Abstract
Background
Giardiasis is one of the most common causes of diarrheal disease worldwide and 5-nitroimidazoles (5-NI) are the most commonly prescribed drugs for the treatment of giardiasis. We evaluated the efficacy of 5-nitroimidazoles (5-NI) in the treatment of giardiasis in a systematic review of randomized controlled trials (RCTs).
Methodology/Principal Findings
We conducted a comprehensive literature search in PubMed-Medline, Scopus, Web of Science and Cochrane Library for RCTs evaluating the efficacy of 5-NI vs. control (placebo or active treatment) on parasitological cure in patients with parasitologically-demonstrated giardiasis. The search was performed in May 2013 with no language restriction by two authors independently. The efficacy outcome was parasitological cure, and harmful outcomes were abdominal pain, bitter or metallic taste, and headache. We included 30 RCTs (n = 3,930). There was a significant and slightly higher response rate with 5-NI in giardiasis treatment (RR 1.06, 95%CI 1.02–1.11, p = 0.005). There was high heterogeneity among studies (I2 = 72%). The response rates for metronidazole, tinidazole and secnidazole were similar (RR 1.05, 95%CI 1.01–1.09, p = 0.01; RR 1.32 95%CI 1.10–1.59, p = 0.003; and RR 1.18 95%CI 0.93–1.449, p = 0.18, respectively). On subgroup analyses, the response rates did not vary substantially and high heterogeneity persisted (I2 = 57%–80%). Harmful outcomes were uncommon, and 5-NIs were associated with lower risk of abdominal pain, and higher risk of both bitter or metallic taste and headache.
Conclusions
Studies investigating the efficacy of 5-NI in giardiasis treatment are highly heterogeneous. 5-NIs have a slightly better efficacy and worse profile for mild harmful outcomes in the treatment of giardiasis in comparison to controls. Larger high quality RCTs are needed to further assess efficacy and safety profiles of 5-NI.
Author Summary
Giardiasis is a major diarrheal disease with worldwide distribution. 5-nitroimidazoles, which include metronidazole and tinidazole, are the most commonly used drugs in the treatment of giardiasis. In recent years, many other drugs with variable efficacies and adverse effects have been proposed for the treatment of giardiasis. No systematic review has evaluated efficacy of 5-nitroimidazoles as a group in comparison to the other antigiardial drugs. In this context, we performed a systematic review of the literature to identify randomized controlled trials comparing the efficacies of 5-nitroimidazoles with a control drug with the aim of assessing effectiveness of 5-nitroimidazoles in the treatment of giardiasis. Four research databases were searched; 30 trials with 3,930 subjects met our inclusion criteria. Results show that there was a high variation of study outcomes between included studies. 5-nitroimidazoles were associated with higher giardiasis cure rates than controls; also, 5-nitroimidazoles are associated with lower risk of abdominal pain, and higher risks of bitter or metallic taste and headache than controls.
Introduction
Giardiasis is an intestinal illness caused by a flagellated protozoan parasite, Giardia lamblia (syn. G.intestinalis and G.duodenalis). The World Health Organization (WHO) estimates that 3 billion people reside in places with giardiasis prevalence of around 30%, and suggests that there are almost one billion cases of giardiasis, contributing to 2.5 million deaths annually from diarrheal disease [1]. In recent years, epidemiology of giardiasis in developed countries has been changing with increasing international travel and migration from highly endemic countries [2]. Approximately, 20,000 new cases of giardiasis are reported annually in the United States [3]. Due to its increasing global burden, and its developmental and socio-economic impact on infected individuals, Giardia has been included in the ‘Neglected Disease Initiative’ of the WHO since 2004 [4].
The most common antibiotics used for the treatment of giardiasis are the 5-Nitroimidazoles (5-NIs); these include metronidazole, tinidazole, secnidazole and ornidazole, of which metronidazole is the most common [5]. Alternative agents which are less commonly used in giardiasis treatment are quinacrine, furazolidone, benzimidazoles (albendazole and mebendazole), paromomycin, bacitracin zinc, chloroquine and nitazoxanide [5]. Depending on local epidemiology, availability, and cost, these drugs have been widely available for the curative treatment of cases; however, several reports of treatment failure have been reported [6], [7], [8]. With the advent of newer agents which might have similar efficacies as 5-NIs,and also offer an added advantage of more simplified regimens, fewer adverse effects or less drug resistance, it is of considerable interest to determine whether 5-NIs are still the best available option in the treatment of giardiasis.
Three previous systematic reviews and/or meta-analyses [9], [10], [11] have evaluated efficacies of antigiardial drugs in the treatment of giardiasis. All three varied in study designs and study aims, but none compared efficacy of 5-NIs as a group in comparison to other antigiardial drugs. Against this background, we performed a systematic review of the literature to identify RCTs comparing the efficacies of 5-NIs with a control with the aim of assessing effectiveness of 5-NIs in the treatment of giardiasis. We hope to provide policymakers and practitioners with a convenient and evidence-based summary of the primary literature on which to base their decisions.
Methods
Data sources and searches
A comprehensive literature search using PubMed-Medline from database inception through May 13, 2013, The Cochrane library from database inception through May 13, 2013, The Web of Science from database inception through May 13, 2013 and Scopus from database inception through May 13, 2013 was conducted by three investigators (AVH, VP and AD). The following keywords were used: metronidazole, tinidazole, secnidazole, ornidazole, 4-nitroimidazole, 5-nitroimidazole, Giardia, G. lamblia, giardiasis, randomized controlled trial and clinical trial.
PubMed search strategy
((“metronidazole”[MeSH Terms] OR “metronidazole”[All Fields]) OR (“tinidazole”[MeSH Terms] OR “tinidazole”[All Fields]) OR (“secnidazole”[Supplementary Concept] OR “secnidazole”[All Fields]) OR (“ornidazole”[MeSH Terms] OR “ornidazole”[All Fields]) OR (“4-nitroimidazole”[Supplementary Concept] OR “4-nitroimidazole”[All Fields] OR “5 nitroimidazole”[All Fields])) AND ((“Giardia”[MeSH Terms] OR “Giardia”[All Fields]) OR G.lamblia[All Fields] OR (“giardiasis”[MeSH Terms] OR “giardiasis”[All Fields])) AND ((“randomized controlled trial”[Publication Type] OR “randomized controlled trials as topic”[MeSH Terms] OR “randomised controlled trial”[All Fields] OR “randomized controlled trial”[All Fields]) OR (“randomized controlled trial”[Publication Type] OR “randomized controlled trials as topic”[MeSH Terms] OR “randomized controlled trial”[All Fields] OR “randomised controlled trial”[All Fields]) OR (“clinical trial”[Publication Type] OR “clinical trials as topic”[MeSH Terms] OR “clinical trial”[All Fields]))
The following predetermined inclusion criteria were used: (i) RCTs evaluating the efficacy of 5-NI in comparison with a control (placebo, active treatment); (ii) study population of patients with parasitologically-demonstrated giardiasis; (iii) study in any language. An active treatment group is a control group receiving comparator drug (5-NI or non-5-NI) for the treatment of giardiasis. Our exclusion criteria were: (i) no control group; (ii) efficacy data (parasitological cure rates) were not available or could not extracted for the study groups.
Study selection and data extraction
A list of retrieved articles was reviewed independently by 3 investigators (AVH, VP and AD) in order to choose potentially relevant articles, and disagreements about particular studies were discussed and resolved by consensus.
Two reviewers (VP and AD) independently extracted data from studies. The following information was extracted: age, gender, geographic location, study setting, diagnostic test for giardiasis, type of 5-NI and dose/duration, comparator drug and dose/duration, follow up time. Extracted beneficial outcome was parasitological cure rate and harmful outcomes were abdominal pain, bitter or metallic taste, and headache. One other author (AVH) reviewed the extractions for inconsistencies, and the three investigators (AVH, VP and AD) reached consensus.
Evaluation of study quality
The quality of all included trials was assessed using a 5-item instrument developed and validated by Jadad [12]. The 5 items in this scale include i) description of randomization, ii) appropriateness of randomization, iii) description of blinding, iv) adequacy and appropriateness of blinding, and v) description of withdrawals and dropouts. Study quality was assessed independently by two investigators (VP and AVH). Disagreements were resolved by consensus. A score of 0–2 was considered as low quality trial and a score of 3–5 was considered high quality trial.
Data synthesis and analysis
Our systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Text S1, available as supporting data) [13]. A high degree of heterogeneity among studies was expected and therefore a formal meta-analysis was a secondary aim. Taking into account the sources of heterogeneity, several subgroup meta-analyses were pre-specified: (i) type of 5-NI used, (ii) excluding studies with two types of 5-NI comparisons, (iii) study setting (outpatient vs hospitalized), (iv) Jadad score (≥3 vs <3), (v) type of main analysis (intention-to-treat vs per-protocol), (vi) sample size (<100 vs ≥100 patients), (vii) ordered by year of publication. DerSimonian and Laird random effects models were used for meta-analyses [14]. We used the inverse variance (IV) or Mantel-Haenzel (MH) method to calculate pooled RRs and 95% CIs, depending on the absence or presence of scarce outcomes, respectively. When efficacy of two 5-NIs was compared, the 5-NI arm was the arm with the larger sample size. Statistical heterogeneity was evaluated with the Cochran χ2 and the I2 statistics. I2 values of 30–60% represented a moderate level of heterogeneity. A P value of <0.1 for χ2 was defined as indicating the presence of heterogeneity. To examine bias in the results of the meta-analyses, the Egger's test was used to evaluate asymmetry of the funnel plots. Asymmetry of the funnel plots should not be equated with publication bias, as asymmetry can be caused by true heterogeneity among study results, poor methodological quality, reporting biases, and chance. We used Review Manager (RevMan 5.0, Oxford, UK; The Cochrane Collaboration, 2008).
Results
Eligible studies
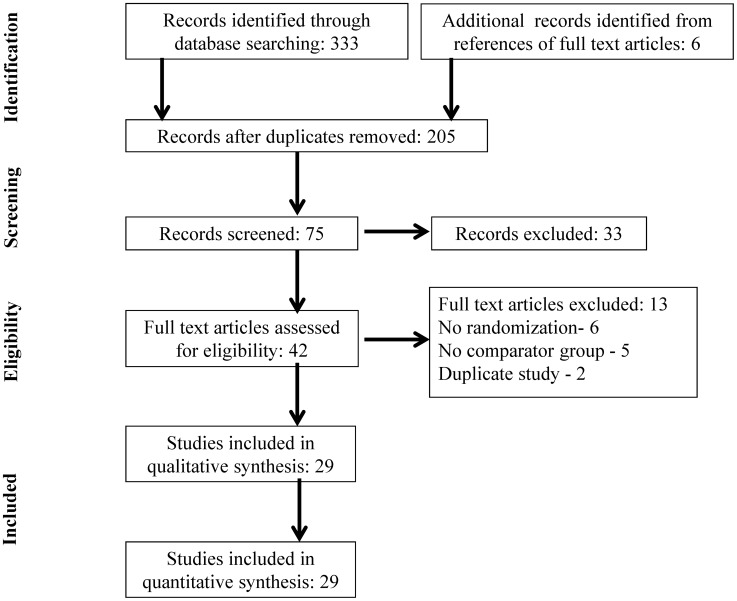
Our search identified 333 publications (Figure 1). After removing duplicates and screening titles of the studies, 75 articles were selected based on relevance to the study topic. After screening the abstracts of these potentially relevant articles, 42 were selected for full-text review based on relevance to the study topic (Figure 1). Thirty randomized controlled trials (n = 3,930) in twenty-nine studies that reported efficacy data of 5-NIs in comparison to a control were included in the systematic review and meta-analyses. The reasons for exclusion of the remaining 13 articles are listed in Figure 1.
Figure 1. Flow diagram of selected studies.
Study characteristics
Table 1 summarizes the main characteristics of the included studies. All trials were conducted in countries endemic to giardiasis. Of the 30 trials included, 22 were in outpatient population; 7 in hospitalized population; one trial did not report the study setting. All patients included in the trials had parasitologically-demonstrated giardiasis. Twenty-four trials were in pediatric population (<18 yrs); 4 trials in both adult and pediatric population; two trials in patients of age >17 yrs. The post-treatment follow-up time varied from 3 days to 5 weeks. A total of 3,930 patients were included in the meta-analysis with sample size ranging from 23 to 502; all but one of the trials included active treatment controls (Table 1).
Table 1. Basic characteristics of included studies.
| First author, Year published | Study location | Sample size | Study Population | Diagnostic test | Patient age category | Age Mean (SD) | Male (%) | Type of 5-NI, dose | Comparator drug, dose | Follow up time |
| al Waili NS, 1992 [22] | Iraq | 44 | NA | Stool exam for cysts and trophozoites | <18 yrs | NA | 63.6 | Metronidazole, 200 mg tid, 5 days | Mebendazole, 200 mg tid, 5 days | Day 7 & 14 |
| Alizadeh A, 2006 [23] | Iran | 120 | outpatient | Trophozoites in iodine-stained wet stool preparations | Both | 22.3 (11.0) | 50.8 | Metronidazole, 250 mg tid, 5 days | Albendazole, 400 mg daily, 5 days | 10 days |
| Bassily S, 1970 [24] | Egypt | 80 | hospitalized | Stools by MIFC technique | Both | NA | NA | Metronidazole€, 250 mg bid daily, 10 days | Mepacrine€ 100 mg tid, 5 days; 2) Furazolidone€ 100 mg qd, 10 days; 3) Placebo 1 capsule tid, 7 days | 5 wks |
| Bulut BU, 1996 [25] | Turkey | 60 | outpatient | Microscopic exam for parasites | <18 yrs | 8.7 (1.6) | 62.5 | Metronidazole, 15 mg/kg daily, 7 days | Mebendazole, 100 mg tid, 7 days; 2) Mebendazole, 100 mg tid, 1 day, 3) Ornidazole, 40 mg/kg, single dose | 2 wks |
| Canete R, 2006 [26] | Cuba | 122 | outpatient | Microscopic exam of fecal samples as wet mounts and/or after Ritchie concentration | <18 yrs | NA | 51.6 | Tinidazole, 50 mg/kg, single dose | Mebendazole, 200 mg tid, 1 day | Day 3, 5 & 7 |
| Cimerman B, 1997 [27] | Brazil | 267 | outpatient | Proto-parasitologic feces test | <18 yrs | 5.8 (2.8) | 60.3 | Tinidazole, 50 mg/kg, single dose | Secnidazole, 30 mg/kg, single dose | Day 7, 14 & 21 |
| Dutta AK, 1994 [28] | India | 150 | hospitalized | Trophozoites/cyst in stool specimens | <18 yrs | NA | 52.7 | Metronidazole, 7.5 mg/kg/dose, tid 5 days | Albendazole, 400 mg once daily, 5 days | Day 1–7, 14 & 21 |
| Escobedo AA, 2003 [29] | Cuba | 146 | outpatient | Microscopic exam of fecal samples as wet mounts and/or after Ritchie concentration | <18 yrs | 8.3 (2.3) | 54.8 | Secnidazole, 30 mg/kg single dose | Mebendazole, 200 mg tid, 3 days | Day 3, 5 & 7 |
| Escobedo AA, 2003 [30] | Cuba | 165 | outpatient | Stool exam as direct wet mounts and after formol-ether concentration | <18 yrs | 6.4 (3.5) | 52.7 | Tinidazole, 50 mg/kg single dose | Chloroquine, 100 mg/kg bd, 5 days; 2) Albendazole, 400 mg daily, 5 days | Day 7 & 10 |
| Escobedo AA, 2008 [31] | Cuba | 166 | outpatient | Microscopic exam of fecal samples as wet mounts and/or after Ritchie concentration | <18 yrs | 7.8 | 52.4 | Tinidazole, 50 mg/kg single dose | Nitazoxanide, 7.5 mg/kg bd, 3 days | Day 5 & 7 |
| Fallah M, 2007 [32] | Iran | 106 | hospitalized | Stool exam by formalin ether concentration technique | <18 yrs | NA | 65.1 | Metronidazole, 15 mg/kg tid, 7 days | Tinidazole, 50 mg/kg single dose | 1–2 wks |
| Gascon J, 1989 [33] | Spain | 23 | outpatient | Stool exam | >18 yrs | NA | 73.9 | Metronidazole, 250 mg tid, 7 days | Mebendazole, 200 mg tid, 1 day | Day 3, 7 & 30 |
| Gazder AJ, 1978 [34] | India | 100 | hospitalized | Stool exam-hanging drop preparation as well as formalin-ether concentration technique | <18 yrs | 5.5 | 65.0 | Metronidazole, 50 mg/kg single dose | Tinidazole, 50 mg/kg single dose | Day 4, 8, 12 & 16 |
| Hall A, 1993 [35] | Bangladesh | 502 | outpatient | Microscopic exam of stool-direct smear in saline and also fixed in 10% v/v formalin-saline and processed by ether sedimentation technique | <18 yrs | 7.1 | 52.5 | Metronidazole, 125 mg tid, 5 days | Albendazole, 400 mg once daily, 3 days; 2) Albendazole, 600 mg single dose | 10 days |
| Hall A, 1993 [35] | Bangladesh | 351 | outpatient | Microscopic exam of stool-direct smear in saline and also fixed in 10% v/v formalin-saline and processed by ether sedimentation technique | <18 yrs | 7.1 | 52.5 | Metronidazole, 125 mg tid, 5 days | Albendazole, 400 mg once daily, 5 days; 2) Albendazole, 800 mg single dose | 10 days |
| Karabay O, 2004 [36] | Turkey | 67 | outpatient | Stool exam for cysts/trophozoites | >18 yrs | 39.5 (13.0) | 42.1 | Metronidazole, 500 mg tid, 5 days | Albendazole, 400 mg/day, 5 days | Day 7–15 |
| Kavousi S, 1979 [37] | Iran | 160 | outpatient | Stool exam - direct smear and zinc sulfate concentration techniques | <18 yrs | 5.4 | 53.8 | Metronidazole, <2 yrs: 125 mg qd; 2–4 yrs: 125 mg bd, 5 days; 5–8 yrs: 125 mg tid, 5 days 9–13 yrs:250 mg tid, 5 days | Quinacrine, 8 mg/kg/day, 5 days | Day 5, 30 & 180 |
| Leite EV, 1976 [38] | Brazil | 30 | hospitalized | Stool exam; centrifugation/fluctuation in zinc sulphate; centrifugation/sedimentation in formol-ether | Both | 19 (1–63)* | 66.6 | Metronidazole, 1–3 yrs: 250 mg qd; 4–7 yrs: 250 mg bid; 8–12 yrs: 250 mg tid; Adults: 500 mg bid | Ornidazole, 1–3 yrs: 250 mg qd; 4–7 yrs: 250 mg bid; 8–12 yrs: 250 mg tid; Adults: 500 mg bid | 10 days |
| Misra PK, 1995 [39] | India | 64 | hospitalized | Trophozoites and/or cysts in stool specimens | <18 yrs | NA | 59.4 | Metronidazole, 7.5 mg/kg/dose tid, 5 days | Albendazole, 400 mg once daily, 5 days | Day 1–7, 14 & 21 |
| Ortiz JJ, 2001 [40] | Peru | 110 | outpatient | Cysts in stool | <18 yrs | 5.7 (2.6) | 49.1 | Metronidazole, 125/250 mg bd, 5 days | Nitazoxanide, 100/200 mg bd, 3 days | 7–10 days |
| Pengsaa K, 1999 [41] | Thailand | 113 | outpatient | Stool exam: direct smear in 0.9% saline and ether sedimentation method | <18 yrs | NA | 52.9 | Tinidazole, 50 mg/kg single dose | Albendazole, 400 mg once daily, 3 days | 1–2 wks |
| Pine MC, 1999 [42] | Peru | 79 | outpatient | Parasitological examination | <18 yrs | 7.8 (2.7) | NA | Metronidazole, 15 mg/kg/day tid, 10 days | 1) Albendazole, 400 mg once daily, 5 days; 2) Furazolidone, 5 mg/kg/day qd, 10 days; 3) Tinidazole, 50 mg/kg/day single dose; 4) Secnidazole, 30 mg/kg/day, single dose | Day 7, 14 & 21 |
| Quiros-Buelna E, 1989 [43] | Mexico | 100 | outpatient | Stool examination | <18 yrs | NA | NA | Metronidazole, <10 kg: 187.5 mg/day; 10–19.9 kg: 375 mg/day; 20–29.9 kg: 562.5 mg/day; ≥30 kg: 750 mg/day | Furazolidone, <10 kg: 66.6 mg/day; 10–19.9 kg: 133.2 mg/day; 20–29.9 kg: 199.8 mg/day ≥30 kg: 266.4 mg/day | Day 1–3 |
| Romero-Cabello R, 1995 [44] | Mexico | 100 | outpatient | Stool exam, flotation/concentration method | <18 yrs | 8 (4–11)# | 49.0 | Metronidazole, 7.5 mg/kg/dose tid, 5 days | Albendazole, 400 mg qd, 5 days | Day 1–7, 14 & 21 |
| Sadjjadi SM, 2001 [45] | Iran | 100 | outpatient | Microscopic exam for ova/parasites by formalin-ether concentration technique | <18 yrs | NA | 70.0 | Metronidazole, 5 mg/kg/dose tid, 5 days | Mebendazole, 200 mg tid, 3 days | Day 7 & 14 |
| Yereli K, 2004 [46] | Turkey | 107 | hospitalized | Saline-Lugol, formalin ethyl acetate concentration and trichrome staining | <18 yrs | 8.3 (3.4) | 47.7 | Metronidazole, 6.7 mg/kg/dose tid, 7 days | Albendazole, 10 mg daily, 5 days | Day 7, 14 & 21 |
| Canete R, 2010 [47] | Cuba | 122 | outpatient | Microscopic exam of fecal samples, as direct wet mounts and/or after Ritchie concentration | <18 yrs | NA | NA | Metronidazole, 5 mg/kg/dose tid, 5 days | Chloroquine, 10 mg/kg bd, 5 days | Day 3, 5 and 10 |
| Teles NSB, 2011 [48] | Brazil | 100 | outpatient | Hoffman and Ritchie sedimentation method | Both | 18.7 (13.4) | 54.0 | Secnidazole, Adults: 2000 mg single dose; Children: 30 mg/kg | Mentha crispa, 2000 mg single dose | 7 days |
| Almirall P, 2011 [49] | Cuba | 126 | outpatient | Examination of fecal samples as direct wet mounts and/or after Ritchie concentration | >17 yrs | 35.0 (13.5) | 69.1 | Secnidazole, 2000 mg single dose | Mebendazole, 200 mg tid, 3 days | Day 3, 5 & 10 |
| Canete R, 2012 [50] | Cuba | 150 | outpatient | microscopic exam of fecal samples, as direct wet mounts and/or after Ritchie concentration | >18 yrs | 29.5 | 48.7 | Metronidazole, 250 mg tid, 5 days | Albendazole, 400 mg once daily, 5 days | Day 3, 5 & 7 |
NA = not available; * = Mean (range); # = median (range); € = children got half the adult dose.
Study quality assessment and publication bias
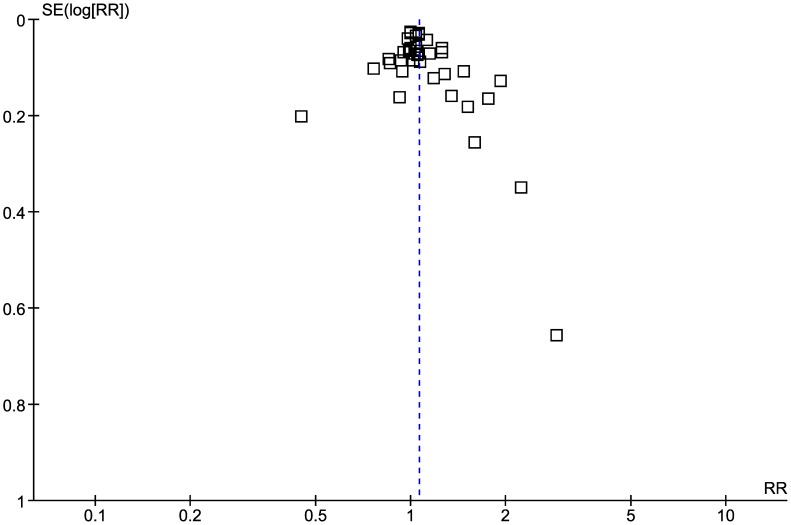
Using the Jadad scale, 13 trials were identified as high quality (Table S1, available as supporting data). All studies were described as randomized, 13 of them appropriately described the generation of the sequence of randomization, but none of studies were appropriately blinded. Twenty-nine studies appropriately described withdrawal and dropouts. Publication bias assessed by funnel plot showed some asymmetry around the point estimate, especially for small sample size studies indicating presence of bias (Figure 2). The Egger test did not suggest asymmetry of the funnel plot (p = 0.1).
Figure 2. Funnel plot assessing publication bias.
Efficacy of 5-NIs in the treatment of giardiasis and meta-analyses of subgroups of studies
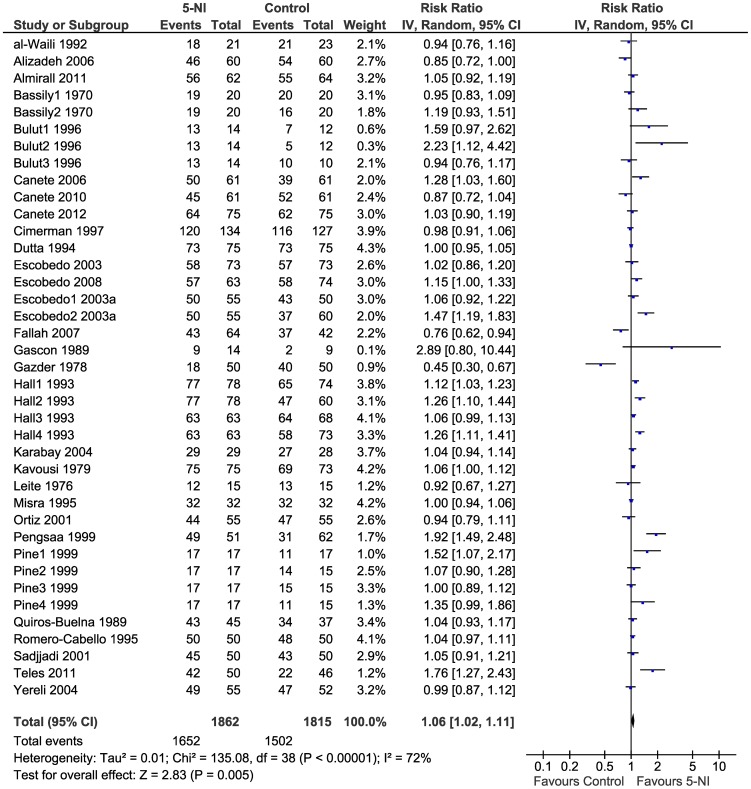
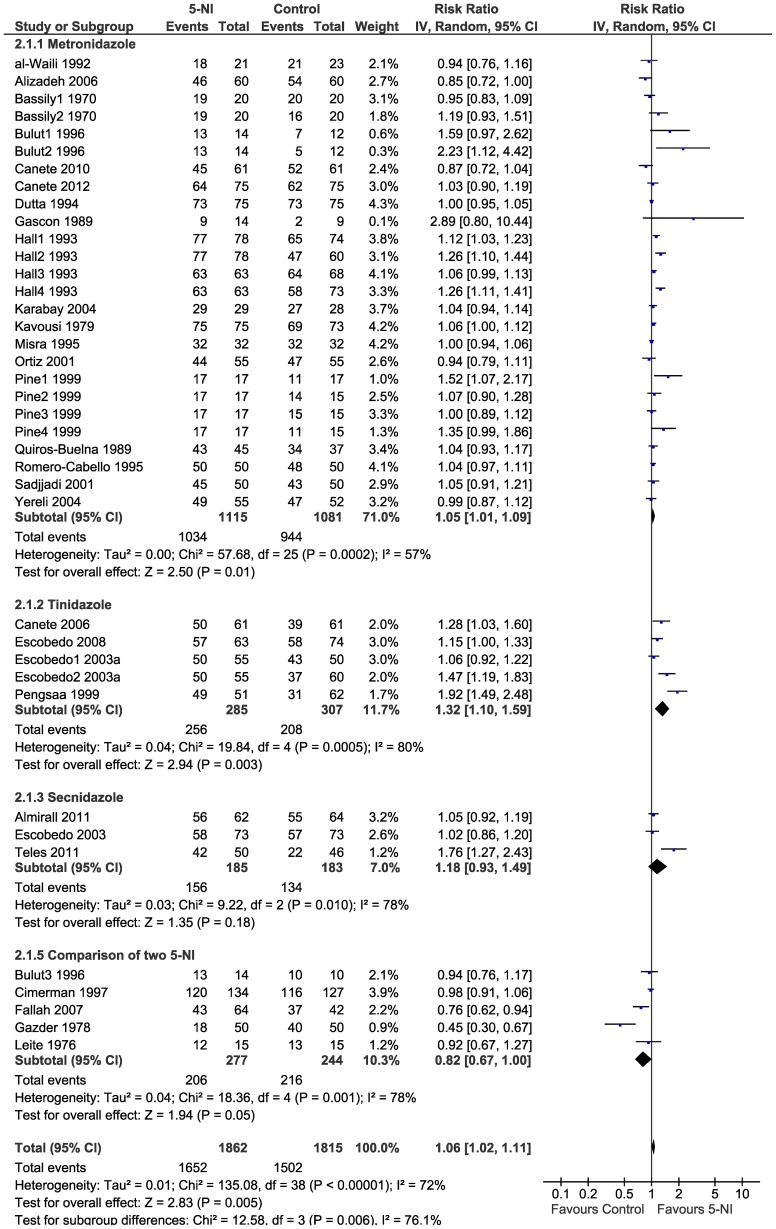
We found a significant and slightly higher cure rates (RR 1.06, 95%CI 1.02–1.11, p = 0.005) (Figure 3). There was high heterogeneity among studies (I2 = 72%). When stratified by type of drug, efficacy of 5-NIs did not vary significantly and high heterogeneity persisted (I2 = 57%–80%): metronidazole (RR 1.05, 95%CI 1.01–1.09, p = 0.01); tinidazole (RR 1.32 95%CI 1.10–1.59 p = 0.003); and secnidazole (RR 1.18 95%CI 0.93–1.49 p = 0.18) (Figure 4). There was no study comparing the drug ornidazole to a control group.
Figure 3. Forest plot showing efficacy of 5-NIs in the treatment of giardiasis.
Figure 4. Forest plot showing efficacy of 5-NIs in the treatment of giardiasis; stratified by type of drug.
On excluding studies comparing two 5-NIs the pooled risk ratio did not vary significantly (RR 1.08, 95%CI 1.04–1.13, p<0.0001) (Figure S1). On subgroup analysis based on study setting, 5-NI efficacy in outpatients was RR 1.10 95%CI 1.05–1.15 p<0.0001 and in hospitalized patients was RR 0.94 95%CI 0.87–1.03 p = 0.18 (Figure S2). When only high quality studies (Jadad score ≥3) were pooled, 5-NI efficacy was RR 1.07 95%CI 1.02–1.13 p = 0.01 (Figure S3). When trials with ITT analysis were pooled RR was 1.01, 95%CI 0.96–1.06 p = 0.62 and trials with PP analysis were pooled RR was 1.15, 95%CI 1.07–1.23 p = 0.0001 (Figure S4). On pooling data from only large sample size studies (n >100), 5-NI efficacy was RR 1.06, 95%CI 1.01–1.12, p = 0.02 (Figure S5). When all trials were pooled in the order of year of publication no significant shift was observed in the trend of 5-NI efficacy in treatment of giardiasis over the years (Figure S6).
Harmful outcomes associated with the use of 5-NI
Abdominal pain, bitter or metallic taste and headache were uncommon harmful outcomes. The use of 5-NI was associated with a lower risk of abdominal pain (RR 0.72, 95%CI 0.57–0.91; p = 0.007, I2 = 51%; Figure S7), higher risk of bitter or metallic taste (RR 3.27, 95%CI 2.66–4.01; p<0.00001, I2 = 100%; Figure S8), and higher risk of headache (RR 1.97, 95%CI 1.37–2.83; p = 0.0003, I2 = 46%; Figure S9).
Discussion
We found that RCTs evaluating the efficacy of 5-NIs for giardiasis treatment are highly heterogeneous in terms of study design and outcomes. Heterogeneity could not be diminished after performing several pre-specified subgroup analyses. The quality of RCTs is mostly low, especially because of lack of double blinding. 5-NIs are associated with a slightly higher giardiasis cure rates than controls; also, 5-NIs are associated with lower risk of abdominal pain, and higher risks of bitter or metallic taste and headache than controls.
Since the first publication of metronidazole in the treatment of giardiasis by Schneider et al [15] more than 50 years ago, 5-NI compounds (mainly metronidazole and tinidazole) have become an important component of the antigiardial armamentarium in many parts of the world, owing to their efficacy, relative safety, universal availability, and cost-effectiveness. However, during these last five decades, 5-NI compounds, principally metronidazole, have also been usually prescribed for several other indications, including gingivitis, bacterial vaginitis, part of the combination treatment for H. pylori, infections with Clostridium difficile and other anaerobic bacteria, amebiasis, and as prophylaxis in colorectal surgery [16], [17].This wide spectrum usage of 5-NIs could have led to an increased occurrence of G. lamblia resistance; in fact, Giardia resistance towards common antigiardials has been demonstrated or induced in vitro [18] and also cross-resistance between metronidazole and tinidazole has been demonstrated [19]. Most of the therapeutically used antigiardial drugs, including metronidazole cause severe side effects and are not well tolerated by many patients and clinical resistance to medication has been observed for all common drugs in up to 20% of giardiasis cases. Treatment failure may be due to both host factors (e.g. low patient compliance due to side effects) and parasite resistance [5], [20]. The present study systematically reviewed all available data from trials examining parasitological outcomes, and comparing efficacy of different 5-NI drugs, doses and regimens. The results obtained, suggest that 5-NI continue to be efficacious for giardiasis. However, it should be stated that we did not find any trial comparing ornidazole to a control group, and so we cannot make recommendations for this drug.
Three previous systematic reviews on the treatment of giardiasis have been published (Table S2). In a systematic review by Zaat and colleagues [10], 31 RCTs published up to 1997 were included (n = 2,988). Three databases were searched, pseudo-randomized trials were included and no language restriction was used. Any trial of treatment of giardiasis comparing drugs or treatment regimens with placebo or other drugs/regimens were included. Metronidazole was found to be equally effective in parasitological cure as other longer therapies such as furazolidone. Tinidazole seems to be the most effective single-dose therapy in terms of parasitological cure compared to other short therapies, having, at the same time, relatively fewer harmful effects such as diarrhea at the end of follow-up. Trials were also found to be clinically and statistically heterogenous. Majority of the included studies were low on methodological quality as per Cochrane Collaboration guidelines. A recent meta-analysis by Solaymani-Mohammadi et al [11] included 8 RCTs (n = 900) published up to 2010 comparing effectiveness and safety of metronidazole vs. albendazole for the treatment of giardiasis. Six databases were searched, and no language restriction was applied. Effectiveness of albendazole was found to be comparable to metronidazole. Patients treated with albendazole also tended to have fewer side effects such as metallic taste and anorexia. Included trials were found to have moderate heterogeneity of effects and the quality was low in 7 of them (Jadad score 0–2).
A recent systematic review by Granados et al [9] included 19 studies (n = 1,817) published up to 2012 and evaluated the relative effectiveness of alternative antibiotic regimens for treating adults or children with symptomatic giardiasis. Six databases were searched, and no language restriction was used. All RCTs comparing metronidazole administered for five to 10 days with any of the following drugs: metronidazole (single dose), tinidazole, albendazole, mebendazole, and nitazoxanide were included. The primary outcomes were parasitological and clinical cure. They concluded that albendazole may be of similar effectiveness to metronidazole, may have fewer gastrointestinal and neurological side effects, and has the advantage of a simplified regimen; evidence was considered to be of moderate quality based on GRADE methodology. Included trials had moderate heterogeneity of effects.
Our study is the first to examine the efficacy of 5-NIs as a group in comparison to other antigiardial drugs in the treatment of giardiasis. Search criteria in our review were not restricted by language thereby avoiding ‘tower of babel bias’ [21]. Several RCTs included in our meta-analysis were deficient in quality (i.e. high risk of bias) in included trials. Given that most of studies did not appropriately use blinding, the probability of information bias and inflation of efficacy of 5-NIs is present. Also, since heterogeneity among studies was expected, we had pre-specified an extensive list of specific study variables for subgroup analyses (Figure S1–S5). On subgroup analysis both low quality studies and high quality studies (by Jadad scoring) showed higher efficacy of 5-NIs vs. controls, while only results from high quality studies achieved significance. Effect sizes remained fairly constant on the rest of subgroup analyses suggesting that heterogeneity of included trials might not have adversely affected our results. In our meta-analysis 28 trials included pediatric population and 6 trials included adult population. Including subjects of different age groups as well as with varied clinical manifestations and a variety of clinical settings allows us to generalize our findings of effectiveness of 5-NI treatment to all age groups and all kinds of symptomatic giardiasis. 5-NIs were associated with higher risk of bitter or metallic taste and headache. Though, these would be considered as minor adverse effects, drug tolerability and adverse events could potentially impact patient compliance. Our findings suggest that 5-NIs have high cure rates and reasonable safety profiles and in absence of better alternative drugs remain the drug the choice in the treatment of giardiasis.
Our study has specific limitations that need to be considered in the interpretation of our findings. There was heterogeneity in some of the relevant study design aspects (time of follow-up, different doses of the drugs, heterogeneity of participants, number of parasitological exams at follow-up, and laboratory techniques across the studies) which made some results difficult to interpret and precluded us from a more confident and robust conclusions about benefits and harms associated with the use of 5-NIs in the treatment of giardiasis. The Egger's test did not suggest asymmetry of the forest plot, but some degree of publication bias can be expected. Results should also be interpreted with caution in light of high proportion of low quality RCTs. Also, we used one of the several tools to evaluate risk of bias, the Jadad score. Unfortunately, this tool does not evaluate other potential and important sources of bias such as concealment of the randomized allocation and selective reporting of outcomes. Despite these limitations, this present review represents the most up-to-date, comprehensive, and systematic attempt to assess the efficacies of 5-NI drugs as in group in the treatment of giardiasis.
Studies investigating the efficacy of 5-NIs in the treatment of giardiasis are highly heterogenous. Though information available from RCTs on the use of 5-NI allow us to confirm that these drugs are still a good option for the treatment of giardiasis, there is a need for well designed, high quality RCTs to further assess safety and efficacy profiles of 5-NIs.
Supporting Information
Forest plot showing efficacy of 5-NIs in the treatment of giardiasis; excluding studies comparing two 5-NI.
(TIFF)
Forest plot showing efficacy of 5-NIs in the treatment of giardiasis; stratified by type of patient (outpatient vs hospitalized).
(TIF)
Forest plot showing efficacy of 5-NIs in the treatment of giardiasis; stratified by Jadad score (≥3 vs <3).
(TIF)
Forest plot showing efficacy of 5-NIs in the treatment of giardiasis; stratified by type of main analysis (ITT vs PP).
(TIF)
Forest plot showing efficacy of 5-NIs in the treatment of giardiasis; stratified by sample size (<100 vs ≥100 patients).
(TIF)
Forest plot showing efficacy of 5-NIs in the treatment of giardiasis; ordered by year of publication.
(TIF)
Forest plot showing abdominal pain associated with 5-NI in the treatment of giardiasis.
(TIF)
Forest plot showing bitter or metallic taste associated with 5-NI in the treatment of giardiasis.
(TIF)
Forest plot showing headache associated with 5-NI in the treatment of giardiasis.
(TIF)
Jadad scoring of included studies.
(DOC)
Description of systematic reviews on drugs for treating giardiasis.
(DOCX)
PRISMA guidelines checklist.
(DOC)
Funding Statement
The authors received no specific funding for this study.
References
- 1. Upcroft P, Upcroft JA (2001) Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev 14: 150–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ekdahl K, Andersson Y (2005) Imported giardiasis: impact of international travel, immigration, and adoption. Am J Trop Med Hyg 72: 825–830. [PubMed] [Google Scholar]
- 3. Yoder JS, Gargano JW, Wallace RM, Beach MJ (2012) Giardiasis surveillance–United States, 2009-2010. MMWR Surveill Summ 61: 13–23. [PubMed] [Google Scholar]
- 4. Savioli L, Smith H, Thompson A (2006) Giardia and Cryptosporidium join the 'Neglected Diseases Initiative'. Trends Parasitol 22: 203–208. [DOI] [PubMed] [Google Scholar]
- 5. Gardner TB, Hill DR (2001) Treatment of giardiasis. Clin Microbiol Rev 14: 114–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Munoz Gutierrez J, Aldasoro E, Requena A, Comin AM, Pinazo MJ, et al. (2013) Refractory giardiasis in Spanish travellers. Travel Med Infect Dis 11: 126–129. [DOI] [PubMed] [Google Scholar]
- 7. Nash TE, Ohl CA, Thomas E, Subramanian G, Keiser P, et al. (2001) Treatment of patients with refractory giardiasis. Clin Infect Dis 33: 22–28. [DOI] [PubMed] [Google Scholar]
- 8. Lopez-Velez R, Batlle C, Jimenez C, Navarro M, Norman F, et al. (2010) Short course combination therapy for giardiasis after nitroimidazole failure. Am J Trop Med Hyg 83: 171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Granados CE, Reveiz L, Uribe LG, Criollo CP (2012) Drugs for treating giardiasis. Cochrane Database Syst Rev 12: CD007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zaat JO, Mank TG, Assendelft WJ (1997) A systematic review on the treatment of giardiasis. Trop Med Int Health 2: 63–82. [DOI] [PubMed] [Google Scholar]
- 11. Solaymani-Mohammadi S, Genkinger JM, Loffredo CA, Singer SM (2010) A meta-analysis of the effectiveness of albendazole compared with metronidazole as treatments for infections with Giardia duodenalis. PLoS Negl Trop Dis 4: e682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, et al. (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 15. Schneider J (1961) [Treatment of giardiasis (lambliasis) by metronidazole]. Bull Soc Pathol Exot Filiales 54: 84–95. [PubMed] [Google Scholar]
- 16. Samuelson J (1999) Why metronidazole is active against both bacteria and parasites. Antimicrob Agents Chemother 43: 1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lofmark S, Edlund C, Nord CE (2010) Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis 50 Suppl 1S16–23. [DOI] [PubMed] [Google Scholar]
- 18. Arguello-Garcia R, Cruz-Soto M, Romero-Montoya L, Ortega-Pierres G (2009) In vitro resistance to 5-nitroimidazoles and benzimidazoles in Giardia duodenalis: variability and variation in gene expression. Infect Genet Evol 9: 1057–1064. [DOI] [PubMed] [Google Scholar]
- 19. Upcroft JA, Dunn LA, Wright JM, Benakli K, Upcroft P, et al. (2006) 5-Nitroimidazole drugs effective against metronidazole-resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrob Agents Chemother 50: 344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lalle M (2010) Giardiasis in the post genomic era: treatment, drug resistance and novel therapeutic perspectives. Infect Disord Drug Targets 10: 283–294. [DOI] [PubMed] [Google Scholar]
- 21. Gregoire G, Derderian F, Le Lorier J (1995) Selecting the language of the publications included in a meta-analysis: is there a Tower of Babel bias? J Clin Epidemiol 48: 159–163. [DOI] [PubMed] [Google Scholar]
- 22. al-Waili NS, Hasan NU (1992) Mebendazole in giardial infection: a comparative study with metronidazole. J Infect Dis 165: 1170–1171. [DOI] [PubMed] [Google Scholar]
- 23. Alizadeh A, Ranjbar M, Kashani KM, Taheri MM, Bodaghi M (2006) Albendazole versus metronidazole in the treatment of patients with giardiasis in the Islamic Republic of Iran. East Mediterr Health J 12: 548–554. [PubMed] [Google Scholar]
- 24. Bassily S, Farid Z, Mikhail JW, Kent DC, Lehman JS Jr (1970) The treatment of Giardia lamblia infection with mepacrine, metronidazole and furazolidone. J Trop Med Hyg 73: 15–18. [PubMed] [Google Scholar]
- 25. Bulut BU, Gulnar SB, Aysev D (1996) Alternative treatment protocols in giardiasis: a pilot study. Scand J Infect Dis 28: 493–495. [DOI] [PubMed] [Google Scholar]
- 26. Canete R, Escobedo AA, Gonzalez ME, Almirall P, Cantelar N (2006) A randomized, controlled, open-label trial of a single day of mebendazole versus a single dose of tinidazole in the treatment of giardiasis in children. Curr Med Res Opin 22: 2131–2136. [DOI] [PubMed] [Google Scholar]
- 27. Cimerman B, Camilo Coura L, JM CS, Gurvitz R, Rocha RS, et al. (1997) Evaluation of Secnidazole Gel and Tinidazole Suspension in the Treatment of Giardiasis in Children. Braz J Infect Dis 1: 241–247. [PubMed] [Google Scholar]
- 28. Dutta AK, Phadke MA, Bagade AC, Joshi V, Gazder A, et al. (1994) A randomised multicentre study to compare the safety and efficacy of albendazole and metronidazole in the treatment of giardiasis in children. Indian J Pediatr 61: 689–693. [DOI] [PubMed] [Google Scholar]
- 29. Escobedo AA, Canete R, Gonzalez ME, Pareja A, Cimerman S, et al. (2003) A randomized trial comparing mebendazole and secnidazole for the treatment of giardiasis. Ann Trop Med Parasitol 97: 499–504. [DOI] [PubMed] [Google Scholar]
- 30. Escobedo AA, Nunez FA, Moreira I, Vega E, Pareja A, et al. (2003) Comparison of chloroquine, albendazole and tinidazole in the treatment of children with giardiasis. Ann Trop Med Parasitol 97: 367–371. [DOI] [PubMed] [Google Scholar]
- 31. Escobedo AA, Alvarez G, Gonzalez ME, Almirall P, Canete R, et al. (2008) The treatment of giardiasis in children: single-dose tinidazole compared with 3 days of nitazoxanide. Ann Trop Med Parasitol 102: 199–207. [DOI] [PubMed] [Google Scholar]
- 32. Fallah M, Rabiee S, Moshtaghu AA (2007) Comparison between efficacy of a single dose of tinidazole with a 7-day standard dose course of metronidazole in giardiasis. Pakistan Journal of Medical Sciences 23: 43–46. [Google Scholar]
- 33. Gascon J, Moreno A, Valls ME, Miro JM, Corachan M (1989) Failure of mebendazole treatment in Giardia lamblia infection. Trans R Soc Trop Med Hyg 83: 647. [DOI] [PubMed] [Google Scholar]
- 34. Gazder AJ, Banerjee M (1978) Single dose therapy of giardiasis with tinidazole and metronidazole. Drugs 15 Suppl 130–32. [DOI] [PubMed] [Google Scholar]
- 35. Hall A, Nahar Q (1993) Albendazole as a treatment for infections with Giardia duodenalis in children in Bangladesh. Trans R Soc Trop Med Hyg 87: 84–86. [DOI] [PubMed] [Google Scholar]
- 36. Karabay O, Tamer A, Gunduz H, Kayas D, Arinc H, et al. (2004) Albendazole versus metronidazole treatment of adult giardiasis: An open randomized clinical study. World J Gastroenterol 10: 1215–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kavousi S (1979) Giardiasis in infancy and childhood: a prospective study of 160 cases with comparison of quinacrine (Atabrine) and metronidazole (Flagyl). Am J Trop Med Hyg 28: 19–23. [DOI] [PubMed] [Google Scholar]
- 38. Leite EV, Goncalves AL, da Costa DG, da Costa Filho RL, Podkameni N (1976) [Comparison between ornidazole and metronidazole: double blind clinical therapeutical trial in intestinal giardiasis]. Rev Inst Med Trop Sao Paulo 18: 28–35. [PubMed] [Google Scholar]
- 39. Misra PK, Kumar A, Agarwal V, Jagota SC (1995) A comparative clinical trial of albendazole versus metronidazole in children with giardiasis. Indian Pediatr 32: 779–782. [PubMed] [Google Scholar]
- 40. Ortiz JJ, Ayoub A, Gargala G, Chegne NL, Favennec L (2001) Randomized clinical study of nitazoxanide compared to metronidazole in the treatment of symptomatic giardiasis in children from Northern Peru. Aliment Pharmacol Ther 15: 1409–1415. [DOI] [PubMed] [Google Scholar]
- 41. Pengsaa K, Sirivichayakul C, Pojjaroen-anant C, Nimnual S, Wisetsing P (1999) Albendazole treatment for Giardia intestinalis infections in school children. Southeast Asian J Trop Med Public Health 30: 78–83. [PubMed] [Google Scholar]
- 42. Pine MC CL, Troyes LR (1999) Comparison of Albendazole with nitrofurans and nitroimidazoles in the treatment of giardiasis in children. Peru Journal of Gastroenterology 19: 95–108. [PubMed] [Google Scholar]
- 43.Quiros-Buelna E (1989) Furazolidone and metronidazole for treatment of giardiasis in children. Scand J Gastroenterol Suppl 169: 65–69. [DOI] [PubMed]
- 44. Romero-Cabello R, Robert L, Munoz-Garcia R, Tanaka J (1995) [Randomized study comparing the safety and efficacy of albendazole and metronidazole in the treatment of giardiasis in children]. Rev Latinoam Microbiol 37: 315–323. [PubMed] [Google Scholar]
- 45. Sadjjadi SM, Alborzi AW, Mostovfi H (2001) Comparative clinical trial of mebendazole and metronidazole in giardiasis of children. J Trop Pediatr 47: 176–178. [DOI] [PubMed] [Google Scholar]
- 46. Yereli K, Balcioglu IC, Ertan P, Limoncu E, Onag A (2004) Albendazole as an alternative therapeutic agent for childhood giardiasis in Turkey. Clin Microbiol Infect 10: 527–529. [DOI] [PubMed] [Google Scholar]
- 47. Canete R, Rivas DE, Escobedo AA, Gonzalez ME, Almirall P, et al. (2010) A randomized, controlled, open-label trial evaluating the efficacy and safety of chloroquine in the treatment of giardiasis in children. West Indian Med J 59: 607–611. [PubMed] [Google Scholar]
- 48. Teles NS, Fechine FV, Viana FA, Viana IO, Nascimento DF, et al. (2011) Evaluation of the therapeutic efficacy of Mentha crispa in the treatment of giardiasis. Contemp Clin Trials 32: 809–813. [DOI] [PubMed] [Google Scholar]
- 49. Almirall P, Escobedo AA, Ayala I, Alfonso M, Salazar Y, et al. (2011) Mebendazole compared with secnidazole in the treatment of adult giardiasis: a randomised, no-inferiority, open clinical trial. J Parasitol Res 2011: 636857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Canete R, Rodriguez P, Mesa L, Brito K, Prior A, et al. (2012) Albendazole versus metronidazole in the treatment of adult giardiasis: a randomized, double-blind, clinical trial. Curr Med Res Opin 28: 149–154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plot showing efficacy of 5-NIs in the treatment of giardiasis; excluding studies comparing two 5-NI.
(TIFF)
Forest plot showing efficacy of 5-NIs in the treatment of giardiasis; stratified by type of patient (outpatient vs hospitalized).
(TIF)
Forest plot showing efficacy of 5-NIs in the treatment of giardiasis; stratified by Jadad score (≥3 vs <3).
(TIF)
Forest plot showing efficacy of 5-NIs in the treatment of giardiasis; stratified by type of main analysis (ITT vs PP).
(TIF)
Forest plot showing efficacy of 5-NIs in the treatment of giardiasis; stratified by sample size (<100 vs ≥100 patients).
(TIF)
Forest plot showing efficacy of 5-NIs in the treatment of giardiasis; ordered by year of publication.
(TIF)
Forest plot showing abdominal pain associated with 5-NI in the treatment of giardiasis.
(TIF)
Forest plot showing bitter or metallic taste associated with 5-NI in the treatment of giardiasis.
(TIF)
Forest plot showing headache associated with 5-NI in the treatment of giardiasis.
(TIF)
Jadad scoring of included studies.
(DOC)
Description of systematic reviews on drugs for treating giardiasis.
(DOCX)
PRISMA guidelines checklist.
(DOC)