Abstract
The purpose of the study was to test the hypothesis that sound context modulates the magnitude of auditory distraction, indexed by behavioral and electrophysiological measures. Participants were asked to identify tone duration, while irrelevant changes occurred in tone frequency, tone intensity, and harmonic structure. Frequency deviants were randomly intermixed with standards (Uni-Condition), with intensity deviants (Bi-Condition), and with both intensity and complex deviants (Tri-Condition). Only in the Tri-Condition did the auditory distraction effect reflect the magnitude difference among the frequency and intensity deviants. The mixture of the different types of deviants in the Tri-Condition modulated the perceived level of distraction, demonstrating that the sound context can modulate the effect of deviance level on processing irrelevant acoustic changes in the environment. These findings thus indicate that perceptual contrast plays a role in change detection processes that leads to auditory distraction.
Keywords: Auditory distraction, Magnitude effect, Deviance level, Mismatch negativity (MMN), P3a, Attention
1. Introduction
A couple talking next to you while reading a book and sipping coffee at your local Starbucks will likely be perceived as less distracting than a couple talking next to you at the same level of loudness while reading at your local library. That is, the perceived degree of distraction will be influenced by the context of the ambient noise. The goal of the current study was to test the hypothesis that contextual factors of the overall sound environment modulate auditory distraction effects.
Previous investigations measuring the degree of auditory distraction have focused on the magnitude of a distracting stimulus (Berti, Roeber, & Schröger, 2004; Doeller et al., 2003; Gomes et al., 2000; Rinne et al., 2007; Tse & Penney, 2008; Wetzel, Widmann & Schröger, 2006). In general, these studies have shown a positive relationship between an increase in magnitude of the physical sound and an increase in both behavioral and electrophysiological measures of distraction. The purpose of the current study was to determine whether the physical magnitude of a distracting stimulus would be perceptually modulated by the contextual environment, such that the same physical magnitude of a sound may be elicit more or less distraction depending upon the context it occurs, not by the magnitude of the stimulus itself.
When an unexpected sound event occurs, further evaluation is needed to determine its relevancy to current behavior (Friedman, Cycowicz, & Gaeta, 2001; Ruhnau, Wetzel, Widmann, & Schröger, 2010; Sussman, Winkler, & Schröger, 2003; Sussman, 2007). This “further evaluation” causes a measure of distraction due to the momentary reorienting of attention, which has been observed as a longer reaction time in behavioral performance on the target task, and by a lower accuracy for the target tones that also contain the distracting element (Schröger, Giard, & Wolff, 2000). Furthermore, a corresponding neurophysiologic marker of attentional orienting called the P3a component is observed to the irrelevant, distracting event (Friedman et al., 2001). Additionally, the irrelevant sound change elicits the mismatch negativity (MMN) component, prior in time to the P3a component. It is generally thought that the MMN component reflects the sound change detection (Näätänen, 1990), and the P3a component reflects the attention-switching to the change for further evaluation (Friedman et al., 2001).
Schröger and Wolff (1998) also reported a component following the P3a they called the “reorienting negativity” (RON), and defined it as a measure of reorienting back to the main task (Berti et al., 2004; Schröger & Wolff, 1998). From their observations, Berti et al. (2004) suggested a “three-stage model of auditory distraction” linked to this chronological sequence of event-related potentials (ERPs): the change detection (indexed by MMN), orienting to the distracting event (indexed by P3a) and then reorienting back to the main task (indexed by RON). Taken together, Berti et al. suggest that these neurophysiologic measures provide a temporal ‘tracking’ ultimately reflected in the measures of behavioral distraction. According to this model, the neurophysiologic measures should concordantly reflect the amount of distraction induced by a stimulus. And consistency between stimulus magnitude and measures of distraction has been reported in studies investigating distraction via irrelevant frequency changes (Berti et al., 2004; Gomes et al., 2000; Rinne et al., 2007), irrelevant intensity increments (Rinne, Särkkä, Degerman, Schröger, & Alho, 2006), irrelevant location changes (Sonnadara, Alain, & Trainor, 2006), and irrelevant temporal changes (Kisley et al., 2004).
However, temporal tracking indices of distraction have not been found for magnitude of intensity decrements (Rinne et al., 2006). Rinne et al. (2006) reported only an MMN component elicited by intensity decrements, without any P3a. This occurred even though in the same study intensity increments elicited both MMN and P3a components, as well as an N1 enhancement (a larger N1 amplitude to the louder intensity compared to the standard intensity tones). This result led Rinne et al. to conclude that the P3a component indexes attentional orienting only when the N1 mechanism is involved (i.e., only when an N1 enhancement is also observed). Horváth, Czigler, et al. (2008) went one step further, to suggest that the change in MMN amplitude associated with the magnitude of deviance observed in previous studies was actually a confound of the N1 mechanism and not a magnitude effect at all. Horváth et al. reported that after minimizing the contribution of the N1 component, there was no significant change of MMN amplitude with the magnitude of deviance level. These results argue against a ‘three-stage model’ or a magnitude effect for processing distracting auditory events (Horváth, Winkler, & Bendixen, 2008; Rinne et al., 2006).
In the current study, we explored an alternative hypothesis to these explanations, that is, the sound environment (or context) primarily influences the perceived magnitude of distracting events. From this perspective, the divergence of results found among the studies of Schröger and colleagues may be explained, at least in part, by a change in the sound context. For example, Berti et al. (2004) and Rinne et al. (2006) used a mixed-block design: all of the deviants were mixed together in each run. They then analyzed the magnitude of the responses in comparison with the tones occurring together within the block. In these study designs, the surrounding stimulus environment was the same in all presentation blocks. In contrast, in Horváth, Czigler, et al. (2008) and Horváth, Winkler, et al. (2008), a single-block design was used: each distracting deviant stimulus was presented separately, singly in separate stimulus blocks. Using this design, no magnitude effect was found (after removing the influence of the N1 effect). Thus, our proposed hypothesis would explain these seemingly disparate results in terms of the context difference. The magnitude effect found in Rinne et al. would be due to being able to compare differences in stimulus magnitude for stimuli presented together in the same block, whereas the absence of a magnitude effect in Horváth et al. would be due there being no comparison to be made among the different stimuli because they was only one distracting event in each block. The single-block design of Horváth et al. may have induced a different expectation, or attentional bias, compared to when processing the different deviant stimuli altogether in one block.
Context effects may also explain the seemingly contradictory pattern of results observed for the intensity increment vs. decrement effect of deviants in the Rinne et al. (2006) study. That is, when intensity decrements were mixed together with the intensity increments a comparison was set up that reduced (or modified) the saliency of the decrements. Magnitude effects or the absence of them, in both of these studies may thus be explained by mechanisms of perceptual contrast. The context of the auditory environment allows or precludes an ability to compare stimulus magnitude.
To test the context hypothesis contributing to effects of auditory distraction, we conducted two experiments, one that used a mixed-block design with three levels of frequency deviation, three levels of intensity deviation, and one level of stimulus complexity (Experiment 1), and another that manipulated the levels of comparison (Experiment 2). This design allowed us to test whether distraction effects initiated by increasing levels of frequency and intensity changes would be consistent with a linear increase in the stimulus deviance level, as well as the influence of a qualitatively different and salient tone on the magnitude effect.
2. Experiment 1 (Tri-Condition)
Experiment 1 tested the hypothesis that behavioral and electro-physiological indices of auditory distraction will increase linearly with increasing magnitude of a stimulus.
2.1. Method
2.1.1. Participants
Fifteen right-handed healthy adults were paid for their participation (9 females, M = 26 years, SD = 4.3). All participants provided written informed consent prior to testing, in accordance with the Declaration of Helsinki and approval from the Internal Review Board of the Albert Einstein College of Medicine, where the study was conducted. All participants passed a hearing screen at 20 dB HL for 500, 1000, 2000, and 4000 Hz in both ears, and reported no history of neurological disorders.
2.1.2. Stimuli and procedure
Table 1 details the experimental conditions and stimulus parameters. Pure tone and complex stimuli were created using Neuroscan STIM software™ (Compumedics, El Paso, TX). The standard pure tone (p = 0.79) had a frequency of 1046.5 Hz, and an intensity level of 70 dB SPL (called ‘standards’). There were seven different deviants (p = 0.03, each). Three pure tones were ‘frequency’ deviants; three pure tones were ‘intensity’ deviants; and one complex tone was deviant in spectral quality (‘complex’ tone). The frequency deviants differed from the standard tone only along the frequency dimension (1108.7 Hz, 1174.7 Hz, and 1244.5 Hz, called F1, F2, and F3, respectively). Intensity deviants differed from the standard only along the intensity dimension (74.2 dB, 78.6 dB, and 83.3 dB, called I1, I2, and I3, respectively). The amount of change from the standard was calculated by a log scale (5.94% for level 1 [small deviance], 12.25% for level 2 [medium deviance], 18.92% for level 3 [large deviance]). Thus, this nomenclature (e.g., F1 or I2) denotes the distance of the deviant from the standard, with “1” indicating the distance closest to the standard, and “3” indicating distance furthest from the standard. The deviant spectral tone had the same fundamental frequency of the standard tones (1046.5 Hz) but with 3 harmonic partials. This complex tone was therefore deviant qualitatively but not in frequency or intensity. All tones were presented bilaterally through insert earphones (E-a-rtone® 3A, Indianapolis, IN) once every 1200 ms (onset-to-onset). Half of all the tones were 100 ms duration and the other half of all the tones were 200 ms duration, regardless of their standard or deviant status.
Table 1.
Stimulus parameters.
| Stimulus | Stimulus dimension
|
Probability within the sequence Condition
|
|||
|---|---|---|---|---|---|
| Frequency (Hz) | Intensity (dB) | Tri- | Bi- | Uni- | |
| Standard | 1046.5 | 70 | 0.79 | 0.80 | 0.80 |
| F1 | 1108.7 | 70 | 0.03 | 0.05 | 0.10 |
| F2 | 1174.7 | 70 | 0.03 | 0.05 | 0.10 |
| F3 | 1244.5 | 70 | 0.03 | ||
| I1 | 1046.5 | 74.2 | 0.03 | 0.05 | |
| I2 | 1046.5 | 78.6 | 0.03 | 0.05 | |
| I3 | 1046.5 | 83.3 | 0.03 | ||
| Complex tone | f0 1046.5a | 70 | 0.03 | ||
Tones were randomized within each sequence type, 50% had 100 ms duration and 50% had 200 ms duration.
Three harmonics: 2093 Hz, 3139.5 Hz, and 4186 Hz.
Participants were seated in a comfortable chair in a sound-attenuated recording booth. Participants were instructed to listen to each tone and press a designated button if it was the shorter tone and a different button if it was the longer tone. The frequency, intensity, and spectral changes were irrelevant to the task. Thus, participants were to identify whether they detected the shorter or longer sound on every trial, including the ‘deviant’ tones that varied in frequency, intensity, and spectral quality. The session began with a short practice for the tone duration discrimination task, followed by 20 experimental blocks of stimuli. Each experimental block contained 150 tones that yields a total of 2370 standard tones and 90 deviants of each type. The standard and deviants were pseudorandomly mixed in each block, so that there were at least two standard tones between any two deviant stimuli. The experimental session was approximately 2.5 h, which included electrode placement and breaks and a snack.
2.1.3. Electroencephalogram (EEG) recording and data analysis
EEG was recorded using a 32-channel electrode cap placed according to the modified International 10–20 System (Jasper, 1958) from FPz, Fz, Cz, Pz, Oz, FP1, FP2, F7, F8, F3, F4, FC5, FC6, FC1, FC2, T7, T8, C3, C4, CP5, CP6, CP1, CP2, P7, P8, P3, P4, O1, O2, and from the left (LM) and right mastoids (RM). Horizontal eye movements were measured by recording the horizontal electro-oculogram (HEOG) in a bipolar configuration between F7 and F8 electrodes. Vertical electro-oculogram (VEOG) was monitored using the FP1 electrode in a biopolar configuration with an external electrode placed below the left eye. The reference electrode was placed at tip of the nose. Impedance was maintained below 5 kΩ across all sites. The EEG and EOG were digitalized at 500 Hz (0.05–100 Hz) and then filtered offline (1–30 Hz). Epochs with activity exceeding ±75 μV at any recorded channel were excluded from further analysis. ERP epochs that contained incorrect responses were also excluded from further analyses. The remaining epochs were then averaged separately for standards and for each of the deviant types. Difference waveforms were computed by subtracting the ERP waveform evoked by the standard from the ERP waveform evoked by the deviant. Statistical calculations were performed on the difference waveforms because there was one standard and seven deviant types in the block.
A 50 ms window, centered on the peak latency of the P3a component as observed in the grand-mean deviant-minus-standard difference waveforms at the Fz electrode, was used to statistically measure its amplitude. The Fz electrode was chosen as having the greatest signal-to-noise ratio, consistent with previous studies (Friedman et al., 2001; Knight & Scabini, 1998). The peak latencies of the seven deviant types used to calculate the P3a component were 338 ms (F1), 326 ms (F2), 316 ms (F3), 354 ms (I1), 324 ms (I2), 324 ms (I3) and 314 ms (complex tone). For each participant, the mean amplitude in the corresponding 50 ms window of the individual grand-mean difference waveforms was computed. To statistically measure the P3a peak latency, a peak detection program (Neuroscan 4.2) was used in the same window, for each individual difference waveform.
For MMN, the difference waves at Fz were re-referenced to the average of the mastoids, and then the amplitude and latency of the MMN component was obtained using a 40 ms window centered on the peak latency of the grand mean difference waveforms. MMN amplitude and latency were calculated the same way as for the P3a component. The peak latencies used to calculate MMN were 156 ms (F1), 142 ms (F2), 132 ms (F3), 168 ms (I1), 152 ms (I2), 120 ms (I3) and 94 ms (complex tone).
Behavioral responses were considered correct if they were responded to by the corresponding response key within 100–900 ms interval from stimulus onset. Errors were calculated if either no response was made or if a wrong response was made (e.g., a long tone was identified as short or a short tone as long). Error rate (ER) was calculated, separately for each type of stimulus in percentage of errors. Statistical analyses for behavioral responses were calculated on the distraction effects: the difference between the response to the deviant tone and the response to the standard tone to be consistent with the ERP measures (MMN and P3a), which were calculated from the difference waveforms (unless otherwise specified).
Three levels of statistical analyses were conducted. First, one-sample two-tailed t-tests were used to verify the presence of behavioral distraction effects (RT and ER), the MMN, and P3a components. These tests determined whether the response difference between the deviant and standard stimulus was significantly different from zero. To further compare distraction effects among all seven deviant types, a one-way Analysis of Variance (ANOVA) for repeated measures was calculated (F1, F2, F3, I1, I2, I3 and complex tone), followed by post hoc comparisons which compared the effects elicited by complex tone with the effects by each of the frequency and intensity deviants. At this level of the analysis, only a significant difference between the complex tone and the other deviants were reported. Differences among the levels of frequency and intensity deviants were reported in the second level.
The second level of analysis was conducted to test the linearity of the distraction effects. For this calculation, the data from the complex tone was omitted, and a two-way repeated-measures ANOVA with factors of deviance level (small ‘1’, medium ‘2’, and large ‘3’) and deviant dimension (frequency vs. intensity) was calculated. Polynomial linear trend analyses were subsequently conducted to test if each measure increased linearly with deviance level.
Finally, a third level analysis was used to test whether the behavioral and electrophysiological measures of distraction correlated with one another. The Pearson product correlation coefficient was calculated with all seven deviants. For example, to calculate the correlation between behavioral distraction and MMN amplitude, one pair of group means (RT distraction, MMN amplitude) were obtained by averaging across all participants, separately for each deviant, resulting in seven pairs of group means. Pearson product correlation coefficient was then calculated based on the seven pairs of data to examine the association between RT distraction and MMN amplitude. All other correlations were calculated in the same way.
As appropriate, Greenhouse–Geisser corrections were applied to correct for violations of sphereicity and p values reported for main effects and interactions. Post hoc calculations were conducted using Tukey HSD.
2.2. Results
2.2.1. Existence of distraction effects (level 1 analysis)
2.2.1.1. Behavioral distraction effects
Table 2 summarizes the behavioral distraction effects. Significant distraction effects on RT means that the RT to the deviant stimulus was significantly longer than the RT to the standard stimulus. Significant distraction effects on ER means that percentage of errors made on the deviant stimulus was higher than percentage of errors made on the standard stimulus. Six of the seven deviant types elicited significant behavioral distraction effects on RT. Only the smallest intensity deviant (I1) did not. Significant distraction effects on ER were observed only for the two larger frequency level deviants (F2 and F3) and for the complex tone.
Table 2.
Behavioral distraction effects on RT and ER (SD in parenthesis) in Tri-, Bi- and Uni-Conditions.
| Deviant | RT (ms)
|
ER (%)
|
||||
|---|---|---|---|---|---|---|
| Tri- | Bi- | Uni- | Tri- | Bi- | Uni- | |
| F1 | 34 (17)** | 19 (11)** | 21 (13)** | 2.9 (5.6) | 1.3 (2.2) | 1.5 (2.7) |
| F2 | 22 (19)** | 23 (15)** | 33 (17)** | 3.3 (5.0)* | 5.7 (4.9)** | 2.8 (3.6)* |
| F3 | 46 (26)** | 12.8 (7.5)** | ||||
| I1 | −11 (10)** | 4 (11) | −1.0 (3.4) | −1.5 (2.7) | ||
| I2 | 9 (13)* | 8 (16) | −1.1 (2.9) | 0.7 (3.0) | ||
| I3 | 31 (13)** | 3.3 (6.1) | ||||
| Complex tone | 55 (29)** | 14.8 (11.8)** | ||||
p < 0.05 on the two-tail one sample t-tests.
p < 0.01 on the two-tail one sample t-tests.
A comparison of the distraction effect on the seven deviants revealed a significant main effect of deviant type on RT (F(6, 84) = 27.65, ε = 0.533, p < 0.001). Post hoc comparisons showed that the complex tone (55 ms) elicited a larger distraction effect (longer RT) to all of the other deviant types (−11 to 34 ms) except for F3 (46 ms). There was no significant difference in RT between the complex tone and F3 (Table 2). Similarly, there was a significant main effect of deviant type on ER (F(6, 84) = 16.16, ε = 0.460, p < 0.001), with the complex tone (0.15) having significantly larger ERs than all other deviants (ranging between −0.01 and 0.033) except F3 (0.13). There was no significant difference in ER between complex tone and F3 (Table 2).
2.2.1.2. ERP distraction effects
Figs. 1 and 2 display the grand-averaged ERP waveforms at Fz, and Fig. 3 displays the voltage distribution maps of the corresponding components. Amplitudes and latencies are summarized in Table 3 (MMN component) and Table 4 (P3a component).
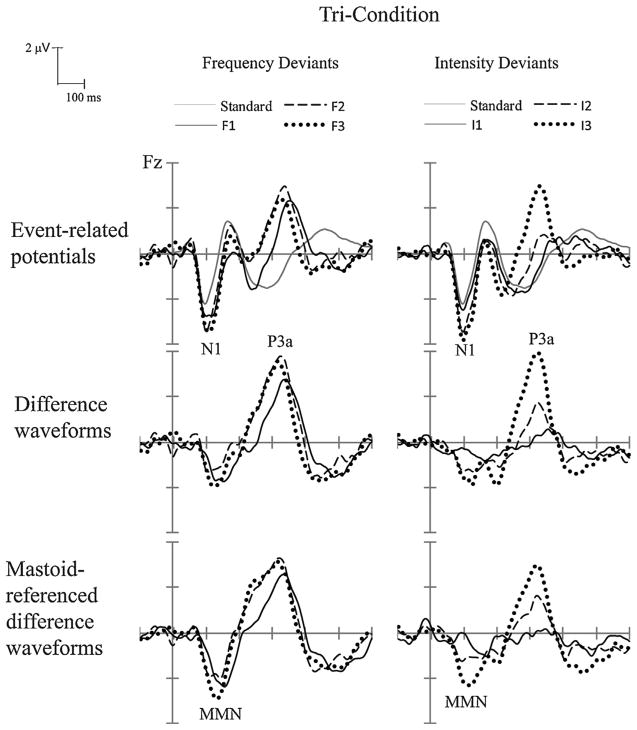
Fig. 1.
Experiment 1: Grand-averaged event-related potentials (ERPs) (top row), difference waveforms (deviant-minus-standard) (middle row), and mastoid-referenced difference waveforms (bottom row) are shown for frequency deviants (left column) and intensity deviants (right column). Small-level deviants (F1 and I1) are denoted with a solid black line; medium-level deviants (F2 and I2) with a dashed black line, and large-level deviants (F3 and I3) with a dotted black line. The ERP response evoked by the standard tones is shown with a solid gray line (top row only). ERP components (N1, MMN, and P3a) are labeled.
Fig. 2.
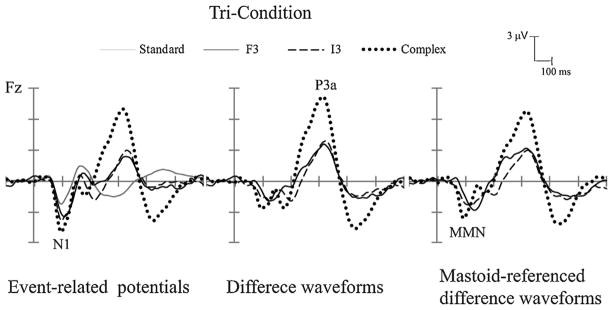
Complex tone and large-level deviants for Experiment 1 (Tri-Condition). Grand-averaged event-related potentials (ERPs) (left column), difference waveforms (deviant-minus-standard) (middle column), and mastoid-referenced difference waveforms (right column) are displayed for the Complex deviant (dotted black line), overlain with the large-level frequency deviant (F3) (solid black line) and large-level intensity deviant (I3) (dashed black line). The ERP response evoked by the standard tones is shown with a solid gray line (left column only). ERP components (N1, MMN, and P3a) are labeled. Positive polarity is upward.
Fig. 3.
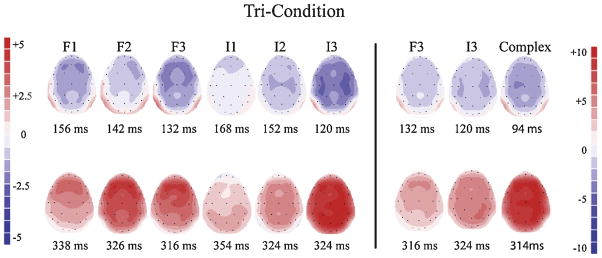
Experiment 1: Tri-Condition. Scalp voltage maps for each deviant displaying the peak latency of the difference waveforms used to measure the MMN (top) and P3a (bottom) components for the frequency deviants (F1/F2/F3), intensity (I1/I2/I3) deviants, and complex tone. The right panel provides a comparison of the complex tone with the largest frequency (F3) and intensity (I3) deviants. Note the difference in scale for the comparison with the complex tone (right panel) and the comparison among the frequency and intensity deviants (left panel). Red indicates positive polarity and blue indicates negative polarity. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 3.
MMN amplitudes and latencies (SD in parenthesis) in Tri-, Bi- and Uni-Conditions.
| Deviant | Amplitude (μV)
|
Latency (ms)
|
||||
|---|---|---|---|---|---|---|
| Tri- | Bi- | Uni- | Tri- | Bi- | Uni- | |
| F1 | −2.20 (0.88)** | −1.83 (0.92)** | −1.75 (1.14)** | 149 (12) | 132 (15) | 133 (14) |
| F2 | −1.80 (1.42)** | −2.25 (1.43)** | −2.24 (0.66)** | 135 (10) | 142 (15) | 129 (14) |
| F3 | −2.68 (1.05)** | 131 (13) | ||||
| I1 | −0.84 (0.85)** | −0.68 (0.91)* | 161 (12) | 141 (13) | ||
| I2 | −1.06 (0.73)** | −2.07 (1.65)** | 148 (15) | 145 (15) | ||
| I3 | −2.20 (1.63)** | 115 (14) | ||||
| Complex tone | −3.04 (1.75)** | 93 (10) | ||||
p < 0.05 on the two-tail one sample t-tests.
p < 0.01 on the two-tail one sample t-tests.
Table 4.
P3a amplitudes and latencies (SD in parenthesis) in Tri-, Bi- and Uni-Conditions.
| Deviant | Amplitude (μV)
|
Latency (ms)
|
||||
|---|---|---|---|---|---|---|
| Tri- | Bi- | Uni- | Tri- | Bi- | Uni- | |
| F1 | 2.51 (1.98)** | 1.72 (1.60)** | 1.46 (2.37) | 333 (17) | 323 (18) | 333 (17) |
| F2 | 3.56 (2.19)** | 2.83 (1.63)** | 2.24 (1.28)** | 322 (19) | 316 (17) | 310 (15) |
| F3 | 3.25 (2.57)** | 313 (17) | ||||
| I1 | 0.43 (0.97) | 0.50 (0.98) | 351 (19) | 330 (16) | ||
| I2 | 1.44 (1.56)** | 2.72 (1.94)** | 326 (15) | 328 (20) | ||
| I3 | 3.59 (1.68)** | 329 (16) | ||||
| Complex tone | 7.69 (4.02)** | 310 (15) | ||||
p < 0.01 on the two-tail one sample t-tests.
All seven deviants elicited significant MMNs (Table 3). Comparison of the amplitude and latency of the components across the deviant types showed a significant main effect of deviant type on MMN amplitude (F(6, 84) = 7.84, ε = 0.551, p < 0.001) and on MMN latency (F(6, 84) = 56.45, ε = 0.667, p < 0.001). Post hoc comparisons revealed that the MMN amplitude was larger when evoked by the complex tone (−3.04 μV) than by I1 and I2 deviants (Table 3, amplitude). MMN latency evoked by the complex tone was shorter in latency (93 ms) than all frequency and intensity deviants (Table 3, latency).
The P3a component was significantly elicited by six of the deviant types (Table 4), but was not elicited by the smallest intensity deviant (I1). In comparing amplitudes and latencies of the deviant types, there was a main effect of deviant type on both P3a amplitude (F(6, 84) = 27.41, ε = 0.394, p < 0.001) and on P3a latency (F(6, 84) = 14.36, ε = 0.672, p < 0.001). Post hoc comparisons showed that the complex tone evoked larger P3a amplitude (−7.69 μV, Table 4, amplitude) than did all other deviants, and that P3a peak latency was shorter (310 ms) than the F1, I1 and I2 deviants (Table 4, latency).
Voltage maps (Fig. 3) indicate a similarity in scalp distribution for the MMN and P3a components evoked by the frequency deviants and the complex tone, which was fronto-central for both stimuli and components. On the other hand, the scalp distribution for the intensity deviants was dissimilar to frequency, where there was a broader, more centrally-defined distribution for both components.
2.2.2. Effects of deviance level (level 2 data analyses)
2.2.2.1. Behavioral distraction
There was a main effect of deviant dimension (frequency/intensity) on both RT (F(1, 14) = 24.46, p < 0.001) and ER (F(1, 14) = 17.02, p = 0.001). Frequency deviants had larger RT distraction effects than intensity deviants (34 ms vs. 9 ms, respectively), and larger ER distraction effects (0.06 vs. 0.004, respectively). The main effect of deviance level (1/2/3) was also significant on RT (F(2, 28) = 64.50, ε = 0.932, p < 0.001) and on ER (F(2, 28) = 26.90, ε = 0.724, p < 0.001). Post hoc calculations revealed that level 3 deviants (F3/I3) had larger RT distraction effects (38 ms) than both level 2 (F2/I2, 15 ms) and level 1 deviants (F1/I1, 12 ms). Larger ER distraction effects were also found for level 3 deviants (0.081) compared to levels 1 and 2 (0.01 and 0.01, respectively). Finally, there was a significant interaction between deviant dimension and deviance level on RT (F(2, 28) = 12.03, ε = 0.895, p < 0.001), but not quite on ER (F(2, 28) = 3.11, ε = 0.748, p = 0.078). Subsequent linear tread analysis (Table 5) showed that the distraction effect on RT increased linearly with deviance level for intensity deviants (F(1, 14) = 110.00, p < 0.001), and not for frequency (F(1, 14) = 4.43, p = 0.054), which explains the significant interaction between deviant dimension and deviance level on RT. The distraction effect on ER increased linearly with deviance level for both frequency (F(1, 14) = 30.06, p < 0.001) and intensity deviants (F(1, 14) = 4.88, p = 0.044).
Table 5.
Linear trend analysis for Experiment 1.a
| Measure | Frequency deviants (F1/F2/F3) | Intensity deviants (I1/I2/I3) |
|---|---|---|
| Behavioral distraction | ||
| RT | F = 4.43, p = 0.054 | F = 110.00, p < 0.001 |
| ER | F = 30.06, p < 0.001 | F = 4.88, p = 0.044 |
| MMN | ||
| Amplitude | F = 2.03, p = 0.176 | F = 6.61, p = 0.022 |
| Latency | F = 26.20, p < 0.001 | F = 78.67, p < 0.001 |
| P3a | ||
| Amplitude | F = 3.19, p = 0.096 | F = 32.23, p < 0.001 |
| Latency | F = 13.42, p = 0.003 | F = 22.71, p < 0.001 |
df = 1,14.
2.2.2.2. ERP measures
Table 3 displays the mean amplitude and peak latency for the MMN component elicited by all deviant types. There was a main effect of deviant dimension (frequency/intensity) on MMN amplitude (F(1, 14) = 44.13, p < 0.001), but not on MMN latency (F(1, 14) = 1.08, p = 0.32). Post hoc calculations revealed that frequency deviants had larger amplitudes than intensity deviants (−2.23 μV vs. −1.37 μV, respectively), but statistically similar latencies (138 ms vs. 141 ms). There was also a main effect of deviance level (1/2/3) on MMN amplitude (F(2, 28) = 7.82, ε = 0.730, p = 0.006). Post hoc calculations showed larger MMN amplitude for deviance level 3 (−2.44 μV) than for deviance level 2 (−1.43 μV) and 1 (−1.52 μV), with no significant amplitude difference between levels 1 and 2. The linear trend analysis on MMN amplitude with deviance level was significant for intensity deviants (F(1, 14) = 6.61, p = 0.022), but not for frequency levels (F(1, 14) = 2.03, p = 0.176) (Table 5). There was also a main effect of deviance level on MMN latency (F(2, 28) = 63.28, ε = 0.953, p < 0.001). Post hoc analysis revealed a shorter peak latency with higher level deviants (3 > 2 > 1). Linear trend analysis revealed a significant linear decrease in peak MMN latency with deviance level for both frequency (F(1, 14) = 26.20, p < 0.001) and intensity deviants (F(1, 14) = 78.67, p < 0.001).
Finally, there was no interaction on MMN amplitude (F(2, 28) = 1.66, ε = 0.959, p = 0.210). There was a significant interaction between deviant dimension and deviance level on MMN latency (F(2, 28) = 16.47, ε = 0.945, p < 0.001). Post hoc calculations revealed shorter MMN latency with increasing deviance level for intensity (3 < 2 < 1), but not for frequency. F1 had a significantly longer peak latency than both F2 and F3, and the difference between F2 and F3 was not significant (Table 3).
Table 4 displays the mean amplitudes and latencies evoked by all deviants for the P3a component. There was a main effect of deviant dimension (frequency/intensity) on P3a amplitude (F(1, 14) = 13.86, p = 0.002) and on P3a latency (F(1, 14) = 13.77, p = 0.002). Post hoc analyses showed larger P3 amplitude for frequency deviants than intensity deviants (3.11 μV vs. 1.82 μV, respectively), and shorter peak latency for frequency than intensity deviants (322 ms vs. 335 ms, respectively). There was also a main effect of deviance level (1/2/3) on P3a amplitude (F(2, 28) = 19.86, ε = 0.733, p < 0.001), and on P3a latency (F(2, 28) = 22.86, ε = 0.914, p < 0.001). Post hoc calculations revealed that P3a amplitude elicited by deviance level 3 (3.42 μV) was significantly larger than deviance level 2 amplitude (2.50 μV), and amplitudes at both levels 3 and 2 were larger than level 1 (1.47 μV). P3a latencies evoked by deviance levels 3 and 2 (317 ms and 324 ms, respectively) were significantly shorter than those evoked by deviance level 1(342 ms). There was no significant latency difference between level 3 and level 2. The linear trend analysis on P3a latency for deviance level was significant for frequency (F(1, 14) = 13.42, p = 0.003) and for intensity deviants (F(1, 14) = 22.71, p < 0.001).
Finally, there was an interaction between deviance dimension and level on P3a amplitude (F(2, 28) = 10.44, ε = 0.995, p < 0.001), but not on P3a latency (F(2, 28) = 1.75, ε = 0.752, p = 0.202). Post hoc calculations showed that the interaction was due to the P3a amplitude for I3 being significant larger than the amplitudes evoked by both the I2 and I1. In contrast, there was no significant P3a amplitude difference among the three levels of frequency. Consistent with this, the linear trend analysis revealed a significant increase of P3a amplitude with deviance level for intensity deviants (F(1, 14) = 32.23, p < 0.001), but not for frequency (F(1, 14) = 3.19, p = 0.096) (Table 5).
2.2.3. Correlations among behavioral distractions, MMN and P3a (level 3 data analysis)
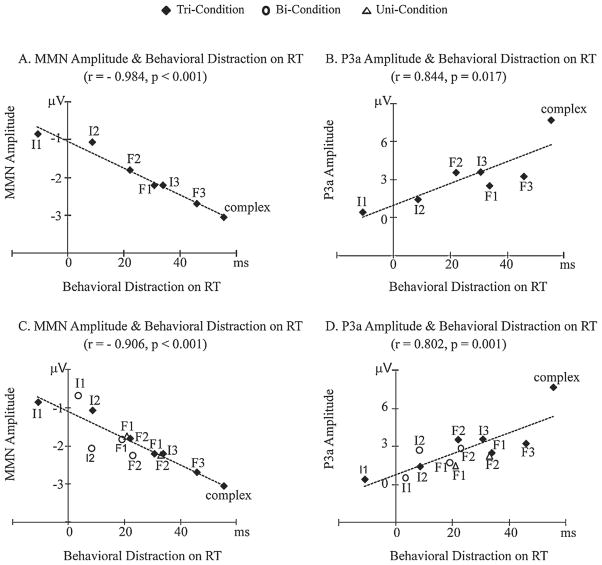
Table 6 and Fig. 4 display the results of the correlation analyses. Pearson’s correlation analysis showed a close relationship among the three measures of auditory distraction, with significant correlations being observed between MMN and behavioral distraction, between P3a and behavioral distraction, and between MMN and P3a. Deviants that evoked larger MMN amplitudes were significantly associated with larger P3a amplitudes; deviants that evoked shorter MMN latencies were significantly associated with shorter P3a latencies; larger amplitude MMN was significantly associated with larger behavioral distraction effects; and larger P3a amplitude was positively associated with larger behavioral distraction effects. Interestingly, the largest correlation was between MMN amplitude and behavioral distraction on RT (r = −0.984, p < 0.001).
Table 6.
Pearson correlation coefficients among different measures of auditory distraction.
| Measure | MMN amplitude | MMN latency | P3a amplitude | P3a latency |
|---|---|---|---|---|
| Experiment 1 alone | ||||
| RT | −.984** | −.800* | .844* | −.869* |
| ER | −.911** | −.772* | .824* | −.781* |
| MMN amplitude | −.851* | .811* | ||
| MMN latency | −.947** | .822* | ||
| Experiment 1 and 2 combined | ||||
| RT | −.906** | −.781** | .802** | −.792** |
| ER | −.847** | −.708** | .839** | −.681** |
| MMN amplitude | −.819** | .741** | ||
| MMN latency | −.831** | .705** | ||
p ≤ 0.05.
p ≤ 0.01.
Fig. 4.
Correlation analyses. (A) MMN amplitude and behavioral RT distraction effects for the Tri-Condition (Experiment 1) only. (B) P3a amplitude and behavioral RT distraction effects for the Tri-Condition (Experiment 1) only. (C) MMN amplitude and behavioral RT distraction effects from both experiments. (D) P3a amplitude and behavioral RT distraction effects from both experiments.
2.3. Discussion
Experiment 1 investigated effects of context on the magnitude response of frequency and intensity when there were multiple deviant types mixed in a blocked presentation. The complex tone, which was qualitatively different than the frequency standard but with the same f0, elicited larger behavioral and electrophysiological distraction effects than all other deviants except for those effects induced by the largest frequency deviance level (F3). This suggests that the complex tone was a largely salient stimulus in the sequence. Behavioral and electrophysiological distraction effects induced by the 3 levels of frequency deviants did not increase linearly with the increase in deviance level. This finding is in contrast to previous findings reported for frequency levels (Berti et al., 2004; Gomes et al., 2000; Rinne et al., 2007). In the previous studies, frequency deviants were presented together but without any other spectral contrast such as the harmonically rich contrast of the complex tone presented in the current study. Thus, the spectral richness of the complex tone likely had a modulating effect on the perceived magnitude of the distracting stimuli, such that physical magnitude of the stimulus was not reflected in the behavioral and electrophysiological indices of distraction. In contrast, a magnitude effect was observed for the levels of intensity deviants, reflected in both behavioral and ERP measures. This result is consistent with other studies that presented multiple levels of intensity increment deviants. Thus, the spectrally rich complex tone modulated the magnitude effect for frequency deviants but had no effect on the magnitude effect along the intensity dimension.
One explanation is that the specific context influences the magnitude effect, in that a comparison amongst the tones occurs. In the current case, the highly salient complex tone could have exerted asymmetrical influence on the processing of frequency and intensity deviants due to the fact that the complex tone was generated by modifying the harmonic structure along the frequency dimension, and thus affecting the spectral but not the intensity dimension. The scalp distribution of the P3a is also consistent with this interpretation, in that a similar frontal–central distribution was observed for P3a to frequency deviants and complex tones (no significant difference was found), but the difference in scalp distribution between intensity and the complex tone was significant.
Alternatively, it is possible that the perceived deviance level among the frequency deviants was different than the perceived level of deviance of the intensity differences, even though the sounds incremented by a logarithm change in both dimensions. Experiment 2 addressed this possibility.
A second main finding, which was unexpected, is that behavioral distraction was more closely associated with the MMN component than with the P3a component. Initially, the significant correlations between MMN and P3a, between MMN and behavioral distraction effects, and between P3a and behavioral distraction effects appeared to be consistent with the three-stage model of auditory distraction. However, the highest correlation coefficient was between MMN amplitude and behavioral distraction effects on RT. This result is not consistent with the three-stage model because the output of the three stages, the behavioral effect, should have a higher correlation with the later of the three stages (the P3a) than with the first of them, due to the inevitable accumulation of random brain noise from each stage. Thus, the current results suggest that auditory distraction may engage parallel mechanisms to the output (behavioral response), which contrasts a simple, successive three stage model.
Alternatively, the smaller correlation between P3a and behavior than between MMN and behavior could have been possible if there were overlap of an N2 component with the P3a component. However, this possibility does not seem likely in our study because there appears to be at least 100 ms time between a negativity (if it is present in the waveform) and the P3a component, suggesting that N2b, if it was elicited, was unlikely to have contributed to the measurement of P3a.
Another possible explanation for the higher correlation between MMN and behavioral distraction effects on RT is that it reflects an association between the N1 and behavioral distraction, as would be the case suggested by Horváth, Czigler, et al. (2008) and Horváth, Winkler, et al. (2008) and by Jacobsen and Schröger (2001). However, this explanation also seems unlikely because the correlation between N1 amplitude and behavioral distraction (r = −0.85, p = 0.015) was comparable to the correlation between P3a amplitude and behavioral distraction (r = 0.84, p = 0.017), and not comparable with the high correlation between MMN and behavioral distraction (r = −0.98, p < 0.001). Thus the N1 and MMN are distinguished as having different albeit overlapping contributions. The relationships between MMN, P3a, and behavioral distraction effects will be further addressed in Experiment 2.
3. Experiment 2 (Bi- and Uni-Conditions)
Experiment 2 explored whether the results of Experiment 1 showing an asymmetrical distraction effect (the absence of a linear effect for frequency but not for intensity) could be explained by the contextual influence of spectral quality of the complex tone on distraction due to frequency deviants. Experiment 2 thus tested the hypothesis that the presence of the complex harmonic tone modulated the magnitude effect for frequency. To that end, we predicted that if the complex tone were removed from the sequences, the frequency magnitude effect should then be observed. Experiment 2 therefore presented one condition in which frequency and intensity deviants were presented with the standard tone (Bi-Condition), and a second condition that contained only frequency deviants and the standard tone (Uni-Condition). The Uni-Condition thus provided a measure of the frequency magnitude effect without any potential influence of intensity deviants. Considering that a magnitude effect on intensity was found in the presence of frequency deviants in the Tri-Condition of Experiment 1, the Uni-Condition was expected to provide a control for the Bi-Condition, by showing similar magnitude effects. A secondary goal of Experiment 2 was to assess the relationship between the behavioral and electro-physiological measures, especially to determine whether the high correlation between MMN and behavioral distraction effects would be replicated under the different contextual conditions of Experiment 2. If the unexpected findings in Experiment 1 were reduced or eliminated in Experiment 2, then it may be attributed to the unique context presentation of sounds in Experiment 1 rather than to an over-all relationship between MMN and behavioral measures of distraction.
3.1. Method
3.1.1. Participants
Thirteen healthy adults participated in Experiment 2 (9 females and 4 males, M = 28 y, SD = 5.4 y) in a separate session approximately three weeks after Experiment 1. One participant did not finish the experiment due to excessive response errors, and a second participant’s data was excluded from analysis due to excessive eye artifact. The data from the remaining 11 participants were analyzed and are reported. Eight of the eleven subjects also participated in Experiment 1. All participants gave written informed consent prior to the testing session, after the study protocol was explained. The study protocol was conducted in agreement with the Declaration of Helsinki guidelines, and was approved by the Albert Einstein College of Medicine Internal Review Board. All new participants passed a hearing screen at 20 dB HL for 500, 1000, 2000, and 4000 Hz in both ears, and reported no history of neurological disorders.
3.1.2. Stimuli and procedure
Table 1 provides a schematic of the stimulus conditions of Experiment 2. Two conditions were conducted (Uni- and Bi-Conditions), requiring participants to perform the same forced-choice task of Experiment 1: for each tone, press a designated response key when the shorter tone is detected, and a different key when the longer tone is detected. In the Bi-Condition, standard tones (80%) were randomly mixed together with two of the frequency deviants (F1 and F2) and two of the intensity deviants (I1 and I2) from Experiment 1. Each deviant occurred with 0.05 probability. F3 and I3 deviants were not included in Experiment 2 because the main influence exerted by the complex tone in Experiment 1 affected the two smaller level deviants, and there was no significant difference in the behavioral distraction effect between the F3 deviant and complex tone. Thus, we sought to explore magnitude effects among the smaller level deviants that significantly differed from both the larger level deviants and the complex tone. In the Uni-Condition, the standard tones (80%) were mixed with two frequency deviants (F1 and F2), which occurred with 10% probability each. No intensity deviants were presented in the Uni-Condition. The experimental session was approximately 2.5 h, which included electrode placement and breaks.
After practice, 12 test runs were presented for the Bi-Condition and 6 test runs for the Uni-Condition, yielding 90 tones for each deviant type in each condition, 1440 standard tones in the Bi-Condition and 900 standard tones in the Uni-Condition. The order of Bi-and Uni-Conditions was balanced across participants to avoid order effects. All other procedures and data recording parameters were the same as described for Experiment 1.
3.1.3. Data analysis
Data analysis proceeded in the same way as described in Experiment 1. Peak latencies used to statistically measure the MMN amplitude were 136 ms (F1), 144 ms (F2), 150 ms (I1) and 150 ms (I2) in the Bi-Condition; and 138 ms (F1) and 130 ms (F2) in the Uni-Condition. Peak latencies used to statistically measure the P3a component were 330 ms (F1), 320 ms (F2), 330 ms (I1) and 336 ms (I2) in the Bi-Condition; and 338 ms (F1) and 312 ms (F2) in the Uni-Condition.
One-sample two-tailed t-tests were conducted to determine whether distraction effects (the difference between the response to the deviant and the response to the standard) were significantly different from zero. Two-way repeated measures ANOVAs were then conducted to (1) compare distraction effects induced by frequency and intensity deviants within the Bi-Condition, using factors of deviant dimension (frequency vs. intensity) and deviance level (1 vs. 2); and (2) to compare distraction effects for frequency deviant responses across the Bi- and Uni-Conditions using factors of condition (Bi- vs. Uni-) and deviance level (1 vs. 2). Behavioral and ERP indices were calculated in separate ANOVAs.
Additionally, distraction effects induced by the frequency deviants were compared across both experiments (Tri-/Bi-/Uni-Conditions), but only for those who participated in both experiments thus using a within-subjects design. Behavioral performance (RT and ER) were first calculated using one-way repeated measures ANOVA on the behavioral measures obtained by the standard tones in all conditions to verify that there were no performance differences between the two experiments. Two-way repeated-measures ANOVAs were then calculated on the participants that participated in both experiments to compare effects across experiments. ANOVAs assessed (1) distraction effects of frequency using factors of condition (Tri-, Bi- and Uni-Condition) and deviance level (1/2); and (2) distraction effects of intensity using factors of condition (Tri- and Bi-Condition) and deviance level (1/2).
Finally, Pearson correlation coefficients were calculated to test the association between the ERP indices and behavioral distraction effects, using the combined data of Experiments 1 and 2. The grand-mean amplitude was obtained separately for each of the 7 deviants from Experiment 1 and for each of the 6 deviants from Experiment 2, resulting in 13 data points for each measure. The 13 pairs of data were used to calculate each correlation coefficient.
3.2. Results
Table 2 summarizes the behavioral distraction effects for all conditions. In both the Bi- and Uni-Conditions, the one-sample t-tests revealed that both levels of frequency deviance (F1 and F2) elicited significant behavioral distraction effects on RT (RT to deviants were longer than to standards). Only F2 elicited a significant behavioral distraction effect on ER (ER to deviants was higher than ER to standards).
Fig. 5 displays the grand-mean ERP waveforms and the deviant-minus-standard difference waveforms at the Fz electrode. Fig. 6 displays the scalp voltage maps from the difference waveforms. Table 3 (MMN) and Table 4 (P3a) summarize the ERP component amplitudes and latencies. As in Experiment 1, all deviants elicited significant MMNs in both conditions (Table 3). In the Bi-Condition, P3a was significantly elicited by both levels of frequency deviance (F1 and F2) and by the larger intensity deviant (I2). In the Uni-Condition, P3a was elicited by the F2 deviant but not by the F1 deviant.
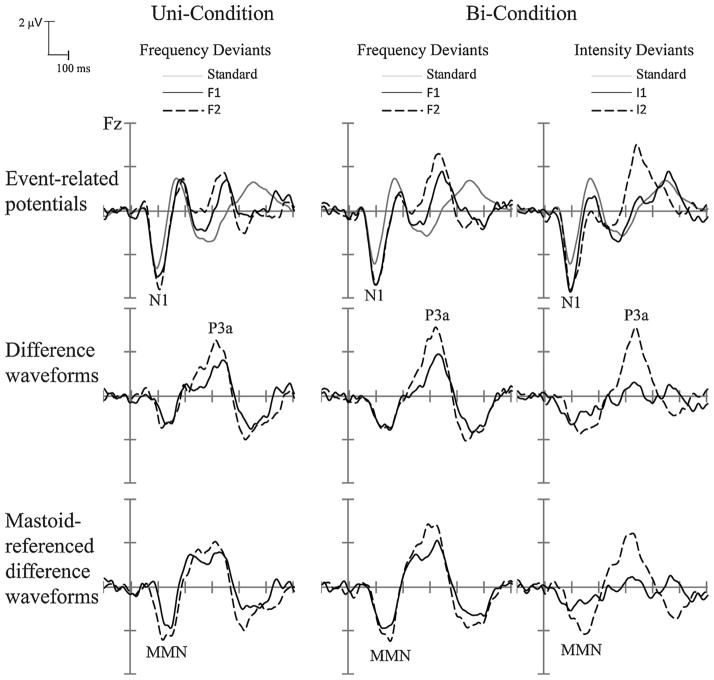
Fig. 5.
Experiment 2: Grand-mean ERP waveforms (top row) elicited by F1 deviant (solid black line), F2 deviant (dashed black line), and the standard (solid gray line) tones in the Uni-Condition (left column), and Bi-Condition (middle column); and for the I1 deviant (solid black line), I2 deviant (dashed black line), and the standard tones (solid gray line) in the Bi-Condition (right), with N1 components labeled. Deviant-minus-standard difference waveforms (middle row) are displayed for the F1 (solid black line) and F2 (dashed black line) deviants and for I1 (solid black line) and I2 (dashed black line) deviants to delineate P3a components (labeled). Mastoid-referenced difference waves (bottom row) are displayed to delineate the MMN components (labeled).
Fig. 6.
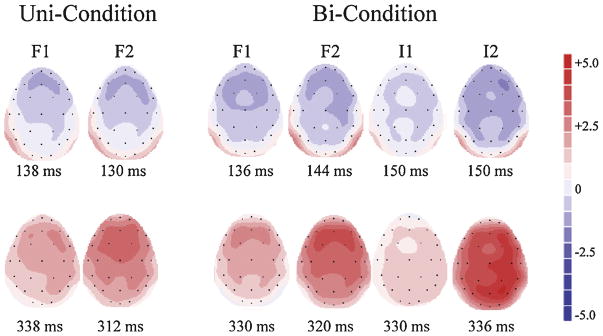
Experiment 2: Scalp voltage maps are displayed, showing the peak latency in the difference waveforms used to measure the MMN (top) and P3a (bottom) components for the F1/F2 deviants in the Uni-Condition (left two columns), and for the F1/F2 deviants in the Bi-Condition (middle two columns), and the I1/I2 deviants in the Bi-Condition (right two columns).
3.2.1. Comparing frequency and intensity deviants within the Bi-Condition
3.2.1.1. Behavioral distraction effects
There was a significant main effect of deviant dimension (frequency/intensity) on RT (F(1, 10) = 9.21, p = 0.013) and on ER (F(1, 10) = 11.02, p = 0.008). Post hoc calculations showed that frequency deviants had larger RT distraction effects than intensity deviants (21 ms vs. 6 ms, respectively), as well as larger ER distraction effects (0.035 vs. −0.004, respectively). There was also a main effect of deviance level (1/2) on ER (F(1, 10) = 13.08, p = 0.005), with post hoc analysis showing that level 2 deviance had higher ER than level 1 deviance (0.032 vs. −0.001, respectively). There were no other main effects or interactions (p > 0.20).
3.2.1.2. ERP distraction effects
MMN amplitude elicited by level 2 deviants was numerically larger than by level 1 deviants (−2.2 μV vs. −1.3 μV, respectively), but this difference did not reach significance (F(1, 10) = 4.89, p = 0.051). The main effect of deviance level on the P3a amplitude was significant (F(1, 10) = 34.18, p < 0.001). Post hoc calculations showed that level 2 deviants were larger in amplitude than P3a evoked by level 1 deviants (2.8 μV vs. 1.1 μV, respectively). There was also a main effect of deviant dimension (frequency/intensity) on P3a latency (F(1, 10) = 8.34, p = 0.016). Post hoc calculations showed that frequency deviants had shorter latencies than intensity deviants (319 ms vs. 329 ms, respectively). There were no other main effects or interactions (p > 0.075).
3.2.2. Comparing frequency deviants across the Bi- and Uni-Conditions
3.2.2.1. Behavioral distraction effects
There was a main effect of condition on RT (F(1, 10) = 9.02, p = 0.013). Post hoc calculations showed that RT distraction effects were larger in the Uni-Condition than in the Bi-Condition (27 ms vs. 21 ms, respectively). In contrast, ER was not significantly different between the two conditions (F(1, 10) = 2.77, p = 0.127) (0.035 vs. 0.021). There was also a main effect of deviance level (1/2) on RT (F(1, 10) = 9.84, p = 0.011) and on ER (F(1, 10) = 9.27, p = 0.012). Post hoc analysis revealed that F2 elicited a larger RT distraction effect than F1 (28 ms vs. 20 ms, respectively). F2 also evoked a larger ER distraction effect than F1 (0.042 vs. 0.014, respectively). There were no other main effects or interactions (p > 0.10).
3.2.2.2. ERP distraction effect
There was a main effect of deviance level (1/2) on P3a amplitude (F(1, 10) = 7.88, p = 0.019) and on P3a latency (F(1, 10) = 18.46, p = 0.002). F2 deviants elicited larger P3a amplitudes than F1 deviants (2.54 μV vs. 1.59 μV, respectively). P3a latency was shorter to F2 than F1 deviants (313 ms vs. 328 ms, respectively). There were no other main effects or interactions (p > 0.10).
3.2.3. Comparing distraction effects among Tri/Bi/Uni-Conditions (Experiments 1 and 2)
3.2.3.1. Behavioral results
Behavioral performance did not differ as a function of condition for the standard tones in either RT (F(2, 14) = 0.40, p = 0.59) or ER (F(2, 14) = 1.89, p = 0.20): RT to the standard tones in Tri-Condition: M = 469 ms; Bi-Condition: M = 479 ms; and Uni-Condition: M = 482 ms. ER to standard tones in the Tri-Condition: M = 0.097; Bi-Condition: M = 0.069; and Uni-Condition: M = 0.061. These results verify that differences in distraction effects were not due to differences in the standard in the different conditions obtained in different sessions.
Comparing behavioral distraction effects for frequency deviants for the eight participants common to both experiments, there was a significant interaction between condition (Tri/Bi/Uni) and deviance level (1/2) on RT (F(2, 14) = 11.98, ε = 0.813, p = 0.002). Post hoc calculations showed that the interaction was due to F1 having a larger RT distraction effect than F2 in the Tri-Condition, but not in the Bi- or in the Uni-Condition (Table 2). ANOVAs calculated on the common intensity level deviants (I1 and I2) in the Bi-and Tri-Condition also revealed a significant interaction between condition and deviance level (F(1, 7) = 15.52, p < 0.006). Post hoc calculations revealed a smaller distraction effect on I1 than I2 in the Tri-Condition, but not in the Bi-Condition. There were no other significant effects involving condition.
3.2.3.2. ERP results
There was a main effect of condition on frequency deviants for P3a amplitude (F(2, 14) = 8.03, ε = 0.932, p = 0.006). Post hoc calculations showed that frequency deviants had larger P3a amplitude in the Tri-Condition (3.2 μV) than in the Bi- (2.0 μV) or Uni- (1.5 μV) conditions (Table 4 and Fig. 7). There were no other significant effects involving condition for frequency or intensity deviants on P3a amplitude. There were no significant effects of condition on MMN when comparing across the three conditions.
Fig. 7.
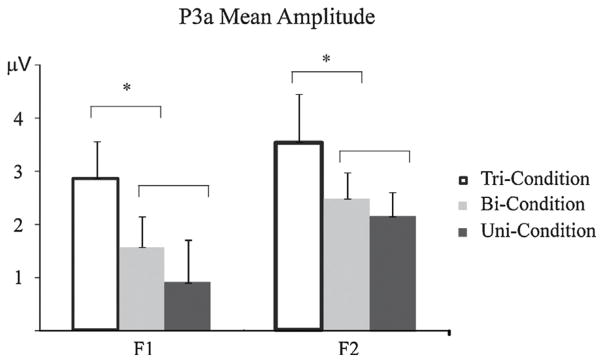
Comparisons of the P3a mean amplitudes for both experiments. F1 (left) and F2 (right) deviants elicited in the Tri-Condition (unfilled), Bi-Condition (light filled), and Uni-Condition (dark filled). Error bars denote standard error of the mean. P3a mean amplitude was calculated and is displayed for those subjects who participated in both experiments (n = 8). P3a amplitude was significantly larger in the Tri-Condition than in the Bi- and Uni-Conditions for both level frequency deviants.
3.2.4. Correlation results
Correlation analysis showed significant associations among MMN, P3a and behavioral distraction effects (Table 6 and Fig. 4). As with the Tri-Condition of Experiment 1, the strongest correlation was found between MMN amplitude and behavioral distraction on RT.
3.3. Discussion
Experiment 2 further tested whether distraction effects found in Experiment 1 were modulated by the sound context. Specifically, we tested whether the asymmetrical effect of magnitude, between frequency and intensity deviants, found in Experiment 1 was contributed by influences from the complex tone being present in the sequence. The results of Experiment 2 were consistent with this hypothesis. Frequency deviants in Experiment 2 showed a magnitude effect, an increase in the magnitude of the distraction effect along with an increase in the magnitude of the stimulus difference between the standard and deviant, which was not found in the presence of the complex tone in Experiment 1. Thus, results from Experiment 2 indicate that context modulates behavioral and electrophysiological indices of distraction. The observed asymmetrical effect on distraction found in Experiment 1 is likely attributed to the spectral complexity of the complex tone exerting influence on the frequency dimension but not on intensity.
3.3.1. Bi-Condition
The main result of the Bi-Condition was that larger magnitude deviants evoked larger distraction effects. Both MMN and P3a amplitudes were larger for the larger magnitude deviants, irrespective of dimension (frequency or intensity). Behavioral indices of distraction, on the other hand, were less straightforward. Behavioral distraction effects reflected in ER were consistent with the ERP indices: larger magnitude deviants resulted in higher error rates for deviants compared to standards. However, there was an interaction with stimulus dimension on RT in that frequency deviants, overall, evoked larger behavioral distraction effects in RT than did intensity deviants. This means that reaction time was longer to frequency deviants compared to standards, whereas reaction time to intensity deviants was sometimes shorter than to standards. For example, the I1 RT distraction effect in the Tri-Condition was −11 ms, which means there was a shorter RT to the deviant than to the standard; and the corresponding I1 ER distraction effect in the Tri-Condition was also a negative value (−0.01), indicating a lower error rate to deviants than standards. One possible explanation is that there is a confound in response between loudness and reaction time. That is, it is possible that participants responded faster to sounds with higher intensity values, regardless of whether the tones were standards or deviants. Because all intensity deviants were louder sounds, this could have resulted in faster responses to the louder intensity deviants than to the standards, which would have offset any distraction effect. To confirm this explanation, a test of magnitude for loudness decrements is needed. Nonetheless, a faster response to louder tones in the current study could have confounded the distraction effects elicited by intensity deviants, leading to what would appear to be a smaller behavioral distraction effect for intensity deviants compared to frequency.
However, the main result pertained to the frequency dimension: when the complex tone was removed from the sequence, distraction effects increased with deviance level for the frequency deviants. This is the same pattern that was observed for intensity deviants in both the Bi- and Tri-Condition. This result indicates that the presence of the complex tone exerted influence on the spectral dimension, modulating the magnitude effect, irrespective of the perceived deviance level between frequency and intensity deviants.
3.3.2. Uni-Condition
The Uni-Conditions also tested whether the mix of frequency and intensity deviants (without the complex tone) may have affected the magnitude response observed in Experiment 1. However, we found the same magnitude effects for frequency level deviants presented without intensity intermixed in the sequence in the Uni-Condition as we did in the Bi-Condition when frequency was mixed together with intensity. Further, the magnitude effect found for frequency in the Bi- and Uni-Conditions is consistent with previous studies that also presented differing levels of frequency (Berti et al., 2004; Gomes et al., 2000; Rinne et al., 2007). Thus, the results indicate that intermixing intensity with frequency deviants was not the crucial factor modulating the magnitude effect for frequency that was found in the Tri-Condition (Experiment 1).
3.3.3. Correlation analysis
Consistent with Experiment 1, the strongest correlation was found between MMN amplitude and RT. This again suggests that MMN amplitude is more strongly associated with behavioral distraction than is P3a amplitude, and contradict predictions made by a three-stage model of auditory distraction.
4. General discussion
The purpose of the study was to investigate whether distraction effects elicited by irrelevant changes in auditory input would be modulated by the surrounding context. The results from both experiments demonstrate influence of the sound context on the magnitude effect. That is, sounds were perceived as more or less distracting depending upon what other sounds were presented in the sequence. Specifically in this study, mixing a complex tone with different levels of pure tone frequency deviants (Tri-Condition) modulated the degree of distraction evoked by the pure tone frequency deviants found when the complex tone was not mixed into the sequence (Bi- and Uni-Conditions). In the Tri-Condition, in which frequency, intensity, and the complex tone were intermixed, there was a linear increase in the distraction effect but only for the intensity deviants, not for frequency. In the Bi-Condition, in which the complex tone was removed from the sequence, the magnitude effect, the linear effect of distraction was observed for both frequency and intensity deviants. The magnitude effect was observed also when frequency deviants were presented without intensity deviants (Uni-Condition). Together, these findings indicate that the complex tone influenced processing of the spectral dimension of the pure tones.
4.1. Attentional bias
One explanation for how the complex tone modified the processing of frequency deviants is that there was an attentional bias toward the frequency dimension when the complex tone was present. The complex tone was not deviant in frequency but rather was different qualitatively, and was highly salient amongst the pure tones. This may have set up an attentional bias toward processing changes along the spectral dimension of the sounds considering that the complex deviant was generated by modifying the spectral content of the tone. Further indication that there was an attentional bias toward the frequency dimension in the presence of the complex tone is suggested by the larger P3a amplitude elicited by frequency deviants in the Tri-Condition compared to the Bi- and Uni-Conditions. In contrast, this effect was not found for MMN amplitude. MMN amplitude did not significantly change when elicited by frequency deviants across the three conditions. This latter result indicates that an attentional bias may not affect the deviance detection process, consistent with the literature that MMN elicitation is generally not affected by attending to the deviants (Rinne, Antila, & Winkler, 2001; Sussman, 2007; Sussman et al., 2003).
4.2. Perceptual contrast
Although attentional bias may explain the larger P3a amplitude for frequency deviants in the Tri-Condition compared to the Bi- and Uni-Conditions, this mechanism alone is not sufficient to explain the absence of the linear increase in distraction with deviance level for frequency deviants in the Tri-Condition. Another mechanism, perceptual contrast, may have interacted with attention to modulate processing of the frequency deviants in the Tri-Condition. That is, the inclusion of the highly salient complex tone in the Tri-Condition may have reduced the perceptual difference amongst the three frequency deviants (F1, F2 and F3), reducing or eliminating the magnitude effects otherwise observed, as shown in results of the Bi- and Uni-Conditions. The complex tone may have acted as an extreme value level along the frequency dimension, thus having no effect on the intensity level dimension. Thus, taken together, the results may be explained by the presence of the complex tone setting up an attentional bias toward the frequency dimension, and the saliency of the complex tone in the mix altering the perceived magnitude for frequency.
4.3. Correlation between MMN and RT
Another issue still to be considered is the unexpected finding that behavioral distraction was more strongly associated with the MMN than with the P3a component. Significant correlations were found between P3a and behavior, as well as between MMN and P3a. However, the strongest correlations of the current study were between MMN amplitude and behavioral distraction effects, replicated in both experiments. This result suggests that there are parallel processes influencing distraction effects observed in the electrophysiological responses. Several findings from the current study support an association between MMN and behavior. Firstly, in the Tri-Condition, the complex tone evoked a significantly larger P3a amplitude than the largest frequency deviant (F3), but no such MMN amplitude difference or behavioral distraction effects were found between the complex tone and F3. Secondly, P3a amplitude to the frequency deviants was larger in the Tri-Condition than in the Bi- or Uni-Conditions. However, behavioral distraction effects and MMN amplitudes were not larger in the Tri- compared to Bi-and Uni-Conditions. Thirdly, F2, which had a numerically smaller behavioral distraction effect and smaller MMN amplitude than F1 and F3 in the Tri-Condition, elicited a numerically larger P3a amplitude compared to F1 and F3. Together, these findings indicate a consistency between MMN and behavioral distraction effects, and a dissociation between P3a and behavioral distraction effects, suggesting that MMN, and not P3a, may be more strongly associated with behavioral distraction.
The current findings are consistent with Rinne et al. (2006), who reported significant MMN components and behavioral distraction effects elicited by intensity decrements even though no P3a component was elicited by them. Although Rinne et al. also tested intensity increments, the relationship among MMN, P3a and behavioral distraction was less clear. In contrast, P3a amplitude has been reported to co-vary with behavioral distraction effects in previous studies (Berti et al., 2004; Gomes et al., 2000). That is, studies have reported that P3a was present along with behavioral distraction effects, and the absence of P3a when behavioral distraction effects were absent (Sussman et al., 2003; Horváth, Sussman, Winkler, & Schröger, 2011). To accommodate these, and also the current findings, we propose that the P3a may not be on the serial processing pathway from MMN to behavioral distraction, even if P3a and behavioral distraction may follow some shared attentional processes occurring after MMN.
Acknowledgments
This research was funded by the Department of Defense (BAA-080001) and the National Institutes of Health (DC004263).
References
- Berti S, Roeber U, Schröger E. Bottom-up influences on working memory: Behavioral and electrophysiological distraction varies with distractor strength. Experimental Psychology. 2004;51:249–257. doi: 10.1027/1618-3169.51.4.249. [DOI] [PubMed] [Google Scholar]
- Doeller C, Opitz B, Mecklinger A, Krick C, Reith W, Schröger E. Prefrontal cortex involvement in preattentive auditory deviance detection: Neuroimaging and electrophysiological evidence. Neuroimage. 2003;20:1270–1282. doi: 10.1016/S1053-8119(03)00389-6. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: An event-related brainpotential (ERP) sign of the brain’s evaluation of novelty. Neuroscience and biobehavioral reviews. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Gomes H, Molholm S, Ritter W, Kurtzberg D, Cowan N, Vaughan HG., Jr Mismatch negativity in children and adults, and effects of an attended task. Psychophysiology. 2000;37:807–816. [PubMed] [Google Scholar]
- Horváth J, Czigler I, Jacobsen T, Maess B, Schröger E, Winkler I. MMN or no MMN: No magnitude of deviance effect on the MMN amplitude. Psychophysiology. 2008;45:60–69. doi: 10.1111/j.1469-8986.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- Horváth J, Winkler I, Bendixen A. Do N1/MMN, P3a, and RON form a strongly coupled chain reflecting the three stages of auditory distraction? Biological Psychology. 2008;79:139–147. doi: 10.1016/j.biopsycho.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Horváth J, Sussman E, Winkler I, Schröger E. Preventing distraction: Assessing stimulus-specific and general effects of the predictive cueing of deviant auditory events. Biological Psychology. 2011;87:35–48. doi: 10.1016/j.biopsycho.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen T, Schröger E. Is there pre-attentive-based comparison of pitch? Psychophysiology. 2001;38:723–727. [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Knight RT, Scabini D. Anatomic bases of event-related potentials and their relationship to novelty detection in humans. Journal of Clinical Neurophysiology. 1998;15:3–13. doi: 10.1097/00004691-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Kisley MA, Davalos DB, Layton HS, Pratt D, Ellis JK, Seger CA. Small changes in temporal deviance modulate mismatch negativity amplitude in humans. Neuroscience Letters. 2004;358:197–200. doi: 10.1016/j.neulet.2004.01.042. [DOI] [PubMed] [Google Scholar]
- Näätänen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. The Behavioral and Brain Sciences. 1990;13:201–288. [Google Scholar]
- Rinne T, Kirjavainen S, Salonen O, Degerman A, Kang X, Woods DL, et al. Distributed cortical networks for focused auditory attention and distraction. Neuroscience Letters. 2007;416:247–251. doi: 10.1016/j.neulet.2007.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne T, Särkkä A, Degerman A, Schröger E, Alho K. Two separate mechanisms underlie auditory change detection and involuntary control of attention. Brain Research. 2006;1077:135–143. doi: 10.1016/j.brainres.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Rinne T, Antila S, Winkler I. Mismatch negativity is unaffected by top-down predictive information. Neuroreport. 2001;12:2209–2213. doi: 10.1097/00001756-200107200-00033. [DOI] [PubMed] [Google Scholar]
- Ruhnau P, Wetzel N, Widmann A, Schröger E. The modulation of auditory novelty processing by working memory load in school age children and adults: A combined behavioral and event-related potential study. BMC Neuroscience. 2010;11:126–139. doi: 10.1186/1471-2202-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröger E, Giard MH, Wolff C. Auditory distraction: Event-related potential and behavioral indices. Clinical Neurophysiology. 2000;111:1450–1460. doi: 10.1016/s1388-2457(00)00337-0. [DOI] [PubMed] [Google Scholar]
- Schröger E, Wolff C. Attentional orienting and reorienting is indicated by human event-related brain potentials. Neuroreport. 1998;9:3355–3358. doi: 10.1097/00001756-199810260-00003. [DOI] [PubMed] [Google Scholar]
- Sonnadara RR, Alain C, Trainor LJ. Effects of spatial separation and stimulus probability on the event-related potentials elicited by occasional changes in sound location. Brain Research. 2006;1071:175–185. doi: 10.1016/j.brainres.2005.11.088. [DOI] [PubMed] [Google Scholar]
- Sussman E. A new view on the MMN and attention debate: The role of context in processing auditory events. Journal of Psychophysiology. 2007;21:164–175. [Google Scholar]
- Sussman E, Winkler I, Schröger E. Top-down control over involuntary attention switching in the auditory modality. Psychonomic Bulletin & Review. 2003;10:630–637. doi: 10.3758/bf03196525. [DOI] [PubMed] [Google Scholar]
- Tse CY, Penney TB. On the functional role of temporal and frontal cortex activation in passive detection of auditory deviance. Neuroimage. 2008;41:1462–1470. doi: 10.1016/j.neuroimage.2008.03.043. [DOI] [PubMed] [Google Scholar]
- Wetzel N, Widmann A, Schröger E. The development of involuntary and voluntary attention from childhood to adulthood: A combined behavioral and event-related potential study. Clinical Neurophysiology. 2006;117:2191–2203. doi: 10.1016/j.clinph.2006.06.717. [DOI] [PubMed] [Google Scholar]