Abstract
Purpose
We sought to reduce the risk of infectious complications and non-relapse mortality (NRM) associated with the use of antithymocyte globulin (ATG) without compromising control of acute graft-versus-host disease (GVHD) in patients undergoing reduced intensity conditioning (RIC) transplantation.
Methods
As part of an ongoing quality improvement effort, we lowered the dose of rabbit ATG from 7.5 mg/kg of ATG (R-ATG) (n=39) to 6.0 mg/kg of ATG (r-ATG) (n=33) in association with fludarabine and busulfan RIC transplantation and then monitored patients for adverse events, relapse, and survival.
Results
Of the 72 mostly high risk (82%) patients studied, 89% received unrelated donor allografts, 25% of which were HLA-mismatched. No differences in post-transplantation full donor-cell chimerism rates were observed between the two ATG-dose groups (p>0.05). When R-ATG vs. r-ATG patients were compared, we observed no significant difference in the cumulative incidence of grade II–IV acute GVHD (32% vs. 27%; p-=0.73) or grade III–IV acute GVHD (23% vs. 11%; p=0.28). However, the r-ATG group had significantly less CMV reactivation (64% vs. 30%; p=0.005) and bacterial infections (56% vs. 18%; p=0.001), a better 1-year cumulative incidence of NRM (18% vs. 3%; p=0.03) and a trend for better 1-year overall survival (64% vs. 84%; p=0.07) compared to R-ATG patients.
Conclusions
A seemingly modest reduction in the dose of rabbit ATG did not compromise control of acute GVHD or achievement of donor chimerism but led to a significant decrease in the risk of serious infections and NRM in high risk RIC allograft recipients.
Keywords: Fludarabine, busulfan, thymoglobulin, antithymocyte globulin, allogeneic stem cell transplantation, graft-versus-host disease
Introduction
Graft-versus-host disease (GVHD) remains one of the main factors limiting the wider applicability of allogeneic hematopoietic stem cell transplantation (HSCT) [1–3]. A variety of strategies have been developed to prevent severe GVHD following either myeloablative or reduced-intensity conditioning (RIC) transplantation, including ex-vivo T cell depletion [4,5], in-vivo T cell depletion with ATG [1,6–10], calcineurin inhibitors [11], and monoclonal antibodies [12–16], but no consensus has been reached on the superiority of one modality over another.
In-vivo T cell depletion by ATG administration is performed by using one of the three commercially available ATG products (Thymoglobulin®, ATGAM® and ATG-Fresenius®) [17]. Despite reducing the risk for GVHD, preliminary data suggest that higher ATG doses seem to be associated with increased risk of disease relapse, infectious complications and non-relapse mortality (NRM) [6,10]. However, only few studies have tried to evaluate the optimal dose-intensity of ATG following myeloablative [17–19], or RIC HSCT [20], and the results of these trial have mostly been inconclusive due to the heterogeneity of the utilized conditioning regimens and ATG administration schedules and the relatively few patients analyzed.
We report here the results of quality improvement effort designed to compare outcomes associated with two moderate doses of rabbit ATG in patients undergoing HSCT following uniform RIC with fludarabine and busulfan. Our data suggest that a seemingly ‘modest’ reduction in rabbit ATG dose from 7.5 mg/kg to 6 mg/kg leads to a marked reduction in the rates of infectious complications, and provides a significant NRM benefit without compromising GVHD control, engraftment kinetics, and post-transplantation survival.
Patients and Methods
Patient population
Seventy-two consecutive patients with hematological malignancies undergoing allogeneic HSCT following uniform RIC with fludarabine, busulfan and rabbit ATG, between January 2006 and December 2008, were included. All patients had adverse-risk (precluding the use of myeloablative conditioning) that was defined by the presence of at least one of the following features: (i) age >55-years; (ii) Karnofsky performance score (KPS) ≤ 70; (iii) hematopoietic cell transplantation-comorbidity index >2) (28); (iv) baseline diagnosis of Hodgkin’s disease, or chronic lymphocytic leukemia (CLL); and (v) prior history of autologous transplantation. This retrospective analysis was approved by the OSU Institutional Review Board and Clinical Scientific Review Committee.
Quality Improvement Program
As part of our continuous quality improvement program, we audit the rates of serious bacterial, fungal, and viral infections as well as rates of NRM on a quarterly and semi-annual basis, respectively. We seek to maintain rates of serious infections (defined as cytomegalovirus (CMV)/Epstein-Barr virus (EBV) reactivation requiring pre-emptive therapy, bacteremias, or invasive fungal/viral/protozoal infections) below 50% and NRM below 15% at one year in recipients of RIC transplantation. Our standard of care (SOC) protocol for recipients of RIC transplantation and unrelated donor or mismatched allografts incorporated a total dose of ATG of 7.5mg/kg (R-ATG) given over three days. When our quality review committee observed that these patients had rates of serious infections above 50% and NRM approaching or greater than 15%, we made a programmatic decision to reduce the total dose of rabbit ATG to 6.0mg/kg and continued to monitor clinical events in these patients.
Conditioning regimen, GVHD prophylaxis, and supportive care
The conditioning regimen consisted of fludarabine 30mg/m2 intravenously on days −7 to −3 (total dose; 150mg/m2) and intravenous busulfan 0.8 mg/kg/dose x 8 doses, on days −4 to −3 (total dose; 6.4mg/kg) [21]. The first cohort of 39 consecutive patients received rabbit ATG (Thymoglobulin®, Genzyme; Cambridge, MA) (R-ATG group) at 2.5 mg/kg/day, on days −4 to −2 (total dose; 7.5mg/kg). The second cohort of 33 patients (r-ATG) were given ATG at 2.0mg/kg/day on the same schedule (total dose; 6.0mg/kg)
All patients received standard prophylaxis of GVHD with tacrolimus (0.03 mg/kg/day IV, commencing on day −2) and mini-dose methotrexate (5 mg/m2 on days +1, +3, +6 and +11) as previously described [22]. Blood levels of tacrolimus were monitored weekly till day +90 to maintain levels between 5–15 ng/ml. From day +90 onwards tacrolimus was tapered at the discretion of the treating physician if no GVHD appeared.
All patients were treated in HEPA-filtered rooms, and received fungal (fluconazole, or posaconazole), herpes zoster/herpes simplex (intravenous acyclovir or oral valacyclovir), bacterial and Pneumocystis jiroveci prophylaxis (trimethoprim/sulfamethoxazole or dapsone). Weekly monitoring for CMV and EBV reactivation by quantitative RT-PCR was conducted. Preemptive ganciclovir or valganciclovir were administered to patients with CMV reactivation (defined as ≥4000copies/ml, reconfirmed within 24 hours from initial detection); preemptive single intravenous dose of rituximab (375mg/m2) was administered to all patients with evidence of EBV reactivation (defined as ≥4000copies/ml, reconfirmed within 24 hours from initial detection). EBV PCR was rechecked 1 week after rituximab administration. For patients with negative EBV PCR at this stage, no further rituximab doses were given, while patients with increasing or persistently positive EBV PCR received three additional weekly rituximab doses. Urine and/or serum BK-virus PCR was obtained in all suspected cases of hemorrhagic cystitis. The time of neutrophil engraftment was considered the first of three successive days with ANC (absolute neutrophil count) ≥0.5 x 109/L after post-transplantation nadir; the time of platelet engraftment was considered the first of three consecutive days with platelet count ≥20 x 109/L, in the absence of platelet transfusion.
GVHD assessment and treatment
Patients achieving neutrophil engraftment were evaluable for acute GVHD that was graded using standard criteria [23]. Patients were evaluable for chronic GVHD if engraftment occurred and the patient survived for 100 days post-transplantation. The diagnoses of chronic and extensive chronic GVHD were made as previously described [24–26]. Corticosteroids comprised the first-line therapy of acute (grade II-IV) and extensive chronic GVHD. Second-line treatment was at the discretion of treating physicians and included mycophenolate mofetil, extracorporeal photopheresis, and infliximab.
Statistical analysis
Baseline categorical variables were compared by using Fisher’s exact test, while continuous variables were compared by Wilcoxon rank-sum test or two-sample t-test as appropriate. Overall survival (OS) and progression free survival (PFS) were estimated using the Kaplan-Meier method. OS was defined as the time from transplant to death, and surviving patients were censored at last follow-up. PFS was defined as the time from transplantation to disease progression/relapse and/or death. OS and PFS data were analyzed by the log-rank test. NRM was defined as death from any cause other than disease progression or relapse. Cumulative incidences of NRM and relapse were calculated with relapse or death as a competing event, respectively [27]. Comparisons between estimates of cumulative incidence were made by using the Gray’s test. The cumulative incidence of acute or chronic GVHD was calculating with relapse or death without relapse or GVHD as competing events [20,27]. Cox proportional hazards models were constructed for a cumulative incidence of acute GVHD, chronic GVHD or NRM, relapse, and for OS and PFS, using a limited backward selection procedure. Variables considered in the model were those significant at α=0.20 level from the univariable models. Variables remaining in the final models were significant at α=0.05 level. Estimates for hazard ratios (HR) and corresponding 95% confidence intervals (CI) were obtained for each significant prognostic factor. All p-values are two sided. All analyses were run using Stata 10.1, Stata Corporation, College Station, Texas. P-values based on Gray’s test were estimated using R-project version 2.8.1, The R Foundation for Statistical Computing, 2008.
Results
Patient characteristics
The baseline characteristics of 72 consecutive patients included in this analysis are shown in Table 1. The two ATG groups did not differ significantly for age, histological diagnosis, donor source, KPS, co-morbidity scores, donor/recipient CMV status and stem-cell dose infused. Approximately 25% of the patients in each group received allografts from HLA-mismatched unrelated donors. More patients in the r-ATG group had high-risk disease at the time of transplantation (p=0.02), while a higher proportion of R-ATG patients received filgrastim (G-CSF) to promote neutrophil engraftment (p=0.001). Routine use of G-CSF administration following allografting was discontinued at our center (in October 2006), after the publication of large registry data showing no improvement in allogeneic transplantation outcomes with growth factor administration [28].
Table 1.
Patient characteristics at the time of transplantation.
| ATG (7.5mg/Kg) N=39 |
ATG (6mg/kg) N=33 |
p-Value | |
|---|---|---|---|
|
| |||
| Median Age; years (range) | 56 (24–70) | 55 (24–69) | 0.66 |
|
| |||
| Male (%) | 71.8 | 63.6 | 0.61 |
|
| |||
| Diagnosis | |||
| AML/MDS | 18 (46.2%) | 9 (27.3%) | 0.06 |
| NHL/Hodgkin’s Disease | 12 (30.8%) | 9 (27.3%) | |
| Chronic lymphocytic leukemia | 4 (10.3%) | 12 (36.4%) | |
| Others | 5 (12.8%) | 3 (9.1%) | |
|
| |||
| Disease risk‡‡ | |||
| Standard-risk | 11 (28%) | 2 (6%) | 0.02 |
| High-risk | 28 (72%) | 31 (94%) | |
|
| |||
| Prior autografting | 6 (15%) | 1 (3%) | 0.11 |
|
| |||
| Donors | |||
| Sibling | 5 (12.8%) | 3 (9.1%) | 0.71 |
| Unrelated | 34 (87.2%) | 30 (90.9%) | |
|
| |||
| Degree of HLA match | |||
| 8/8 match† | 31 (79%) | 29 (87%) | 0.34 |
| 10/10 match‡ | 29 (74%) | 25 (75%) | 0.99 |
|
| |||
| Median KPS; (range) | 90 (70–100) | 90 (80–100) | 0.17 |
|
| |||
| Median HCT-CI; (range) | 1 (0–4) | 2 (0–4) | 0.11 |
|
| |||
| Cytomegalovirus status | |||
| Patient and/or donor seropositive | 27 (69.2%) | 22 (66.7%) | 0.99 |
| Both patient and donor seronegative | 12 (30.8%) | 11 (33.3%) | |
|
| |||
| Graft source | |||
| Bone marrow | 4 (10.3%) | - | 0.12 |
| Peripheral blood stem cells | 35 (89.7%) | 33 (100%) | |
|
| |||
| Patients receiving G-CSF | 29 (74.4%) | 11 (33.3%) | 0.001 |
|
| |||
| Median CD34+ cell dose (106 cells/kg recipient), (range) | 7.02 (0.69 – 10.2) | 7.2 (2.13 – 10.0) | 0.93 |
|
| |||
| Median CD3+ cell dose (107 cells/kg recipient), (range) | 2.33 (0.19 – 4.24) | 2.32 (0.45 – 4.83) | 0.64 |
Abbreviations: AML=acute myeloid leukemia; G-CSF=granulocyte colony stimulating factor; KPS=Karnofsky performance score; HCT-CI= Hematopoietic cell transplantation-comorbidity index; HLA=human leukocyte antigen; MDS=myelodysplastic syndrome; NHL=non-Hodgkin’s lymphoma.
8/8 match defined by high-resolution allele-level matching at HLA-A, -B, -C and –DRB1.
10/10 match defined by high-resolution allele-level matching at HLA-A, -B, -C, –DRB1 and –DQB1.
Patients with chronic myeloid leukemia in first chronic phase, acute leukemia in first complete remission, myelodysplastic syndrome with refractory anemia or refractory anemia with ringed sideroblasts were considered to have low-risk disease. All other patients were placed in the high-risk disease category.
Engraftment and chimerism
Median time to neutrophil engraftment was significantly longer in the r-ATG group compared to the R-ATG group (18 vs. 15 days; p=0.01), likely due to the omission of G-CSF in the r-ATG group (Table 2). A trend for longer median time to platelet engraftment was seen in R-ATG group compared to the r-ATG group (17.5 vs. 15 days; p=0.06). One patient each in both groups experienced secondary graft failure, which resolved with G-CSF administration. One R-ATG patient had secondary graft rejection, with no such events observed in the r-ATG group. Primary graft failure was not observed. Rates of complete donor-cell chimerism at days +30, +60, +180 and +360 for R-ATG patients were 59%, 63%, 84% and 91% respectively, and for r-ATG patients were 43%, 78%, 92% and 100% respectively (p=NS).
Table 2.
Engraftment Kinetics and donor-cell chimerism post-transplantation.
| ATG (7.5mg/Kg) | ATG (6mg/kg) | p-Value | |
|---|---|---|---|
|
| |||
| Neutrophil engraftment (days), Median (range) | 15 (12–20) | 18 (8–32) | 0.01 |
|
| |||
| Platelet engraftment (days), Median (range) | 17.5 (10–292) | 15 (10–49) | 0.06 |
|
| |||
| Secondary Graft failure | 1 | 1 | 0.99 |
| Secondary Graft rejection | 1 | - | NA |
|
| |||
| Day +30 Chimerism; Median | |||
| T-cell | 97% | 91.5% | 0.15 |
| Myeloid | 100% | 100% | 0.78 |
|
| |||
| Day +90 Chimerism; Median | |||
| T-cell | 99% | 100% | 0.24 |
| Myeloid | 100% | 100% | 0.76 |
|
| |||
| Day +180 Chimerism; Median | |||
| T-cell | 100% | 100% | 0.56 |
| Myeloid | 100% | 100% | 0.03 |
|
| |||
| Day +360 Chimerism; Median | |||
| T-cell | 100% | 100% | 0.84 |
| Myeloid | 100% | 100% | 0.11 |
GVHD
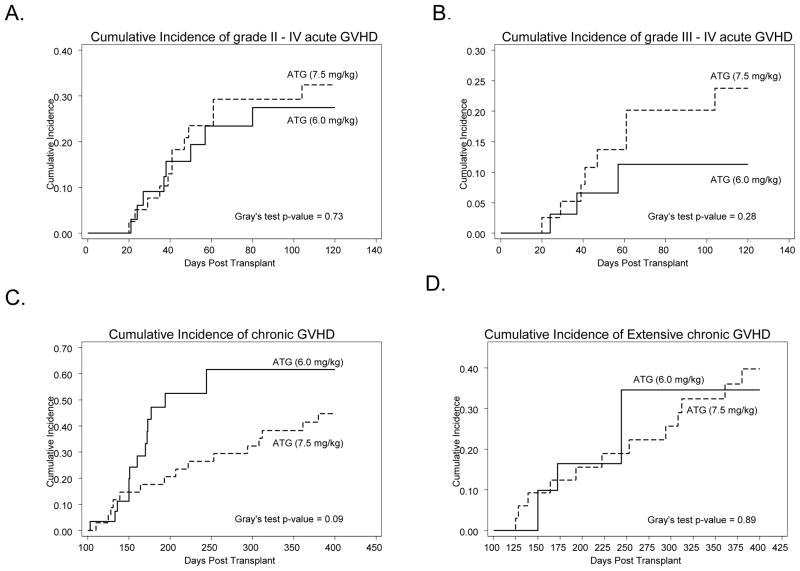
All 72 patients were evaluable for acute GVHD (Table 3). The median time to onset of acute GVHD was 44 days and 38 days for the R-ATG and r-ATG groups respectively. While accounting for competing events, the day +120-cumulative incidence of grade II-IV acute GVHD was 32% for R-ATG group and 27% for r-ATG group (p=0.73) (Figure 1A). The day +120-cumulative incidence of grade III-IV acute GVHD for R-ATG patients and r-ATG patients was 23% and 11% respectively (p=0.28) (Figure 1B). On univariate analysis, baseline diagnosis of lymphoma (Hodgkin’s and non-Hodgkin’s) was the only variable associated with acute GVHD (HR=3.44; 95% CI=1–11; p=0.04).
Table 3.
Assessment of graft-versus-host disease according to dose of antithymocyte globulin used.
| ATG (7.5mg/kg) N=39 |
ATG (6mg/kg) N=33 |
p-value | |
|---|---|---|---|
|
| |||
| Rates of acute GVHD*; %(N) | |||
| Grade I–IV | 41% (16) | 48.4% (16) | 0.52 |
| Grade II–IV | 30.7% (12) | 24.2% (8) | 0.53 |
| Grade III–IV | 20.5% (8) | 9% (3) | 0.17 |
|
| |||
| Cumulative incidence of acute GVHD at day +120† (%) | |||
| Grade II–IV | 27% | 32% | 0.73 |
| Grade III–IV | 23% | 11% | 0.28 |
|
| |||
| Rates of chronic GVHD*; %(N) | |||
| Overall chronic GVHD | 44.1% (15) | 44.8% (13) | 0.95 |
| Limited chronic GVHD | 8.8% (3) | 31% (9) | 0.02 |
| Extensive chronic GVHD | 35.2% (12) | 13.7% (4) | 0.05 |
|
| |||
| Cumulative incidence of chronic GVHD at day +400† (%) | |||
| Overall chronic GVHD | 44% | 61% | 0.09 |
| Limited chronic GVHD | 9% | 44% | 0.01 |
| Extensive chronic GVHD | 40% | 34% | 0.89 |
Abbreviations: GVHD=graft-versus-host disease.
Represents simple rates of GVHD to provide comparison with studies not reporting cumulative incidence of GVHD.
Represents cumulative incidence of GVHD (at specified time points), while adjusting for competing events (for details please refer to statistical methods)
Figure 1.
Cumulative incidence of GVHD according to the ATG dosage group. (A) cumulative incidence of grade II–IV acute GVHD, (B) cumulative incidence of grade III–IV acute GVHD, (C) cumulative incidence of chronic GVHD and (D) cumulative incidence of extensive chronic GVHD. Solid curves represent patients receiving ATG at 6.0mg/kg, while the dashed curves represent patient getting the 7.5mg/kg ATG dose.
Sixty-three patients surviving for at least 100 days post-transplantation were evaluable for chronic GVHD (R-ATG group=34, r-ATG group=29) (Table 3). The median time to onset of chronic GVHD for R-ATG and r-ATG groups was 207 days and 160 days respectively. The day +400-cumulative incidence of chronic GVHD was 44% (n=15) for R-ATG group and 61% (n=13) for r-ATG group (p=0.09) (Figure 1C). The day +400-cumulative incidence of extensive chronic GVHD for the patients in R-ATG group vs. those in the r-ATG groups was 40% (n=12) vs. 34% (n=4) respectively (p=0.89) (Figure 1D). On univariable analysis, baseline diagnosis of CLL was the only variable associated with chronic GVHD (HR=2.82; 95% CI=1–7.8; p=0.04).
Infectious complications
Compared to R-ATG group, the patients in the r-ATG group had significantly fewer episodes of CMV reactivations (64.1% vs. 30.3%; p=0.005) and bacterial infections (56.4% vs. 18.2% p=0.001) (Table 4). There was a non- significant trend to less BK-virus associated hemorrhagic cystitis in the r-ATG cohort compared to R-ATG patients (9.1% vs. 25.6%; p=0.12) (Table 4). In the R-ATG group two patients developed post-transplant lymphoproliferative disorder (PTLD) and two additional patients developed adenoviral infections, while no such events were seen the r-ATG group. Three and one invasive fungal infections were reported in the R-ATG and r-ATG patients respectively.
Table 4.
Infectious complications post allogeneic transplantation.
| ATG (7.5mg/Kg) N (%) |
ATG (6mg/kg) N (%) |
p-Value | |
|---|---|---|---|
|
| |||
| CMV reactivation | 25 (64.1) | 10 (30.3) | 0.005 |
|
| |||
| EBV reactivation | 10 (25.6) | 7 (21.2) | 0.78 |
|
| |||
| Adenovirus infections | 2 (5.1) | 0 | 0.49 |
|
| |||
| BK-virus associated hemorrhagic cystitis | 10 (25.6) | 3 (9.1) | 0.12 |
|
| |||
| Bacterial infections | 22 (56.4) | 6 (18.2) | 0.001 |
| Gram positive bacteria | 14 | 3 | |
| Gram negative bacteria | 8 | 3 | |
|
| |||
| Invasive fungal infections | 3 (7.7) | 1 (3) | 0.62 |
| Aspergillus Fumigatus | 1 | 1 | |
| Histoplasma Capsulatum | 1 | - | |
| Candida Albicans | 1 | - | |
Abbreviations: CMV=cytomegalovirus; EBV=Epstein-Barr virus.
Non-relapse mortality and Relapse rate
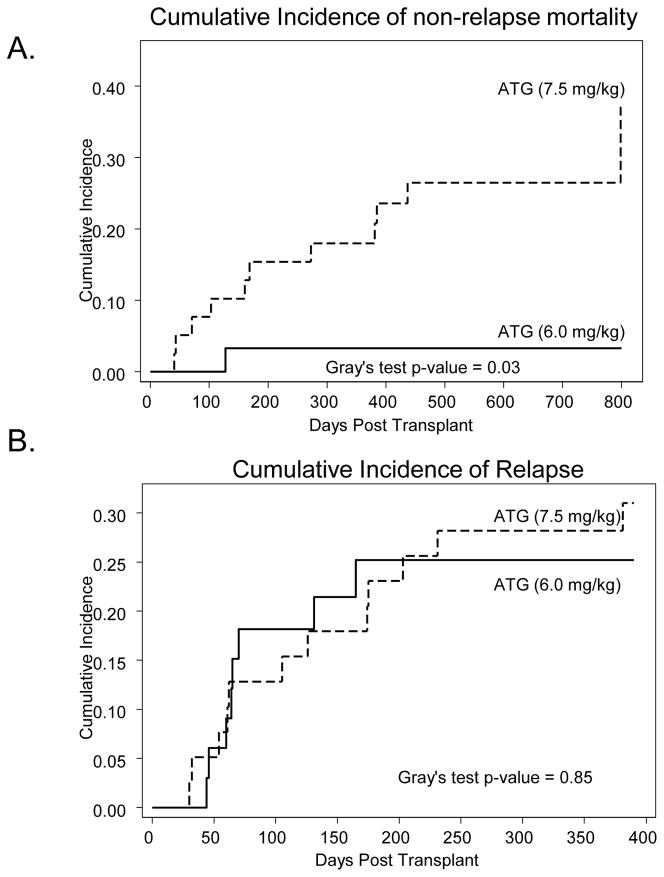
Median follow-up of surviving patients following HSCT is 15 months. The day 100-cumulative incidence of NRM rate was significantly lower in r-ATG group compared to R-ATG group (0% vs. 7.7%; p=0.03). Similarly, the 1-year cumulative incidence of NRM was significantly lower in r-ATG group compared to R-ATG group (3% vs. 18%; p=0.03) (Figure 2A). On univariate analysis no other clinical variable was significantly associated with NRM risk. Causes of NRM are listed in Table 5.
Figure 2.
(A) Cumulative incidence of non-relapse mortality according to the ATG dosage group. (B) Cumulative incidence of disease relapse according to the ATG dosage group. Solid curves represent patients receiving ATG at 6.0mg/kg, while the dashed curves represent patient getting the 7.5mg/kg ATG dose.
Table 5.
Causes of non-relapse mortality according to dose of antithymocyte globulin administered.
| Cause of death | ATG (7.5mg/Kg) N=11 |
ATG (6mg/kg) N=1 |
|---|---|---|
| GVHD with sepsis | 1 | 1 |
| GVHD without sepsis | 1 | - |
| Sepsis | 1 | - |
| Post-transplant lymphoproliferative disorder | 2 | - |
| Second malignancy | 2 | - |
| Adenoviral pneumonia | 1 | - |
| Cerebral toxoplasmosis | 1 | - |
| JC-viral progressive multifocal leukoencephalopathy | 1 | - |
| Cardiac toxicity | 1 | - |
At last follow-up 20 patients relapsed (R-ATG=12; r-ATG=8). The cumulative incidence of relapse for R-ATG and r-ATG patients was not significantly different (p=0.85); the 1- and 2-year rates for the two groups were 28% vs. 25% and 25% vs. 31% respectively (Figure 2B). On univariate analysis baseline diagnosis of CLL (HR=0.09; 95% CI=0.01–0.68; p=0.02) was the only factor associated with the relapse risk.
Overall and Progression free survival
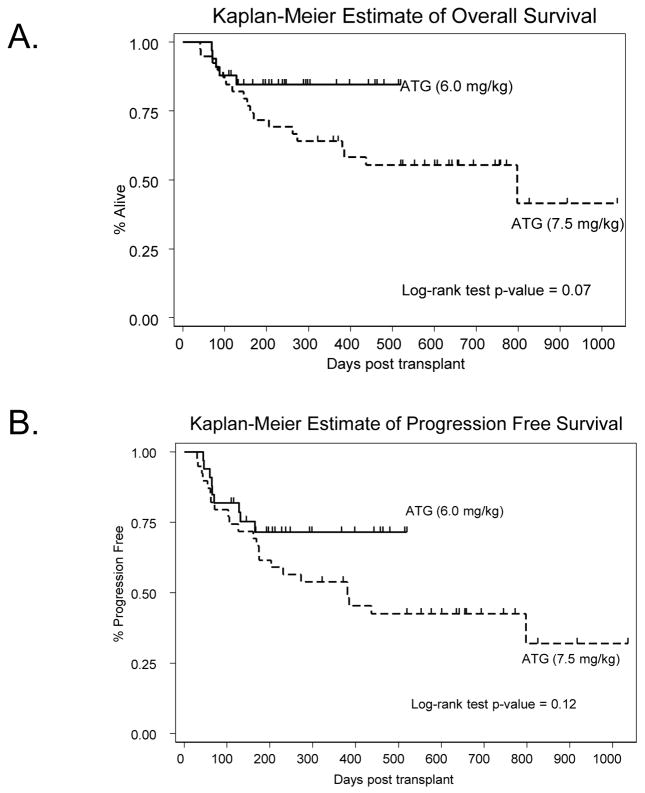
At last follow-up 49 patients were alive (R-ATG group=21, r-ATG group=28). Patients in the r-ATG group showed a trend for better 1-year OS compared to patients in the R-ATG group (84% vs. 64%; log-rank p=0.07) (Figure 3A; Online only); the 2-year expected OS rates were 84% and 55% respectively. Univariate analysis identified rabbit ATG dose (HR=2.40; p-value=0.08), baseline diagnosis of CLL (HR=0.11; p-value=0.03), and KPS ≥90 (HR=0.50; p-value=0.09) as variables of interest for multivariable analysis. However, none of these variables demonstrated independent prognostic significance on multivariate Cox regression analysis (p-value>0.05). The expected 1-year PFS rates were 53% and 71% for patients in R-ATG group and those in r-ATG group respectively (log-rank p-value=0.12) (Figure 3B; Online only). The corresponding estimates of 2 year PFS rates were 42% and 71% respectively. In a multivariable analysis, baseline diagnosis of CLL (HR=0.10; 95% CI=0.02–0.45; p-value=0.003) and KPS ≥90 (HR=0.91; 95% CI=0.85–0.97; p-value=0.008) were the only factors independently associated with a better PFS.
Figure 3.
Overall survival and progression free survival according to ATG dosage group. (A) Kaplan-Meier estimates of overall survival following allogeneic transplantation, (B) Kaplan-Meier estimates of progression free survival following allogeneic transplantation. Solid curves represent patients receiving ATG at 6.0mg/kg, while the dashed curves represent patient getting the 7.5mg/kg ATG dose.
Discussion
As part of our continuous quality improvement program, we sought to reduce the risk of infectious complications and NRM observed in recipients of mostly unrelated RIC allografts by means of a planned dose reduction of rabbit ATG associated with our SOC protocol. We then retrospectively analyzed the impact of this modest ATG dose reduction on clinical outcomes and have made several interesting observations. First our data support that a relatively small ATG dose reduction to 6mg/kg had no deleterious effect on engraftment kinetics or donor chimerism. Second, the lower ATG dose did not appear to increase the risk of either acute or chronic GVHD. Third, the dose reduction was associated with significantly less viral and bacterial infections. These results ultimately translated into a reduction in NRM and similar OS and PFS for patients that received the lower dose of ATG compared with those that received the higher dose. These findings we feel validate the importance of continuous quality monitoring associated with blood and marrow transplant programs.
Over the last two decades various investigators have reported encouraging transplantation outcomes with the inclusion of ATG in transplant conditioning regimens [6,9,29–31], while others have reported comparable transplantation results without the use of ATG [32,33]. These divergent data have created considerable controversy about the role (if any) and indications of ATG use with allogeneic HSCT. The lack of randomized data addressing the role of ATG with transplant conditioning has been the key factor fueling this controversy for decades. Recently a phase III trial, reported in abstract form only [34], has shown significant reduction in the rates of acute and chronic GVHD for patients randomized to receiving ATG with transplant conditioning. This key study supports the use of ATG, at least for patients receiving unrelated donor allografts following myeloablative conditioning.
ATG prevents development of GVHD not only through in vivo donor effector T cell depletion, but also via pleiotropic effects on the immune system including depletion and modulation of antigen presenting cells, modulation of cell surface molecules that mediate leukocyte/endothelium interactions and induction of regulatory T-cells [35–40]. In addition to dose-intensity, the efficacy of ATG in preventing GVHD is intricately dependent on the type of ATG preparation used and on the timing of ATG administration before HSCT [1,17,41]. Unfortunately in majority of the studies assessing ATG dose-intensity, different ATG doses were administered on different schedules, making interpretation of the efficacy results difficult [6,17,20][6]. Thus, in our study, when we elected to reduce the 7.5mg/kg total dose of rabbit ATG in patients undergoing RIC HSCT, we decided to keep the ATG preparation and administration schedule uniform, to facilitate comparison of the two ATG dose levels. This allowed us to investigate the impact of an apparently lower ATG dose on GVHD control rates, graft function, infection, and other transplantation outcomes.
Numerous studies have shown the efficacy of ATG for GVHD prophylaxis [4,6–10,19,31,42][4,6]. However, higher ATG doses (thymoglobulin dose-equivalents ≥7.5–15mg/kg) have been associated with increased risk of relapse, infectious complications and NRM [8,31,43]. Despite these data, only a handful of studies have attempted to define the optimal dose-intensity of ATG. Bacigalupo et al [6] reported outcomes of patients receiving either 15mg/kg or 7.5mg/kg of thymoglobulin (along with myeloablative conditioning) compared to patients not receiving thymoglobulin. Although no direct dose comparisons were planned in that study, rates of acute GVHD appeared worse (69%) with the lower 7.5mg/kg ATG dose, compared to the higher 15mg/kg dose (37%). However the 15mg/kg ATG was associated with significantly more lethal infectious complications.
Meijer et al [18] compared progressively lower doses of thymoglobulin (8mg/kg, 6mg/kg, and 4mg/kg) in patients undergoing myeloablative HSCT and reported significantly higher rates of acute GVHD and trends towards higher rates of chronic GVHD with the lower thymoglobulin doses. A limitation of this study, however was the relatively small number of patients receiving the lower 4mg/kg (n=9) and 6mg/kg (n=13) ATG dose. In contrast Ayuk et al [17] reported no difference in acute and chronic GVHD rates in patients receiving two different doses of ATG-Fresenius (30mg/kg vs. 60mg/kg) with myeloablative conditioning. In the only series comparing ATG dose-intensity in patients undergoing (matched sibling) RIC-HSCT, Mohty et al [20] observed a significant increase in rates of acute and chronic GVHD when thymoglobulin dose was reduced from ≥7.5mg/kg to 2.5mg/kg. Our analysis, in contrast shows that a less aggressive thymoglobulin dose reduction may not compromise acute GVHD control. This is a potentially important observation given that most of our patients received allografts from unrelated donors, had high-risk disease at baseline, and approximately a quarter of the patients received transplants from HLA-mismatched unrelated donors.
Significantly fewer r-ATG patients in our analysis received G-CSF post-allografting, which may explain acceptable acute GVHD control rates in this group. It must however be pointed out that while some studies suggest an increased risk of acute GVHD with post-HSCT G-CSF administration [44], others have found no such association [28,45]. Moreover, in our analyses G-CSF was not independently associated with an increased risk of acute GVHD (p=0.63). We also found no negative correlation between attainment of full donor chimerism and lower ATG dose. The median time to neutrophil engraftment was longer in the r-ATG patients, which is likely due to less frequent use of G-CSF in this group.
In our study the higher 7.5mg/kg ATG dose was associated with frequent CMV reactivations, bacterial infections and lethal infectious complications, leading to significantly higher NRM rates. However unlike previous studies, where the benefit of fewer infectious complications attained with lower ATG doses (2.5–4mg/kg) was off-set by unacceptable rates of GVHD [18,20,43], we demonstrated here that ‘conservative’ ATG dose de-escalation to 6mg/kg can significantly reduce the rates of infectious events and NRM while maintaining acceptable GVHD control rates. The 1 year NRM rate of 3% in the r-ATG cohort is encouraging, especially when considering the high-risk characteristics of patients included at baseline.
In summary, our findings suggest that for patients undergoing RIC HSCT, a total dose of ATG as low as 6mg/kg dose appears to be associated with significantly fewer infectious complications and lower rates of NRM, without compromising GVHD control or survival when compared to higher administered doses. Our data highlight the fact that the relative ATG dose intensity has significant impact on transplantation outcomes, underscoring the need to systematically determine and employ the ATG dose with ‘best’ therapeutic index, not only in clinical practice, but also in future clinical trials. We must acknowledge that these findings were made in the context of a quality improvement effort rather than a prospective hypothesis driven research protocol. It also involved a heterogeneous group of high-risk patients. Nevertheless, the present study is one of the larger analyses that have attempted to address the question of defining the best therapeutic index for ATG in patients undergoing HSCT. Based on our analysis, we conclude that validation of these results would be warranted in a larger, prospective trial evaluating the impact of varying doses of ATG.
Footnotes
Financial Disclosure and Propriety Statement: Nothing to disclose
Presented in part as an oral presentation at 2009 ASBMT Tandem Meetings, Tampa, FL (abstract #9)
References
- 1.Bacigalupo A, Lamparelli T, Gualandi F, et al. Prophylactic antithymocyte globulin reduces the risk of chronic graft-versus-host disease in alternative-donor bone marrow transplants. Biol Blood Marrow Transplant. 2002;8:656–661. doi: 10.1053/bbmt.2002.v8.abbmt080656. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara JL, Levy R, Chao NJ. Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood Marrow Transplant. 1999;5:347–356. doi: 10.1016/s1083-8791(99)70011-x. [DOI] [PubMed] [Google Scholar]
- 3.Hamadani M, Awan FT, Devine SM. The impact of HMG-CoA reductase inhibition on the incidence and severity of graft-versus-host disease in patients with acute leukemia undergoing allogeneic transplantation. Blood. 2008;111:3901–3902. doi: 10.1182/blood-2008-01-132050. [DOI] [PubMed] [Google Scholar]
- 4.Lee KH, Lee JH, Lee JH, et al. Hematopoietic cell transplantation from an HLA-mismatched familial donor is feasible without ex vivo-T cell depletion after reduced-intensity conditioning with busulfan, fludarabine, and antithymocyte globulin. Biol Blood Marrow Transplant. 2009;15:61–72. doi: 10.1016/j.bbmt.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Marmont AM, Horowitz MM, Gale RP, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78:2120–2130. [PubMed] [Google Scholar]
- 6.Bacigalupo A, Lamparelli T, Bruzzi P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO) Blood. 2001;98:2942–2947. doi: 10.1182/blood.v98.10.2942. [DOI] [PubMed] [Google Scholar]
- 7.Basara N, Baurmann H, Kolbe K, et al. Antithymocyte globulin for the prevention of graft-versus-host disease after unrelated hematopoietic stem cell transplantation for acute myeloid leukemia: results from the multicenter German cooperative study group. Bone Marrow Transplant. 2005;35:1011–1018. doi: 10.1038/sj.bmt.1704957. [DOI] [PubMed] [Google Scholar]
- 8.Bredeson CN, Zhang MJ, Agovi MA, et al. Outcomes following HSCT using fludarabine, busulfan, and thymoglobulin: a matched comparison to allogeneic transplants conditioned with busulfan and cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:993–1003. doi: 10.1016/j.bbmt.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duggan P, Booth K, Chaudhry A, et al. Unrelated donor BMT recipients given pretransplant low-dose antithymocyte globulin have outcomes equivalent to matched sibling BMT: a matched pair analysis. Bone Marrow Transplant. 2002;30:681–686. doi: 10.1038/sj.bmt.1703674. [DOI] [PubMed] [Google Scholar]
- 10.Kroger N, Zabelina T, Kruger W, et al. In vivo T cell depletion with pretransplant anti-thymocyte globulin reduces graft-versus-host disease without increasing relapse in good risk myeloid leukemia patients after stem cell transplantation from matched related donors. Bone Marrow Transplant. 2002;29:683–689. doi: 10.1038/sj.bmt.1703530. [DOI] [PubMed] [Google Scholar]
- 11.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 12.Juckett M, Rowlings P, Hessner M, et al. T cell-depleted allogeneic bone marrow transplantation for high-risk non-Hodgkin’s lymphoma: clinical and molecular follow-up. Bone Marrow Transplant. 1998;21:893–899. doi: 10.1038/sj.bmt.1701209. [DOI] [PubMed] [Google Scholar]
- 13.Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111:2470–2475. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamadani M, Hofmeister CC, Jansak B, et al. Addition of infliximab to standard acute graft-versus-host disease prophylaxis following allogeneic peripheral blood cell transplantation. Biol Blood Marrow Transplant. 2008;14:783–789. doi: 10.1016/j.bbmt.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peggs KS, Sureda A, Qian W, et al. Reduced-intensity conditioning for allogeneic haematopoietic stem cell transplantation in relapsed and refractory Hodgkin lymphoma: impact of alemtuzumab and donor lymphocyte infusions on long-term outcomes. Br J Haematol. 2007;139:70–80. doi: 10.1111/j.1365-2141.2007.06759.x. [DOI] [PubMed] [Google Scholar]
- 16.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayuk F, Diyachenko G, Zabelina T, et al. Comparison of two doses of antithymocyte globulin in patients undergoing matched unrelated donor allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:913–919. doi: 10.1016/j.bbmt.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Meijer E, Cornelissen JJ, Lowenberg B, Verdonck LF. Antithymocyteglobulin as prophylaxis of graft failure and graft-versus-host disease in recipients of partially T-cell-depleted grafts from matched unrelated donors: a dose-finding study. Exp Hematol. 2003;31:1026–1030. doi: 10.1016/s0301-472x(03)00204-2. [DOI] [PubMed] [Google Scholar]
- 19.Remberger M, Svahn BM, Mattsson J, Ringden O. Dose study of thymoglobulin during conditioning for unrelated donor allogeneic stem-cell transplantation. Transplantation. 2004;78:122–127. [PubMed] [Google Scholar]
- 20.Mohty M, Bay JO, Faucher C, et al. Graft-versus-host disease following allogeneic transplantation from HLA-identical sibling with antithymocyte globulin-based reduced-intensity preparative regimen. Blood. 2003;102:470–476. doi: 10.1182/blood-2002-12-3629. [DOI] [PubMed] [Google Scholar]
- 21.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 22.Przepiorka D, Ippoliti C, Khouri I, et al. Tacrolimus and minidose methotrexate for prevention of acute graft-versus-host disease after matched unrelated donor marrow transplantation. Blood. 1996;88:4383–4389. [PubMed] [Google Scholar]
- 23.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 24.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 26.Farmer ER. The histopathology of graft-versus-host disease. Adv Dermatol. 1986;1:173–188. [PubMed] [Google Scholar]
- 27.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 28.Khoury HJ, Loberiza FR, Jr, Ringden O, et al. Impact of posttransplantation G-CSF on outcomes of allogeneic hematopoietic stem cell transplantation. Blood. 2006;107:1712–1716. doi: 10.1182/blood-2005-07-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byrne JL, Stainer C, Cull G, et al. The effect of the serotherapy regimen used and the marrow cell dose received on rejection, graft-versus-host disease and outcome following unrelated donor bone marrow transplantation for leukaemia. Bone Marrow Transplant. 2000;25:411–417. doi: 10.1038/sj.bmt.1702165. [DOI] [PubMed] [Google Scholar]
- 30.Finke J, Bertz H, Schmoor C, et al. Allogeneic bone marrow transplantation from unrelated donors using in vivo anti-T-cell globulin. Br J Haematol. 2000;111:303–313. doi: 10.1046/j.1365-2141.2000.02305.x. [DOI] [PubMed] [Google Scholar]
- 31.Russell JA, Turner AR, Larratt L, et al. Adult recipients of matched related donor blood cell transplants given myeloablative regimens including pretransplant antithymocyte globulin have lower mortality related to graft-versus-host disease: a matched pair analysis. Biol Blood Marrow Transplant. 2007;13:299–306. doi: 10.1016/j.bbmt.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Sierra J, Storer B, Hansen JA, et al. Unrelated donor marrow transplantation for acute myeloid leukemia: an update of the Seattle experience. Bone Marrow Transplant. 2000;26:397–404. doi: 10.1038/sj.bmt.1702519. [DOI] [PubMed] [Google Scholar]
- 33.Hansen JA, Gooley TA, Martin PJ, et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med. 1998;338:962–968. doi: 10.1056/NEJM199804023381405. [DOI] [PubMed] [Google Scholar]
- 34.A Randomized Prospective Multicenter Phase III Trial Comparing Standard GvHD Prophylaxis with Cyclosporine and Methotrexate with Additional Pretransplant ATG Fresenius (ATG-F) in Allogeneic Stem Cell Transplantation from Matched Unrelated Donors -- Finke et al. 112 (11): 57 -- ASH Annual Meeting Abstracts.;2009.
- 35.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21:1387–1394. doi: 10.1038/sj.leu.2404683. [DOI] [PubMed] [Google Scholar]
- 36.Feng X, Kajigaya S, Solomou EE, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008;111:3675–3683. doi: 10.1182/blood-2008-01-130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang L, Fehse B, Engel M, Zander A, Kroger N. Antithymocyte globulin induces ex vivo and in vivo depletion of myeloid and plasmacytoid dendritic cells. Transplantation. 2005;79:369–371. doi: 10.1097/01.tp.0000150210.77543.1b. [DOI] [PubMed] [Google Scholar]
- 38.Grullich C, Ziegler C, Finke J. Rabbit anti T-lymphocyte globulin induces apoptosis in peripheral blood mononuclear cell compartments and leukemia cells, while hematopoetic stem cells are apoptosis resistant. Biol Blood Marrow Transplant. 2009;15:173–182. doi: 10.1016/j.bbmt.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 39.Beiras-Fernandez A, Walther S, Kaczmarek I, et al. In vitro influence of polyclonal anti-thymocyte globulins on leukocyte expression of adhesion molecules. Exp Clin Transplant. 2005;3:370–374. [PubMed] [Google Scholar]
- 40.Haidinger M, Geyeregger R, Poglitsch M, et al. Antithymocyte globulin impairs T-cell/antigen-presenting cell interaction: disruption of immunological synapse and conjugate formation. Transplantation. 2007;84:117–121. doi: 10.1097/01.tp.0000266677.45428.80. [DOI] [PubMed] [Google Scholar]
- 41.Dominietto A, Van Lint MT, Gualandi F. Is timing of anti-thymocyte globulin (ATG) – pre and post hemopoietic stem cell transplants (HSCT) – relevant for graft vs host disease (GvHD)? Blood. 2003;102:242a. [Google Scholar]
- 42.Crawley C, Szydlo R, Lalancette M, et al. Outcomes of reduced-intensity transplantation for chronic myeloid leukemia: an analysis of prognostic factors from the Chronic Leukemia Working Party of the EBMT. Blood. 2005;106:2969–2976. doi: 10.1182/blood-2004-09-3544. [DOI] [PubMed] [Google Scholar]
- 43.Mohty M, Jacot W, Faucher C, et al. Infectious complications following allogeneic HLA-identical sibling transplantation with antithymocyte globulin-based reduced intensity preparative regimen. Leukemia. 2003;17:2168–2177. doi: 10.1038/sj.leu.2403105. [DOI] [PubMed] [Google Scholar]
- 44.Ringden O, Labopin M, Gorin NC, et al. Treatment with granulocyte colony-stimulating factor after allogeneic bone marrow transplantation for acute leukemia increases the risk of graft-versus-host disease and death: a study from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2004;22:416–423. doi: 10.1200/JCO.2004.06.102. [DOI] [PubMed] [Google Scholar]
- 45.Ho VT, Mirza NQ, Junco DdD, Okamura T, Przepiorka D. The effect of hematopoietic growth factors on the risk of graft-vs-host disease after allogeneic hematopoietic stem cell transplantation: a meta-analysis. Bone Marrow Transplant. 2003;32:771–775. doi: 10.1038/sj.bmt.1704228. [DOI] [PubMed] [Google Scholar]