Abstract
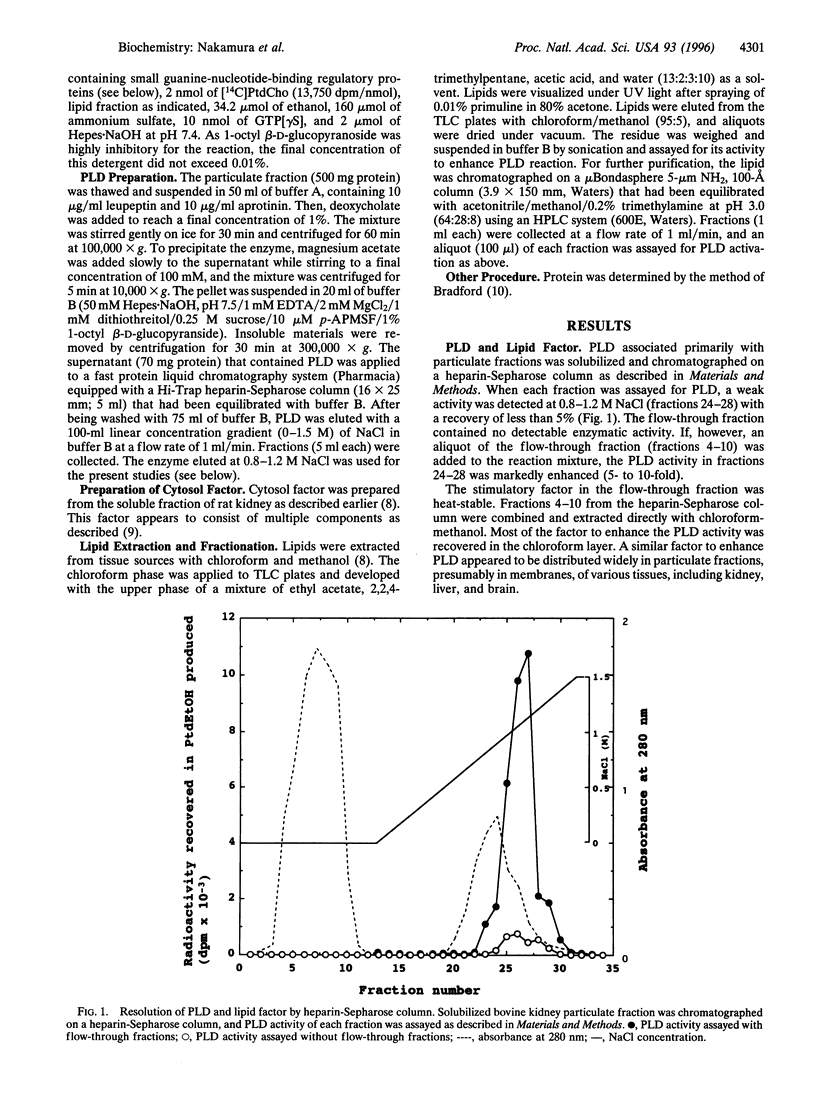
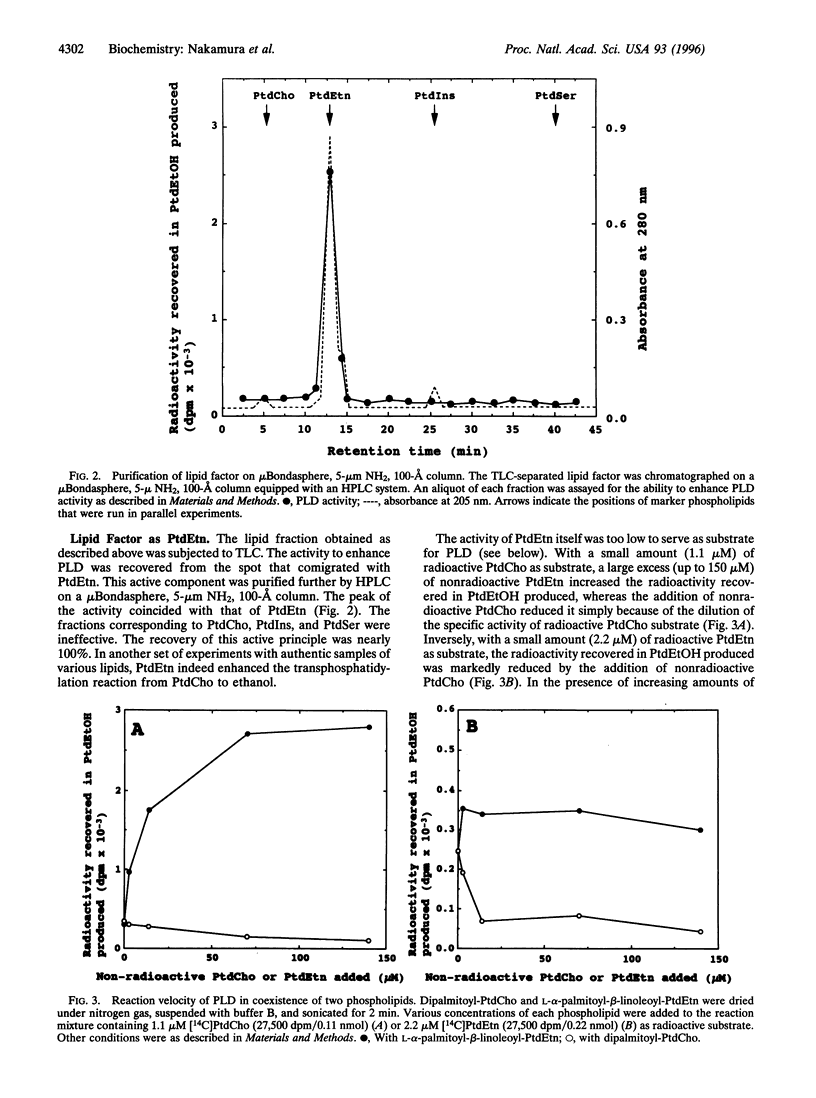
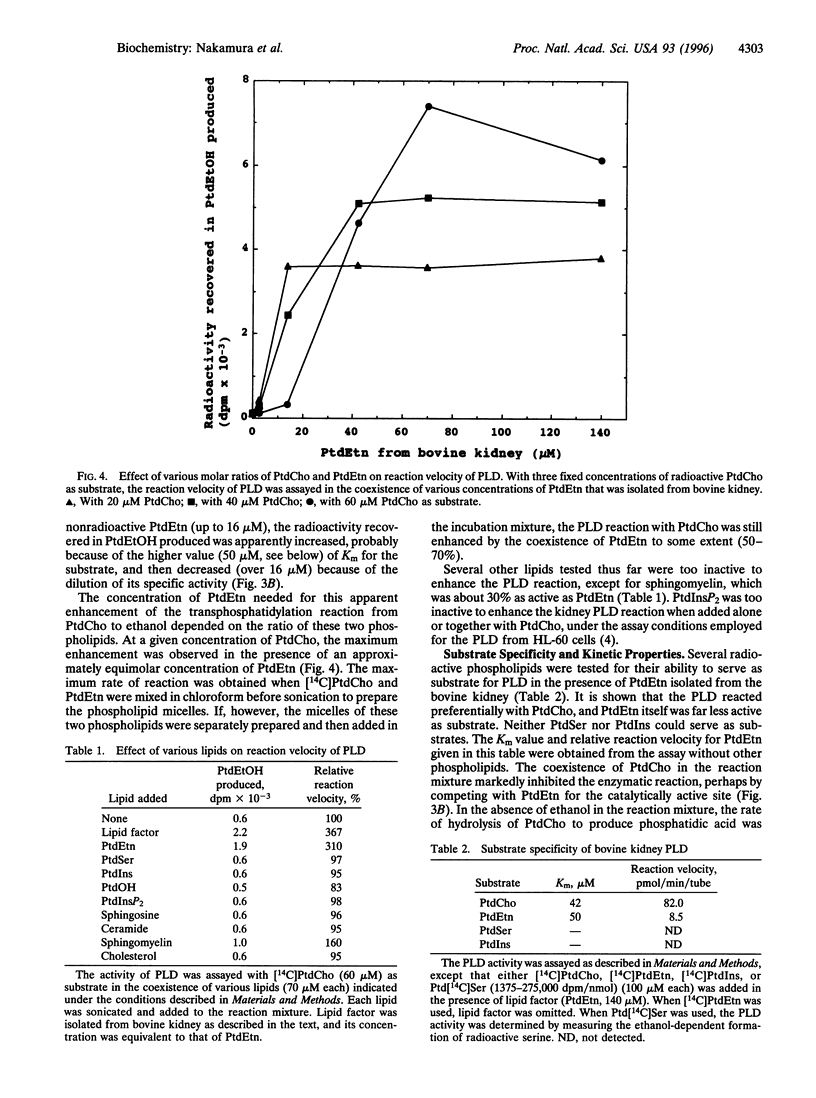
Bovine kidney phospholipase D (PLD) was assayed by measuring the formation of phosphatidylethanol from added radioactive phosphatidylcholine (PtdCho) in the presence of ethanol, guanosine 5'-[gamma-thio]triphosphate, ammonium sulfate, and cytosol factor that contained small GTP-binding regulatory proteins. The PLD enzyme associated with particulate fractions was solubilized by deoxycholate and partially purified by chromatography on a heparin-Sepharose column. This PLD preferentially used PtdCho as substrate. After purification, the enzyme per se showed little or practically no activity but required an additional factor for the enzymatic reaction. This factor was extracted with chloroform/methanol directly from particulate fractions of various tissues, including kidney, liver, and brain, and identified as phosphatidylethanolamine (PtdEtn), although this phospholipid did not serve as a good substrate. Plasmalogen-rich PtdEtn, dioleoyl-PtdEtn, and L-alpha-palmitoyl-beta-linoleoyl-PtdEtn were effective, but dipalmitoyl-PtdEtn was inert. Sphingomyelin was 30% as active as PtdEtn. The results suggest that mammalian PLD reacts nearly selectively with PtdCho in the form of mixed micelles or membranes with other phospholipids, especially PtdEtn.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown H. A., Gutowski S., Moomaw C. R., Slaughter C., Sternweis P. C. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993 Dec 17;75(6):1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- Chalifa V., Möhn H., Liscovitch M. A neutral phospholipase D activity from rat brain synaptic plasma membranes. Identification and partial characterization. J Biol Chem. 1990 Oct 15;265(29):17512–17519. [PubMed] [Google Scholar]
- Cockcroft S., Thomas G. M., Fensome A., Geny B., Cunningham E., Gout I., Hiles I., Totty N. F., Truong O., Hsuan J. J. Phospholipase D: a downstream effector of ARF in granulocytes. Science. 1994 Jan 28;263(5146):523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Hammond S. M., Altshuller Y. M., Sung T. C., Rudge S. A., Rose K., Engebrecht J., Morris A. J., Frohman M. A. Human ADP-ribosylation factor-activated phosphatidylcholine-specific phospholipase D defines a new and highly conserved gene family. J Biol Chem. 1995 Dec 15;270(50):29640–29643. doi: 10.1074/jbc.270.50.29640. [DOI] [PubMed] [Google Scholar]
- Huang C., Wykle R. L., Daniel L. W., Cabot M. C. Identification of phosphatidylcholine-selective and phosphatidylinositol-selective phospholipases D in Madin-Darby canine kidney cells. J Biol Chem. 1992 Aug 25;267(24):16859–16865. [PubMed] [Google Scholar]
- Liscovitch M., Chalifa V., Pertile P., Chen C. S., Cantley L. C. Novel function of phosphatidylinositol 4,5-bisphosphate as a cofactor for brain membrane phospholipase D. J Biol Chem. 1994 Aug 26;269(34):21403–21406. [PubMed] [Google Scholar]
- Massenburg D., Han J. S., Liyanage M., Patton W. A., Rhee S. G., Moss J., Vaughan M. Activation of rat brain phospholipase D by ADP-ribosylation factors 1,5, and 6: separation of ADP-ribosylation factor-dependent and oleate-dependent enzymes. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11718–11722. doi: 10.1073/pnas.91.24.11718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Shimooku K., Akisue T., Jinnai H., Hitomi T., Kiyohara Y., Ogino C., Yoshida K., Nishizuka Y. Mammalian phospholipase D: activation by ammonium sulfate and nucleotides. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12319–12322. doi: 10.1073/pnas.92.26.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura S., Yamashita S. Purification and characterization of phosphatidylcholine phospholipase D from pig lung. J Biol Chem. 1994 Dec 9;269(49):31207–31213. [PubMed] [Google Scholar]
- Pertile P., Liscovitch M., Chalifa V., Cantley L. C. Phosphatidylinositol 4,5-bisphosphate synthesis is required for activation of phospholipase D in U937 cells. J Biol Chem. 1995 Mar 10;270(10):5130–5135. doi: 10.1074/jbc.270.10.5130. [DOI] [PubMed] [Google Scholar]
- Singer W. D., Brown H. A., Bokoch G. M., Sternweis P. C. Resolved phospholipase D activity is modulated by cytosolic factors other than Arf. J Biol Chem. 1995 Jun 23;270(25):14944–14950. doi: 10.1074/jbc.270.25.14944. [DOI] [PubMed] [Google Scholar]
- Wang P., Anthes J. C., Siegel M. I., Egan R. W., Billah M. M. Existence of cytosolic phospholipase D. Identification and comparison with membrane-bound enzyme. J Biol Chem. 1991 Aug 15;266(23):14877–14880. [PubMed] [Google Scholar]



