Abstract
A long-standing puzzle in developmental psychology is how infants imitate gestures they cannot see themselves perform (facial gestures). Two critical issues are: (a) the metric infants use to detect cross-modal equivalences in human acts and (b) the process by which they correct their imitative errors. We address these issues in a detailed model of the mechanisms underlying facial imitation. The model can be extended to encompass other types of imitation. The model capitalizes on three new theoretical concepts. First, organ identification is the means by which infants relate parts of their own bodies to corresponding ones of the adult’s. Second, body babbling (infants’ movement practice gained through self-generated activity) provides experience mapping movements to the resulting body configurations. Third, organ relations provide the metric by which infant and adult acts are perceived in commensurate terms. In imitating, infants attempt to match the organ relations they see exhibited by the adults with those they feel themselves make. We show how development restructures the meaning and function of early imitation. We argue that important aspects of later social cognition are rooted in the initial cross-modal equivalence between self and other found in newborns.
Keywords: imitation, faces, cross-modal, memory, motor coordination, self
Imitation is a mechanism for the intergenerational transmission of acquired characteristics. Before explicit linguistic instruction, infants learn many of the skills, customs, and behaviour patterns of their culture through imitation. In imitating, infants use another’s behaviours as a basis for their own, despite differences in body size, perspective of view, and modality through which self and other can be perceived. As ubiquitous and useful as imitation is, how imitation is accomplished poses one of the deeper puzzles in infancy.
All imitative acts are not of the same kind. There are distinctions among manual, vocal, and facial imitation. In manual imitation the infant sees an adult hand movement and must generate a matching movement. One possible mechanism would be for the infant to look at his or her own hand and use visual guidance as a way of achieving a match between self and other. Vocal imitation also capitalizes on intramodal comparisons, because infants use auditory guidance to help achieve the match (Kuhl and Meltzoff, 1996). However, for the case of facial imitation, a mechanism based on intramodal guidance would be useless. Infants can see the adult’s face but cannot see their own faces. They can feel their own faces move, but have no access to the feelings of movement in the other. By what mechanism can they connect the felt but unseen movements of the self with the seen but unfelt movements of the other?
Classical theories such as Piaget’s (1962) answered this question through learning experiences with mirrors and tactile exploration of one’s own and others’ faces. Mirrors made the unseen visible, rendering one’s own body and that of the other in visual terms. Tactile exploration of faces rendered both self and other in tangible terms. In the last 20 years, empirical work from many laboratories has revealed that infants too young to have learned from such experience none the less imitate facial gestures.
This work raises several new questions. What motivates young infants to imitate? What functions does early imitation serve? What psychological mechanisms underlie it? Elsewhere we have discussed the questions of motivation and function (Meltzoff and Moore, 1992, 1994, 1995b). The aim of this paper is to tackle the mechanism question. We will consider not only how infants can imitate in the first place, but also how they correct their imitative efforts to more faithfully match what they perceive.
Until recently there was insufficient empirical evidence to resolve the mechanism question. This has now changed, and we capitalize on the new discoveries to propose a model of the mechanism underlying early facial imitation. The paper contains six sections. (1) A synopsis of our viewpoint is provided. (2) We organize 10 key phenomena from recent research allowing us to characterize early imitation as goal directed, generative, and representationally mediated. (3) New theoretical concepts are introduced to encompass these characteristics. (4) These concepts are used to provide a detailed model of the psychological mechanisms underlying facial imitation, specifying the means by which infants render the acts of self and other in commensurate terms. (5) The explanatory value, parsimony, and coherence of the model are assessed. (6) The implications of the model for developmental theory are considered.
CONCEPTUAL SCHEMATIC OF THE AIM HYPOTHESIS
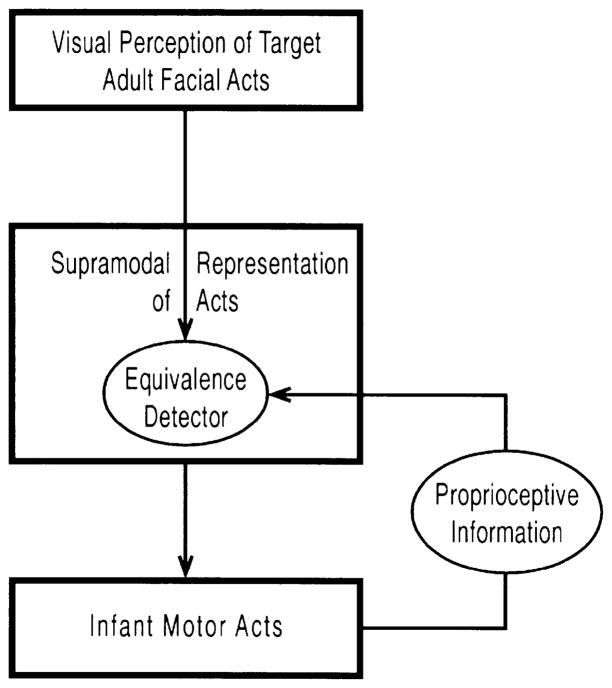
We think that early facial imitation is based on ‘active intermodal mapping’ (AIM) (Meltzoff and Moore, 1977, 1983, 1994). Figure 1 provides a conceptual schematic of the AIM hypothesis. The key claim is that imitation is a matching-to-target process. The active nature of the matching process is captured by the proprioceptive feedback loop. The loop allows infants’ motor performance to be evaluated against the seen target and serves as a basis for correction. According to this view, the perceived and produced human acts are coded within a common (supramodal) framework which enables infants to detect equivalences between their own acts and ones they see. AIM posits an intermodal mechanism for imitation, in contrast to a reflexive or a conditioned basis for generating the matching response.
Figure 1.
A conceptual schematic of the active intermodal mapping hypothesis (AIM).
ORGANIZING THE EMPIRICAL EVIDENCE
New empirical evidence now allows us to enrich the AIM hypothesis. Ten phenomena bearing on the mechanisms underlying early facial imitation are displayed in Table 1. The entries listed as numbers 1–9 have been demonstrated in infants under 2 months of age. However, there is developmental change in the expression of these competences, as noted in no. 10. For example, neonates can imitate a broad range of facial and manual gestures (no. 1), but young infants do not imitate everything. There is a progression in imitation from pure body actions, to actions on objects, to using one object as a tool for manipulating other objects. Similarly, neonates imitate novel acts (no. 6), but research on older infants reveals a generative imitation of novelty that is beyond the scope of younger infants (Bauer and Mandler, 1992; Barr et al., 1996; Meltzoff, 1988, 1995b). Any adequate theory of early imitation will have to account for at least the 10 phenomena in Table 1.
Table 1.
Ten characteristics of early imitation
Note: Superscripts in the table refer to the following papers:
Meltzoff and Moore (this paper);
The phenomena in Table 1 support at least three inferences about the nature of early imitation. We use them to argue that imitation is: (a) representationally mediated, (b) goal directed, and (c) generative and specific.
Imitation is Representationally Mediated
One way of conceiving of early imitation is that there is a perception–production transducer that directly converts visual input to specific motor output. In this view, a seen tongue protrusion yields a tongue protrusion movement by virtue of particular built-in connections between visual and motor centres of the young brain. Two lines of evidence suggest that a more differentiated process than simple transduction is required: (a) the response need not be temporally coupled to the stimulus and (b) imitation is not compulsory; infants need not produce what is given to perception.
In an early study, mouth-opening and tongue-protrusion gestures were shown to 3-week-old infants while they were engaged in the competing motor activity of sucking on a pacifier (Meltzoff and Moore, 1977). The adult terminated the gestural demonstration, assumed a neutral face, and only then removed the pacifier. Three-week-old infants differentially imitated both gestures despite the fact that the adult was no longer showing them. Infants have also been shown to imitate with longer delays. In Meltzoff and Moore (1994) 6-week-old infants saw a person perform a specific gesture on day 1, and then after a 24-hour delay saw the same adult in a neutral pose. Different groups of infants saw different gestures on the first day, and they all saw the same neutral pose on the second day. What differed across the groups was not their current perception but what they had seen the adult do in the past. The results showed that infants differentially imitated the gestures they saw 24 hours earlier. It is difficult to see how direct transduction could account for these data, because the target guiding the infants’ action had been absent for 24 hours. Evidently, information gained from vision can be stored and accessed at a later time. One way of achieving this is to represent the adult’s act.
There is further evidence that infants’ responses are not stimulus bound. Infants sometimes override their current perception. If the situation is arranged correctly, infants will imitate a gesture from the past even when it mismatches what they currently see. A study of 6-week-olds used two people who came and went in front of the infants (Meltzoff and Moore, 1992). One person always demonstrated one gesture and the other a different gesture. After the exchange of people occurred, infants often shut down their ongoing activity stared intently at the new person, and then responded by reproducing the first person’s acts. Infants were not imitating what was in current perception; they were overriding what they saw and imitating what the first person did. Imitation of what was in the perceptual field was not compulsory. We have previously noted that this phenomenon has implications for how infants use imitation to individuate people and determine identity across contacts with multiple individuals (Meltzoff and Moore, 1992, 1994, 1995b). For the present purpose, these data show a flexibility between the stimulus and response that is not explained by direct transduction.
Implications
Early imitation is not entirely stimulus bound, directly triggered, or reflexive. There is flexibility inasmuch as infants can imitate the past when shown a neutral face and even override a currently perceived gesture to do something else. We hypothesize that some form of representation stands between perception and production.
Imitation is Goal Directed
Empirical evidence from several independent laboratories shows that the infants’ initial imitative responses are often similar to the target but not a complete reproduction. Partial matches along several dimensions are commonly observed (Abravanel and Sigafoos, 1984; Heimann et al., 1989; Jacobson, 1979; Maratos, 1982; Meltzoff and Moore, 1977, 1983). To understand why infants respond with the partial matches, we conducted a study of how 6-week-olds temporally organize their behaviour. A microanalysis of the response showed that infants gradually corrected their imitative attempts over time in a sequence of ordered steps (Meltzoff and Moore, 1994). Correction was neither a ‘random walk’ nor a paring away of irrelevant components from high activity levels, but a generation of novel behaviours not found in baseline activity.
One interpretation that makes sense of this pattern is that the responses are goal directed. The goal organizes these actions by providing a criterion for success and governing the sequence of attempts. The hypothesis that neonatal imitation is organized by a goal is also compatible with other recent findings of primitive goal directedness in newborn motor movements (Butterworth and Hopkins, 1988; van der Meer et al., 1995).
We also discovered something about the nature of the mistakes infants make. When observing an adult sticking his tongue out of the corner of his mouth, 6-week-olds displayed a ‘creative error’. Infants poked out their tongues and simultaneously turned their heads to the side, achieving a new kind of ‘tongue to the side’. A coder who was blind to the stimulus reviewed the video records and scored every instance in which infants produced a head turn (>30° off midline) simultaneously with a tongue protrusion (HT+TP). The results were that 70% (7 of 10) of the infants shown the tongue protrusion-to-the-side gesture produced the HT+TP response. Only 30% (9 of 30) of control infants shown other facial gestures did so, p<0.05 (see Meltzoff and Moore, 1994, for details of the study).1 This HT+TP response was not the infants’ final effort but occurred early in the test period, as a step towards a more faithful tonge-protrusion-to-the-side response.
The head movement was not in the stimulus, but was an alternative way of getting their bodies to do an act involving both tongue protrusion and an off-midline direction. It is only at the level of goals that head turn is relevant to tongue-protrusion-to-the-side. The adult’s movement was a tongue thrust diagonally out of the mouth and the infant’s movement was a head rotation to the side. These are different as specific muscle movements, but the tongue protrusion ends up off midline in both cases. Although the literal movements were very different, the final result in terms of the orientation of the tongue was similar, and in this sense it can be seen as an act organized by a goal.
Implications
Both of the foregoing phenomena suggest infants’ imitative responses are not motor units akin to reflexes that are simply released by the appropriate input. Rather, early imitation is a goal-directed response whose aim is ‘matching the target’. Infants recruit multiple means, some of them not directly given in the stimulus, in attempting to achieve that aim.
Imitation is Generative and Specific
Imitation within the first 2 months of life is not limited to a few privileged gestures. The wide range of gestures that can be imitated suggests a generative process. The list continues to lengthen with successive studies, and to date includes: tongue protrusion, lip protrusion, mouth opening, hand gestures, head movements, eye blinking, cheek and brow motions, and components of emotional expressions (Abravanel and DeYong, 1991; Abravanel and Sigafoos, 1984; Field et al., 1982, 1983, 1986; Fontaine, 1984; Heimann, 1989; Heimann et al., 1989; Heimann and Schaller, 1985; Jacobson, 1979; Kaitz et al., 1988; Legerstee, 1991; Kugiumutzakis, 1985; Maratos, 1982; Meltzoff and Moore, 1977, 1983, 1989, 1992, 1994; Reissland, 1988; Vinter, 1986).
Meltzoff and Moore (1977) selected four gestures to test the specificity of the mapping between the adult’s and the infant’s bodies: lip protrusion, mouth opening, tongue protrusion, and finger movement. The results showed that infants confused neither organs nor actions. They differentially responded to tongue protrusion with tongue protrusion and not lip protrusion, thus showing that the specific organ could be identified. Infants also differentially imitated two different movements of the same body part, for example, lip opening led to lip opening, not lip protrusion. Such specificity can also be seen in the data from other laboratories (Fontaine, 1984; Maratos, 1982).
Implications
Tongue protrusion is the most studied imitative gesture, and this has sometimes been misinterpreted to mean that it is the only facial gesture that can be imitated. However, as in statistics, the mode does not tell you about the range—and the range of gestures is large. There are limits on what neonates will imitate, but the critical point for theory construction is that early imitation is not limited to a few privileged body parts or salient movement patterns. Moreover, the response is quite specific. There is no global confusion either on the organ side or the movement side. A generative matching-to-target process is indicated.
THEORETICAL CONCEPTS NEEDED TO ACCOUNT FOR FACIAL IMITATION
We are now in a position to flesh out the AIM hypothesis previously presented as a conceptual schematic (Figure 1). An adequate account of the mechanisms underlying early imitation should specify what is present at birth, what is based on early experience, and how cross-modal equivalence can be established. We describe these three aspects next.
Organ Identification
The newborns’ first response to seeing a particular facial gesture is activation of the corresponding body part. For example, when they see tongue protrusion, there is often a quieting of the movements of other body parts and an activation of tongue. They do not necessarily protrude the tongue during this initial phase, but may elevate it, wiggle it, or move it slightly in the oral cavity. Likewise, when shown lip protrusion, they produce a marked tension of the lips and even press them together before there is imitation of the movement. It is as if young infants isolate what part of their body to move before how to move it. We call this ‘organ identification’.
Because organ identification occurs in newborns and precedes other imitative efforts, we think that it is the first step in generating an imitative response. We note that an ability to identify corresponding body parts renders self and other in commensurate terms—as movements of tongues, lips, hands, etc. In this view, organs are the cross-modal units of analysis.2
We can envision two accounts of how organ identification occurs. The first account is that a delimited set of organs is recognized at birth on the basis of their form (‘organs as forms’). It may be that the perceptual context of a face helps identify the whole whose internal parts infants can then parse into facial organs. Some research is compatible with the notion that facial organs may be perceptually identifiable by human newborns, though it is not definitive. Goren et al. (1975), Morton and Johnson (1991), and others report evidence that faces are preferred by newborns over other patterns matched in sensory characteristics. Their claim is that through evolution the structure of a face has become a distinctive perceptual unit responded to at birth.3 Moreover, neurophysiological data show that visual displays of particular organs, notably parts of the face and hands in monkeys, activate specific brain sites (Desimone, 1991; Gross et al., 1969, 1972; Gross and Sargent, 1992; Perrett et al., 1987, 1992; Rolls, 1992). Gross has also discovered that ‘some [face-selective cells] will respond to face components in isolation’ (Gross, 1992, p. 5). Thus, specific organs could be neurally represented at birth.
The second account of organ identification is that organs are differentiable because each organ has a unique spatiotemporal pattern of movement (‘organs from motion’). Musculature and skeletal structure restrict what body parts can do. Tongues move in and out, but do not flex. Arms are jointed appendages and typically flex. Fingers are a set of three-jointed appendages that can contract on themselves. It may be that infants come to individuate their organs through proprioceptive monitoring of their own actions. The claim is that these unique spatiotemporal movement patterns, which we call ‘kinetic signatures’, are recognized as cross-modally equivalent when done by self and other. The data are not definitive in showing that infants can map facial organs in this way, but there is compatible evidence. For example, the literature on biological motion using point-light displays shows that the spatiotemporal pattern produced by human movement (such as gait) is a pattern to which infants are acutely attuned (e.g., Bertenthal, 1996).
Both of these accounts serve to differentiate and identify organs. On the first account, organ identification is a perceptual given, preadapted by evolution; on the second account, organ identification emerges from the unique movements each organ can make. Much as we would like to determine which alternative obtains, the available data do not allow a firm decision. The first account perhaps is more compatible with young infants’ rapid activation of the correct organ and the findings that they can imitate static facial postures in the absence of kinetic information (Meltzoff and Moore, 1992). The remainder of the essay is written from this perspective, but the account of imitation we are providing is compatible with both alternatives.4
Body Babbling: Mapping Movements to End States
An imitative act is not one indissociable unit. It can be differentiated into organ identification and movement components. This section concerns the movement component. We do not think that infants know a priori what muscle movements achieve a particular state of organ relations, such as tongue protrusion, mouth opening, or lip protrusion. This could be learned through experience.
We call this experiential process ‘body babbling’. In body babbling, infants move their limbs and facial organs in repetitive body play analogous to vocal babbling. In the more familiar notion of vocal babbling the muscle movements are mapped to the resulting auditory consequence; infants are learning this articulatory–auditory relation (Kuhl and Meltzoff, 1996). Our notion of body babbling works in the same way, a principal difference being that the process can begin in utero. What is acquired through body babbling is a mapping between movements and the organ-relation end states that are attained.
By organ-relation end states (‘OR end states’) we mean a configural relation between organs. For example, three differentiable OR end states differing in extension might be: tongue-to-lips, tongue-between-lips, tongue-beyond-lips. Because both the dynamic patterns of movement and the body end states achieved can be monitored proprioceptively, infants’ body babbling builds up a ‘directory’ mapping movements to OR end states. On this view, the links between specific OR end states and the muscle movements needed to achieve them come from experience rather than being innately given.
Studies of fetal and neonatal behaviour have documented self-generated activity that could serve this hypothesized body babbling function (Hooker, 1952; Humphrey, 1971; Patrick et al., 1982; Prechtl, 1969, Vries et al., 1982, 1985). This is not to say that every possible organ relation is already mapped in the neonatal period. However, the possibility of an elementary directory prepared by prenatal experience means that we have identified a developmental process by which newborns could coordinate OR end states with the movements needed to achieve them, without invoking strong nativist claims.5
Organ Relations as the Cross-Modal Metric of Equivalence
We now come to the cross-modal metric of equivalence used in imitation. Regarding the visual target, infants parse the adult act they see into the organ relations it exhibits. Regarding infants’ own bodies, the consequences of their self-generated movements can be proprioceptively coded in terms of the relations between organs that are attained. Our hypothesis is that organ relations provide the common framework in which the acts of self and other are registered. ‘Tongue-to-lips’, which is an organ relation, would be a description that cuts across modality of perception and could describe both the target and the self. Thus organ relations render commensurate the seen but unfelt act of the adult and the felt but unseen facial act of the infant.
Such mechanisms would allow infants to imitate behaviours practised in body babbling as though looking up the target’s OR end state in the ‘movement–end state directory’ and executing the specified movements. However, results show that infants are not restricted to the imitation of well-practised acts that can be imitated on first try. As we have seen, they are not always satisfied with their initial motor performance. They correct their movements over time. Thus, the infant’s criterion for success seems to be achieving ‘a match of organ relations’, rather than reading out a specific pattern of muscle movements from the directory. Moreover, correction toward a more veridical match implies that representation of the target’s organ relations is independent of the infant’s motor attempts. In this sense, there is a differentiation between the representation of self and other.
In brief, our hypothesis is that the configural relation between organs serves as the cross-modal equivalence underlying imitation. Infants can perceive organ relations as applying both to adults and themselves. These perceived organ relations are the targets infants attempt to match—an activity observers see as behavioural imitiation.
A MODEL OF FACIAL IMITATION
Architecture
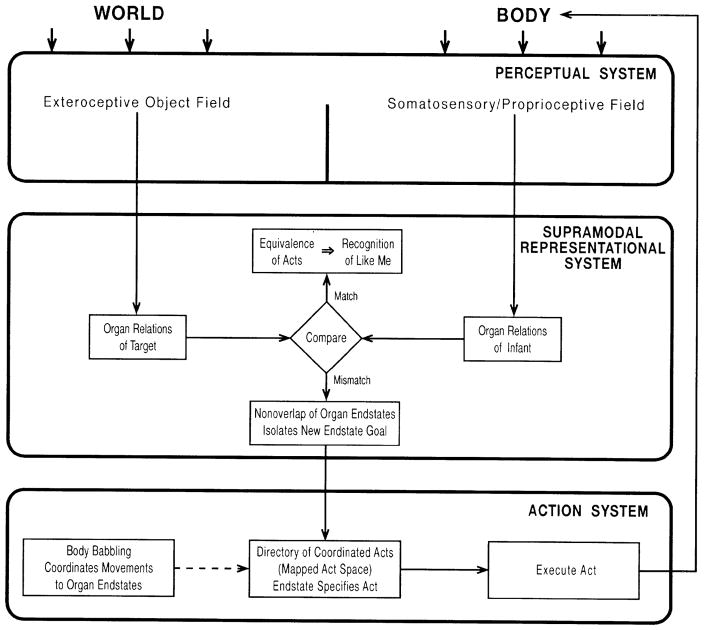
Figure 2 provides a detailed model of the mechanism underlying early facial imitation. This model fleshes out the general AIM framework schematized in Figure 1. Figure 2 shows how infants could generate an act that matches a visually perceived facial target. In the model, the equivalence between the infant and adult acts is specified in terms of organ relations. The model depicts the mechanisms underlying facial imitation in the first 2 months of life. Issues of development are addressed in the last section of the paper.
Figure 2.
AIM model of the mechanisms underlying early facial imitation. The model depicts the functional relations among the external world, perceptual system, representational system, and action system. Representations of the external target (the adult demonstration) and the infant’s body are compared in terms of organ relations (see text). The solid arrows indicate current processing. The dotted arrow indicates prior learning from body babbling experience.
The major components of the model are portrayed by the three bold boxes. The bold box labelled perceptual system functions to provide the perception of the infant’s own body and the external world. Comparisons between the organ relations of an external target and the current position of the infant’s own body are computed in the box labelled supramodal representational system. This comparison yields two possible outcomes: a match or mismatch. (1) A mismatch specifies a new configuration of the body, which serves as a goal for the next imitative attempt and is enacted by the box labelled action system. The arrow from the action system to the infant’s body shows that the effect of the act is a change in body configuration. (As already described, the action system box also includes the process of learning to map muscle movements to OR end states through prior body babbling experience.) (2) A match indicates that the motor act seen and the motor act done by the self are equivalent. This recognition of the equivalence of acts is grounds for infants’ apprehension that the other is, in some primitive sense, ‘like me’ (Meltzoff and Moore, 1995a, 1998).6
Operation of the Supramodal Representational System
One challenge for theories of early imitation is the finding that infants correct imitative errors. Consider what happens when a novel gesture such as tongue-protrusion-to-the side is presented to 6-week-olds, as was done in Meltzoff and Moore (1994). This target might initially be coded as the familiar organ relation of ‘tongue-to-lips’. The movement-end state directory would generate a first response of small tongue movement (assuming this was previously performed in body babbling). In fact, the data from the 1994 study show that 9 of the 10 infants who saw the tongue-protrusion-to-the-side target initially produced a small tongue movement with no lateral component. According to our model, this initial effort is corrected by computing the non-overlap between the organ relations of the visual target and those achieved by these first attempts. This comparison isolates the lateral dimension as a missing component, setting the goal for the next act. Repeated cycles of this process, isolating aspects of the target not captured in the last attempt, give the imitative progression its non-random character.
Meltzoff and Moore’s (1994) microanalysis of the correction process revealed four monotonically ordered steps in infants’ convergence toward the tongue-protrusion-to-the-side target. As diagrammed in Figure 3, the first step is a lateral movement of the tongue. Step 2 adds a small outward component to the lateral one. Step 3 produces a full tongue protrusion far beyond the lips. Step 4 integrates the lateral component into the full tongue protrusion. This process involves differentiating several dimensions of the target from one another (laterality, forwardness, and extent) and then integrating them into a single act. The process is not trial and error or even a simple progression from small to large, but rather an ordered, constructive process.7 Figure 4 shows the highest-level match to the adult’s gesture, which was achieved after the correction process already described.
Figure 3.
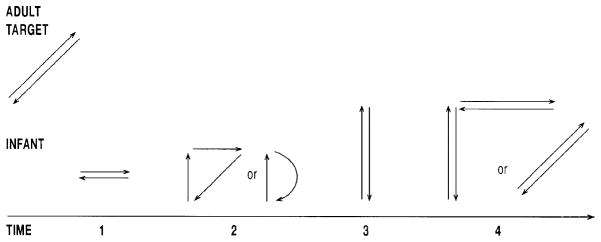
A diagram of infants’ correction process. Infants who are shown a novel gesture of tongue-protrusion-to-the-side (‘adult target’) progress through an ordered series of tongue movements (indicated by ‘1–4’). The direction and extent of the arrows depict the corresponding dimensions of the infants’ tongue protrusion responses. The responses are not randomly ordered, but rather exhibit a systematic convergence toward a more faithful match of the adult target (see text).
Figure 4.

Six-week-old infants imitating the large tongue-protrusion-to-the side gesture demonstrated in the Meltzoff and Moore (1994) study. Such behaviour rarely occurs in baseline activity when infants have not been shown the gesture. Infants produce these imitative matches after correcting earlier approximations. The AIM model of early imitation accounts for such correction by postulating that infants are monitoring their unseen actions through proprioception (see text). The acts shown here correspond to step 4 in Figure 3.
The correction process results in a novel behaviour that was not initially present. In our terms, it takes infants beyond entries in the directory formed by previous body babbling. Infants do not seem able to generate the novel response de novo, on first try, by inferring what movements to make from seeing a new OR end state alone. None the less, imitation is powerful and generative in the sense that a match to novel targets can be achieved without extrinsic reinforcement.
EXPLANATORY VALUE OF THE MODEL
Assessing Parsimony and Coherence
Infant imitation involves an active matching to target in which infants can, at some level, use equivalences between self and other in generating their response. Our initial AIM hypothesis argued that this equivalence is mediated by a supramodal representational system (Meltzoff and Moore, 1977, 1983). Over the past twenty years, we have designed studies investigating the nature of this system. We now think that organ relations are the metric infants use in determining cross-modal equivalence. Organ relations are the lingua franca by which acts of self and other can be commonly coded. They are the terms in which a tongue-to-lips by the adult and by the infant can be represented commensurately.
The concept of organ relations unifies three phenomena of early imitation. First, the notion that the adult model is analysed in terms of OR end states would allow infants to imitate static gestures, which has been demonstrated (Meltzoff and Moore, 1992). Second, infants’ coding of their own actions in terms of organ relations provides a parsing of their own acts. This creates a directory or ‘act space’ in which multiple muscle movements are mapped as equivalent paths to the same OR end state (addressing the motor equivalence problem). Such coding of infants’ own bodies would allow them to recognize when they are being imitated by another, which has also been reported (Meltzoff, 1990; Meltzoff and Moore, 1995a). Third, given that organ relations represent both self and other in commensurate terms, infants could detect imitative mismatches and correct subsequent imitative attempts (Meltzoff and Moore, 1994).
Assessing Alternatives
Imitation and Nativism
Facial imitation is demonstrated by newborns, but it is not completely explained by nativism. We propose important roles for learning and cognition. This may seem counterintuitive: if newborns do it, why invoke learning, no less cognition? The traditional argument seems to be that newborns = built in = reflexive transduction. However, the research findings suggest that this conflates separable terms.
There is no logical necessity that everything newborns do has to be built in, preformed, genetically specified. This ignores any contribution from prenatal experience. In fact, we have argued that intrauterine body babbling experience provides an initial mapping between movements and the OR end states they produce. Similarly, there is no logical necessity that all newborn behaviour—anymore than all 8-month-old behaviour—has to be an automatic, reflexive transduction. This cannot be decided by fiat. It is an empirical issue. For this reason we have carefully investigated newborns. The findings are that early imitation displays both goal-directed correction and temporal flexibility, which are more compatible with interpretive/cognitive processes than direct transduction.
Imitation and Amodal Perception: The Differentiation Problem
Imitation requires some cross-modal metric of equivalence. This raises a new problem. If infants can perceive self and other in equivalent terms, can they be told apart? Is there any perceptual distinction between self and other for the young baby? Piaget and Freud wrote elegantly of the initial ‘confusion’, ‘adualism’, and lack of distinction between self and other. In more modern terms, if one invokes a thoroughly amodal perceptual system to mediate imitation, this (ironically) recreates a Piagetian lack of differentiation.
A little data goes a long way in sorting this out. As we have seen, imitative acts can be corrected to achieve a more veridical match. Thus information about the infant’s acts is available for comparison to a representation of the adult’s act. More importantly for the problem at hand, representation of the target derived from the external world is not confused with or modified by the infants’ own motor attempts. This suggests a differentiation such that representation of the other’s body is separate from representation of the infant’s body. Although both representations use the supramodal ‘language’ of organ relations, self and other are not just one undifferentiated whole.
IMPLICATIONS FOR DEVELOPMENTAL THEORY
Early imitation is not the same as the more mature imitation displayed at 18 months. We believe there are profound developmental restructurings over this period especially in the meaning that imitation has for infants and the functions that it serves. We will discuss how imitation, broadly construed, serves as a ‘discovery procedure’ for understanding persons. We will also argue that several important aspects of childhood social cognition can be traced back to early imitation and will sketch such development8.
Four Developmental Changes in Imitation
According to our model, the newborn initially construes adult behaviours in terms of the organ relations they exhibit. With development, infants come to interpret the behaviour of other people at a higher level, in terms of human acts. An elementary human act is not just a movement or an organ relation, but rather a goal-directed organ transformation. Thus the first developmental change is an integration of the OR end state and the movements-to-produce-it into a single unit. Human acts (not simply OR end states in and of themselves) become the new terms of analysis, the meaningful units for parsing the behaviour of self and other. Human acts themselves have characteristics that can be imitated such as speed, duration, and manner. Thus, imitation at the level of acts can yield an increase in fidelity. For example, infants are not confined to imitating mouth opening, but can also imitate the duration of mouth openings. Such imitation has been reported in 6-week-olds (Meltzoff and Moore, 1994). Imitation rapidly shifts in the first weeks of life from being the matching of OR end states to the matching of acts.9
A subsequent development is from construing imitation as a matching of specific acts to the more abstract notion of a matching relationship. This developmental change is illustrated by infants’ reactions to being imitated by adults. Both 1-month-olds and 1-year-olds show marked interest in being copied, but react differently. After a period of being imitated, older infants gleefully ‘test’ whether they are being copied by abruptly changing acts while staring at the adult to see what he will do (Meltzoff, 1990). Younger infants do not switch to a new behaviour as if testing. They treat the other’s particular behaviour as a consequent of their own behaviour—as if their tongue protrusion causes the seen tongue protrusion—and they become upset if the adult starts to do another behaviour. In sum, older infants interpret imitation at a more abstract level than younger infants. The matching relationship transcends particular acts: it is not the notion that (infant) tongue protrusion causes (adult) tongue protrusion—x leads to x—but rather that the other is doing ‘the same as’ I do. Interpersonal matching becomes a meaningful unit of analysis, parsing interactions in terms of relationships rather than particular behaviours per se.
At about 1 year of age infants show a new interest in imitating the facial actions of others (Uzgiris and Hunt, 1975; Piaget, 1962). We hypothesize that this reinvigoration is due to a new modality-specific understanding of themselves —’my tongue protrusion looks like that seen tongue protrusion’ (Moore and Meltzoff, 1978). This idea makes sense of the observation that 1-year-olds do more than strictly imitate; they hold out their tongue in exaggerated fashion for an extended period of time. They treat the other as a biological mirror, inducing the adult to hold out his/her tongue for visual inspection. Infants at this age also tactually compare the unseen parts of their bodies with those of adults, feeling the adult’s mouth before reaching to their own. This appears to be an active tactual exploration of the similarity between self and the other. In both cases, the developmental change is infants’ new interest in the unseen parts of their own bodies. Imitation allows infants to enrich the supramodal perception of themselves with sense-specific information such as what their tongue might look like and how their tongue and another’s may feel the same. This enrichment of the already-established supramodal information with sense-specific information transforms their understanding of themselves and what it means to be like the other.
A further developmental change in imitation involves construing human acts so that the goal of an act can be identified even if it is not seen. This allows imitation of an inferred act. The best evidence comes from studies in which 18-month-olds are shown an adult trying, but failing, to perform an act (Meltzoff, 1995a). For example, when an adult’s hand slips off a dumbbell he is trying to pull apart, infants infer the adult goal and pull apart the dumbbell themselves. Imitation has developed to the point that infants no longer imitate what they literally see, but what the adult tried to do, a step toward understanding the intentions of others.
Imitation as a Discovery Procedure in Human Social Development
The foregoing examples show development in infants’ understanding of themselves, others, and interpersonal relationships. This takes us well beyond neonatal imitation as a behaviour, and highlights the significance of imitation in the growth of social cognition.
In our model, the underlying components of newborn imitation are organ identification, body babbling, and supramodal representation. Imitation, in turn, can be deployed as something like a discovery procedure for understanding the actions of people. Through interactions with others and the concomitant growth in self-understanding infants are engaged in an open-ended developmental process.
Such open-ended development continues beyond the changes sketched above and beyond infancy (Gopnik and Meltzoff, 1997). Further developments along this line include taking the perspective of others, role-taking and, eventually, the uniquely human capacity to form moral judgements based on the fundamental equality of persons. Thus development can be characterized as a process of creating equilibrium between self and other at increasing levels of abstraction (Meltzoff and Moore, 1995b). The outcome is a concept of person in which the self is understood as an objective entity in a world of others, and the other is ascribed a subjectivity as rich as one’s own. It is our thesis that this developmental pathway is grounded in the initial equivalence of self and other manifest by early imitation.
Acknowledgments
The order of authorship is alphabetical; the article is a co-equal creation. We thank Pat Kuhl, Scott Johnson, and Alan Slater for insightful suggestions on an earlier draft. We are also grateful to Calle Fisher and Craig Harris for help on the experiments. Funding was provided by a grant from NIH (HD-22514).
Footnotes
The results remained the same when the data were analysed in a different manner. In the alternative analysis, we calculated for each S (N=40) the number of HT+TP responses as a proportion of the total number of tongue protrusions emitted by each S [HT+TP] ÷ TP). The results showed that these proportional scores were significantly greater for the tongue-protrusion-to-the-side group compared to the controls, Mann–Whitney U=92.5, p<0.05.
The reader may wonder what body parts would constitute ‘organs’. Preliminary and published studies suggest that the following body parts may be organs that can be identified by young infants: head, brows, jaw, lips, tongue, arms, hands, fingers, trunk, legs, and feet.
There has long been interest in infants’ visual preference for faces, and recent progress has been made in understanding this phenomenon (de Boysson-Bardies et al., 1993; Morton and Johnson, 1991; Walton et al., 1992). It is now established that newborns prefer faces over other patterns of about the same size and complexity, scrambled faces, etc. It remains to be determined whether the preference is based on a dedicated ‘face detector’ or general sensory characteristics such as the visual amplitude spectrum (Kleiner, 1993; Slater, 1993). Regardless of how the preference is mediated, there is a consensus that a face recruits more visual attention than other patterns newborns regularly encounter in the real world.
A third possibility is that biologically relevant human acts are redundantly coded, specified both by the configural relations between organs and by kinetic signatures. Such redundancy would allow infants to imitate static gestures as we have reported (Meltzoff and Moore, 1992) and also the dynamic displays discussed by Bower (1982).
Presumably the easiest actions and the most salient proprioceptive outcomes are coordinated first. Simple tongue protrusion may be easily imitated due to prior experience with this movement in the intrauterine environment. It would be a familiar action for neonates, practised in body babbling. Maratos (1982) advanced similar arguments.
Because the adult act is coded in the representational system, and representations persist, the proposed model accommodates the findings of early deferred imitation (e.g., Meltzoff and Moore, 1994). Even after the display has disappeared from view, the representation can be used as a target whenever the infant’s attention is drawn to it.
The model assumes that infants’ comparisons between representations of their own body and the adult target isolate the discrepancies between them (the non-overlap in OR end states), making them salient and recruiting infant attention. Such highlighting, or ‘pop out’, at the representational level may be similar to infants’ increased attention when there is a discrepancy between perception and representation (as in visual dishabituation). In the tongue-protrusion-to-the-side case, the missing lateral component is the first dimension isolated (Figure 3, Time 1). After performing a lateral tongue, what is more discrepant is the missing forward dimension. Having established the relevant directions of the tongue movements (lateral and forward), the degree of forward extent beyond the lips next becomes salient. Such a process could underlie the systematic ordering of infants’ corrections that was documented in Meltzoff and Moore (1994) and diagrammed in Figure 3.
As in all developmental theories, an interesting case is presented by infants with sensory or motor deficits such as blindness or motor paralysis. Because the present model postulates organ identification and a supramodal framework, the deficits can be compensated for. Development may be slowed, but it would not be blocked. Supramodal representation allows one modality to substitute for another; for example, facial organs may be identified by tactual exploration in the case of blindness. Similarly, as long as the motor deficit does not extend to movements of every organ of the infant’s body, some channels for elaborating self–other relations will exist. Even in the Gedanken experiment of a non-comatose infant with complete motor paralysis, the capacity for organ identification would still provide grounds for treating other people as special. As long as the integrity of the central supramodal representational system is not compromised, motor impairments affecting individual body parts can be overcome.
The notion that infants come to construe behaviour in terms of human acts deserves further analysis. Of course even the youngest infants perceive spatiotemporal movements of the face and the OR end states attained. Our point is that newborns do not initially construe the movements and end states as a unitary whole combining them as a single act (the difference between seeing a display as either: tongue movements vs, the configuration of tongue-between-lips vs. the unitary act of tongue protrusion). This developmental change of processing the act as a unitary whole is probably connected to the emergence of an ‘act space’ from the more primitive movement-to-end state directory. We think that the observation of social others plays an important role in this development. Initially, OR end states are known only in terms of self movement (as a result of producing the movement); observation of others presents OR end states differentiated from the infants’ accompanying movements. These differentiated end states help provide the limits and extent (dimensions) of an organ’s act space. Given a dimensionalized act space, infants could interpolate new points that lie within the already established space. For example, they could directly and fluidly imitate a novel act which lies within the space, even though they have never practised this particular movement pattern before. Act space thus organizes and represents an organ’s transformations (the range of possible end states that can be attained, calibrated with muscle movements).
References
- Abravanel E, DeYong NG. Does object modeling elicit imitative-like gestures from young infants? Journal of Experimental Child Psychology. 1991;52:22–40. doi: 10.1016/0022-0965(91)90004-c. [DOI] [PubMed] [Google Scholar]
- Abravanel E, Sigafoos AD. Exploring the presence of imitation during early infancy. Child Development. 1984;55:381–392. [PubMed] [Google Scholar]
- Barr R, Dowden A, Hayne H. Developmental changes in deferred imitation by 6- to 24-month-old infants. Infant Behavior and Development. 1996;19:159–170. [Google Scholar]
- Bauer PJ, Mandler JM. Putting the horse before the cart: the use of temporal order in recall of events by one-year-old children. Developmental Psychology. 1992;28:441–452. [Google Scholar]
- Bertenthal BI. Origins and early development of perception, action, and representation. Annual Review of Psychology. 1996;47:431–459. doi: 10.1146/annurev.psych.47.1.431. [DOI] [PubMed] [Google Scholar]
- Bower TGR. Development in Infancy. 2. San Francisco: W. H. Freeman; 1982. [Google Scholar]
- Butterworth G, Hopkins B. Hand–mouth coordination in the new-born baby. British Journal of Developmental Psychology. 1988;6:303–314. [Google Scholar]
- de Boysson-Bardies B, de Schonen S, Jusczyk P, MacNeilage P, Morton J. Developmental Neurocognition: Speech and Face Processing in the First Year of Life. Dordrecht, Netherlands: Kluwer; 1993. [Google Scholar]
- Desimone R. Face-selective cells in the temporal cortex of monkeys. Journal of Cognitive Neuroscience. 1991;3:1–8. doi: 10.1162/jocn.1991.3.1.1. [DOI] [PubMed] [Google Scholar]
- Field TM, Goldstein S, Vaga-Lahr N, Porter K. Changes in imitative behavior during early infancy. Infant Behavior and Development. 1986;9:415–421. [Google Scholar]
- Field TM, Woodson R, Cohen D, Greenberg R, Garcia R, Collins E. Discrimination and imitation of facial expressions by term and preterm neonates. Infant Behavior and Development. 1983;6:485–489. [Google Scholar]
- Field TM, Woodson R, Greenberg R, Cohen D. Discrimination and imitation of facial expressions by neonates. Science. 1982;218:179–181. doi: 10.1126/science.7123230. [DOI] [PubMed] [Google Scholar]
- Fontaine R. Imitative skills between birth and six months. Infant Behavior and Development. 1984;7:323–333. [Google Scholar]
- Gopnik A, Meltzoff AN. Words, Thoughts, and Theories. Cambridge, MA: MIT Press; 1997. [Google Scholar]
- Goren CC, Sarty M, Wu PYK. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics. 1975;56:544–549. [PubMed] [Google Scholar]
- Gross CG. Representation of visual stimuli in inferior temporal cortex. In: Bruce V, Cowey A, Ellis AW, editors. Processing the Facial Image. New York: Oxford University Press; 1992. pp. 3–10. [DOI] [PubMed] [Google Scholar]
- Gross CG, Bender DB, Rocha-Miranda CE. Visual receptive fields of neurons in inferotemporal cortex of the monkey. Science (Wash) 1969;166:1303–1306. doi: 10.1126/science.166.3910.1303. [DOI] [PubMed] [Google Scholar]
- Gross CG, Rocha-Miranda CE, Bender DB. Visual properties of neurons in inferotemporal cortex of the macaque. Journal of Neurophysiology. 1972;35:96–111. doi: 10.1152/jn.1972.35.1.96. [DOI] [PubMed] [Google Scholar]
- Gross CG, Sargent J. Face recognition. Current Opinion in Neurobiology. 1992;2:156–161. doi: 10.1016/0959-4388(92)90004-5. [DOI] [PubMed] [Google Scholar]
- Heimann M. Neonatal imitation, gaze aversion, and mother–infant interaction. Infant Behavior and Development. 1989;12:495–505. [Google Scholar]
- Heimann M, Nelson KE, Schaller J. Neonatal imitation of tongue protrusion and mouth opening: methodological aspects and evidence of early individual differences. Scandinavian Journal of Pscyhology. 1989;30:90–101. doi: 10.1111/j.1467-9450.1989.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Heimann M, Schaller J. Imitative reactions among 14–21 day old infants. Infant Mental Health Journal. 1985;6:31–39. [Google Scholar]
- Hooker D. The Prenatal Origin of Behavior. Lawrence, KS: University of Kansas Press; 1952. [Google Scholar]
- Humphrey T. Development of oral and facial motor mechanisms in human fetuses and their relation to craniofacial growth. Journal of Dental Research. 1971;50:1428–1441. doi: 10.1177/00220345710500061301. [DOI] [PubMed] [Google Scholar]
- Jacobson SW. Matching behavior in the young infant. Child Development. 1979;50:425–430. [PubMed] [Google Scholar]
- Kaitz M, Meschulach-Sarfaty O, Auerbach J, Eidelman A. A reexamination of newborn’s ability to imitate facial expressions. Developmental Psychology. 1988;24:3–7. [Google Scholar]
- Kleiner KA. Specific vs. non-specific face recognition device. In: de Boysson-Bardies B, de Schonen S, Jusczyk P, MacNeilage P, Morton J, editors. Developmental Neurocognition: Speech and Face Processing in the First Year of Life. Dordrecht, Netherlands: Kluwer; 1993. [Google Scholar]
- Kugiumutzakis J. Unpublished doctoral dissertation. Uppsala University; Sweden: 1985. The Origin, Development, and Function of the Early Infant Imitation. [Google Scholar]
- Kuhl PK, Meltzoff AN. Infant vocalizations in response to speech: vocal imitation and developmental change. Journal of the Acoustical Society of America. 1996;100:2425–2438. doi: 10.1121/1.417951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerstee M. The role of person and object in eliciting early imitation. Journal of Experimental Child Psychology. 1991;51:423–433. doi: 10.1016/0022-0965(91)90086-8. [DOI] [PubMed] [Google Scholar]
- Maratos O. Trends in the development of imitation in early infancy. In: Bever TG, editor. Regressions in Mental Development: Basic Phenomena and Theories. Hillsdale, NJ: Erlbaum; 1982. pp. 81–101. [Google Scholar]
- Meltzoff AN. Foundations for developing a concept of self: the role of imitation in relating self to other and the value of social mirroring, social modeling, and self practice in infancy. In: Cicchetti D, Beeghly M, editors. The Self in Transition: Infancy to Childhood. Chicago: University of Chicago press; 1990. pp. 139–164. [Google Scholar]
- Meltzoff AN. Infant imitation after a 1-week delay: long-term memory for novel acts and multiple stimuli. Developmental Psychology. 1988;24:470–476. doi: 10.1037/0012-1649.24.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. Understanding the intentions of others: re-enactment of intended acts by 18-month-old children. Developmental Psychology. 1995a;31:838–850. doi: 10.1037/0012-1649.31.5.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. What infant memory tells us about infantile amnesia: long-term recall and deferred imitation. Journal of Experimental Child Psychology. 1995b;59:497–515. doi: 10.1006/jecp.1995.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198:75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Newborn infants imitate adult facial gestures. Child Development. 1983;54:702–809. [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Imitation in newborn infants: exploring the range of gestures imitated and the underlying mechanisms. Developmental Psychology. 1989;25:954–962. doi: 10.1037/0012-1649.25.6.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Early imitation within a functional framework: the importance of person identity, movement, and development. Infant Behavior and Development. 1992;15:479–505. doi: 10.1016/0163-6383(92)80015-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Imitation, memory, and the representation of persons. Infant Behavior and Development. 1994;17:83–99. doi: 10.1016/0163-6383(94)90024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. A theory of the role of imitation in the emergence of self. In: Rochat P, editor. The Self in Early Infancy: Theory and Research. New York:North-Holland: 1995a. pp. 73–93. [Google Scholar]
- Meltzoff AN, Moore MK. Infants’ understanding of people and things: from body imitation to folk psychology. In: Bermúdez J, Marcel AJ, Eilan N, editors. The Body and the Self. Cambridge, MA: MIT Press; 1995b. pp. 43–69. [Google Scholar]
- Meltzoff AN, Moore MK. Infant intersubjectivity: broadening the dialogue to include intention, identity, and imitation. In: Bråten S, editor. Intersubjective Communication and Emotion in Early Ontogeny: A Sourcebook. Cambridge, UK: Cambridge University Press; 1998. [Google Scholar]
- Moore MK, Meltzoff AN. Object permanence, imitation, and language development in infancy: toward a neo-Piagetian perspective on communicative and cognitive development. In: Minifie FD, Lloyd LL, editors. Communicative and Cognitive Abilities: Early Behavioral Assessment. Baltimore: University Park Press; 1978. pp. 151–184. [Google Scholar]
- Morton J, Johnson MH. CONSPEC and CONLEARN: a two-process theory of infant face recognition. Psychological Review. 1991;98:164–181. doi: 10.1037/0033-295x.98.2.164. [DOI] [PubMed] [Google Scholar]
- Patrick J, Campbell K, Carmichael L, Natale R, Richardson B. Patterns of gross fetal body movement over 24-hour observation intervals during the last 10 weeks of pregnancy. American Journal of Obstetrics and Gynecology. 1982;142:363–371. doi: 10.1016/s0002-9378(16)32375-4. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Hietanen JK, Oram MW, Benson PJ. Organization and functions of cells responsive to faces in the temporal cortex. In: Bruce V, Cowey A, Ellis AW, editors. Processing the Facial Image. New York: Oxford University Press; 1992. pp. 23–30. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Mistlin AJ, Chitty AJ. Visual neurons responsive to faces. Trends in Neuroscience. 1987;10:358–364. [Google Scholar]
- Piaget J. Play, Dreams and Imitation in Childhood. New York: Norton; 1962. [Google Scholar]
- Prechtl HFR. Brain and behavior mechanisms in the human newborn infant. In: Robinson RJ, editor. Brain and Early Behavior. New York: Free Press; 1969. pp. 289–360. [Google Scholar]
- Reissland N. Neonatal imitation in the first hour of life: observations in rural Nepal. Developmental Psychology. 1988;24:464–469. [Google Scholar]
- Rolls ET. Neurophysiological mechanisms underlying face processing within and beyond the temporal cortical visual areas. In: Bruce V, Cowey A, Ellis AW, editors. Processing the Facial Image. New York: Oxford University Press; 1992. pp. 11–21. [DOI] [PubMed] [Google Scholar]
- Slater AM. Visual perceptual abilities at birth: implications for face perception. In: de Boysson-Bardies B, de Schonen S, Jusczyk P, MacNeilage P, Morton J, editors. Developmental Neurocognition: Speech and Face Processing in the First Year of Life. Dordrecht, Netherlands: Kluwer; 1993. [Google Scholar]
- Trevarthen C. The foundations of intersubjectivity: development of interpersonal and cooperative understanding in infants. In: Olson DR, editor. The Social Foundations of Language and Thought: Essays in Honor of Jerome S Bruner. New York: Norton; 1980. pp. 316–342. [Google Scholar]
- Uzgiris IC, Hunt JM. Assessment in Infancy: Ordinal Scales of Psychological Development. Urbana: University of Illinois Press; 1975. [Google Scholar]
- van der Meer ALH, van der Weel FR, Lee DN. The functional significance of arm movements in neonates. Science. 1995;267:693–695. doi: 10.1126/science.7839147. [DOI] [PubMed] [Google Scholar]
- Vinter A. The role of movement in eliciting early imitations. Child Development. 1986;57:66–71. [Google Scholar]
- de Vries JIP, Visser GHA, Prechtl HFR. The emergence of fetal behaviour. I. Qualitative aspects. Early Human Development. 1982;7:301–322. doi: 10.1016/0378-3782(82)90033-0. [DOI] [PubMed] [Google Scholar]
- de Vries JIP, Visser GHA, Prechtl HFR. The emergence of fetal behaviour. II. Quantitative aspects. Early Human Development. 1985;12:99–120. doi: 10.1016/0378-3782(85)90174-4. [DOI] [PubMed] [Google Scholar]
- Walton GE, Bower NJA, Bower TGR. Recognition of familiar faces by newborns. Infant Behavior and Development. 1992;15:265–269. [Google Scholar]