40, 41], although considerable improvement in patients with subacute SCI was also reported [42]. Allogeneic umbilical cord and cord-blood MSCs were demonstrated to cause a slight improvement in patients with incomplete [43] and complete [44] chronic SCI, respectively, and considerable improvement was seen in patients with incomplete SCI when several rounds of cord-blood CD34+ and cord-tissue MSC cells were transplanted [45]. The single or multiple use of OECs produced slight [46] or considerable [47] improvements, respectively, in patients with chronic complete SCI. The transplantation of olfactory mucosa in patients with complete chronic SCI caused slight improvement [48] or considerable improvement [49] in several patients. The administration of Schwann cells was shown to be safe and to produce slight improvement [50, 51]. Similarly, slight sensation improvement was observed in several patients after the single administration of a combination of MSCs and Schwann cells [52]. Considerable improvement in patients with chronic SCI was achieved using a combination of MSCs and the patients’ autoimmune T cells [53].
The objective of the current study was to assess the safety and efficacy of combining multiple intravenous and intrathecal administrations of BM nucleated cells (BMNCs) for the treatment of a group of five children with chronic SCI characterized by the complete interruption of SC continuity.
Materials and Methods
Patients
The presented study was a prospective, longitudinal medical experiment. The study was performed at University Children’s Hospital (UCH) in Cracow, Poland, from 2005 to 2011. The trial was approved by the Jagiellonian University Medical College bioethical committee (KBET/13/B/2005). The parents of all eligible pediatric patients provided written informed consent according to the Helsinki Declaration. Pediatric patients with chronic SCI were included in the study. The general data collected before the experimental therapy consisted of age, gender, the cause of injury, the injury level, the length of time since the injury, previous medical treatment for SCI, and past medical history.
Enrollment Criteria
The study included patients with SCI with a complete interruption of SC continuity confirmed by imaging studies (computed tomography and magnetic resonance imaging [MRI]) and with American Spinal Injury Association (ASIA) impairment scale grade A. No patient selection was performed depending on the level of SCI. Only children with a minimum of a 6-month time interval between the trauma and the initiation of experimental therapy were enrolled (classified by the authors as “chronic SCI”), with no improvement in their medical condition after standard and obligatory therapeutic methods and with no deterioration or significant improvement of SC function within the 1-month period preceding the experimental therapy. Patients with focal central nervous system lesions (neoplastic lesions, untreated vascular malformations) or chronic diseases (e.g., systemic diseases) that would require long-term pharmacotherapy were excluded from the study.
Prior to the treatment, the patients were examined by a neurologist.
The BM-derived cell implantation procedure was performed when the patients were stable, without contraindications for general anesthesia from the viewpoint of internal medicine and cardiology and without any serious infectious diseases, including sepsis, immediately prior to the procedure.
Ultimately, five patients were enrolled in the study. In total, these patients were subjected to 19 implantation procedures with BM-derived cells.
Procedures
BM Aspiration
Autologous BM from patients who qualified for the experimental treatment was harvested from the posterior superior iliac crest under general anesthesia. With the patient placed in the prone position, with a slightly elevated pelvis, the BM was aspirated from multiple punctures of the iliac ala. BM was collected in medium consisting of 0.9% sodium chloride, heparin, and gentamicin.
BMNC Isolation, Evaluation, and Preparation for Transplantation
The isolation of BMNCs from BM suspension was performed at the Department of Transplantation, Jagiellonian University Medical College, using 10% hydroxyethyl starch solution (Fresenius Kabi, Kutno, Poland, http://www.fresenius-kabi.pl). Hydroxyethyl starch was added to the BM until a final concentration of 3% was reached. Next, the obtained solution was mixed and left in a sterile bottle for 40–50 minutes for distribution. After all erythrocytes had sedimented at the bottom of the bottle, the upper cell suspension was collected into 50-ml tubes and centrifuged. The supernatant was discarded, and the cell suspension was washed with phosphate-buffered saline (PBS) solution (PAA Laboratories, Goetzis, Austria, http://www.paa.at) and resuspended in PBS. The cell number was evaluated, and the cells were divided into two parts.
The number of the cells was assessed using a hematocytometer. The viability of the cells was checked using a trypan blue exclusion assay (0.4% solution; Sigma-Aldrich, Seelze, Germany, http://www.sigmaaldrich.com). The percentages of CD34+ and CD133+ cells were assessed according to the International Society of Hematotherapy and Graft Engineering protocol using monoclonal anti-CD34-PE and anti-CD133-PE antibodies (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) and cytofluorimetric evaluation on a FACSCalibur (Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com). The sterility of the material was confirmed by microbiological evaluation.
The first sample of BMNCs, comprising 0.5 × 109 cells, was centrifuged and resuspended in 5 ml of 5% glucose solution (Fresenius Kabi) for intraspinal injection. The second sample, comprising the rest of the cells, was prepared in 30 ml of PBS for intravenous injection.
Surgical Procedure and BM-Derived Cell-Transplantation Procedure
The surgical procedure was performed by a team of specialists from the Department of Children's Neurosurgery at UCH in Cracow. With the patient under general anesthesia, the SC was exposed by a laminectomy. The injury was verified under a microscope, and the necrotic tissues were resected. The BMNC suspension was administered directly to the site of the SCI cavity and peripherally via cubital vein for 10 minutes using a drip infusion set. The wound was closed in a typical manner, with the meninx closed with a watertight suture and the resected lamella of the vertebral arch restored. Subsequent BMNC implantations were performed at variable intervals, the length of which depended on the general condition of the patient, the prognosis regarding possible further effectiveness of the experimental therapy, and the parent’s consent. Cells were infused via lumbar puncture and intravenously except in case 1, in which the cells were administered only intramedullary. All of the patients underwent intensive neurorehabilitation (4-week duration) after every cycle of the experimental treatment.
Pretreatment Neurological Examination
Pretreatment neurological examinations were performed according to a protocol that included an evaluation of motor function according to the ASIA scale and the Frankel grading system. Sensory deficits were evaluated using dermatome sensory maps. Post-traumatic spasticity was assessed using the Ashworth scale, and bladder function evaluation was based on subjective parental evaluation. Similarly, quality of life was assessed on the basis of parental evaluation according to the Spinal Cord Independence Measure (SCIM) scale.
Safety Evaluation Criteria
The safety criteria for the transplantation procedure included the appearance of infection, fever, headache, pain, an increased level of C-reactive protein, increased leukocytosis, allergic reaction or shock, and perioperative complications (anesthesia- and analgesia-related complications, infections of the wound) for 7–14 days after the procedure. The safety criteria for using BMNCs included infection, neuropathic pain, cancer development, and deterioration of the neurological state and were assessed for a 1- to 6-year follow-up period.
Follow-up Assessment of Treatment Success
The follow-up evaluation consisted of a neurological examination evaluating sensation and motor improvement measured by the ASIA scale and the Frankel grading system. Sensory deficits were evaluated using dermatome sensory maps. Post-traumatic spasticity was assessed using the Ashworth scale, and bladder function evaluation was based on subjective parental evaluation. Similarly, quality of life was assessed based on the functional recovery estimated by the SCIM method [54].
In addition, an evaluation of the development of neuropathic pain, secondary infections, urinary tract infections, or pressure ulcers of the skin was performed.
Results
Case Presentation
Case 1
A boy experienced a perinatal injury of the cranio-occipital junction in the course of a forceps delivery. He was admitted to UCH. The injury resulted in damage to the SC involving the C1-C2 segment and the clinical symptoms of a complete transverse SC lesion, such as tetraparesis, apnea, a preserved brainstem reaction, and bulbar syndrome. The patient was submitted to continuous mechanical ventilation.
At the age of 7 months, the boy was subjected to the experimental therapy. BMNC implantation was performed into the injury cavity only once, and the treatment was discontinued owing to withdrawal of parental consent. After the implantation procedure, the patient underwent 4 weeks of intense neurorehabilitation.
There were no adverse events connected with the transplantation procedure and no later adverse events.
The patient, who had vegetative symptoms, died several years after the implantation from severe pneumonia and concomitant respiratory failure.
Case 2
A boy was hit by a motor vehicle at the age of 7 years. MRI of the thoracic spine demonstrated a concussion of the anterior SC (hematomyelia) at Th9-Th10. Blood was also present in the subarachnoid space at Th10. A follow-up MRI of the SC showed its complete destruction at the aforementioned level. A computed tomography scan of the head did not show any abnormalities. Conservative treatment and motor rehabilitation in the initial phase improved the medical condition of the patient; the muscles of the shoulder girdle and upper extremities were strengthened, active function of the oblique and straight abdominal muscles was restored, and the boy had better trunk control. Neurologically, spastic paralysis of the lower extremities persisted, and superficial and deep sensation was preserved up to Th12. The boy presented with a neurogenic bladder without vesicoureteral reflux.
Sixty months later, the boy was admitted to UCH and subjected to the experimental therapy. The boy had two BMNC implantations (Table 1; Fig. 1). In the course of the first procedure, the injured segment of the SC was subjected to revision, an intramedullary scar was resected, and a portion of BMNC suspension was implanted into the injury cavity using a drain. At the same time, the second sample of BMNCs was administered intravenously. After the implantation procedure, the patient underwent 4 weeks of intense neurorehabilitation. After 13.5 months, the transplantation was repeated, and BMNCs were administered intravenously and via lumbar puncture.
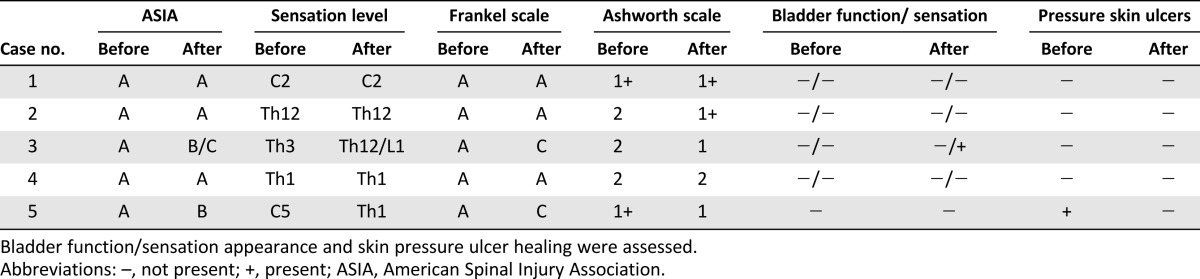
Table 1.
The number of cells in and characteristics of each BMNC transplantation
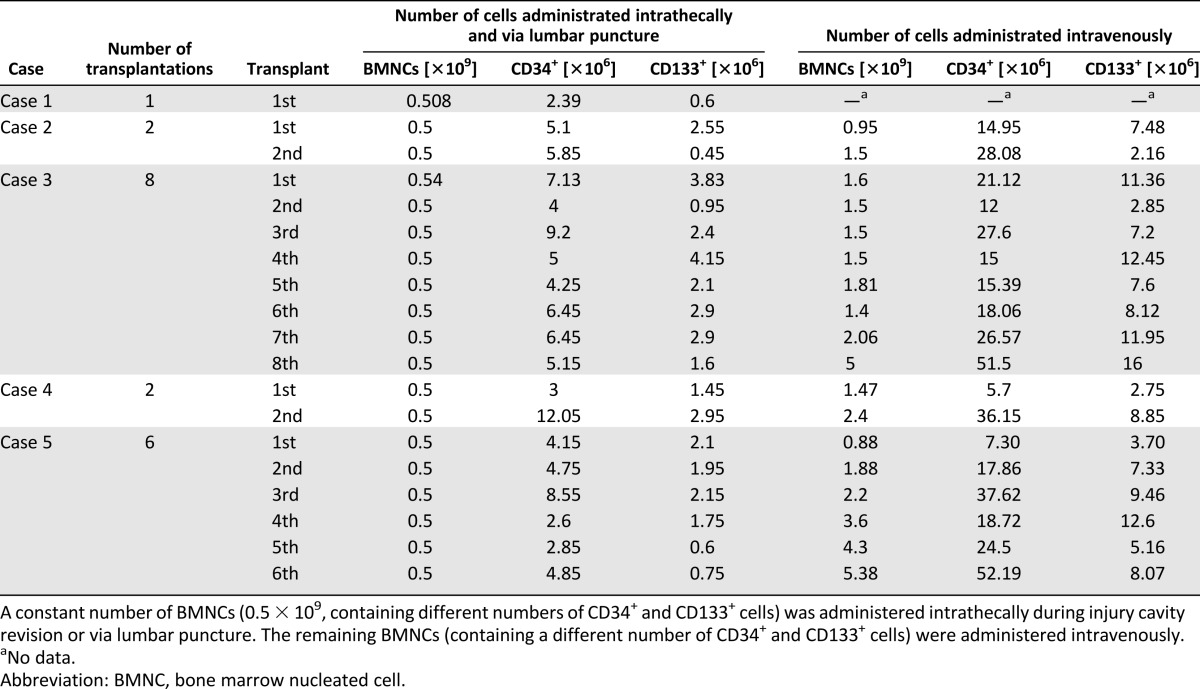
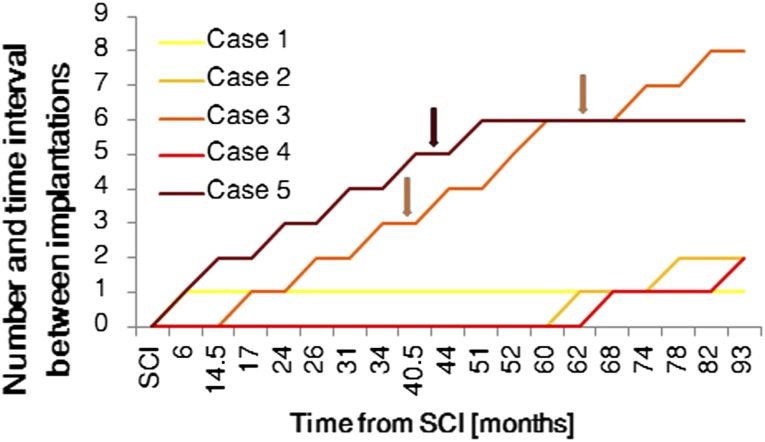
Figure 1.
Time intervals between SCI and subsequent bone marrow nucleated cell implantations. Neurological improvement is shown in the patients from case 3 and case 5. Abbreviation: SCI, spinal cord injury.
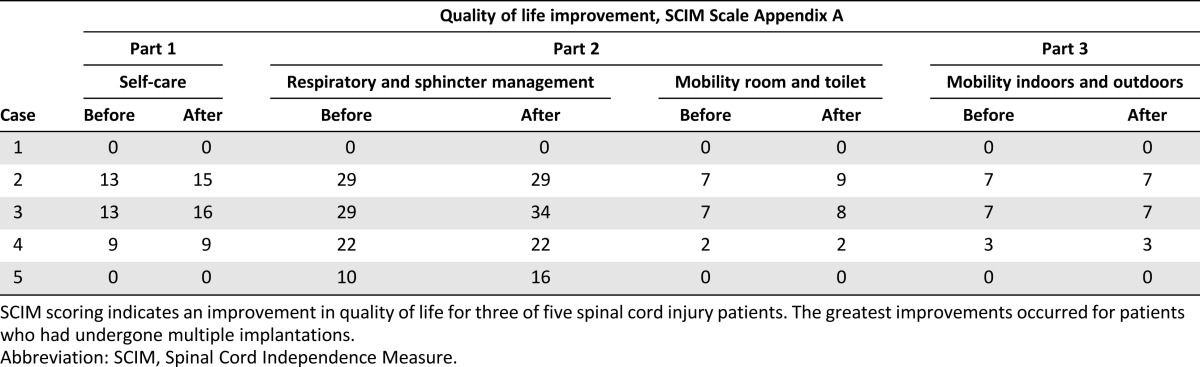
There were no adverse events connected with the transplantation procedures. Within the 5 years of follow-up, no negative effects of the BMNC treatment were noted. Neither clinical nor imaging examinations showed any improvement. The ASIA grade of A did not change; however, the patient reported quality-of-life improvement. The patient’s spasticity was reduced from 2 to 1+ on the Ashworth scale. The SCIM score in appendix A, part 1, regarding the ability to self-service, increased from 13 to 15 points; in part 2, regarding respiratory and sphincter management, the score stayed at 29, and room and toilet mobility improved from 7 to 9 points; and in part 3, regarding indoor and outdoor mobility, the score remained at 7 points (Table 2).
Table 2.
Quality-of-life improvement evaluated with the use of the SCIM scale for five cases
The parents’ consent for the experimental therapy was withdrawn after the second round of BMNC implantation.
The patient was followed up by the Outpatient Department of Neurosurgery and was submitted to regular follow-up examinations. The last examination was performed in March 2012.
Case 3
A 6-year-old boy was hit by a tree branch, which caused injury of the thoracic spine (SCIWORA). On the 10th day after the injury, the boy was admitted to the Department of Neurosurgery at UCH in Cracow. At admission, the child was conscious (Glasgow Coma Scale score of 15) and presented with flaccid paralysis of the lower extremities, obliterated superficial and deep sensation starting at Th3, and trace abdominal medial and inferior skin reflexes. Because MRI was not possible, myelotomography showed a focal concussion of the SC at Th5-Th7 (and especially Th3-Th4), which predominantly involved the posterior horns and lateral columns of the SC. In addition, the patient was diagnosed with cerebral concussion, hemothorax, and a fracture of the left femoral bone. Conservative treatment and motor rehabilitation in the initial phase did not cause any significant neurological improvement.
One year later (in 2005), the patient qualified for the experimental therapy. In the course of a laminotomy of the Th3-Th4 arches performed under general anesthesia, the intramedullary cavity was revised, the necrotic tissues were resected, and the patient received an intrathecal and intravenous BMNC infusion (Table 1; Fig. 1). A follow-up MRI of the thoracic spinal canal demonstrated a 30 × 17-mm intraspinal and intramedullary fluid-filled space situated at Th3-Th4 that extended to the enlarged intervertebral foramens Th3/Th4 and Th4/Th5. At the level of Th5, the medulla was thinned, with a sagittal dimension of up to 3 mm, whereas at Th6, the dimension was 5 mm. There were no adverse events connected with the transplantation procedure. After the implantation procedure, the patient underwent 4 weeks of intense neurorehabilitation. Clinical and imaging examinations did not show any improvement. BMNC implantations were repeated twice via lumbar puncture and intravenous infusion in the next year (2006). The first slight neurological improvement was observed during a neurological examination after the third implantation and manifested as a change in sensation level starting at Th5 (Table 3). The fourth transplantation was performed in 2007. Next, two transplantations were performed in 2008. Further improvement of sensation level, descending by eight segments, was then observed to Th12/L1. The ASIA grade of A changed to B/C, and the Frankel scale score of A changed to C. The patient declared improvement in the sensation of urinary bladder filling. The patient’s spasticity was reduced from 2 to 1 on the Ashworth scale. The patient declared improvement in quality of life (Table 2). The SCIM score in appendix A, part 1, regarding ability to self-service, increased from 13 to 16 points; part 2, regarding respiratory and sphincter management, changed from 29 to 34 points and, regarding room and toilet mobility, changed from 7 to 8 points; and the score in part 3, regarding indoor and outdoor mobility, remained at 7 points. Another two BMNC implantations were performed in the following year (2009). No further improvement was observed. In total, the patient was subjected to eight BMNC transplantations (Table 1; Fig. 1). None of the transplantation procedures caused any adverse events. Within the 3 years of follow-up, no negative effects of the BMNC treatment were noted, and imaging examinations excluded any possibility of lesions that are carcinogenic in character.
Table 3.
Summary of the results of the neurological evaluations before and after the treatment using the ASIA, Frankel, and Ashworth grading systems
The patient is being followed up by the Outpatient Department of Neurosurgery. The last examination was conducted in March 2012.
Case 4
A boy was severely injured at the age of 3 years when he was hit by the wheel of a backing-up cart. On admission to UCH, the child was hospitalized in the intensive care unit. Within the initial 24 hours, his fractured left zygomatic bone was set, and his retrobulbar hematoma was evacuated. He was in a pharmacological coma for the next 3 days. A neurological examination showed flaccid paraparesis of the lower extremities, profound paresis of the upper limbs, obliteration of deep and superficial sensation and lower limb position sense, superficial sensation in the lower limbs from the level of C3-C4, and paralysis of the sphincters. MRI of the spinal canal showed lesions of edematous-malacic character in the cervical SC and interior segment of the medulla oblongata, extending from C7 downward and reaching the terminal cone. The entire cross-section of the SC was filled with an intramedullary hematoma, and a fracture of the C4 vertebral body was observed. Conservative treatment during the first stage caused a change in sensation level to Th1. Motor rehabilitation introduced during the following year did not cause any significant neurological improvement.
The child then qualified for experimental therapy. The patient received two BMNC implantations (Table 1; Fig. 1). In the course of the first procedure, a Th2-Th3 laminectomy was performed, the necrotic tissue was resected, and a suspension of BMNCs was administered to the posthematoma intramedullary cavity and intravenously. A second implantation of BMNCs was performed 2 years later via lumbar puncture and intravenous infusion and was complicated by transient bradycardia caused by the patient’s reaction to the anesthetic drugs, which necessitated interrupting the procedure. One half of the prepared cell volume was administered. Postoperatively, the child demonstrated slight disturbances in short-term memory and subcutaneous liquorrhea at the surgical site. The therapy was terminated. There were no adverse events connected with the transplantation procedures. No late complications following the experimental therapy were observed in a 2-year follow-up period. Improvement in the neurological or clinical condition of the patient was not observed. The parents’ consent for the experimental therapy was withdrawn after the second round of BMNC implantation.
Contact with the patient was lost at the beginning of 2012 as a result of the parents’ decision.
Case 5
A girl experienced an injury involving the medulla oblongata and cervical SC at the age of 2.5 years. At admission to the Department of Neurosurgery at UCH in Cracow, a neurological examination showed flaccid tetraparesis, obliteration of motor function and all types of sensation starting at C5, an absent swallowing reflex, and an absent respiratory drive. The child required a tracheostomy, continuous mechanical ventilation, and percutaneous endoscopic gastrostomy (PEG). MRI demonstrated destruction of the SC involving C2-C4. During the first stage of therapy, conservative treatment was initiated, and the patient was bedridden and motor-rehabilitated at the bedside. Standard postinjury therapy for more than a year-long period caused no improvement. The patient had two pressure ulcers. Both of these ulcers were localized to the iliac crest region, on the left and right sides, and were approximately 1.5 × 2.0 cm in size.
The patient then qualified for experimental therapy. In total, she was subjected to six cell implantations, one each in 2007 and 2008 and two each in 2009 and 2010 (Table 1; Fig. 1). During the first surgery, the necrotic tissues were resected, and the BMNCs were administered into the injury cavity, followed by intravenous infusion. Next, implantations were performed by lumbar puncture and intravenous infusion. After the fifth procedure, a slight improvement in neurological status was observed (Fig. 1). The ASIA grade of A changed to B, and the Frankel scale score of A changed to C. The patient signaled mosaic patterns of pain sensation, active tongue movements appeared, and her swallowing ability and respiratory drive were restored. Thus, the girl was fed orally and through the PEG, and the cough reflex was restored. The sensation level decreased to Th1. The patient continued to be bedridden and to present with tetraparesis. Her skin was pink, without necrotic lesions or pressure ulcers. The patient’s spasticity was reduced from 1+ to 1 on the Ashworth scale. The parents declared significant improvement of the child’s quality of life (Table 2). The SCIM scale score in appendix A, part 1, did not change (score of 0), but in part 2, regarding respiratory and sphincter management, the score increased from 10 to 16 points, although room and toilet mobility did not change (score of 0); part 3, regarding indoor and outdoor mobility, did not change in score (score of 0).
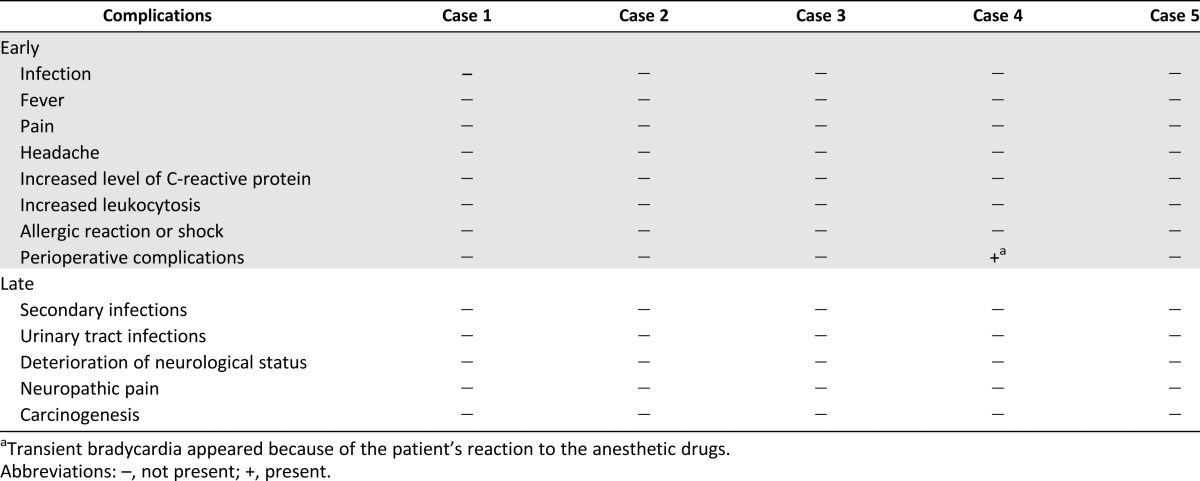
None of the BMC transplantations caused any adverse events (Table 4). No late complications following the experimental therapy were observed in a 6-year follow-up period.
Table 4.
Early and late complications of the implantation procedures and experimental treatment
The patient is monitored at the Department of Children's Neurosurgery.
Case Summary
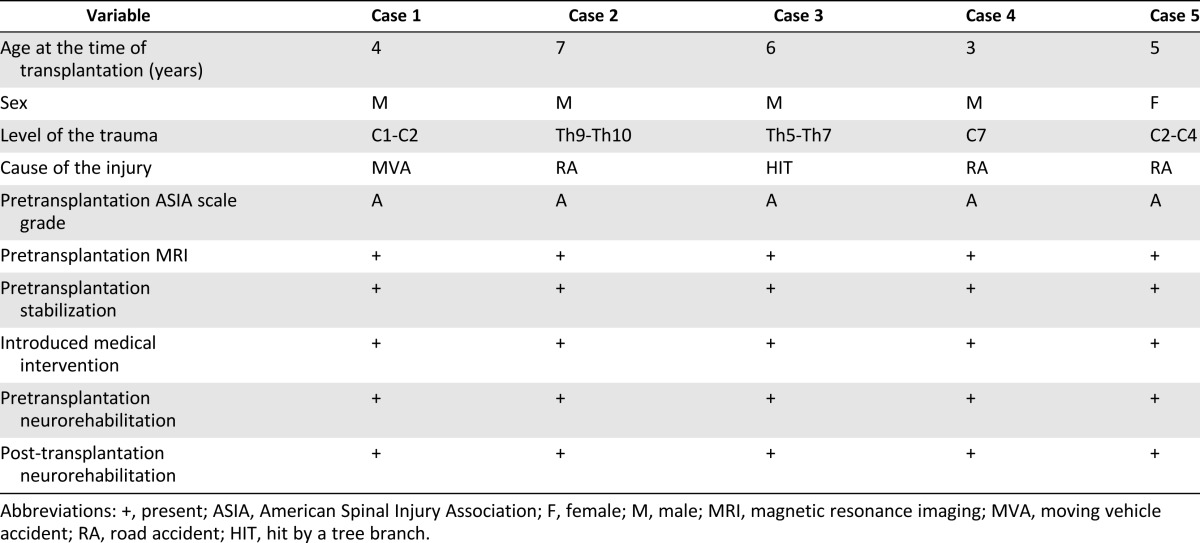
General data collected from the five patients enrolled in the experimental cell therapy are presented in Table 5. Four boys and one girl who were 3–7 years old at the time of admission and who had complete SC interruptions at different levels and from different causes participated in the trial. The patients were included in the trial no earlier than 6 months after injury.
Table 5.
Characteristics of the experimental group, general data, pretrial neurological evaluation, introduced treatment, and neurorehabilitation from five case studies
Collectively, 19 intrathecal and intravenous BMNC implantation procedures were performed. In total, 18 BMNC intrathecal, lumbar puncture, and intravenous transplantation procedures were performed with no adverse events, such as infection, fever, pain, headache, increased level of C-reactive protein, increased leukocytosis, allergic reaction or shock, or other perioperative complications, during 7–14 days of follow-up. In one patient, transient bradycardia appeared (Table 4). The patients were submitted to 1–6 years of long-term follow-up and demonstrated no adverse complications, such as aggravation of neurological status, infection, neuropathic pain, or tumor formation.
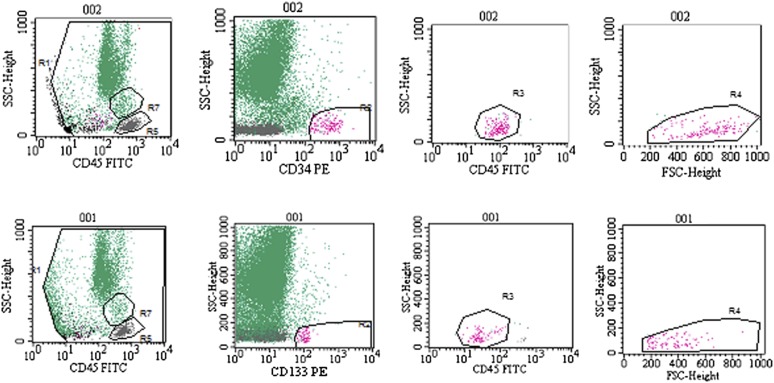
The children were submitted to different numbers of transplantations at different time intervals. The same number of BMNCs (0.5 × 109, containing 2.39–12.05 × 106 CD34+ cells and 0.6–4.15 × 106 CD133+ cells) was transplanted into the SC at each transplantation, and a variable number of BMNCs (0.95–5.38 × 109, containing 5.7–52.19 × 106 CD34+ cells and 2.16–12.45 × 106 CD133+ cells) was transplanted intravenously (Table 1; Fig. 2).
Figure 2.
Fluorescence-activated cell sorting analysis of CD34+ (top) and CD133+ (bottom) cell content in the bone marrow nucleated cells. Representative plots demonstrate the identification and quantification of the subpopulations. Top row: CD34+ cell population: debris exclusion, high CD34 and low CD45 expression, low granularity and lymphocyte size. Bottom row: CD133+ cell population: debris exclusion, CD133 expression, low CD45 expression, low granularity and lymphocyte size. Abbreviations: FITC, fluorescein isothiocyanate; FSC, forward scatter; PE, phycoerythrin; SSC, side scatter.
Two of the five children treated received the highest number of transplantations and demonstrated neurological and quality-of-life improvements (Tables 2, 3). The ASIA grade for case 3 changed from A to B/C, and the Frankel scale score changed to C. The sensation level improved from Th3 to Th12/L1, and spasticity changed from 2 to 1 after the therapy. The patient’s self-care increased from 13 to 16, especially in dressing and bathing abilities. Respiratory and sphincter management increased from 29 to 34, as the sensation of the bladder was partly restored. In addition, room and toilet mobility increased from 7 to 8.
In case 5, the ASIA grade changed from A to B, and the Frankel score changed to C. BMNC therapy caused the appearance of signaled mosaic patterns of pain sensation, and active tongue movements and the respiratory drive were restored. Thus, the girl was fed orally and through the PEG, and the cough reflex was restored. Her sensation level decreased to Th1, and her spasticity changed from +1 to 1 after the therapy. The ulcers that she had at enrollment in the experimental therapy healed, and new ulcers did not appear. Her quality-of-life improvement was reflected by increased respiratory and sphincter management from 10 to 16 as a result of the restored respiratory function. The girl still needs the complete and permanent assistance of her parents.
In addition, in case 2, although no neurological improvement was noted, spasticity was reduced from 2 to +1. The quality of life regarding self-care increased from 13 to 15, and room and toilet mobility increased from 7 to 9.
The time between the injury and the beginning of the experimental therapy was shorter for the patients who showed neurological improvement: 6 or 17 months in comparison with 62–68 months for those patients who did not respond to the treatment. The improvement appeared after multiple implantations (Fig. 1). Three patients withdrew from the therapy after the first or second implantation.
In case 3, neurological improvement appeared 44 months after the beginning of the experimental therapy and after the third and fourth BMNC implantation procedures. In case 5, neurological improvement appeared 50 months after the beginning of the experimental therapy and after the fifth BMC implantation procedure.
None of the patients developed neuropathic pain. Those patients without ulcers at enrollment in the experimental therapy did not develop any ulcers during the few years after BMNC transplantation or in the 6-year follow-up period.
Discussion
In the last two decades, reports have demonstrated the safety and potential efficacy of cell therapy in adult patients with SCIs using cells from different adult tissues, including BM cells [30–38], MSCs [39–45], OECs [46, 47], olfactory mucosa [48, 49], and Schwann cells [50, 51], or cell combinations [52, 53]. More considerable improvements were observed for patients with acute SCIs with multiple transplantations or when a strategy combining different types of cells was applied. Our preliminary study, although conducted on a small number of patients carrying a different injury level and type, is the first one performed on pediatric patients suffering from chronic SCI. Our therapeutic approach was based on the combined intrathecal and intravenous administration of BMNCs.
Because none of our five pediatric patients with complete SCI who were submitted to 19 implantations showed early complications, such as fever, headache, or elevated inflammatory markers, we report the safety and feasibility of multiple intrathecal and intravenous BMNC implantations. The safety and feasibility of BM-derived cells for intrathecal implantation and implantation via lumbar puncture had already been demonstrated for adult patients [30–38], with minor side effects observed in several patients, such as fever, headache, general ache, numbness or tingling, abdominal discomfort, facial flushing, constipation, infections, neuropathic pain, or leukocytosis [30, 35, 37]. Our results expand on these safety and feasibility reports by using BMNC therapy for pediatric SCI patients with both single and multiple implantations. A 1- to 6-year follow-up evaluation demonstrated no long-term side effects after using autologous BM cells. Neuropathic pain, infections, deterioration of neurologic status, and cancer did not appear. Our results, demonstrating the safety of using BMNCs in pediatric SCI patients, extend safety reports for this therapy in adult patients [30–38] with both single and multiple implantations.
A different volume of BM was aspirated according to the different body masses of the children. Our BM preparation methodology was set to obtain all nucleated cells after erythrocyte depletion. The route of administration was both intravenous and intramedullary. The constant value in our therapy approach was 0.5 × 109 BMNCs administered intramedullary and via lumbar puncture. The remaining BMNCs were administered intravenously. Although the percentages and total numbers of CD34+ and CD133+ cells among BMNCs differed among the patients, the numbers (mostly >4 × 106) were similar to the numbers of CD34+ cells (4–9 × 106) in the BM reported to be sufficient to cause improvement in adult chronic SCI patients [37]. Interestingly, the administration of BM cells containing a greater number of CD34+ cells (approximately 90 × 106) by another group did not cause greater improvement [32]. The minimal dose of CD34+ cells in transplanted BM that was reported to cause improvement in acute SCI patients was 2.6 × 106 [33], with no improvement below 1 × 106 CD34+ cells and better improvement with 12 × 106 or 26 × 106 cells.
According to the bioethical committee’s guidelines, patients were included in the trial at least 6 months after SCI. The study approach included multiple implantations for all patients. Unfortunately, only two patients received six to eight rounds of BMNCs, and the three others stopped after the first or second procedure because parental consent was withdrawn. None of the patients demonstrated any improvement after the first BMNC implantation. A lack of improvement after a single BM cell implantation in adult patients with chronic SCI patients and complete SCI has been reported [35], although several groups demonstrated the possibility of improvement after a single procedure [32, 37, 38]. Interestingly, the application of multiple (three or more) rounds of BMNC implantations resulted in the appearance of neurological, motor (respiratory drive, swallowing reflex, and bladder sensation restoration, depending on the level of the injury), sensation, spasticity, and quality-of-life improvements in our pediatric patients. The restoration of the respiratory drive reported in this paper is unique because respiratory dependence is an exclusion criterion in most studies and one of the most frequent causes of mortality in SCI patients [4]. In addition, quality-of-life improvement was observed in one patient after two rounds of BMNC. Thus, we conclude that it is possible to achieve neurological and quality-of-life improvements in pediatric patients with chronic SCI using BMNC therapy, but multiple implantations may be needed. It seems that chronic SCI patients may need prolonged trophic stimulation of the SC to attain the improvement provided only by multiple implantations. A direct comparison has never been performed, but the available data seem to confirm the usefulness of multiple administrations during cell therapy. Better improvement was seen in adults with chronic SCI after four rounds of OEC implantation [46] than after a single implantation [47]. Based on our results, we conclude that when applying multiple implantations, the time interval between subsequent implantation procedures may influence the therapeutic improvement.
Furthermore, none of the patients subjected to BMNC implantations developed any skin lesions in the form of decubitus sores, even during the 6-year follow-up period. In case 5, such lesions were present prior to SC implantation, and after the procedure, the lesions healed faster and did not recur. The intravenous administration of cells was reported not to cause any neurological improvement in SCI patients [34], but we believe that CD34+ cells and/or MSCs present among transplanted BMNCs might act trophically (e.g., via stimulation of angiogenesis), providing better blood supply and better wound healing. The intravenous application of CD34+ BM cells into the peripheral blood or of cultured MSCs has already been described to be useful in the treatment of diabetic critical limb ischemia and decubitus and skin ulcers [55, 56]. Thus, although intravenous injections of BMNCs may not cause SC regeneration, this method may be worth considering as a way of preventing or hastening the healing of pressure ulcers.
We are aware that our preliminary observations are based on a small number of children, but we believe that these findings are worth consideration while designing studies with a higher number of patients. Larger studies with patients segregated according to the type and level of the injury with the same infusion intervals should be performed to obtain more consistent data.
A comparison of the treatment results obtained from a small group of SCI pediatric patients with the results available from adult SCI patients does not suggest an advantage for younger SCI patients [32, 37, 38]. This finding is somehow unexpected because the younger age should provide better ability to regenerate. Because the present study was done on a small number of patients, a larger study using the same methodology for pediatric and adult patients, allowing a direct comparison, should be performed to confirm or contradict the observation.
Conclusion
We report the safety and feasibility of multiple BMNC implantations and of the introduced treatment strategy. Our preliminary results demonstrate the possibility of attaining neurological, motor and sensation, and quality-of-life improvement in children with chronic complete SCI with the use of multiple BMNC implantations. Intravenous implantations of these cells seem to prevent pressure ulcers and/or help them heal. These results should be confirmed in a larger group of patients.
Acknowledgments
This work was partially financed by a KNOW grant to Jagiellonian University School of Medicine and by Jagiellonian University School of Medicine Grant K/ZDS/004044. A.W. is presently working at the Department of Pediatric and Adolescent Endocrinology, Jagiellonian University Medical School of Medicine, Cracow, Poland.
Footnotes
Contributed equally as first authors.
Contributed equally as co-senior authors.
Author Contributions
D.J.: provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing; O.M.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; Z.K. and A.W.: provision of study material or patients; S.K.: conception and design, provision of study material or patients, final approval of manuscript; M.M.: conception and design, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Thuret S, Moon LDF, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 2.Devivo MJ. Epidemiology of traumatic spinal cord injury: Trends and future implications. Spinal Cord. 2012;50:365–372. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 3.Vaccaro AR, Daugherty RJ, Sheehan TP, et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine. 1997;22:2609–2613. doi: 10.1097/00007632-199711150-00006. [DOI] [PubMed] [Google Scholar]
- 4.McKinley WO, Jackson AB, Cardenas DD, et al. Long-term medical complications after traumatic spinal cord injury: A regional model systems analysis. Arch Phys Med Rehabil. 1999;80:1402–1410. doi: 10.1016/s0003-9993(99)90251-4. [DOI] [PubMed] [Google Scholar]
- 5.Rekand T, Hagen EM, Grønning M. Spasticity following spinal cord injury. Tidsskr Nor Laegeforen. 2012;132:970–973. doi: 10.4045/tidsskr.10.0872. [DOI] [PubMed] [Google Scholar]
- 6.Norrbrink C, Löfgren M, Hunter JP, et al. Patients’ perspectives on pain. Top Spinal Cord Inj Rehabil. 2012;18:50–56. doi: 10.1310/sci1801-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollis ER, II, Lu P, Blesch A, et al. IGF-I gene delivery promotes corticospinal neuronal survival but not regeneration after adult CNS injury. Exp Neurol. 2009;215:53–59. doi: 10.1016/j.expneurol.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grill R, Murai K, Blesch A, et al. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novikova LN, Novikov LN, Kellerth J-O. Biopolymers and biodegradable smart implants for tissue regeneration after spinal cord injury. Curr Opin Neurol. 2003;16:711–715. doi: 10.1097/01.wco.0000102620.38669.3e. [DOI] [PubMed] [Google Scholar]
- 10.Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 11.Neumann S, Bradke F, Tessier-Lavigne M, et al. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- 12.Qiu J, Cai D, Dai H, et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- 13.Lu P, Yang H, Jones LL, et al. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikulina E, Tidwell JL, Dai HN, et al. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci USA. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearse DD, Pereira FC, Marcillo AE, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 16.Bradbury EJ, Moon LDF, Popat RJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 17.Freund P, Wannier T, Schmidlin E, et al. Anti-Nogo-A antibody treatment enhances sprouting of corticospinal axons rostral to a unilateral cervical spinal cord lesion in adult macaque monkey. J Comp Neurol. 2007;502:644–659. doi: 10.1002/cne.21321. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Lepski G. Cell transplantation for spinal cord injury: A systematic review. Biomed Res Int. 2013:786475. doi: 10.1155/2013/786475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Civin CI, Strauss LC, Brovall C, et al. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984;133:157–165. [PubMed] [Google Scholar]
- 20.Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: Characteristics and clinical applications. Folia Histochem Cytobiol. 2006;44:215–230. [PubMed] [Google Scholar]
- 21.Kucia M, Zhang YP, Reca R, et al. Cells enriched in markers of neural tissue-committed stem cells reside in the bone marrow and are mobilized into the peripheral blood following stroke. Leukemia. 2006;20:18–28. doi: 10.1038/sj.leu.2404011. [DOI] [PubMed] [Google Scholar]
- 22.Majka M, Janowska-Wieczorek A, Ratajczak J, et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97:3075–3085. doi: 10.1182/blood.v97.10.3075. [DOI] [PubMed] [Google Scholar]
- 23.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shyu W-C, Lin S-Z, Chiang M-F, et al. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. J Neurosci. 2006;26:3444–3453. doi: 10.1523/JNEUROSCI.5165-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao L-R, Duan W-M, Reyes M, et al. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]
- 26.Shen LH, Li Y, Chen J, et al. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393–399. doi: 10.1016/j.neuroscience.2005.08.092. [DOI] [PubMed] [Google Scholar]
- 27.Akiyama Y, Radtke C, Honmou O, et al. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002;39:229–236. doi: 10.1002/glia.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki M, Honmou O, Akiyama Y, et al. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia. 2001;35:26–34. doi: 10.1002/glia.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chopp M, Zhang XH, Li Y, et al. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- 30.Park HC, Shim YS, Ha Y, et al. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 2005;11:913–922. doi: 10.1089/ten.2005.11.913. [DOI] [PubMed] [Google Scholar]
- 31.Deda H, Inci MC, Kürekçi AE, et al. Treatment of chronic spinal cord injured patients with autologous bone marrow-derived hematopoietic stem cell transplantation: 1-year follow-up. Cytotherapy. 2008;10:565–574. doi: 10.1080/14653240802241797. [DOI] [PubMed] [Google Scholar]
- 32.Geffner LF, Santacruz P, Izurieta M, et al. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: Comprehensive case studies. Cell Transplant. 2008;17:1277–1293. doi: 10.3727/096368908787648074. [DOI] [PubMed] [Google Scholar]
- 33.Attar A, Ayten M, Ozdemir M, et al. An attempt to treat patients who have injured spinal cords with intralesional implantation of concentrated autologous bone marrow cells. Cytotherapy. 2011;13:54–60. doi: 10.3109/14653249.2010.510506. [DOI] [PubMed] [Google Scholar]
- 34.Syková E, Homola A, Mazanec R, et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15:675–687. doi: 10.3727/000000006783464381. [DOI] [PubMed] [Google Scholar]
- 35.Yoon SH, Shim YS, Park YH, et al. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells. 2007;25:2066–2073. doi: 10.1634/stemcells.2006-0807. [DOI] [PubMed] [Google Scholar]
- 36.Callera F, do Nascimento RX. Delivery of autologous bone marrow precursor cells into the spinal cord via lumbar puncture technique in patients with spinal cord injury: A preliminary safety study. Exp Hematol. 2006;34:130–131. doi: 10.1016/j.exphem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Kumar AA, Kumar SR, Narayanan R, et al. Autologous bone marrow derived mononuclear cell therapy for spinal cord injury: A phase I/II clinical safety and primary efficacy data. Exp Clin Transplant. 2009;7:241–248. [PubMed] [Google Scholar]
- 38.Chernykh ER, Stupak VV, Muradov GM, et al. Application of autologous bone marrow stem cells in the therapy of spinal cord injury patients. Bull Exp Biol Med. 2007;143:543–547. doi: 10.1007/s10517-007-0175-y. [DOI] [PubMed] [Google Scholar]
- 39.Saito F, Nakatani T, Iwase M, et al. Spinal cord injury treatment with intrathecal autologous bone marrow stromal cell transplantation: The first clinical trial case report. J Trauma. 2008;64:53–59. doi: 10.1097/TA.0b013e31815b847d. [DOI] [PubMed] [Google Scholar]
- 40.Pal R, Venkataramana NK, Bansal A, et al. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy. 2009;11:897–911. doi: 10.3109/14653240903253857. [DOI] [PubMed] [Google Scholar]
- 41.Park JH, Kim DY, Sung IY, et al. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery. 2012;70:1238–1247; discussion 1247. doi: 10.1227/NEU.0b013e31824387f9. [DOI] [PubMed] [Google Scholar]
- 42.Karamouzian S, Nematollahi-Mahani SN, Nakhaee N, et al. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin Neurol Neurosurg. 2012;114:935–939. doi: 10.1016/j.clineuro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Han D, Wang Z, et al. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy. 2013;15:185–191. doi: 10.1016/j.jcyt.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Kang K-S, Kim SW, Oh YH, et al. A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: A case study. Cytotherapy. 2005;7:368–373. doi: 10.1080/14653240500238160. [DOI] [PubMed] [Google Scholar]
- 45.Ichim TE, Solano F, Lara F, et al. Feasibility of combination allogeneic stem cell therapy for spinal cord injury: A case report. Int Arch Med. 2010;3:30. doi: 10.1186/1755-7682-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang H, Xi H, Chen L, et al. Long-term outcome of olfactory ensheathing cell therapy for patients with complete chronic spinal cord injury. Cell Transplant. 2012;21(suppl 1):S23–S31. doi: 10.3727/096368912X633734. [DOI] [PubMed] [Google Scholar]
- 47.Rao Y, Zhu W, Liu H, et al. Clinical application of olfactory ensheathing cells in the treatment of spinal cord injury. J Int Med Res. 2013;41:473–481. doi: 10.1177/0300060513476426. [DOI] [PubMed] [Google Scholar]
- 48.Chhabra HS, Lima C, Sachdeva S, et al. Autologous olfactory [corrected] mucosal transplant in chronic spinal cord injury: An Indian Pilot Study. Spinal Cord. 2009;47:887–895. doi: 10.1038/sc.2009.54. [DOI] [PubMed] [Google Scholar]
- 49.Lima C, Escada P, Pratas-Vital J, et al. Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil Neural Repair. 2010;24:10–22. doi: 10.1177/1545968309347685. [DOI] [PubMed] [Google Scholar]
- 50.Saberi H, Firouzi M, Habibi Z, et al. Safety of intramedullary Schwann cell transplantation for postrehabilitation spinal cord injuries: 2-year follow-up of 33 cases. J Neurosurg Spine. 2011;15:515–525. doi: 10.3171/2011.6.SPINE10917. [DOI] [PubMed] [Google Scholar]
- 51.Zhou XH, Ning GZ, Feng SQ, et al. Transplantation of autologous activated Schwann cells in the treatment of spinal cord injury: Six cases, more than five years of follow-up. Cell Transplant. 2012;21(suppl 1):S39–S47. doi: 10.3727/096368912X633752. [DOI] [PubMed] [Google Scholar]
- 52.Yazdani SO, Hafizi M, Zali A-R, et al. Safety and possible outcome assessment of autologous Schwann cell and bone marrow mesenchymal stromal cell co-transplantation for treatment of patients with chronic spinal cord injury. Cytotherapy. 2013;15:782–791. doi: 10.1016/j.jcyt.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Moviglia GA, Fernandez Viña R, Brizuela JA, et al. Combined protocol of cell therapy for chronic spinal cord injury. Report on the electrical and functional recovery of two patients. Cytotherapy. 2006;8:202–209. doi: 10.1080/14653240600736048. [DOI] [PubMed] [Google Scholar]
- 54.Anderson K, Aito S, Atkins M, et al. Functional recovery measures for spinal cord injury: An evidence-based review for clinical practice and research. Report of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Measures Meeting. J Spinal Cord Med. 2008;31:133–144. doi: 10.1080/10790268.2008.11760704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang P, Li S, Han M, et al. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 2005;28:2155–2160. doi: 10.2337/diacare.28.9.2155. [DOI] [PubMed] [Google Scholar]
- 56.Yoshikawa T, Mitsuno H, Nonaka I, et al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121:860–877. doi: 10.1097/01.prs.0000299922.96006.24. [DOI] [PubMed] [Google Scholar]