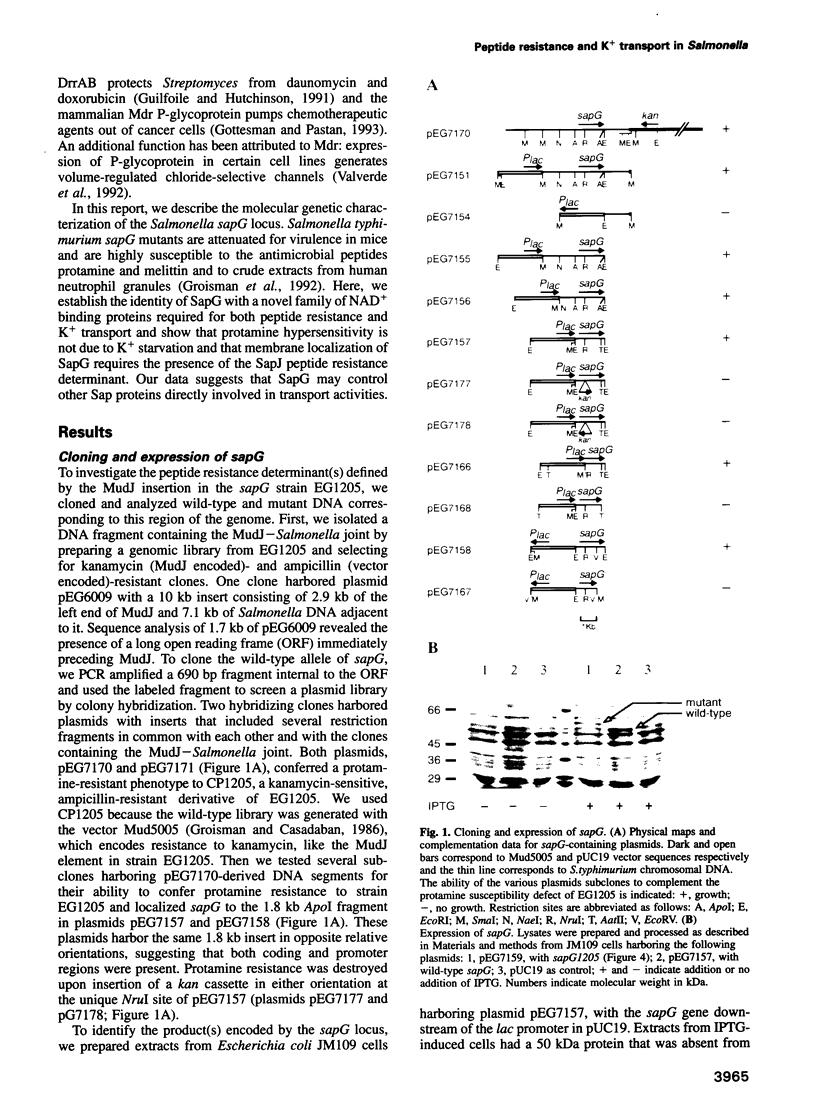
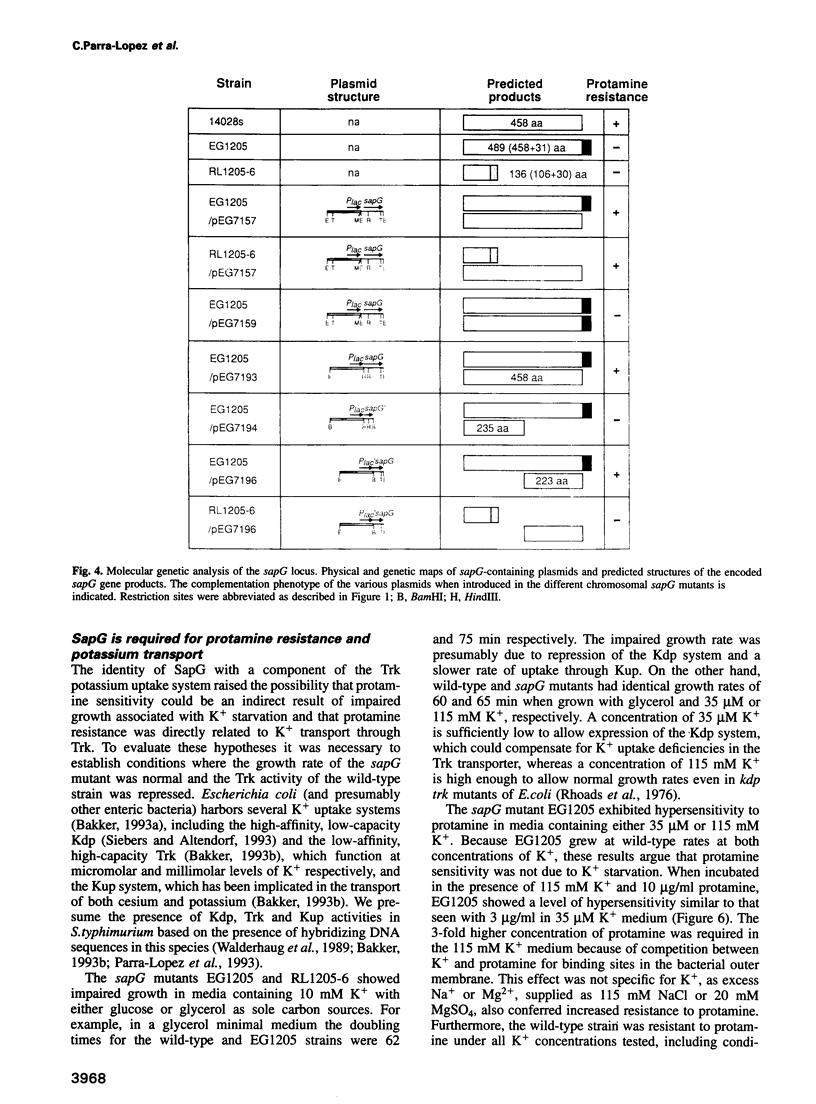
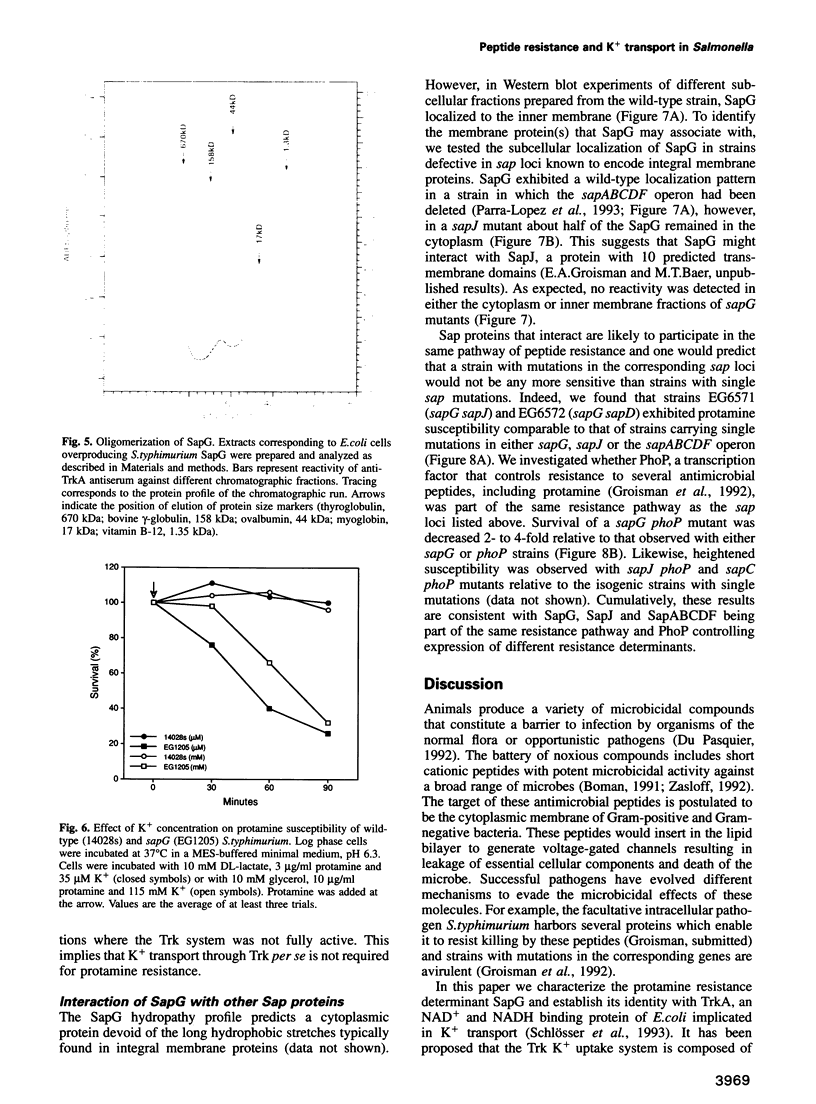
Abstract
The ability of invading pathogens to proliferate within host tissues requires the capacity to resist the killing effects of a wide variety of host defense molecules. sap mutants of the facultative intracellular parasite Salmonella typhimurium exhibit hypersensitivity to antimicrobial peptides, cannot survive within macrophages in vitro and are attenuated for mouse virulence in vivo. We conducted a molecular genetic analysis of the sapG locus and showed that it encodes a product that is 99% identical to the NAD+ binding protein TrkA, a component of a low-affinity K+ uptake system in Escherichia coli. SapG exhibits similarity with other E. coli proteins implicated in K+ transport including KefC, a glutathione-regulated efflux protein, and Kch, a putative transporter similar to eukaryotic K+ channel proteins, sapG mutants were killed by the antimicrobial peptide protamine in the presence of both high and low K+, indicating that protamine hypersensitivity is not due to K+ starvation. Strains with mutations in sapG and either sapJ or the sapABCDF operon were as susceptible as sapG single mutants, suggesting that the proteins encoded by these loci participate in the same resistance pathway. SapG may modulate the activities of SapABCDF and SapJ to mediate the transport of peptides and potassium.
Full text
PDF


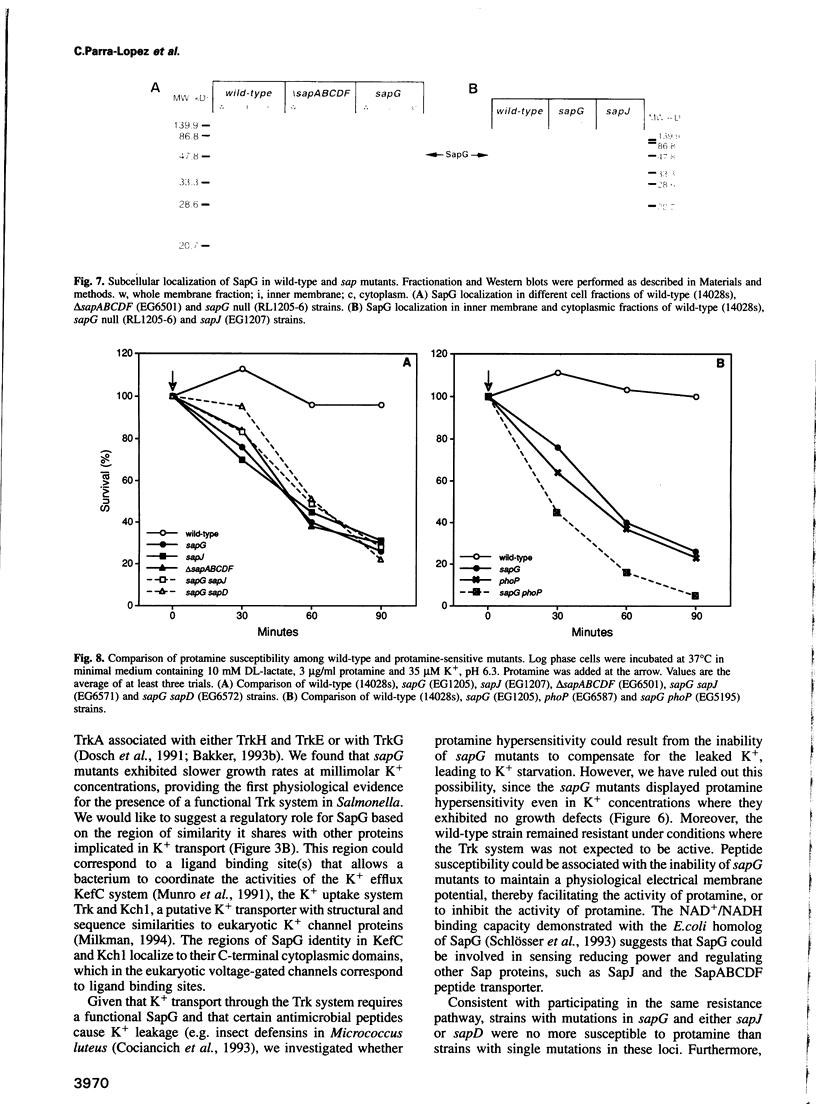


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boman H. G. Antibacterial peptides: key components needed in immunity. Cell. 1991 Apr 19;65(2):205–207. doi: 10.1016/0092-8674(91)90154-q. [DOI] [PubMed] [Google Scholar]
- Cociancich S., Ghazi A., Hetru C., Hoffmann J. A., Letellier L. Insect defensin, an inducible antibacterial peptide, forms voltage-dependent channels in Micrococcus luteus. J Biol Chem. 1993 Sep 15;268(26):19239–19245. [PubMed] [Google Scholar]
- Cruciani R. A., Barker J. L., Zasloff M., Chen H. C., Colamonici O. Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3792–3796. doi: 10.1073/pnas.88.9.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doige C. A., Ames G. F. ATP-dependent transport systems in bacteria and humans: relevance to cystic fibrosis and multidrug resistance. Annu Rev Microbiol. 1993;47:291–319. doi: 10.1146/annurev.mi.47.100193.001451. [DOI] [PubMed] [Google Scholar]
- Dosch D. C., Helmer G. L., Sutton S. H., Salvacion F. F., Epstein W. Genetic analysis of potassium transport loci in Escherichia coli: evidence for three constitutive systems mediating uptake potassium. J Bacteriol. 1991 Jan;173(2):687–696. doi: 10.1128/jb.173.2.687-696.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier L. Origin and evolution of the vertebrate immune system. APMIS. 1992 May;100(5):383–392. doi: 10.1111/j.1699-0463.1992.tb00888.x. [DOI] [PubMed] [Google Scholar]
- Eisenhauer P. B., Harwig S. S., Lehrer R. I. Cryptdins: antimicrobial defensins of the murine small intestine. Infect Immun. 1992 Sep;60(9):3556–3565. doi: 10.1128/iai.60.9.3556-3565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer P. B., Lehrer R. I. Mouse neutrophils lack defensins. Infect Immun. 1992 Aug;60(8):3446–3447. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Kim B. S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P. I., Groisman E. A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Gill D. R., Hyde S. C., Higgins C. F., Valverde M. A., Mintenig G. M., Sepúlveda F. V. Separation of drug transport and chloride channel functions of the human multidrug resistance P-glycoprotein. Cell. 1992 Oct 2;71(1):23–32. doi: 10.1016/0092-8674(92)90263-c. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Groisman E. A., Casadaban M. J. Mini-mu bacteriophage with plasmid replicons for in vivo cloning and lac gene fusing. J Bacteriol. 1986 Oct;168(1):357–364. doi: 10.1128/jb.168.1.357-364.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman E. A. In vivo genetic engineering with bacteriophage Mu. Methods Enzymol. 1991;204:180–212. doi: 10.1016/0076-6879(91)04010-l. [DOI] [PubMed] [Google Scholar]
- Groisman E. A., Parra-Lopez C., Salcedo M., Lipps C. J., Heffron F. Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11939–11943. doi: 10.1073/pnas.89.24.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman E. A., Saier M. H., Jr Salmonella virulence: new clues to intramacrophage survival. Trends Biochem Sci. 1990 Jan;15(1):30–33. doi: 10.1016/0968-0004(90)90128-x. [DOI] [PubMed] [Google Scholar]
- Groisman E. A., Sturmoski M. A., Solomon F. R., Lin R., Ochman H. Molecular, functional, and evolutionary analysis of sequences specific to Salmonella. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1033–1037. doi: 10.1073/pnas.90.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoile P. G., Hutchinson C. R. A bacterial analog of the mdr gene of mammalian tumor cells is present in Streptomyces peucetius, the producer of daunorubicin and doxorubicin. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8553–8557. doi: 10.1073/pnas.88.19.8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemstra P. S., Eisenhauer P. B., Harwig S. S., van den Barselaar M. T., van Furth R., Lehrer R. I. Antimicrobial proteins of murine macrophages. Infect Immun. 1993 Jul;61(7):3038–3046. doi: 10.1128/iai.61.7.3038-3046.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Ganz T., Albert J., Rotrosen D. The opsonizing ligand on Salmonella typhimurium influences incorporation of specific, but not azurophil, granule constituents into neutrophil phagosomes. J Cell Biol. 1989 Dec;109(6 Pt 1):2771–2782. doi: 10.1083/jcb.109.6.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan B. L., Selsted M. E., Ganz T., Lehrer R. I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci U S A. 1990 Jan;87(1):210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Lichtenstein A. K., Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- Macario A. J., Dugan C. B., Conway de Macario E. An archaeal trkA homolog near dnaK and dnaJ. Biochim Biophys Acta. 1993 Dec 14;1216(3):495–498. doi: 10.1016/0167-4781(93)90022-6. [DOI] [PubMed] [Google Scholar]
- Milkman R. An Escherichia coli homologue of eukaryotic potassium channel proteins. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3510–3514. doi: 10.1073/pnas.91.9.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro A. W., Ritchie G. Y., Lamb A. J., Douglas R. M., Booth I. R. The cloning and DNA sequence of the gene for the glutathione-regulated potassium-efflux system KefC of Escherichia coli. Mol Microbiol. 1991 Mar;5(3):607–616. doi: 10.1111/j.1365-2958.1991.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Narva K. E., Feitelson J. S. Nucleotide sequence and transcriptional analysis of the redD locus of Streptomyces coelicolor A3(2). J Bacteriol. 1990 Jan;172(1):326–333. doi: 10.1128/jb.172.1.326-333.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Lopez C., Baer M. T., Groisman E. A. Molecular genetic analysis of a locus required for resistance to antimicrobial peptides in Salmonella typhimurium. EMBO J. 1993 Nov;12(11):4053–4062. doi: 10.1002/j.1460-2075.1993.tb06089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski K., Klosse U., de Bruijn F. J. Characterization of a novel Azorhizobium caulinodans ORS571 two-component regulatory system, NtrY/NtrX, involved in nitrogen fixation and metabolism. Mol Gen Genet. 1991 Dec;231(1):124–138. doi: 10.1007/BF00293830. [DOI] [PubMed] [Google Scholar]
- Raymond M., Gros P., Whiteway M., Thomas D. Y. Functional complementation of yeast ste6 by a mammalian multidrug resistance mdr gene. Science. 1992 Apr 10;256(5054):232–234. doi: 10.1126/science.1348873. [DOI] [PubMed] [Google Scholar]
- Rhoads D. B., Waters F. B., Epstein W. Cation transport in Escherichia coli. VIII. Potassium transport mutants. J Gen Physiol. 1976 Mar;67(3):325–341. doi: 10.1085/jgp.67.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlösser A., Hamann A., Bossemeyer D., Schneider E., Bakker E. P. NAD+ binding to the Escherichia coli K(+)-uptake protein TrkA and sequence similarity between TrkA and domains of a family of dehydrogenases suggest a role for NAD+ in bacterial transport. Mol Microbiol. 1993 Aug;9(3):533–543. doi: 10.1111/j.1365-2958.1993.tb01714.x. [DOI] [PubMed] [Google Scholar]
- Schlösser A., Kluttig S., Hamann A., Bakker E. P. Subcloning, nucleotide sequence, and expression of trkG, a gene that encodes an integral membrane protein involved in potassium uptake via the Trk system of Escherichia coli. J Bacteriol. 1991 May;173(10):3170–3176. doi: 10.1128/jb.173.10.3170-3176.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Miller S. I., Henschen A. H., Ouellette A. J. Enteric defensins: antibiotic peptide components of intestinal host defense. J Cell Biol. 1992 Aug;118(4):929–936. doi: 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S. I., Blount P., Martinac B., Blattner F. R., Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 1994 Mar 17;368(6468):265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- Valverde M. A., Díaz M., Sepúlveda F. V., Gill D. R., Hyde S. C., Higgins C. F. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature. 1992 Feb 27;355(6363):830–833. doi: 10.1038/355830a0. [DOI] [PubMed] [Google Scholar]
- Walderhaug M. O., Litwack E. D., Epstein W. Wide distribution of homologs of Escherichia coli Kdp K+-ATPase among gram-negative bacteria. J Bacteriol. 1989 Feb;171(2):1192–1195. doi: 10.1128/jb.171.2.1192-1195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhoff H. V., Juretić D., Hendler R. W., Zasloff M. Magainins and the disruption of membrane-linked free-energy transduction. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6597–6601. doi: 10.1073/pnas.86.17.6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antibiotic peptides as mediators of innate immunity. Curr Opin Immunol. 1992 Feb;4(1):3–7. doi: 10.1016/0952-7915(92)90115-u. [DOI] [PubMed] [Google Scholar]