This study’s goal was to explore the role of oxygen modulation in the maturation of pancreatic progenitor (PP) cells differentiated from human embryonic stem cells. Results show that oxygen modulation contributes to enhanced maturation of PP cells and confirm its importance in the design of β-cell differentiation protocols. These findings open the door to future strategies for the transplantation of fully mature β cells.
Keywords: Cell transplantation, Cellular therapy, Diabetes, Developmental biology, Pancreas, Embryonic stem cells, Microenvironment, Pancreatic differentiation
Abstract
The possibility of using human embryonic stem (hES) cell-derived β cells as an alternative to cadaveric islets for the treatment of type 1 diabetes is now widely acknowledged. However, current differentiation methods consistently fail to generate meaningful numbers of mature, functional β cells. In order to address this issue, we set out to explore the role of oxygen modulation in the maturation of pancreatic progenitor (PP) cells differentiated from hES cells. We have previously determined that oxygenation is a powerful driver of murine PP differentiation along the endocrine lineage of the pancreas. We hypothesized that targeting physiological oxygen partial pressure (pO2) levels seen in mature islets would help the differentiation of PP cells along the β-cell lineage. This hypothesis was tested both in vivo (by exposing PP-transplanted immunodeficient mice to a daily hyperbaric oxygen regimen) and in vitro (by allowing PP cells to mature in a perfluorocarbon-based culture device designed to carefully adjust pO2 to a desired range). Our results show that oxygen modulation does indeed contribute to enhanced maturation of PP cells, as evidenced by improved engraftment, segregation of α and β cells, body weight maintenance, and rate of diabetes reversal in vivo, and by elevated expression of pancreatic endocrine makers, β-cell differentiation yield, and insulin production in vitro. Our studies confirm the importance of oxygen modulation as a key variable to consider in the design of β-cell differentiation protocols and open the door to future strategies for the transplantation of fully mature β cells.
Introduction
Advances in the directed differentiation of human embryonic stem (hES) cells into insulin-producing β cells present clinicians with the opportunity of progressively phasing out the use of human islets for the treatment of the most severe cases of diabetes [1, 2]. Unlike the latter, hES cells are considered an inexhaustible cell source, and recent developments in the field suggest that even hES cell-derived endodermal progenitors can be expanded in a virtually unlimited fashion for both hepatic and pancreatic regeneration applications [3]. Indeed, the breakthrough definition of the conditions resulting in the specification of hES cells along the definitive endoderm lineage [4] paved the way to the formulation of protocols for the in vitro differentiation of insulin-producing β cells [5–7]. None of the resulting cell products, however, met the necessary criteria for therapeutic scalability, such as the ability to be derived in high yields versus nonendocrine cells and the monohormonal expression of insulin. Because of the perceived limitations of in vitro culture to foster the functional maturation of insulin-producing cells, researchers in the field resorted to the transplantation of partially differentiated hES cell-derivatives (i.e., pancreatic progenitor [PP]-like cells), a strategy that has met with success in preclinical models of diabetes [8, 9]. Albeit valid, the solution was not without shortcomings: first, the fact that the transplanted cells were not mature posed a heightened risk for teratogenic lesions (a concern that was confirmed in a high percentage of transplanted animals [8, 9]). Second, it takes several months for these cells to fully mature in vivo [8, 9]. Also, although there is no reason to suspect that a human microenvironment would be less permissive than the mouse’s to sustain adequate maturation of PP cells, this is an assumption that may or may not prove to be right as we move these findings to the clinic. Because of the above reasons, it would be highly desirable to have instead a fully functional, mature endocrine cell product for transplantation.
We have previously shown that oxygen tension is a critical factor in steering PP differentiation toward endocrine cell (and particularly β cell) differentiation [10]. As first postulated by our team [10, 11] and later confirmed by others [12–14], molecular oxygen acts through hypoxia-inducible factor (HIF)-1α (the main “oxygen sensor” of the cell) to potentially modulate some of the key pathways involved in fate acquisition during pancreatic development, including Notch and Wnt/β-catenin [11]. Here we show that oxygen tension modulation also has a measurable effect on the differentiation of β cells from hES cells, both in vivo (by means of hyperbaric oxygen treatment [HOT] of the host after transplantation) and in vitro, using a novel culture device in which cells are placed atop an air-permeable, liquid-impermeable perfluorocarbon-silicone (PFC/PDMS)-based membrane. This system allows for the fine adjustment of oxygen tension throughout the entirety of cell aggregates while minimizing the formation of diffusion gradients [10, 15]. In the first case, transplanted PPs were able to restore normoglycemia in half of the streptozotocin (stz)-induced diabetic mice when these were subjected to a daily post-transplantation HOT regimen, whereas none of the control animals experienced reversal of diabetes. In the second case, the placement of PPs in conditions that targeted a physiological oxygen tension of 40–80 mmHg (as measured in native islets [16]) resulted in the in vitro generation of monohormonal insulin producing cells that exhibited characteristics of fully mature β cells. In contrast, and as previously reported [9], PPs allowed to mature in standard culture dishes in nonoptimized oxygen conditions yielded populations of polyhormonal cells. These findings strongly suggest that the mere in vitro replication of the physiological pattern of oxygenation that accompany native β-cell development might assist in inducing their complete maturation from hES cells, potentially bypassing the need for lengthy and possibly dangerous in vivo maturation methods.
Materials and Methods
In Vitro Differentiation of hES Cells
The H1 hES cell line was obtained from WiCell Research Institute (Madison, WI, http://www.wicell.org) and cultured as described in [17]. Experiments with H1 cells were approved by the University of Miami Embryonic Stem Cell Research Oversight Committee. Cells were induced to differentiate along the pancreatic endocrine lineage following the well-characterized stages of pancreatic development: definitive endoderm (stage 1), primitive gut tube (stage 2), posterior foregut (stage 3), pancreatic endoderm and endocrine precursors (stage 4), and pancreatic endocrine cells (stage 5). An additional stage 6 (mature endocrine cells) was explored in some versions of the protocol (described below). Two variations of the differentiation method were used. In protocol 1 (based on that published in [17]), undifferentiated H1 cells were grown to 60%–70% confluence on top of 1:30 Matrigel-coated (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) surfaces. At that point, the cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) supplemented with 2% fatty acid-free bovine serum albumin (BSA) (Proliant, Ankeny, IA, http://www.proliantinc.com), 20 ng/ml Wnt3A (R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com), and 100 ng/ml activin-A (Peprotech, Rocky Hill, NJ, http://www.peprotech.com) for day 1, and 8 ng/ml fibroblast growth factor 2 (FGF2) (Peprotech) for the following 3–4 days (stage 1). After the completion of stage 1, cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)-F12 medium (Invitrogen) with 2% fatty acid-free BSA, 0.25 μM Cyclopamine-KAAD (EMD Millipore, Gibbstown, NJ, http://www.emdmillipore.com/chemicals), and 50 ng/ml FGF7 (Peprotech) for 2 days (stage 2). Stage 3 entailed culture in DMEM-F12 medium supplemented with 0.25 μmol/l Cyclopamine-KAAD, 2 μmol/l retinoic acid (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), 100 ng/ml Noggin (R&D Systems), 50 ng/ml FGF7, and 1% (vol/vol) B27 (Invitrogen) for 4 days. In stage 4, cells were cultured for 3 days in DMEM-F12 medium with 1 μmol/l ALK5 inhibitor II (Axxora, San Diego, CA, http://www.axxora.com), 100 ng/ml Noggin, 1 μM 2,4-diamino-5-phenyl thiazole (EMD Millipore) and 1% B27. For stage 5, cells underwent 7 days of culture in DMEM-F12 medium supplemented with 1% B27 and 1 μM ALK5 inhibitor. Finally, in stage 6 cells were further cultured in DMEM-F12 medium with 1% B27 for an additional week.
Protocol 2 was a variation of that already published in [9], which only describes stages 1–4. In short, the method entails the treatment of 60%–70% confluent H1 cells to RPMI 1640 medium (Invitrogen) supplemented with 0.2% fetal bovine serum (FBS) (HyClone, Logan, UT, http://www.hyclone.com), 100 ng/ml activin-A (Peprotech; Rocky Hill, NJ), and 20 ng/ml Wnt3A (R&D Systems) for day 1, and then RPMI 1640 medium with 0.5% FBS and 100 ng/ml activin-A for the following 2 days (stage 1). Stage 2 requires DMEM-F12 medium (Invitrogen) supplemented with 2% FBS and 50 ng/ml FGF7 (Peprotech) for 3 days. Cultures were further cultured for 4 days in DMEM-HG medium (Invitrogen) supplemented with 0.25 μmol/l SANT-1 (Sigma-Aldrich), 2 μmol/l retinoic acid (Sigma-Aldrich), 100 ng/ml Noggin (R&D Systems), and 1% (vol/vol) B27 (Invitrogen) (stage 3). Stage 4 cells were obtained by culturing an additional 3 to 4 days in DMEM-high glucose (HG) medium supplemented with 1 μmol/l ALK5 inhibitor II (ALK5i; Axxora), 100 ng/ml Noggin, 50 nmol/l TPB ((2S,5S)-(E,E)-8-(5-(4-(trifluoromethyl)phenyl)-2,4-pentadienoylamino)benzolactam; EMD Millipore), and 1% B27 in monolayer format. At the end of stage 4, cells were gently scraped to form clusters and further allowed to mature for an additional 15 days in the same culture medium, either in 35-mm ultralow-attachment plates (Corning Enterprises, Corning, NY, http://www.corning.com) at 21% O2 or PFC/PDMS dishes of the same diameter at 9%–12% O2 (stage 5).
PFC/PDMS Dish Manufacture
Thirty-five-millimeter plates featuring a PFC/PDMS bottom membrane were made according to the specifications previously described in [15]. In essence, these are similar to standard 35-mm cell culture dishes in that they feature a rigid plastic structure with transparent sides and a loose-fitting lid. The main difference is that the bottom of the plate is not made of gas-impermeable plastic but of an air-permeable, liquid-impermeable PFC-silicone membrane. This bottom is slightly raised by 1 mm plastic feet so as to allow airflow from underneath when the dish is placed on any rigid surface, such as a tray inside an incubator. Cells are plated covered in culture medium (as in regular dishes), owing to the fact that these membranes are liquid-impermeable. However, the gas permeability and diffusion properties of PFC allow for enhanced oxygen transfer to the cells, which are in direct contact with the PFC-silicone membrane. This is in addition to the conventional flow of air that happens by diffusion through the culture medium from the top.
Oxygen Consumption Rate Measurements
Aliquots of approximately 500 cell aggregates were used for measurements of oxygen consumption rate in temperature-controlled, sealed and stirred microchambers (Instech Co., Plymouth Meeting, PA, http://www.instechlabs.com). Oxygen consumption rate per unit time was quantified as in the equation below:
where oxygen consumption rate (OCR) is the cellular oxygen consumption rate in mol per m3 seconds and Δt is the change in time in seconds, Δ[O2] is the change in oxygen concentration in moles, and V is the chamber volume in liters. After measurements were completed, cells were collected from the chambers, solubilized in AT extraction buffer, and stored at −80°C for later DNA quantification.
DNA Quantification/Tissue Volume Determination
DNA was quantified against double-stranded DNA standards using the Quant-iT pico green assay (Invitrogen). Cell number was estimated using a previously reported value of 6 pg of DNA per single cell. Total tissue volume was then derived using the calculated cell number and single cell volume using the following equation:
where V is the total tissue volume, N is the cell number, and R is the average radius of the cells, 5 × 10−6 m. From the measured consumption rate and tissue volume, the consumption rate per unit volume was determined.
Finite Element Modeling
To determine optimal incubator settings required to maintain physiological oxygen profiles in stem cell aggregates during in vitro culture, finite element modeling was performed with Comsol Multiphysics v3.5a software (Burlington, MA, http://www.comsol.com). Using previously published representative size distributions for islets of Langerhans, an external oxygen concentration was targeted that minimized hypoxia (<0.4 mmHg) and hyperoxia (>80 mmHg) while maintaining maximal tissue volumes in the physiological niche of islets [15]. Maximal and minimal incubator oxygen concentrations were determined using the mean cellular OCR ± SD of the mean (+SD for maximal, −SD for minimal). Additional details about the method are provided in the supplemental online data.
Glucose-Stimulated Insulin Release
Glucose-stimulated insulin release (GSIR) was done by aliquoting 100 hES cell-derived stage 5 clusters suspended in a Sephadex G10 slurry (GE Healthcare Biosciences, Pittsburgh, PA, http://www.gelifesciences.com) within 10-ml microchromatography columns. Equilibration was reached by incubating the cells in low-glucose (2.2 mM)-modified Krebs buffer containing 0.1% wt/vol BSA, 26 mM sodium bicarbonate and 25 mM HEPES buffer. Sequential 1-hour incubations were then conducted in low (2.2 mM), high (16.6 mM), and low (2.2 mM) buffers. Samples were collected for insulin analysis at the end of each hour following the preincubation. Insulin was quantified using the Mercodia Human Insulin ELISA (Winston-Salem, NC, http://www.mercodia.com).
Use of Human Tissue Material
Investigations with human tissues were preceded by University of Miami institutional review board approval. Human islet preparations were processed at the Diabetes Research Institute cGMP Core according to standard methods described elsewhere [18].
Quantitative Reverse Transcription-Polymerase Chain Reaction
Purification of total RNA was done using the miRNA Mirvana kit (Life Technologies, Grand Island, NY, http://www.lifetech.com). Random oligomers were used to create cDNA with the High Capacity Reverse Transcription kit (Life Technologies). The relative expression of selected genes was calculated by means of TaqMan assays in Applied Biosystems thermal cyclers (Life Technologies). The ΔCt method for relative quantification was used for all calculations.
Immunofluorescence
Samples were fixed in 4% paraformaldehyde overnight at 4°C and then embedded in Tissue-Tek O.C.T. Compound (Sakura, Alphen aan den Rijn, The Netherlands, http://www.sakuraeu.com) for 10-μm-thick sectioning using a microtome. Tissues collected from experimental animals were fixed in 10% buffered formalin solution, and paraffin sections were used for hematoxylin and eosin staining (for morphological assessment) and immunofluorescence microscopy analysis [19] done at the Imaging Core of the Diabetes Research Institute. Staining was done with primary antibodies against glucagon (Dako, Glostrup, Denmark, http://www.dako.com) (1:250 dilution), insulin (Dako) (1:250 dilution), Nkx6.1 (LifeSpan Biosciences, Seattle, WA, http://www.lsbio.com) (1:250), Pdx1 (Santa Cruz Biotechnology, Dallas, TX, http://www.scbt.com) (1:50), and Ngn3 (Santa Cruz Biotechnology) (1:100). Secondary antibodies were purchased from Molecular Probes/Invitrogen (Carlsbad, CA, http://probes.invitrogen.com). Nucleus counterstaining was done with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich). Images were captured using a Leica SP5 inverted confocal microscope with motorized stage (×20 dry) (Leica, Heerbrugg, Switzerland, http://www.leica.com). Metamorph imaging software was used to quantify the insulin and glucagon signal.
Animal Studies
All animal procedures were done and monitored under protocols approved by the University of Miami Institutional Animal Care and Use Committee. Athymic (nu/nu) mice (5–6 weeks old; Harlan Laboratories, Indianapolis, IN, http://www.harlan.com) were housed in virus antibody-free rooms at the Division of Veterinary Resources. Personnel at the Translational Models Core of the Diabetes Research Institute performed all surgical procedures. A single stz injection induced selective destruction of islet β cells and onset of diabetes. Animals were monitored and subcutaneous insulin pellets (Linplant; LinShin Canada Inc., Toronto, Ontario, Canada, http://linshincanada.com) used as needed to maintain weight and overall health of the animals prior to transplantation. Under general anesthesia (isoflurane inhalation, to effect), the left kidney was externalized and a small puncture made in its capsule. Approximately 8.5 × 106 cells were injected in a minimal volume of saline, as described in [20]. Buprenorphine was administered subcutaneously to alleviate postsurgical pain. After transplantation, recipients were followed up with blood glucose measurements to monitor graft function on whole blood (tail vein pricking) using portable glucometers (OneTouchUltra2; LifeScan). Hyperbaric oxygen therapy was started on the day of the transplant and consisted of daily 60-min exposure to 100% oxygen (HOT-100%) at 2.0 atm absolute (ATA) into small-animal hyperbaric chambers (RSI-B11; Reimers Systems), as detailed in [19]. Control animals were handled similarly without undergoing HOT [19]. Human C-peptide in serum was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Mercodia) on samples collected at selected time points after transplantation as basal (fasting), nonfasting (fed animals), and/or 60 minutes after glucose bolus stimulation, as described in [20].
Statistical Analyses
Statistical significance of differences between groups was assessed by the two-tailed Student’s t test. In all comparisons, a value of p < .05 was considered statistically significant, and values of p ≤ .01 were considered highly significant. For HOT animal studies, we used n = 4 per group. A group size of n = 3 was used for in vitro analyses.
Results
In Vitro Differentiation of hES Cells Yields Nonfunctional Polyhormonal Cell Types
Using a variation of the protocol recently described in [17], H1 hES cells were induced to differentiate along the pancreatic endocrine cell lineage by progressive adoption of definitive endoderm (stage 1), primitive gut tube (stage 2), posterior foregut (stage 3), pancreatic endoderm (stage 4), endocrine progenitor (stage 5) and mature endocrine (stage 6) fates. A basic fluorescein diacetate/propidium iodide viability assay indicates that the cells at the final stage of differentiation are >99% viable (data not shown). Total insulin content was 48.91 μU of insulin/μg of protein, which is approximately 10% the amount of previously published determinations for human islet preparations [21]. However, these aggregates did not exhibit glucose-regulated insulin secretion, as evidenced both by static incubation and perfusion (data not shown).
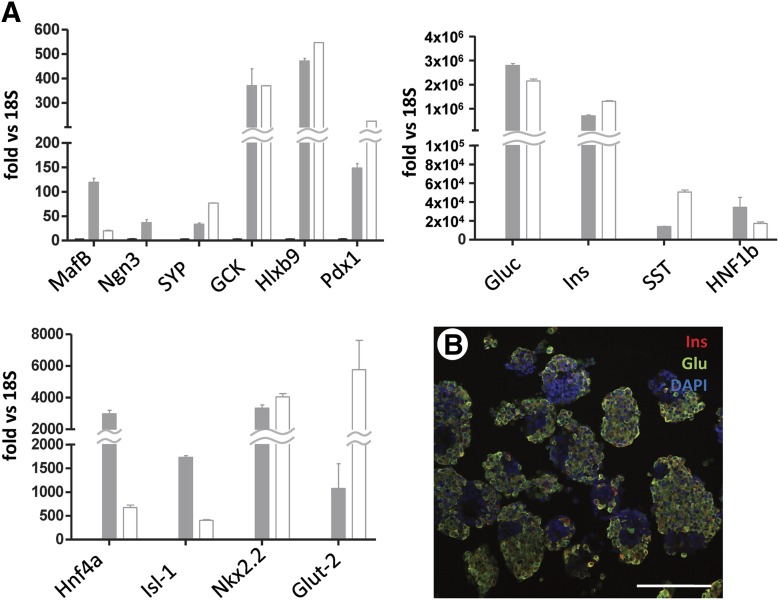
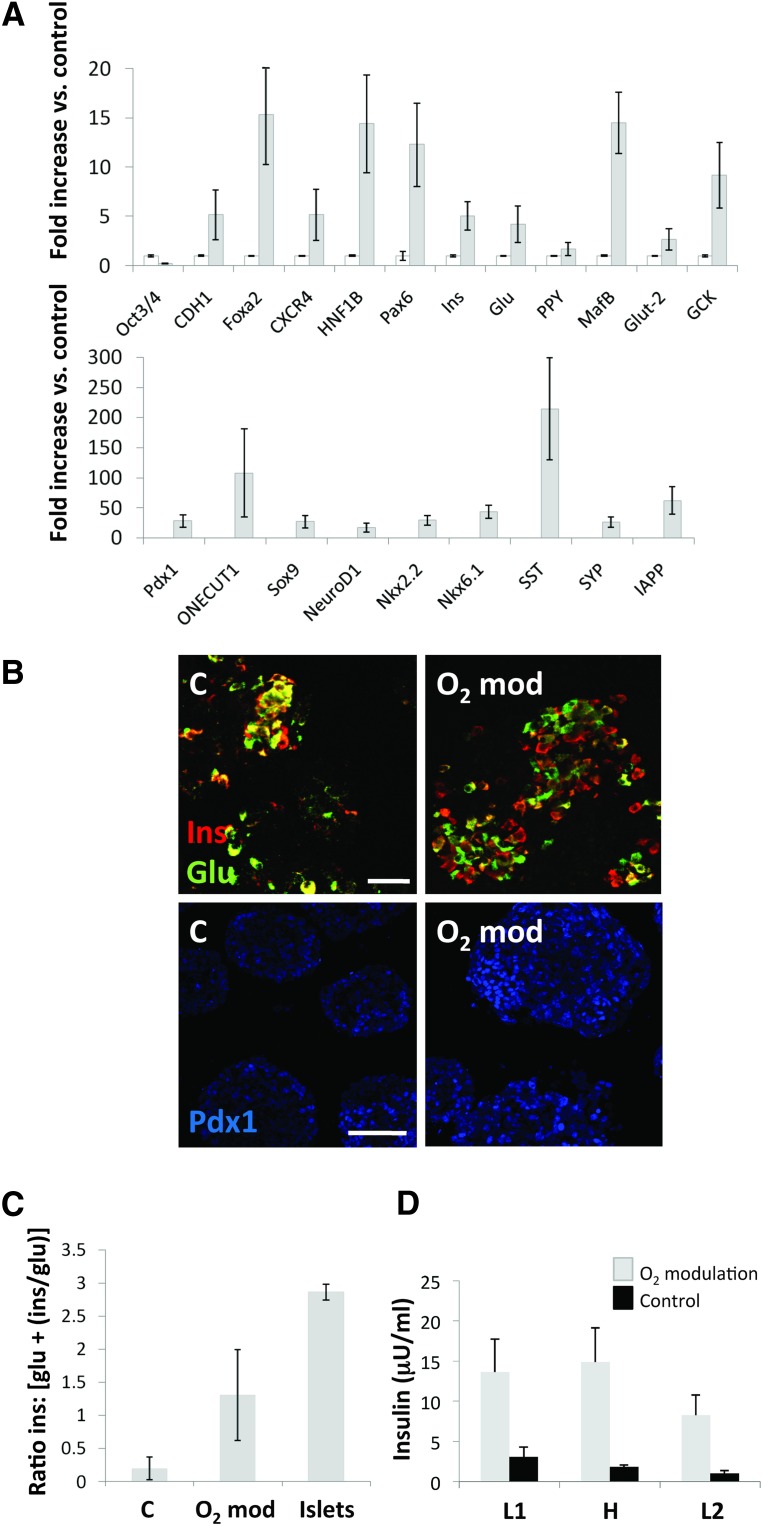
Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) data (Fig. 1A) indicates that stage 6 aggregates express numerous key pancreatic endocrine markers at levels that are almost comparable (GCK, Nkx2.2, Hlxb9, Pdx1, synaptophysin), if not even higher (glucagon, HNF1β, HNF4α) than those detected in a human islet preparation. However, insulin expression as measured by RT-PCR was still approximately half that of native islets. Another marker of β-cell functionality, Glut-2, was expressed at 1/6 the level seen in islets, and Ngn3 (a proendocrine marker of immature pancreatic progenitors, nearly undetectable in adult islets) was >50-fold higher. Insulin and glucagon were largely coexpressed by the same cells, as evidenced by the near total absence of single red (insulin) or green (glucagon) signal (Fig. 1B).
Figure 1.
Gene expression and immunofluorescence profile of stage 6 cells. (A): Quantitative real-time reverse transcription-polymerase chain reaction of 14 representative markers of pancreatic endocrine cell differentiation. Open columns indicate human isolated islets (positive control). Gray columns indicate stage 6 human embryonic stem cell-derived endocrine-like cells. Bars indicate SE. The y-axis indicates fold increase over 18S (endogenous normalizer). (B): Immunofluorescence staining of insulin (red) and glucagon (green). Scale bar = 150 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; Glu, glucagon; Ins, insulin.
The oxygen consumption index (OCI) of human islets has been shown to be highly predictive of islet function in vivo, with indices >1.27 resulting in 90% success in diabetes reversal within 3 days of full mass transplantation in immunodeficient rodents [22]. However, the OCI of stage 5 cells was only 0.835. Paradoxically, the β cell fractional viability index of stage 5 cells was 0.47, with indices >0.3 being 90% predictive of in vivo diabetes reversal using the same model [23].
Transplantation of Stage 6 Aggregates Does Not Result in Diabetes Reversal
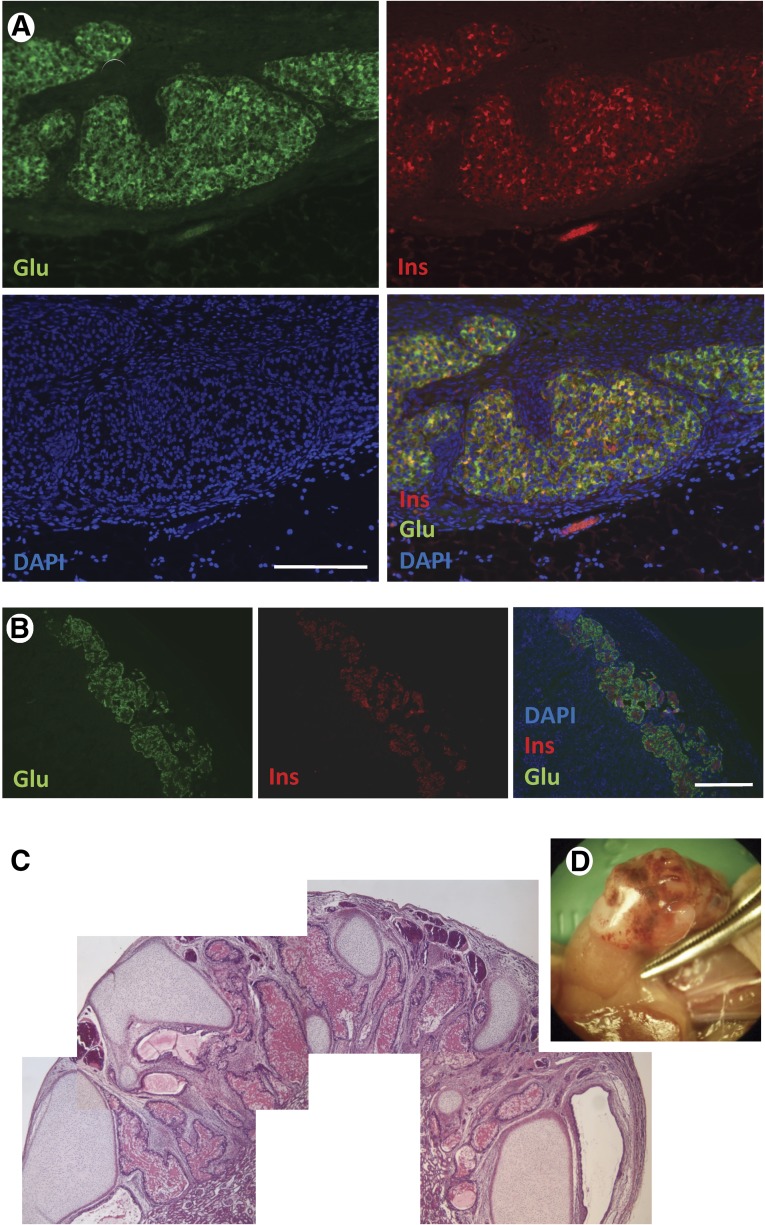
Although the majority of the analyses conducted in the previous section (OCI, colocalization of insulin and glucagon, in vitro glucose irresponsiveness) would be consistent with a prediction of nonfunction in vivo, the relatively high insulin production and intriguing RT-PCR profile led us to assess the ability of stage 6 cells to correct hyperglycemia in streptozotocin-induced diabetic immunodeficient (nu/nu) mice. Transplantation was done as described in the Methods section. At postoperative day (POD) +14, none of the transplanted animals had returned to normoglycemia. One of the mice was humanely sacrificed and the graft explanted for immunohistochemical analysis. As shown in Figure 2A, the transplanted cells engrafted well and exhibited strong insulin and glucagon staining. However, a high degree of colocalization indicated that these cells were still polyhormonal, and as such immature. In contrast, control mice transplanted with human islets, as expected, had monohormonal insulin+ or glucagon+ cells (Fig. 2B). The remaining animal was sacrificed at POD +76, at which point the transplanted kidney had developed a large neoplastic mass (Fig. 2C) and insulin expression was virtually undetectable (data not shown). A pathological analysis of this mass revealed the presence of cartilage tissue, intestinal epithelium, blood vessels, and glandular structures, with no overt evidence of primitive neural epithelium. These features were consistent with those of a teratoma.
Figure 2.
Immunofluorescence and histopathological analysis of stage 5-transplanted cells. (A): Inoculum site under the kidney capsule showing engraftment of stage 5 cells (POD +14) expressing glucagon (green) and insulin (red). Blue: DAPI. Scale bar = 100 μm. (B): Engrafted human islets (positive control). Glucagon (green) and insulin (red). Blue: DAPI. Scale bar = 100 μm. (C): Composite hematoxylin/eosin microphotographs of a histopathological section of the teratoma developed in the graft-bearing kidney explanted on day postoperative day +75 (D). Insulin and glucagon staining were not detectable (not shown). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; Glu, glucagon; Ins, insulin.
Post-Transplantation HOT Supports Functional β-Cell Maturation
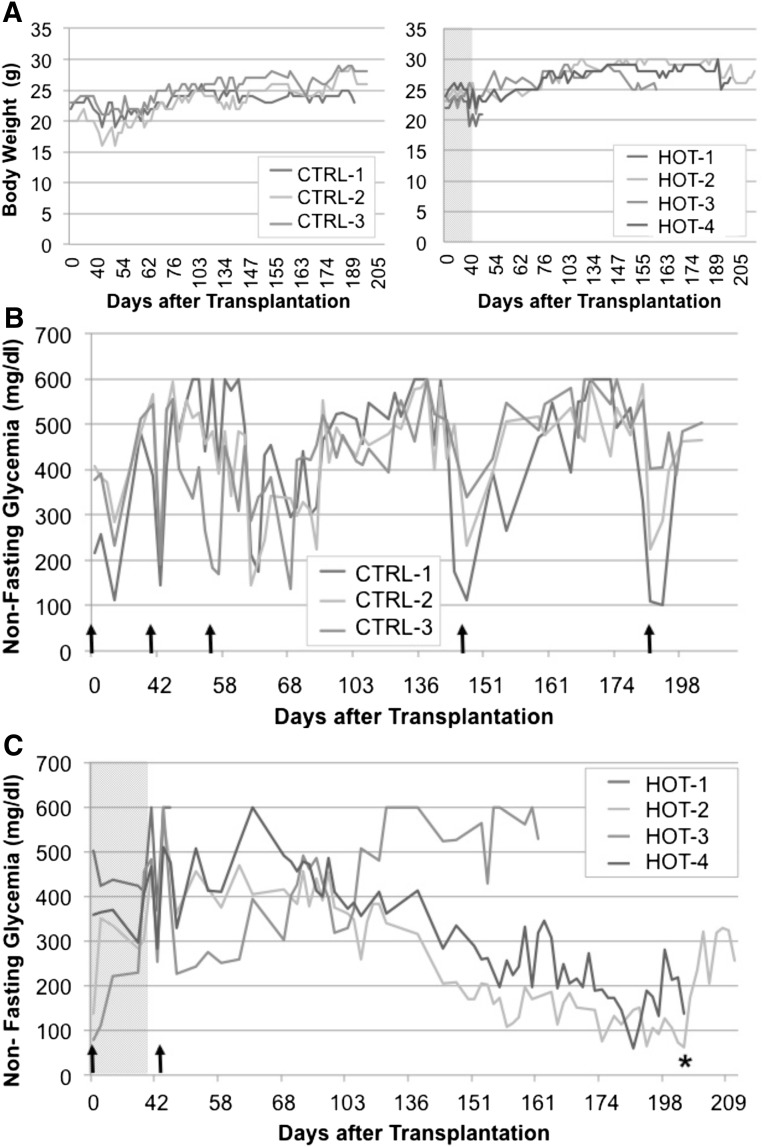
The above experiments suggest that stage 6 cells might have already matured into terminal phenotypes that are not permissive for glucose-regulated insulin secretion. Thus, we decided to transplant instead pancreatic progenitor cells, characterized by the expression of Pdx1 and Nkx6.1 but not significant amounts of endocrine hormones (data not shown). As reported elsewhere, such cells are amenable to functional maturation into monohormonal β cells upon transplantation in mice [8, 9]. As the use of HOT has proven beneficial for islet survival and function during the critical post-transplantation time [24], we reasoned that a daily HOT regimen following the transplantation of stage 4 cells might accelerate their maturation. Eight streptozotocin-treated, nu/nu mice were transplanted with 8.5 × 106 stage 4 PP cells and assigned to one of the following groups: HOT (which received a 1 hour/day hyperbaric oxygen session for 40 days) or control (which were similarly manipulated daily but received no treatment). All mice were diabetic at the time of transplantation, and were maintained in good general condition by subcutaneous insulin pellets (Linplant) as needed throughout the entirety of the experiment. The estimated volume of the grafted cells was 7.47 ± 2.23 μl (range, 5.3–10.6 μm) in the control group and 8.77 ± 1.27 μm (range, 7.9–10.6 μm) in the HOT group (p = not significant). Human C-peptide in serum was measured at POD +42 (stimulated sample, 60 minutes) and PODs +64, +84, +125, and +160 (basal sample, nonfasting, nonstimulated). One mouse from each group died before POD +64 (day of the first nonstimulated C-peptide measurement). Mice in the control group required a second insulin pellet on POD +54 to maintain good general condition. On POD +142 and subsequently POD +170, the pellets had to be replaced with new ones because mice showed significant weight loss (Fig. 3). Mice in the HOT group, in contrast, maintained good weight and general condition after the insulin pellet was placed on POD +43 without the need for new ones until around POD +150, when the only animal with no detectable human C-peptide in this group (mouse HOT-3) started to lose weight and died. The other two mice in this group maintained good body weight throughout the follow-up period (Fig. 3). Mouse HOT-2 became normoglycemic on POD +149 (human C-peptide at POD +160 = 157 pmol/l) and mouse HOT-4 had fluctuating glycemic values for some time, reaching normoglycemia at POD +173 (human C-peptide at POD +160 = 234 pmol/l). A nephrectomy of the graft-bearing kidney was conducted in these two animals at POD +202 to confirm that reversal of diabetes was due to the implanted cells. This was at least the case of mouse HOT-2. Mouse HOT-4, however, was sacrificed at the time of nephrectomy because of the presence of a large (∼1.8 cm), well-defined neoplasm as well as adherences to the adjacent pancreas and splenomegaly (supplemental online Fig. 1a). Both HOT animals had good separation of α and β cells in the site of the graft, whereas insulin+ cells were virtually undetectable in their pancreata (supplemental online Fig. 1a, 1b). Control animals, in contrast, had only fibrotic tissue at the site of the cell inoculum. Mouse CRTL-3 had a renal cyst at caudal renal pole (data not shown).
Figure 3.
Follow up of diabetic nu/nu mice transplanted with stage 4 pancreatic progenitor cells and exposed to a hyperbaric oxygen treatment (HOT) regimen for 40 days. (A): Body weight (g) of animals in the HOT (n = 4, left panel) and control (n = 3; right panel) groups. The x-axis indicates days after transplantation. (B, C): Nonfasting blood glucose measurements (mg/dl) in control (B) and HOT (C) groups. The gray area in the latter represents the 40-day treatment. The x-axis indicates days after transplantation. Upward arrows indicate time of implantation of an insulin (LP) pellet. ∗, Nephrectomy of the graft-bearing kidney. Abbreviations: CTRL, control; HOT, hyperbaric oxygen treatment.
In Vitro Targeting of Physiological Oxygen Partial Pressure Results in Enhanced Endocrine Differentiation and Increased Separation of α and β Cells
The previous set of experiments showed that HOT was able to provide an environment that supported functional β cell maturation from PP cells in vivo. However, it took several months for this to occur, and HOT did not prevent the formation of tumors in the graft site. Because of this, we decided to use oxygen modulation in the context of the original hES cell differentiation method, with the goal of defining a set of conditions conducive to full maturation in vitro. Success at achieving such an outcome, we reasoned, might lead to faster diabetes reversal and potentially lower incidence of tumoral lesions upon transplantation.
We have previously established that the in vitro targeting of an oxygen partial pressure (pO2) known to be in the physiological range for β cells [16] results in increased islet survival and function [15] as well as enhanced endocrine cell differentiation from native murine PP cells [10]. To accomplish this targeting, we made use of culture dishes in which the surface in contact with the cells is made of an air-permeable, liquid impermeable PFC/PDMS membrane [15]. Using the observed size distribution (50–300 μm, with a majority of aggregates in the 100–150 μm range) and the measured range of oxygen consumption rates (maximum and minimum) for the stage 4 stem cell aggregates, COMSOL v3.3 finite element analysis software-assisted computerized modeling of oxygen diffusion through cell aggregates (two-dimensional diffusion/reaction finite element modeling on permutations of control and experimental culture systems) was performed. The goal of this modeling was to determine the needed external (incubator) oxygen partial pressure to maintain the majority of cultured tissue at or near physiological oxygen levels (40–80 mmHg) while avoiding hypoxic or anoxic conditions (0.1–0.4 mmHg), as detailed in prior work with islets and pancreatic precursor tissues [10, 15]. As shown in supplemental online Figure 2, we came up with a range of 9%–12% external (incubator) O2 concentration at which the majority of the aggregates would be at a physiological or near-physiological pO2 range. In contrast, control stage 4 aggregates plated in ultra-low attachment plates at atmospheric 21% O2 exhibited a higher degree of hypoxia. When an oxygen-permeable membrane is used at the bottom, as is the case with PFC/PDMS dishes, even lower-than-atmospheric O2 concentrations are enough to ensure a physiological-like oxygenation throughout the tissue.
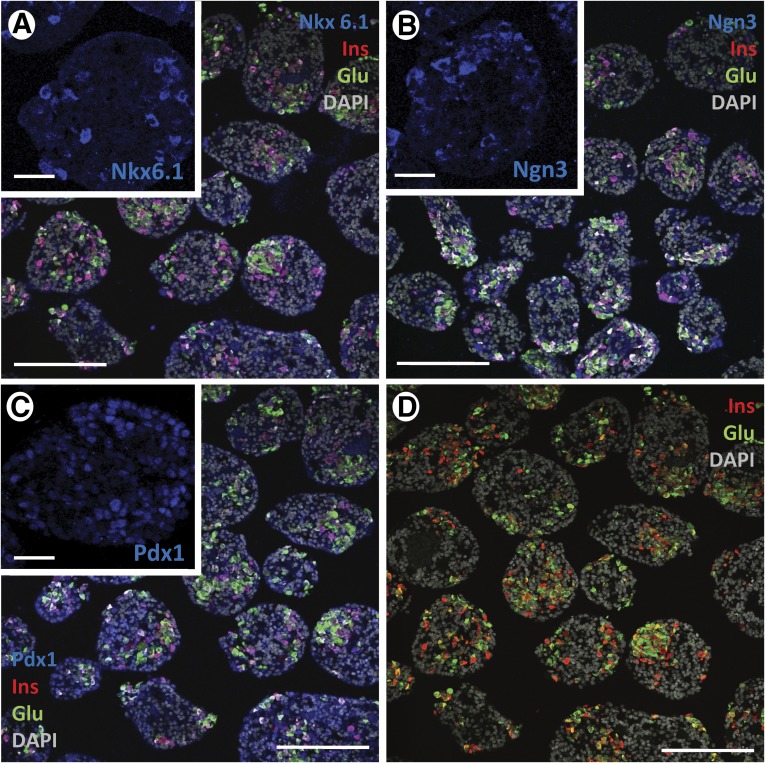
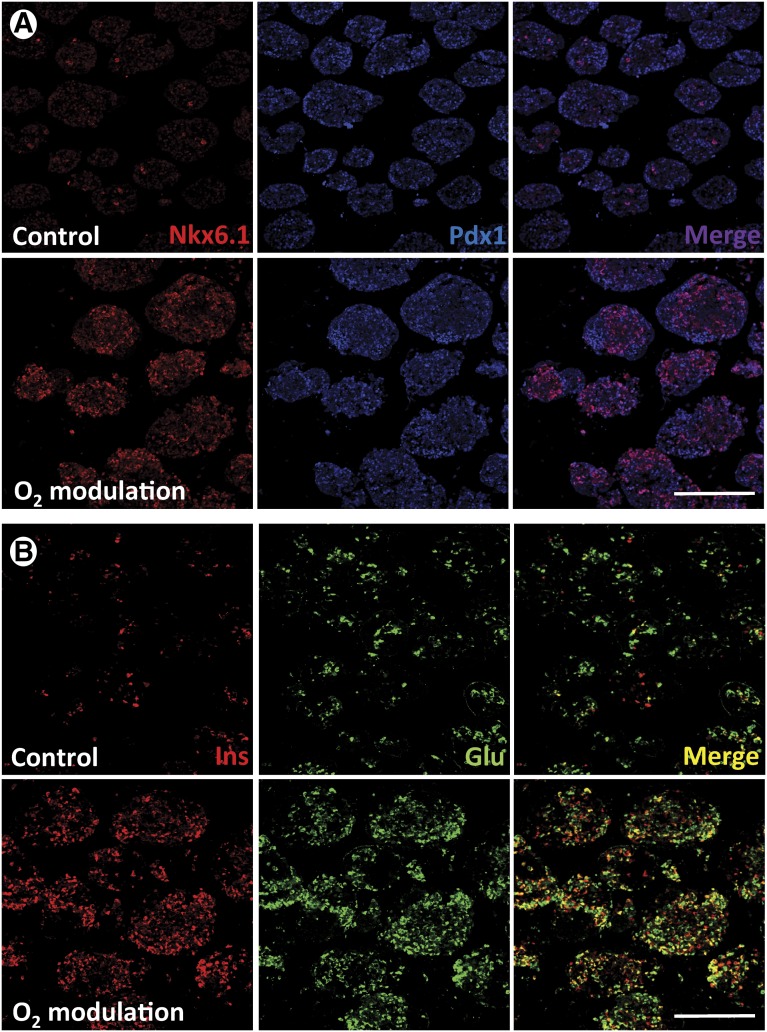
hES cells were allowed to differentiate up to stage 4 (PP) in normal tissue culture-treated 6-well plates. Cell clusters were detached from the plastic by gentle pipetting and subsequently plated either in ultralow-attachment 30-mm dishes at 21% O2 or PFC/PDMS dishes of the same diameter at 9%–12% O2, depending on the oxygen consumption rate of each sample. Aggregate density was maintained in both groups for the remainder of the experiment (15 days). Samples were collected at the end of this period for quantitative RT-PCR and IF analysis. Aliquots of live cells were used to conduct a GSIR assay in vitro. Cells grown in control (21% O2, standard plates) conditions had the general phenotype shown in Figure 4. They expressed markers of pancreatic endocrine specification such as Nkx6.1 (Fig. 4A), Ngn3 (Fig. 4B), and Pdx1 (Fig. 4C), as well as insulin and glucagon (Figs. 4A–4D). Nkx6.1 and Ngn3 expression tended to be cytoplasmic and often colocalized with the islet hormones. Pdx1, in contrast, tended to be nuclear (Fig. 4C, inset) and was detected preferentially in hormone-negative cells. Although to a lesser degree than in the first version of the protocol (Fig. 1), coexpression of insulin and glucagon was still noticeable (Fig. 4D). As shown in Figure 5A, the expression of most key markers of pancreatic development was significantly upregulated in the PFC/PDMS group versus the above control. In addition to the islet hormones, markers of terminal pancreatic endocrine cell maturation such as Pdx1 (28-fold), Nkx6.1 (43-fold), glucokinase (9-fold), synaptophysin (26-fold), and islet amyloid polypeptide (62-fold) were significantly (p < .05) upregulated. Notably, the immunostaining for insulin and glucagon (Fig. 4B) revealed a much better separation of the two hormones in the oxygen-modulated group. Metamorph analysis showed that the ratio between cells that expressed insulin alone versus those that expressed either glucagon or both glucagon and insulin was higher in the oxygen-modulated group than in the controls, although in both instances it remained below that calculated for a human islet preparation (Fig. 5C). Finally, the cultures described above were subjected to GSIR assays. Clusters were exposed to low glucose-containing medium (L1, 2.2 mM) followed by high glucose (H, 16.6 mM) and finally to low glucose again (L2) to rule out that cells might be “dumping” insulin rather than responding in a physiological manner. Medium was collected, and C-peptide levels were measured by ELISA (Mercodia). The results are displayed in Figure 5D. Although no significant responsiveness was noted in the oxygen-modulated cells (stimulation index, 1.1 ± 0.02), the overall levels of C-peptide release were significantly higher (p < .05) than in the control (cell clusters cultured from stage 4 in ultralow-attachment plates at 21% O2). The overall histological appearance and organization of the oxygen-modulated clusters was almost undistinguishable from that of human isolated islets. The effects of this intervention were most noticeable in batches such as the one shown in Figure 6, which at the end of the standard differentiation process was deemed suboptimal due to a low β-cell yield (top panel). The parallel completion of differentiation in a separate aliquot placed in oxygen-optimized conditions, however, led to a significant increase in endocrine cell differentiation (bottom panel).
Figure 4.
Phenotypic analysis of pancreatic progenitor cells allowed to mature in regular conditions. (A–D): Immunofluorescence analysis. Following in vitro maturation, these cells expressed Nkx6.1 (A), Ngn3 (B), Pdx1 (C), insulin, and glucagon (A–D). Nkx6.1 and Ngn3 expression was often seen in the cytosolic compartment ([A] and [B], insets), together with hormone signal. However, Pdx1 tended to be nuclear ([C], inset) and was mostly observed in hormone-negative cells. (A–C): Clockwise from top left (including insets): Nkx6.1, Ngn3, and Pdx1 (blue), insulin (red), glucagon (green), and DAPI (gray). (D): Insulin (red), glucagon (green), and DAPI (blue). Scale bars = 150 μm (main microphotographs) and 50 μm (insets). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; Glu, glucagon; Ins, insulin.
Figure 5.
Oxygen modulation results in immunohistochemical, gene expression, and functional changes consistent with a better maturation of hES cells toward pancreatic endocrine fates. (A): Quantitative reverse transcription-polymerase chain reaction analysis of 20 relevant markers of pancreatic endocrine development plus Oct3/4 (a marker of pluripotency). The y-axis indicates fold increase versus control (cells differentiated in regular cell culture dishes at 21% O2). Open columns: control cells. Gray columns: oxygen-modulated cells. Bars indicate SE. All differences between control and experimental group were statistically significant (p < .05). (B): Representative microphotographs of control (left column) and oxygen-modulated (right column) cultures. Coexpression of insulin (red) and glucagon (green) was more commonly observed in control than in oxygen-modulated conditions. Pdx1 staining (blue) also appeared to be stronger in the latter. Scale bars = 20 μm (top row) and 100 μm (bottom row). (C): Metamorph-calculated ratio of insulin-expressing cells versus cells that expressed either glucagon or insulin + glucagon. Control stage 5 cells, oxygen-modulated stage 5 cells and human isolated islets were examined. Bars indicate SE (n = 3). Differences between the islet ratio and the other two ratios were statistically significant (p < .01). (D): Glucose-stimulated insulin release assay conducted on oxygen-modulated stage 5 cells (gray columns) and control stage 5 cells (black columns). Measurements were taken in triplicate. The y-axis indicates insulin (microunits/ml). x-axis indicates L1 (low glucose 1: 2.2 mM), H (high glucose: 16.6 mM), and L2 (low glucose 2: 2.2 mM). Bars indicate SE. Abbreviations: C, control; glu, glucagon; H, high glucose; L, low glucose; ins, insulin.
Figure 6.
Immunofluorescence comparison of oxygen-modulated and control stage 5 cells. (A): Top row: control cells; bottom row: oxygen-modulation group. The pancreatic progenitor markers Nkx6.1 (red) and Pdx1 (blue) were examined at the same time point. (B): Top row: control cells; bottom row: oxygen-modulation group. The terminal pancreatic endocrine markers insulin (red) and glucagon (green) were analyzed. Scale bars = 150 μm. Abbreviations: Glu, glucagon; Ins, insulin.
Discussion
Last-generation protocols for the differentiation of pluripotent stem cells along the pancreatic endocrine cell lineage have been highly successful up to the PP stage [5, 7–9, 25–29]. but so far have failed to consistently yield functional β cells in vitro. The existence of this bottleneck, which has steered the field toward in vivo maturation strategies [8, 9, 25, 27], indicates that there is a missing element in in vitro settings. Although such a factor could very well be chemical, the failure to detect it among the hundreds of such agents tested through high-throughput screening approaches [9, 17, 30] makes it more likely that the defect is more related to our inability to fully mimic the niche in which β-cell specification takes place.
The role of evolving oxygen tensions in the control of pancreatic endocrine differentiation is now well established [10–15]. The very biology of the adult islet, which receives approximately 25% of the total amount of oxygen that is delivered to the pancreas in spite of its marginal share of the overall mass of the organ [31, 32] hints at the importance of this physical variable in its ontogeny. Our previous studies with human isolated islets [15] and murine pancreatic progenitors [10] strongly suggest that oxygen is a key component of the β-cell niche and as such ought to be carefully adjusted when its differentiation is recapitulated in vitro.
Based on our previous findings on murine PP cells, we hypothesized that oxygen modulation might also have a drastic effect on the outcome of β-cell differentiation from hES cells. We set out to test this hypothesis in two distinct settings. In the first one, mice transplanted with hES-derived PP cells were exposed to a daily HOT. Although we have previously shown other beneficial effects of HOT in adult mice (such as a somewhat reduced rate of autoimmune diabetes and enhanced β cell replication [19]), in this case the rationale was the well-known fact that cells transplanted under the kidney capsule experience a hypoxic environment until vascularization ensues [16, 33]. Such hypoxic conditions would affect PP cells precisely at the time during which enhanced oxygenation is thought to be required for their maturation. As HOT has been shown to improve murine islet survival following transplantation under the kidney capsule [24], the assumption was made that hES-derived PP cells delivered to the same location would also find increased oxygen levels and thus mature faster than in control (not exposed to a hyperbaric regimen) animals. Our findings confirmed this hypothesis. Following transplantation of stage 4 PP cells under the kidney capsule, diabetes was reversed in half of the animals exposed to HOT (none in the control group). In addition, all the animals in the experimental group maintained body weight throughout the course of the experiment and required less exogenous insulin administration than control mice. The analysis of the grafts demonstrated good segregation of α and β cells in the HOT group, whereas the control group lacked mature pancreatic endocrine cells. At the very least, these results suggest that enhanced tissue oxygenation creates an environment that is permissive for in vivo PP engraftment and β-cell maturation. However, this approach did not prevent the formation of teratomous lesions.
The second setting in which we tested our hypothesis was in vitro, the rationale being that completion of maturation prior to transplantation might help not only in the functional characterization of the transplantable tissues, but also, potentially, in minimizing the risk of tumor formation. Our previous research on murine PP cells shows that just ensuring that the pO2 of cultured embryonic day 13.5 (e13.5) dorsal pancreatic buds remains within the physiological range for islets (40–80 mmHg) is sufficient for the faithful recapitulation of in vivo development [10]. Additionally, our experiments demonstrated that the targeting of physiological oxygen levels results in a selective bias of differentiation toward endocrine cell fates. Such fine-tuning of pO2 required the manufacture of specialized culture devices [15] in which air is distributed to the cultured cells in a bidirectional manner, that is, by passive diffusion through the culture medium (top) and by placing the cells atop a PDMS/PFC membrane directly exposed to the atmospheric air (bottom). Conventional means of culture, in contrast, are characterized by a unidirectional flow of air (i.e., from the top) and therefore are unable to prevent the formation of oxygen diffusion gradients across the cultured tissues.
We reasoned that the addition of a final in vitro step to our β-cell differentiation method [9], conducted in PDMS/PFC dishes that ensure that the maturation of PP cells occurs at the right pO2, might result in enhanced β-cell differentiation. A comprehensive redesign of this protocol would require a sequential optimization of each one of its stages, comparing the effect of optimized oxygenation within the appropriate range for each phase. The resulting method would account for the physiological pO2 patterns observed in vivo. However, at this time we decided to focus only on stage 4 cells. Even if it could be argued that standard differentiation up to that point is still subject to improvement, the most critical and well-documented change in pO2 occurs precisely after the generation of PP cells [11, 34]. It is generally accepted that mammalian development occurs at very low O2 levels prior to the onset of blood circulation [35–41]. Therefore, it is reasonable to expect that most of the development of the pancreas occurs in hypoxic conditions, at least until the advent of blood flow in the organ at approximately e13.5 (mouse model) [34]. As standard in vitro conditions tend to be hypoxic at the cell level anyway, the need for oxygen modulation during stages 1–4 might not be as dire. Proof of that, in fact, is that last-generation hES cell differentiation protocols exhibit a high PP generation efficiency [27]. We have proposed a model [11] whereby the onset of circulation/blood oxygenation within the developing pancreas might be ultimately responsible for the molecular changes that lead to endocrine cell differentiation. Indeed, islets have much higher oxygen requirements than the surrounding tissue [31, 32]. PDMS/PFC devices can effectively meet these oxygen requirements whereas conventional ones cannot. hES cell differentiation methods have been traditionally developed with the latter, which are unfit to support optimal islet cell differentiation and survival. Hence, our working hypothesis was that the appropriate specification and maturation of stage 4 PP cells is likely to require a switch from low to high (40–80 mmHg) pO2 [16, 33]. In our experimental setting, aliquots of stage 4 PP cells were allowed to mature in vitro in two different conditions: in regular cell culture plates at 21% O2 and in PFC/PDMS dishes at 9%–12% O2. Perhaps counterintuitively, the latter condition offers a higher and more uniform degree of oxygenation of PP clusters than normal conditions at 21% oxygen because of the minimization of gas diffusion gradients and hypoxic regions within the aggregate. The 9%–12% range was calculated to target a physiological oxygen concentration range across most of the volume of these aggregates when cultured in PFC/PDMS devices, based on their plating density, average diameter, and oxygen consumption rate (OCR) at this stage. The analysis of the differentiation outcome reflected dramatic gains in the expression of key pancreatic endocrine genes when comparing the oxygen-modulated group to the controls, which was confirmed by immunofluorescence. There was also a noticeably higher degree of separation between the two main islet hormones (glucagon and insulin), which appeared to be largely expressed in different cells. Coexpression remained the norm, however, in cultures allowed to mature under regular conditions.
Conclusion
Despite the obvious improvement in endocrine cell differentiation using oxygen modulation, further refinements will be necessary to achieve functional β-like cells in vitro that could be used for transplantation with a reasonable chance of success. This was evidenced by the relative nonresponsiveness of PFC/PDMS-matured cells in a GSIR assay. For all its benefits, the modulation of oxygen is just one of the many components of the ideal β-cell differentiation niche. The synergistic addition of other elements, such as the use of an organ-matched extracellular matrix and/or the presence of other “helper” cell types found in the native niche (such as mesenchymal [42, 43] or endothelial [44–46]), will get us closer to the goal of ex vivo recapitulating islet development in its entirety.
Supplementary Material
Acknowledgments
We acknowledge Drs. Armando Méndez, Hirohito Ichii, George McNamara, and Kevin Johnson and the staff of the Preclinical Cell Processing and Translational Models Core (all at the Diabetes Research Institute) for their technical contributions and helpful discussions. We are also grateful to Dr. Phillip Ruiz Jr. (Transplantation Laboratories and Immunopathology of the Miami Transplant Institute) for kind assistance with the pathological assessment. This work was funded by the Diabetes Research Institute Foundation. This work was also supported in part by NIH Grants DK70460 and NIH U42 RR016603 and by the City of Hope (Duarte, California).
Author Contributions
S.C., S.A.-C., J.A.G., R.D.M., and S.V.: performance of experiments, data analysis; C.R., A.P., and L.I.: discussion and input into the experimental design and data analysis, manuscript writing and editing; C.A.F.: design and performance of experiments, data analysis, manuscript writing; J.D.-B.: coordination of study; experiment design, data analysis, manuscript writing.
Disclosure of Potential Conflicts of Interest
C.R. has uncompensated intellectual property rights, a consultant/advisory role, and uncompensated stock options. A.P. has compensated research funding from Positive ID and ATRM (J&J) and uncompensated employment, intellectual property rights, a consultant/advisory role, and uncompensated ownership interests with Converge Biotech, Inc., NEVA, LLC. L.I. has been awarded an uncompensated patent. C.A.F. has uncompensated intellectual property rights (inventor) and uncompensated ownership interest (shareholder). J.D.-B. is an uncompensated co-inventor in a patent and shareholder of OPhysio Inc.
References
- 1.Fiorina P, Shapiro AM, Ricordi C, et al. The clinical impact of islet transplantation. Am J Transplant. 2008;8:1990–1997. doi: 10.1111/j.1600-6143.2008.02353.x. [DOI] [PubMed] [Google Scholar]
- 2.Domínguez-Bendala J, Inverardi L, Ricordi C. Stem cell-derived islet cells for transplantation. Curr Opin Organ Transplant. 2011;16:76–82. doi: 10.1097/MOT.0b013e32834252b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng X, Ying L, Lu L, et al. Self-renewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell. 2012;10:371–384. doi: 10.1016/j.stem.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Amour KA, Agulnick AD, Eliazer S, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 5.D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 6.Jiang J, Au M, Lu K, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–1953. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 7.McLean AB, D’Amour KA, Jones KL, et al. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- 8.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 9.Rezania A, Bruin JE, Riedel MJ, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraker CA, Alvarez S, Papadopoulos P, et al. Enhanced oxygenation promotes beta-cell differentiation in vitro. Stem Cells. 2007;25:3155–3164. doi: 10.1634/stemcells.2007-0445. [DOI] [PubMed] [Google Scholar]
- 11.Fraker CA, Ricordi C, Inverardi L, et al. Oxygen: A master regulator of pancreatic development? Biol Cell. 2009;101:431–440. doi: 10.1042/BC20080178. [DOI] [PubMed] [Google Scholar]
- 12.Heinis M, Simon MT, Duvillié B. New insights into endocrine pancreatic development: The role of environmental factors. Horm Res Paediatr. 2010;74:77–82. doi: 10.1159/000314894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinis M, Simon MT, Ilc K, et al. Oxygen tension regulates pancreatic beta-cell differentiation through hypoxia-inducible factor 1alpha. Diabetes. 2010;59:662–669. doi: 10.2337/db09-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinis M, Soggia A, Bechetoille C, et al. HIF1α and pancreatic β-cell development. FASEB J. 2012;26:2734–2742. doi: 10.1096/fj.11-199224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraker CA, Cechin S, Álvarez-Cubela S, et al. A physiological pattern of oxygenation using perfluorocarbon-based culture devices maximizes pancreatic islet viability and enhances β-cell function. Cell Transplant. 2013;22:1723–1733. doi: 10.3727/096368912X657873. [DOI] [PubMed] [Google Scholar]
- 16.Carlsson PO, Mattsson G. Oxygen tension and blood flow in relation to revascularization in transplanted adult and fetal rat pancreatic islets. Cell Transplant. 2002;11:813–820. [PubMed] [Google Scholar]
- 17.Rezania A, Riedel MJ, Wideman RD, et al. Production of functional glucagon-secreting α-cells from human embryonic stem cells. Diabetes. 2011;60:239–247. doi: 10.2337/db10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricordi C, Fraker C, Szust J, et al. Improved human islet isolation outcome from marginal donors following addition of oxygenated perfluorocarbon to the cold-storage solution. Transplantation. 2003;75:1524–1527. doi: 10.1097/01.TP.0000058813.95063.7A. [DOI] [PubMed] [Google Scholar]
- 19.Faleo G, Fotino C, Bocca N, et al. Prevention of autoimmune diabetes and induction of β-cell proliferation in NOD mice by hyperbaric oxygen therapy. Diabetes. 2012;61:1769–1778. doi: 10.2337/db11-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prabakar KR, Domínguez-Bendala J, Molano RD, et al. Generation of glucose-responsive, insulin-producing cells from human umbilical cord blood-derived mesenchymal stem cells. Cell Transplant. 2012;21:1321–1339. doi: 10.3727/096368911X612530. [DOI] [PubMed] [Google Scholar]
- 21.Brandhorst H, Brandhorst D, Brendel MD, et al. Assessment of intracellular insulin content during all steps of human islet isolation procedure. Cell Transplant. 1998;7:489–495. doi: 10.1177/096368979800700508. [DOI] [PubMed] [Google Scholar]
- 22.Fraker C, Timmins MR, Guarino RD, et al. The use of the BD oxygen biosensor system to assess isolated human islets of langerhans: Oxygen consumption as a potential measure of islet potency. Cell Transplant. 2006;15:745–758. doi: 10.3727/000000006783981440. [DOI] [PubMed] [Google Scholar]
- 23.Ichii H, Inverardi L, Pileggi A, et al. A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant. 2005;5:1635–1645. doi: 10.1111/j.1600-6143.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 24.Juang JH, Hsu BR, Kuo CH, et al. Beneficial effects of hyperbaric oxygen therapy on islet transplantation. Cell Transplant. 2002;11:95–101. [PubMed] [Google Scholar]
- 25.Bruin JE, Rezania A, Xu J, et al. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia. 2013;56:1987–1998. doi: 10.1007/s00125-013-2955-4. [DOI] [PubMed] [Google Scholar]
- 26.Chetty S, Pagliuca FW, Honore C, et al. A simple tool to improve pluripotent stem cell differentiation. Nat Methods. 2013;10:553–556. doi: 10.1038/nmeth.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rezania A, Bruin JE, Xu J, et al. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells. 2013;31:2432–2442. doi: 10.1002/stem.1489. [DOI] [PubMed] [Google Scholar]
- 28.Kelly OG, Chan MY, Martinson LA, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29:750–756. doi: 10.1038/nbt.1931. [DOI] [PubMed] [Google Scholar]
- 29.Schulz TC, Young HY, Agulnick AD, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS ONE. 2012;7:e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S, Borowiak M, Fox JL, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5:258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 31.Lifson N, Kramlinger KG, Mayrand RR, et al. Blood flow to the rabbit pancreas with special reference to the islets of Langerhans. Gastroenterology. 1980;79:466–473. [PubMed] [Google Scholar]
- 32.Jansson L. The regulation of pancreatic islet blood flow. Diabetes Metab Rev. 1994;10:407–416. doi: 10.1002/dmr.5610100405. [DOI] [PubMed] [Google Scholar]
- 33.Carlsson PO, Palm F, Mattsson G. Low revascularization of experimentally transplanted human pancreatic islets. J Clin Endocrinol Metab. 2002;87:5418–5423. doi: 10.1210/jc.2002-020728. [DOI] [PubMed] [Google Scholar]
- 34.Colen KL, Crisera CA, Rose MI, et al. Vascular development in the mouse embryonic pancreas and lung. J Pediatr Surg. 1999;34:781–785. doi: 10.1016/s0022-3468(99)90373-1. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell JA, Yochim JM. Intrauterine oxygen tension during the estrous cycle in the rat: Its relation to uterine respiration and vascular activity. Endocrinology. 1968;83:701–705. doi: 10.1210/endo-83-4-701. [DOI] [PubMed] [Google Scholar]
- 36.Rodesch F, Simon P, Donner C, et al. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- 37.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miki A, Fujimoto E, Ohsaki T, et al. Effects of oxygen concentration on embryonic development in rats: A light and electron microscopic study using whole-embryo culture techniques. Anat Embryol (Berl) 1988;178:337–343. doi: 10.1007/BF00698664. [DOI] [PubMed] [Google Scholar]
- 39.New DA. Whole-embryo culture and the study of mammalian embryos during organogenesis. Biol Rev Camb Philos Soc. 1978;53:81–122. doi: 10.1111/j.1469-185x.1978.tb00993.x. [DOI] [PubMed] [Google Scholar]
- 40.Burggren WW. What is the purpose of the embryonic heart beat? Or how facts can ultimately prevail over physiological dogma. Physiol Biochem Zool. 2004;77:333–345. doi: 10.1086/422230. [DOI] [PubMed] [Google Scholar]
- 41.Gassmann M, Fandrey J, Bichet S, et al. Oxygen supply and oxygen-dependent gene expression in differentiating embryonic stem cells. Proc Natl Acad Sci USA. 1996;93:2867–2872. doi: 10.1073/pnas.93.7.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duvillié B, Attali M, Bounacer A, et al. The mesenchyme controls the timing of pancreatic beta-cell differentiation. Diabetes. 2006;55:582–589. doi: 10.2337/diabetes.55.03.06.db05-0839. [DOI] [PubMed] [Google Scholar]
- 43.Sneddon JB, Borowiak M, Melton DA. Self-renewal of embryonic-stem-cell-derived progenitors by organ-matched mesenchyme. Nature. 2012;491:765–768. doi: 10.1038/nature11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lammert E, Cleaver O, Melton D. Role of endothelial cells in early pancreas and liver development. Mech Dev. 2003;120:59–64. doi: 10.1016/s0925-4773(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 45.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 46.Ranjan AK, Joglekar MV, Hardikar AA. Endothelial cells in pancreatic islet development and function. Islets. 2009;1:2–9. doi: 10.4161/isl.1.1.9054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.