Abstract
A ligand was cloned for murine OX40, a member of the TNF receptor family, using a T cell lymphoma cDNA library. The ligand (muOX40L) is a type II membrane protein with significant identity to human gp34 (gp34), a protein whose expression on HTLV-1-infected human leukemic T cells is regulated by the tax gene. The predicted structures of muOX40L and gp34 are similar to, but more compact than, those of other ligands of the TNF family. Mapping of the muOX40L gene revealed tight linkage to gld, the FasL gene, on chromosome 1. gp34 maps to a homologous region in the human genome, 1q25. cDNAs for human OX40 receptor were cloned by cross-hybridization with muOX40, and gp34 was found to bind the expressed human receptor. Lymphoid expression of muOX40L was detected on activated T cells, with higher levels found on CD4+ rather than CD8+ cells. The cell-bound recombinant ligands are biologically active, co-stimulating T cell proliferation and cytokine production. Strong induction of IL-4 secretion by muOX40L suggests that this ligand may play a role in regulating immune responses. In addition, the HTLV-1 regulation of gp34 suggests a possible connection between virally induced pathogenesis and the OX40 system.
Full text
PDF


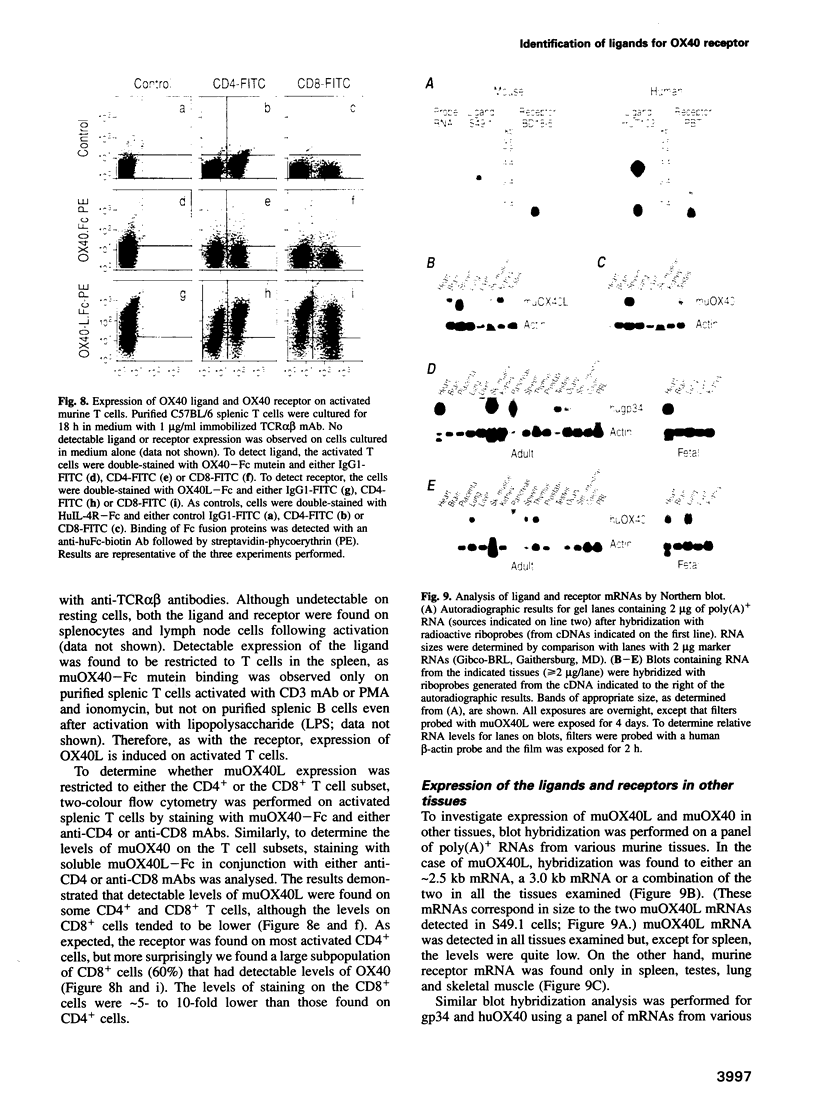
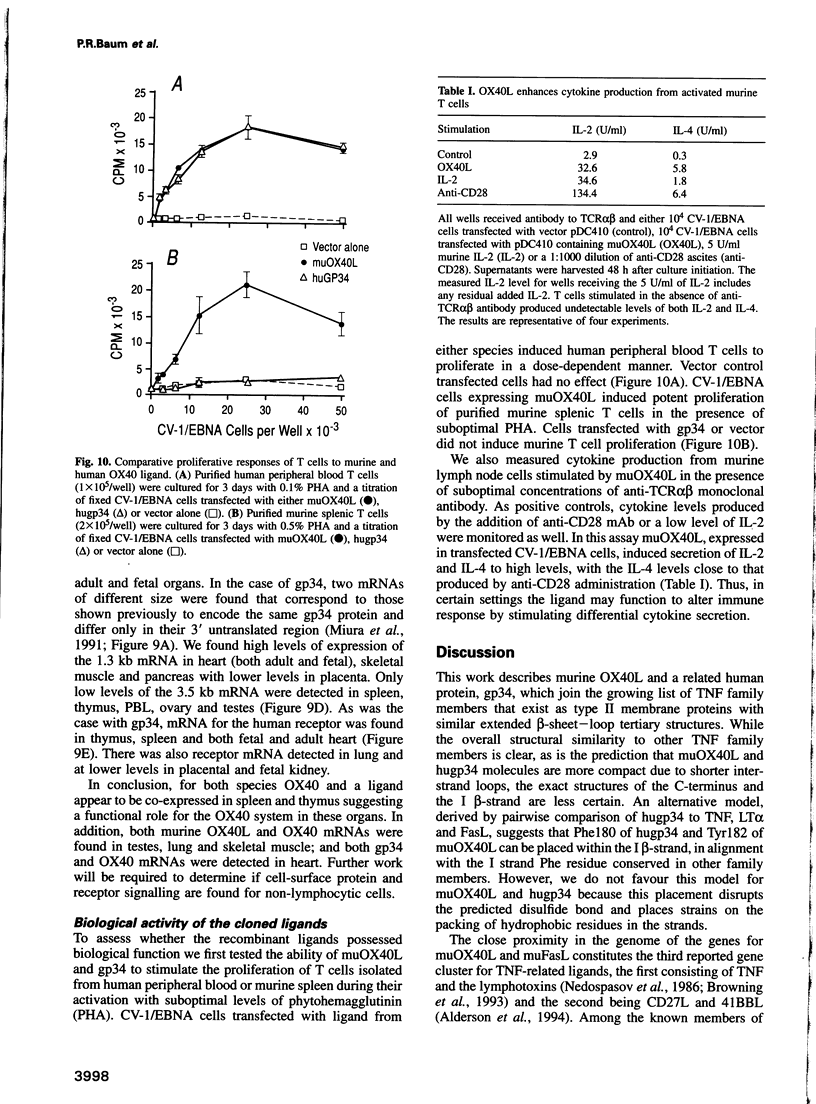



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Marshall J. D., Roths J. B., Sidman C. L. Differences defined by bone marrow transplantation suggest that lpr and gld are mutations of genes encoding an interacting pair of molecules. J Exp Med. 1990 Nov 1;172(5):1367–1375. doi: 10.1084/jem.172.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage R. J., Fanslow W. C., Strockbine L., Sato T. A., Clifford K. N., Macduff B. M., Anderson D. M., Gimpel S. D., Davis-Smith T., Maliszewski C. R. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992 May 7;357(6373):80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- Armitage R. J., Sato T. A., Macduff B. M., Clifford K. N., Alpert A. R., Smith C. A., Fanslow W. C. Identification of a source of biologically active CD40 ligand. Eur J Immunol. 1992 Aug;22(8):2071–2076. doi: 10.1002/eji.1830220817. [DOI] [PubMed] [Google Scholar]
- Armitage R. J., Tough T. W., Macduff B. M., Fanslow W. C., Spriggs M. K., Ramsdell F., Alderson M. R. CD40 ligand is a T cell growth factor. Eur J Immunol. 1993 Sep;23(9):2326–2331. doi: 10.1002/eji.1830230941. [DOI] [PubMed] [Google Scholar]
- Banner D. W., D'Arcy A., Janes W., Gentz R., Schoenfeld H. J., Broger C., Loetscher H., Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993 May 7;73(3):431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Bishop D. T. The information content of phase-known matings for ordering genetic loci. Genet Epidemiol. 1985;2(4):349–361. doi: 10.1002/gepi.1370020404. [DOI] [PubMed] [Google Scholar]
- Browning J. L., Ngam-ek A., Lawton P., DeMarinis J., Tizard R., Chow E. P., Hession C., O'Brine-Greco B., Foley S. F., Ware C. F. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993 Mar 26;72(6):847–856. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- Calderhead D. M., Buhlmann J. E., van den Eertwegh A. J., Claassen E., Noelle R. J., Fell H. P. Cloning of mouse Ox40: a T cell activation marker that may mediate T-B cell interactions. J Immunol. 1993 Nov 15;151(10):5261–5271. [PubMed] [Google Scholar]
- Callen D. F., Baker E., Eyre H. J., Chernos J. E., Bell J. A., Sutherland G. R. Reassessment of two apparent deletions of chromosome 16p to an ins(11;16) and a t(1;16) by chromosome painting. Ann Genet. 1990;33(4):219–221. [PubMed] [Google Scholar]
- Canfield S. M., Morrison S. L. The binding affinity of human IgG for its high affinity Fc receptor is determined by multiple amino acids in the CH2 domain and is modulated by the hinge region. J Exp Med. 1991 Jun 1;173(6):1483–1491. doi: 10.1084/jem.173.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Engers H. D., Macdonald H. R., Brunner T. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):703–717. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W. P., Nickoloff J. A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992 Jan;200(1):81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992 Jun;131(2):423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck M. J., Sprang S. R. The structure of tumor necrosis factor-alpha at 2.6 A resolution. Implications for receptor binding. J Biol Chem. 1989 Oct 15;264(29):17595–17605. doi: 10.2210/pdb1tnf/pdb. [DOI] [PubMed] [Google Scholar]
- Fanslow W. C., Anderson D. M., Grabstein K. H., Clark E. A., Cosman D., Armitage R. J. Soluble forms of CD40 inhibit biologic responses of human B cells. J Immunol. 1992 Jul 15;149(2):655–660. [PubMed] [Google Scholar]
- Fanslow W. C., Clifford K. N., Park L. S., Rubin A. S., Voice R. F., Beckmann M. P., Widmer M. B. Regulation of alloreactivity in vivo by IL-4 and the soluble IL-4 receptor. J Immunol. 1991 Jul 15;147(2):535–540. [PubMed] [Google Scholar]
- Fanslow W. C., Clifford K. N., Seaman M., Alderson M. R., Spriggs M. K., Armitage R. J., Ramsdell F. Recombinant CD40 ligand exerts potent biologic effects on T cells. J Immunol. 1994 May 1;152(9):4262–4269. [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin R. G., Alderson M. R., Smith C. A., Armitage R. J., VandenBos T., Jerzy R., Tough T. W., Schoenborn M. A., Davis-Smith T., Hennen K. Molecular and biological characterization of a ligand for CD27 defines a new family of cytokines with homology to tumor necrosis factor. Cell. 1993 May 7;73(3):447–456. doi: 10.1016/0092-8674(93)90133-b. [DOI] [PubMed] [Google Scholar]
- Gross J. A., Callas E., Allison J. P. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992 Jul 15;149(2):380–388. [PubMed] [Google Scholar]
- Hu-Li J., Ohara J., Watson C., Tsang W., Paul W. E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S). J Immunol. 1989 Feb 1;142(3):800–807. [PubMed] [Google Scholar]
- Hunter K. W., Watson M. L., Rochelle J., Ontiveros S., Munroe D., Seldin M. F., Housman D. E. Single-strand conformational polymorphism (SSCP) mapping of the mouse genome: integration of the SSCP, microsatellite, and gene maps of mouse chromosome 1. Genomics. 1993 Dec;18(3):510–519. doi: 10.1016/s0888-7543(11)80007-8. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latza U., Dürkop H., Schnittger S., Ringeling J., Eitelbach F., Hummel M., Fonatsch C., Stein H. The human OX40 homolog: cDNA structure, expression and chromosomal assignment of the ACT35 antigen. Eur J Immunol. 1994 Mar;24(3):677–683. doi: 10.1002/eji.1830240329. [DOI] [PubMed] [Google Scholar]
- Lynch D. H., Watson M. L., Alderson M. R., Baum P. R., Miller R. E., Tough T., Gibson M., Davis-Smith T., Smith C. A., Hunter K. The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity. 1994 May;1(2):131–136. doi: 10.1016/1074-7613(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Mallett S., Fossum S., Barclay A. N. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes--a molecule related to nerve growth factor receptor. EMBO J. 1990 Apr;9(4):1063–1068. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan C. J., Slack J. L., Mosley B., Cosman D., Lupton S. D., Brunton L. L., Grubin C. E., Wignall J. M., Jenkins N. A., Brannan C. I. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO J. 1991 Oct;10(10):2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S., Ohtani K., Numata N., Niki M., Ohbo K., Ina Y., Gojobori T., Tanaka Y., Tozawa H., Nakamura M. Molecular cloning and characterization of a novel glycoprotein, gp34, that is specifically induced by the human T-cell leukemia virus type I transactivator p40tax. Mol Cell Biol. 1991 Mar;11(3):1313–1325. doi: 10.1128/mcb.11.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey P. J., Goodwin R. G., Nordan R. P., Anderson D., Grabstein K. H., Cosman D., Sims J., Lupton S., Acres B., Reed S. G. Recombinant interleukin 7, pre-B cell growth factor, has costimulatory activity on purified mature T cells. J Exp Med. 1989 Mar 1;169(3):707–716. doi: 10.1084/jem.169.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley B., Beckmann M. P., March C. J., Idzerda R. L., Gimpel S. D., VandenBos T., Friend D., Alpert A., Anderson D., Jackson J. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989 Oct 20;59(2):335–348. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedospasov S. A., Shakhov A. N., Turetskaya R. L., Mett V. A., Azizov M. M., Georgiev G. P., Korobko V. G., Dobrynin V. N., Filippov S. A., Bystrov N. S. Tandem arrangement of genes coding for tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta) in the human genome. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):611–624. doi: 10.1101/sqb.1986.051.01.073. [DOI] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Paterson D. J., Jefferies W. A., Green J. R., Brandon M. R., Corthesy P., Puklavec M., Williams A. F. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987 Dec;24(12):1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- Ray F. A., Nickoloff J. A. Site-specific mutagenesis of almost any plasmid using a PCR-based version of unique site elimination. Biotechniques. 1992 Sep;13(3):342–348. [PubMed] [Google Scholar]
- Rost B., Sander C. Jury returns on structure prediction. Nature. 1992 Dec 10;360(6404):540–540. doi: 10.1038/360540b0. [DOI] [PubMed] [Google Scholar]
- Seldin M. F., Morse H. C., 3rd, Reeves J. P., Scribner C. L., LeBoeuf R. C., Steinberg A. D. Genetic analysis of autoimmune gld mice. I. Identification of a restriction fragment length polymorphism closely linked to the gld mutation within a conserved linkage group. J Exp Med. 1988 Feb 1;167(2):688–693. doi: 10.1084/jem.167.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Gruss H. J., Davis T., Anderson D., Farrah T., Baker E., Sutherland G. R., Brannan C. I., Copeland N. G., Jenkins N. A. CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993 Jul 2;73(7):1349–1360. doi: 10.1016/0092-8674(93)90361-s. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Srinivasan S., March C. J., Sudarsanam S. An automated method for modeling proteins on known templates using distance geometry. Protein Sci. 1993 Feb;2(2):277–289. doi: 10.1002/pro.5560020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Takahashi T., Golstein P., Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993 Dec 17;75(6):1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Brannan C. I., Jenkins N. A., Copeland N. G., Suda T., Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994 Mar 25;76(6):969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Inoi T., Tozawa H., Yamamoto N., Hinuma Y. A glycoprotein antigen detected with new monoclonal antibodies on the surface of human lymphocytes infected with human T-cell leukemia virus type-I (HTLV-I). Int J Cancer. 1985 Nov 15;36(5):549–555. doi: 10.1002/ijc.2910360506. [DOI] [PubMed] [Google Scholar]
- Tozawa H., Andoh S., Takayama Y., Tanaka Y., Lee B., Nakamura H., Hayami M., Hinuma Y. Species-dependent antigenicity of the 34-kDa glycoprotein found on the membrane of various primate lymphocytes transformed by human T-cell leukemia virus type-I (HTLV-I) and simian T-cell leukemia virus (STLV-I). Int J Cancer. 1988 Feb 15;41(2):231–238. doi: 10.1002/ijc.2910410213. [DOI] [PubMed] [Google Scholar]
- Urdal D. L., Mochizuki D., Conlon P. J., March C. J., Remerowski M. L., Eisenman J., Ramthun C., Gillis S. Lymphokine purification by reversed-phase high-performance liquid chromatography. J Chromatogr. 1984 Jul 27;296:171–179. doi: 10.1016/s0021-9673(01)96410-6. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Watson M. L., D'Eustachio P., Mock B. A., Steinberg A. D., Morse H. C., 3rd, Oakey R. J., Howard T. A., Rochelle J. M., Seldin M. F. A linkage map of mouse chromosome 1 using an interspecific cross segregating for the gld autoimmunity mutation. Mamm Genome. 1992;2(3):158–171. doi: 10.1007/BF00302874. [DOI] [PubMed] [Google Scholar]
- van Seventer G. A., Shimizu Y., Shaw S. Roles of multiple accessory molecules in T-cell activation. Curr Opin Immunol. 1991 Jun;3(3):294–303. doi: 10.1016/0952-7915(91)90027-x. [DOI] [PubMed] [Google Scholar]