Abstract
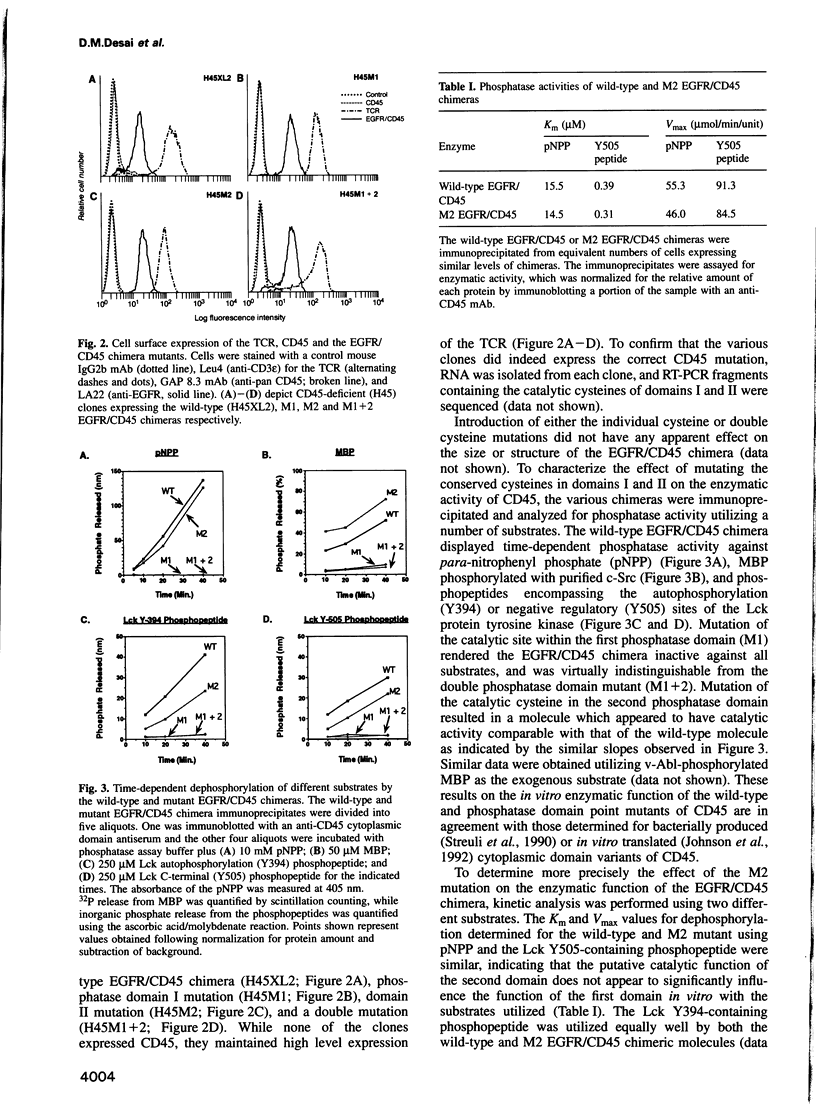
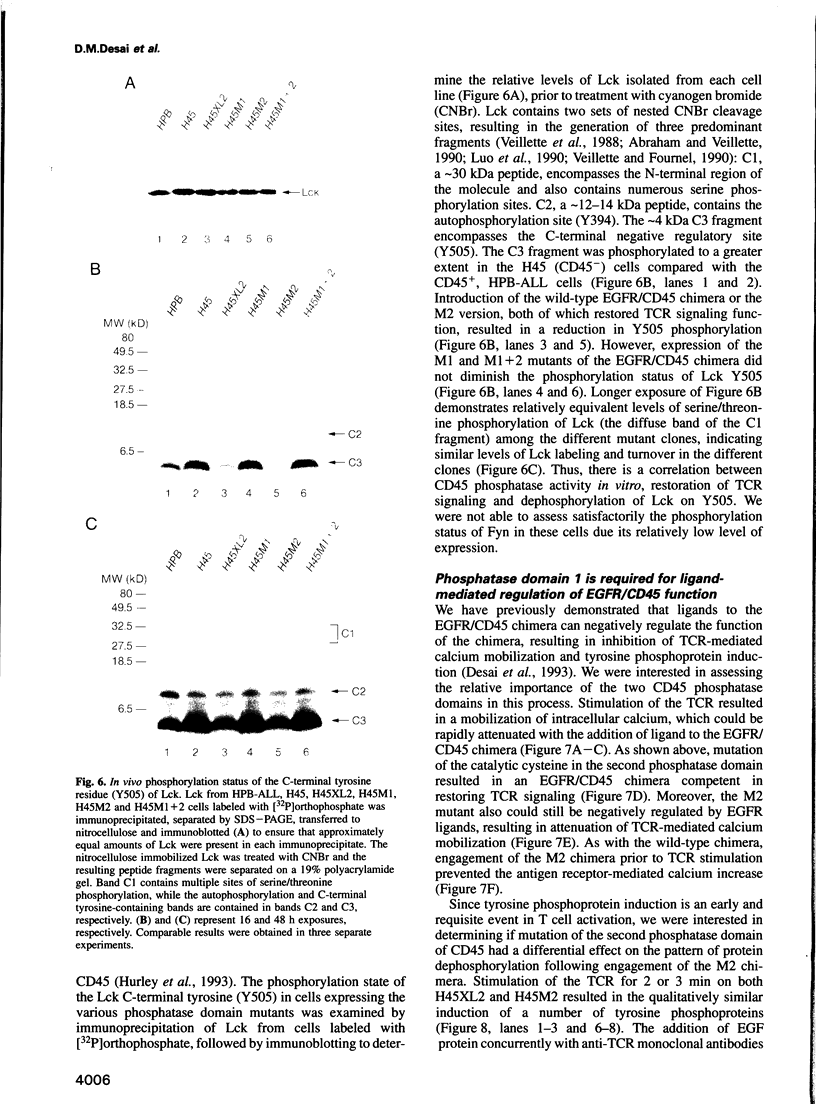
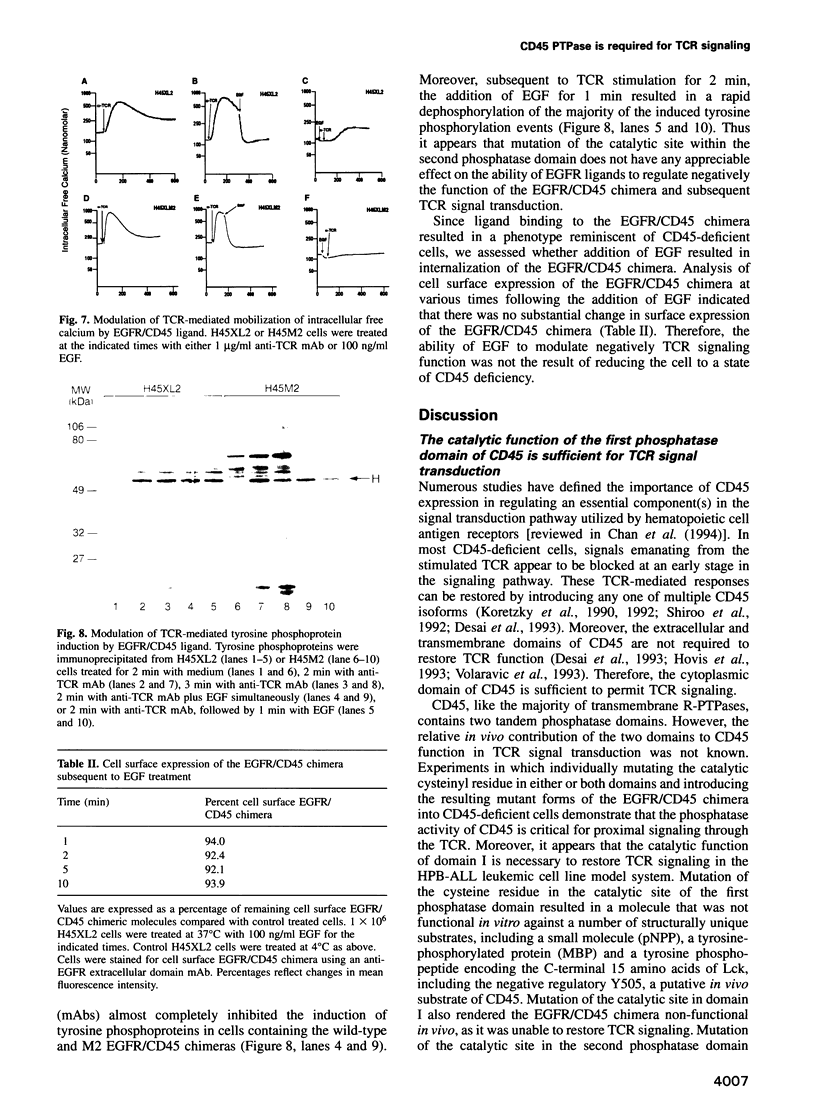
Cell surface expression of CD45, a receptor-like protein tyrosine phosphatase (PTPase), is required for T cell antigen receptor (TCR)-mediated signal transduction. Like the majority of transmembrane PTPases, CD45 contains two cytoplasmic phosphatase domains, whose relative in vivo function is not known. Site-directed mutagenesis of the individual catalytic residues of the two CD45 phosphatase domains indicates that the catalytic activity of the membrane-proximal domain is both necessary and sufficient for restoration of TCR signal transduction in a CD45-deficient cell. The putative catalytic activity of the distal phosphatase domain is not required for proximal TCR-mediated signaling events. Moreover, in the context of a chimeric PTPase receptor, the putative catalytic activity of the distal phosphatase domain is not required for ligand-induced negative regulation of PTPase function. We also demonstrate that the phosphorylation of the C-terminal tyrosine of Lck, a site of negative regulation, is reduced only when CD45 mutants with demonstrable in vitro phosphatase activity are introduced into the CD45-deficient cells. These results demonstrate that the phosphatase activity of CD45 is critical for TCR signaling, and for regulating the levels of C-terminal phosphorylated Lck molecules.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N., Veillette A. Activation of p56lck through mutation of a regulatory carboxy-terminal tyrosine residue requires intact sites of autophosphorylation and myristylation. Mol Cell Biol. 1990 Oct;10(10):5197–5206. doi: 10.1128/mcb.10.10.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A., Kanner S. B., Sgroi D., Ledbetter J. A., Stamenkovic I. CD22-mediated stimulation of T cells regulates T-cell receptor/CD3-induced signaling. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10242–10246. doi: 10.1073/pnas.89.21.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autero M., Saharinen J., Pessa-Morikawa T., Soula-Rothhut M., Oetken C., Gassmann M., Bergman M., Alitalo K., Burn P., Gahmberg C. G. Tyrosine phosphorylation of CD45 phosphotyrosine phosphatase by p50csk kinase creates a binding site for p56lck tyrosine kinase and activates the phosphatase. Mol Cell Biol. 1994 Feb;14(2):1308–1321. doi: 10.1128/mcb.14.2.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. M., Dethloff G. M., Imboden J. B. CD45-negative mutants of a rat natural killer cell line fail to lyse tumor target cells. J Immunol. 1993 Oct 1;151(7):3646–3653. [PubMed] [Google Scholar]
- Burns C. M., Sakaguchi K., Appella E., Ashwell J. D. CD45 regulation of tyrosine phosphorylation and enzyme activity of src family kinases. J Biol Chem. 1994 May 6;269(18):13594–13600. [PubMed] [Google Scholar]
- Cahir McFarland E. D., Hurley T. R., Pingel J. T., Sefton B. M., Shaw A., Thomas M. L. Correlation between Src family member regulation by the protein-tyrosine-phosphatase CD45 and transmembrane signaling through the T-cell receptor. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1402–1406. doi: 10.1073/pnas.90.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Chan A. C., Desai D. M., Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu Rev Immunol. 1994;12:555–592. doi: 10.1146/annurev.iy.12.040194.003011. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K. 1002 protein phosphatases? Annu Rev Cell Biol. 1992;8:463–493. doi: 10.1146/annurev.cb.08.110192.002335. [DOI] [PubMed] [Google Scholar]
- Cho H., Ramer S. E., Itoh M., Kitas E., Bannwarth W., Burn P., Saito H., Walsh C. T. Catalytic domains of the LAR and CD45 protein tyrosine phosphatases from Escherichia coli expression systems: purification and characterization for specificity and mechanism. Biochemistry. 1992 Jan 14;31(1):133–138. doi: 10.1021/bi00116a019. [DOI] [PubMed] [Google Scholar]
- Chow L. M., Fournel M., Davidson D., Veillette A. Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature. 1993 Sep 9;365(6442):156–160. doi: 10.1038/365156a0. [DOI] [PubMed] [Google Scholar]
- Cool D. E., Tonks N. K., Charbonneau H., Fischer E. H., Krebs E. G. Expression of a human T-cell protein-tyrosine-phosphatase in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7280–7284. doi: 10.1073/pnas.87.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Howell B. The when and how of Src regulation. Cell. 1993 Jun 18;73(6):1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- Desai D. M., Sap J., Schlessinger J., Weiss A. Ligand-mediated negative regulation of a chimeric transmembrane receptor tyrosine phosphatase. Cell. 1993 May 7;73(3):541–554. doi: 10.1016/0092-8674(93)90141-c. [DOI] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Frangioni J. V., Beahm P. H., Shifrin V., Jost C. A., Neel B. G. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992 Feb 7;68(3):545–560. doi: 10.1016/0092-8674(92)90190-n. [DOI] [PubMed] [Google Scholar]
- Galaktionov K., Beach D. Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell. 1991 Dec 20;67(6):1181–1194. doi: 10.1016/0092-8674(91)90294-9. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Evidence for protein-tyrosine-phosphatase catalysis proceeding via a cysteine-phosphate intermediate. J Biol Chem. 1991 Sep 15;266(26):17026–17030. [PubMed] [Google Scholar]
- Honegger A. M., Kris R. M., Ullrich A., Schlessinger J. Evidence that autophosphorylation of solubilized receptors for epidermal growth factor is mediated by intermolecular cross-phosphorylation. Proc Natl Acad Sci U S A. 1989 Feb;86(3):925–929. doi: 10.1073/pnas.86.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovis R. R., Donovan J. A., Musci M. A., Motto D. G., Goldman F. D., Ross S. E., Koretzky G. A. Rescue of signaling by a chimeric protein containing the cytoplasmic domain of CD45. Science. 1993 Apr 23;260(5107):544–546. doi: 10.1126/science.8475387. [DOI] [PubMed] [Google Scholar]
- Hurley T. R., Hyman R., Sefton B. M. Differential effects of expression of the CD45 tyrosine protein phosphatase on the tyrosine phosphorylation of the lck, fyn, and c-src tyrosine protein kinases. Mol Cell Biol. 1993 Mar;13(3):1651–1656. doi: 10.1128/mcb.13.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving B. A., Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991 Mar 8;64(5):891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Johnson P., Ostergaard H. L., Wasden C., Trowbridge I. S. Mutational analysis of CD45. A leukocyte-specific protein tyrosine phosphatase. J Biol Chem. 1992 Apr 25;267(12):8035–8041. [PubMed] [Google Scholar]
- Justement L. B., Campbell K. S., Chien N. C., Cambier J. C. Regulation of B cell antigen receptor signal transduction and phosphorylation by CD45. Science. 1991 Jun 28;252(5014):1839–1842. doi: 10.1126/science.1648262. [DOI] [PubMed] [Google Scholar]
- Kaplan R., Morse B., Huebner K., Croce C., Howk R., Ravera M., Ricca G., Jaye M., Schlessinger J. Cloning of three human tyrosine phosphatases reveals a multigene family of receptor-linked protein-tyrosine-phosphatases expressed in brain. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7000–7004. doi: 10.1073/pnas.87.18.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishihara K., Penninger J., Wallace V. A., Kündig T. M., Kawai K., Wakeham A., Timms E., Pfeffer K., Ohashi P. S., Thomas M. L. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993 Jul 16;74(1):143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- Koretzky G. A., Kohmetscher M. A., Kadleck T., Weiss A. Restoration of T cell receptor-mediated signal transduction by transfection of CD45 cDNA into a CD45-deficient variant of the Jurkat T cell line. J Immunol. 1992 Aug 15;149(4):1138–1142. [PubMed] [Google Scholar]
- Koretzky G. A., Kohmetscher M., Ross S. CD45-associated kinase activity requires lck but not T cell receptor expression in the Jurkat T cell line. J Biol Chem. 1993 Apr 25;268(12):8958–8964. [PubMed] [Google Scholar]
- Koretzky G. A., Picus J., Schultz T., Weiss A. Tyrosine phosphatase CD45 is required for T-cell antigen receptor and CD2-mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2037–2041. doi: 10.1073/pnas.88.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretzky G. A., Picus J., Thomas M. L., Weiss A. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyl inositol pathway. Nature. 1990 Jul 5;346(6279):66–68. doi: 10.1038/346066a0. [DOI] [PubMed] [Google Scholar]
- Krueger N. X., Streuli M., Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990 Oct;9(10):3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luo K., Hurley T. R., Sefton B. M. Transfer of proteins to membranes facilitates both cyanogen bromide cleavage and two-dimensional proteolytic mapping. Oncogene. 1990 Jun;5(6):921–923. [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Altman A. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6302–6306. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada S., Okada M., MacAuley A., Cooper J. A., Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991 May 2;351(6321):69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- Okada M., Nada S., Yamanashi Y., Yamamoto T., Nakagawa H. CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J Biol Chem. 1991 Dec 25;266(36):24249–24252. [PubMed] [Google Scholar]
- Ostergaard H. L., Shackelford D. A., Hurley T. R., Johnson P., Hyman R., Sefton B. M., Trowbridge I. S. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8959–8963. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingel J. T., Thomas M. L. Evidence that the leukocyte-common antigen is required for antigen-induced T lymphocyte proliferation. Cell. 1989 Sep 22;58(6):1055–1065. doi: 10.1016/0092-8674(89)90504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pot D. A., Dixon J. E. Active site labeling of a receptor-like protein tyrosine phosphatase. J Biol Chem. 1992 Jan 5;267(1):140–143. [PubMed] [Google Scholar]
- Pot D. A., Woodford T. A., Remboutsika E., Haun R. S., Dixon J. E. Cloning, bacterial expression, purification, and characterization of the cytoplasmic domain of rat LAR, a receptor-like protein tyrosine phosphatase. J Biol Chem. 1991 Oct 15;266(29):19688–19696. [PubMed] [Google Scholar]
- Schraven B., Kirchgessner H., Gaber B., Samstag Y., Meuer S. A functional complex is formed in human T lymphocytes between the protein tyrosine phosphatase CD45, the protein tyrosine kinase p56lck and pp32, a possible common substrate. Eur J Immunol. 1991 Oct;21(10):2469–2477. doi: 10.1002/eji.1830211025. [DOI] [PubMed] [Google Scholar]
- Shiroo M., Goff L., Biffen M., Shivnan E., Alexander D. CD45 tyrosine phosphatase-activated p59fyn couples the T cell antigen receptor to pathways of diacylglycerol production, protein kinase C activation and calcium influx. EMBO J. 1992 Dec;11(13):4887–4897. doi: 10.1002/j.1460-2075.1992.tb05595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieh M., Bolen J. B., Weiss A. CD45 specifically modulates binding of Lck to a phosphopeptide encompassing the negative regulatory tyrosine of Lck. EMBO J. 1993 Jan;12(1):315–321. doi: 10.1002/j.1460-2075.1993.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenkovic I., Sgroi D., Aruffo A., Sy M. S., Anderson T. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and alpha 2-6 sialyltransferase, CD75, on B cells. Cell. 1991 Sep 20;66(6):1133–1144. doi: 10.1016/0092-8674(91)90036-x. [DOI] [PubMed] [Google Scholar]
- Stover D. R., Charbonneau H., Tonks N. K., Walsh K. A. Protein-tyrosine-phosphatase CD45 is phosphorylated transiently on tyrosine upon activation of Jurkat T cells. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7704–7707. doi: 10.1073/pnas.88.17.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. B., Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992 Aug 21;70(4):585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Thai T., Tang M., Saito H. Distinct functional roles of the two intracellular phosphatase like domains of the receptor-linked protein tyrosine phosphatases LCA and LAR. EMBO J. 1990 Aug;9(8):2399–2407. doi: 10.1002/j.1460-2075.1990.tb07415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A., Wu J. J., Maizel A. L. Evidence for monomeric and dimeric forms of CD45 associated with a 30-kDa phosphorylated protein. J Biol Chem. 1992 Aug 15;267(23):16651–16659. [PubMed] [Google Scholar]
- Tan X., Stover D. R., Walsh K. A. Demonstration of protein tyrosine phosphatase activity in the second of two homologous domains of CD45. J Biol Chem. 1993 Apr 5;268(10):6835–6838. [PubMed] [Google Scholar]
- Tian S. S., Tsoulfas P., Zinn K. Three receptor-linked protein-tyrosine phosphatases are selectively expressed on central nervous system axons in the Drosophila embryo. Cell. 1991 Nov 15;67(4):675–685. doi: 10.1016/0092-8674(91)90063-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Thomas M. L. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- Veillette A., Fournel M. The CD4 associated tyrosine protein kinase p56lck is positively regulated through its site of autophosphorylation. Oncogene. 1990 Oct;5(10):1455–1462. [PubMed] [Google Scholar]
- Veillette A., Horak I. D., Bolen J. B. Post-translational alterations of the tyrosine kinase p56lck in response to activators of protein kinase C. Oncogene Res. 1988 May;2(4):385–401. [PubMed] [Google Scholar]
- Volarević S., Niklinska B. B., Burns C. M., June C. H., Weissman A. M., Ashwell J. D. Regulation of TCR signaling by CD45 lacking transmembrane and extracellular domains. Science. 1993 Apr 23;260(5107):541–544. doi: 10.1126/science.8475386. [DOI] [PubMed] [Google Scholar]
- Wang Y., Pallen C. J. The receptor-like protein tyrosine phosphatase HPTP alpha has two active catalytic domains with distinct substrate specificities. EMBO J. 1991 Nov;10(11):3231–3237. doi: 10.1002/j.1460-2075.1991.tb04886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C. T., Pingel J. T., Nelson J. O., Thomas M. L. CD8+ T-cell clones deficient in the expression of the CD45 protein tyrosine phosphatase have impaired responses to T-cell receptor stimuli. Mol Cell Biol. 1991 Sep;11(9):4415–4422. doi: 10.1128/mcb.11.9.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. H., Seow K. T., Bahri S. M., Oon S. H., Chia W. Two Drosophila receptor-like tyrosine phosphatase genes are expressed in a subset of developing axons and pioneer neurons in the embryonic CNS. Cell. 1991 Nov 15;67(4):661–673. doi: 10.1016/0092-8674(91)90062-4. [DOI] [PubMed] [Google Scholar]