Abstract
Carbon catabolite repression in Aspergillus nidulans is mediated by a negative-acting protein coded by the creA gene. We have investigated how CREA controls the expression of the ethanol regulon genes. CREA is a major component of the control of this regulon. Its presence in the cell results in a permanent, albeit partial, repression of the alc genes under all physiological growth conditions, even when the fungus is grown on carbon sources considered to be non-repressing. A crucial step in the control processes is the repression of the positive-acting specific regulatory gene alcR, by the binding of CREA on its cognate target sites on the alcR promoter. The removal of one of these targets, URSA, results in a 50% derepression of the alcR gene. Furthermore, the presence of this sequence contributes directly to the low alcR expression under nonrepressing conditions and reduces alcR promoter function by at least 100-fold. CREA acts both on the regulatory gene alcR and directly on the two structural genes alcA and aldA, as glucose repression of the latter genes occurs in strains where alcR transcription is driven by a strong constitutive and derepressed promoter. In vivo and in vitro competition experiments show that CREA acts by competing directly with the binding of the ALCR activator for the same region of the alcR promoter, a region which encompasses overlapping targets for both regulatory proteins. These data are consistent with a model in which the activating and repressing regulatory proteins compete to regulate expression of the ethanol regulon genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arst H. N., Jr, Tollervey D., Dowzer C. E., Kelly J. M. An inversion truncating the creA gene of Aspergillus nidulans results in carbon catabolite derepression. Mol Microbiol. 1990 May;4(5):851–854. doi: 10.1111/j.1365-2958.1990.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Bailey C., Arst H. N., Jr Carbon catabolite repression in Aspergillos nidulans. Eur J Biochem. 1975 Feb 21;51(2):573–577. doi: 10.1111/j.1432-1033.1975.tb03958.x. [DOI] [PubMed] [Google Scholar]
- Brandl C. J., Furlanetto A. M., Martens J. A., Hamilton K. S. Characterization of NGG1, a novel yeast gene required for glucose repression of GAL4p-regulated transcription. EMBO J. 1993 Dec 15;12(13):5255–5265. doi: 10.1002/j.1460-2075.1993.tb06221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove D. J. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966 Jan 11;113(1):51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- Cubero B., Scazzocchio C. Two different, adjacent and divergent zinc finger binding sites are necessary for CREA-mediated carbon catabolite repression in the proline gene cluster of Aspergillus nidulans. EMBO J. 1994 Jan 15;13(2):407–415. doi: 10.1002/j.1460-2075.1994.tb06275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowzer C. E., Kelly J. M. Analysis of the creA gene, a regulator of carbon catabolite repression in Aspergillus nidulans. Mol Cell Biol. 1991 Nov;11(11):5701–5709. doi: 10.1128/mcb.11.11.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felenbok B., Sequeval D., Mathieu M., Sibley S., Gwynne D. I., Davies R. W. The ethanol regulon in Aspergillus nidulans: characterization and sequence of the positive regulatory gene alcR. Gene. 1988 Dec 20;73(2):385–396. doi: 10.1016/0378-1119(88)90503-3. [DOI] [PubMed] [Google Scholar]
- Felenbok B. The ethanol utilization regulon of Aspergillus nidulans: the alcA-alcR system as a tool for the expression of recombinant proteins. J Biotechnol. 1991 Jan;17(1):11–17. doi: 10.1016/0168-1656(91)90023-o. [DOI] [PubMed] [Google Scholar]
- Gill G., Ptashne M. Negative effect of the transcriptional activator GAL4. Nature. 1988 Aug 25;334(6184):721–724. doi: 10.1038/334721a0. [DOI] [PubMed] [Google Scholar]
- Griggs D. W., Johnston M. Regulated expression of the GAL4 activator gene in yeast provides a sensitive genetic switch for glucose repression. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8597–8601. doi: 10.1073/pnas.88.19.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwynne D. I., Buxton F. P., Sibley S., Davies R. W., Lockington R. A., Scazzocchio C., Sealy-Lewis H. M. Comparison of the cis-acting control regions of two coordinately controlled genes involved in ethanol utilization in Aspergillus nidulans. Gene. 1987;51(2-3):205–216. doi: 10.1016/0378-1119(87)90309-x. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Giguere V., Segui P., Evans R. M. Colocalization of DNA-binding and transcriptional activation functions in the human glucocorticoid receptor. Cell. 1987 Apr 10;49(1):39–46. doi: 10.1016/0092-8674(87)90753-7. [DOI] [PubMed] [Google Scholar]
- Kulmburg P., Judewicz N., Mathieu M., Lenouvel F., Sequeval D., Felenbok B. Specific binding sites for the activator protein, ALCR, in the alcA promoter of the ethanol regulon of Aspergillus nidulans. J Biol Chem. 1992 Oct 15;267(29):21146–21153. [PubMed] [Google Scholar]
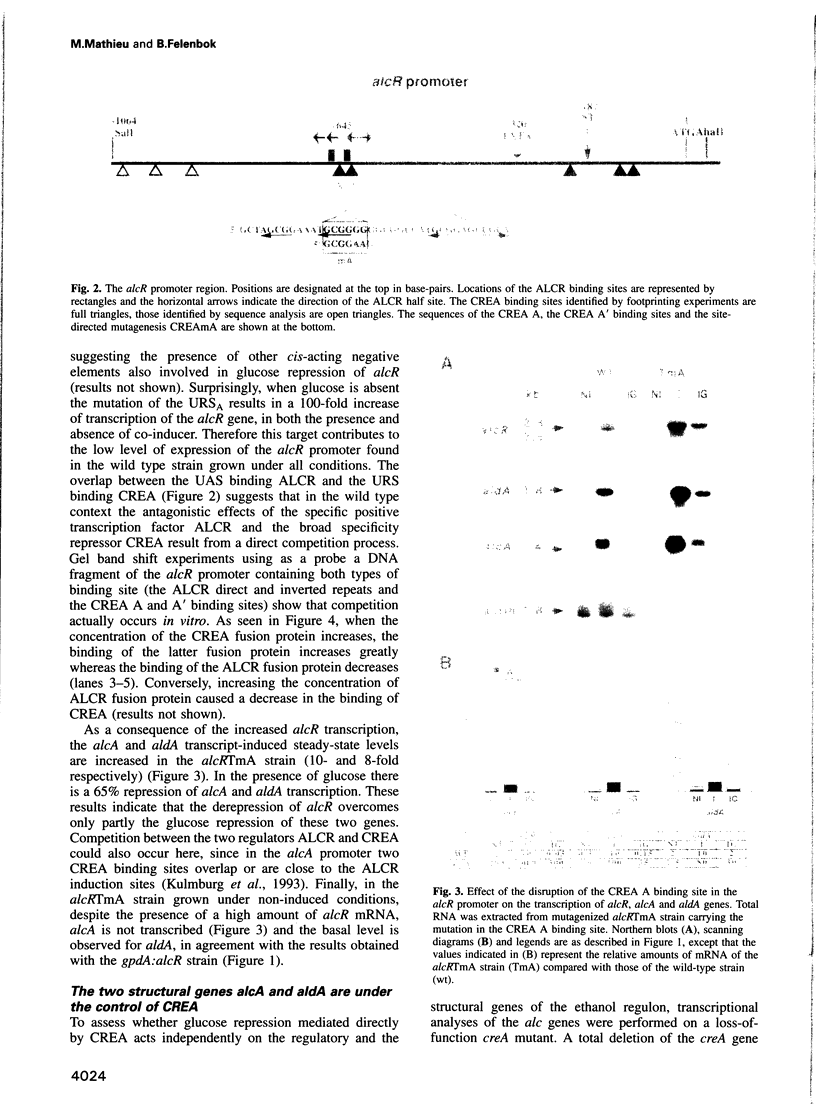
- Kulmburg P., Mathieu M., Dowzer C., Kelly J., Felenbok B. Specific binding sites in the alcR and alcA promoters of the ethanol regulon for the CREA repressor mediating carbon catabolite repression in Aspergillus nidulans. Mol Microbiol. 1993 Mar;7(6):847–857. doi: 10.1111/j.1365-2958.1993.tb01175.x. [DOI] [PubMed] [Google Scholar]
- Kulmburg P., Prangé T., Mathieu M., Sequeval D., Scazzocchio C., Felenbok B. Correct intron splicing generates a new type of a putative zinc-binding domain in a transcriptional activator of Aspergillus nidulans. FEBS Lett. 1991 Mar 11;280(1):11–16. doi: 10.1016/0014-5793(91)80193-7. [DOI] [PubMed] [Google Scholar]
- Kulmburg P., Sequeval D., Lenouvel F., Mathieu M., Felenbok B. Identification of the promoter region involved in the autoregulation of the transcriptional activator ALCR in Aspergillus nidulans. Mol Cell Biol. 1992 May;12(5):1932–1939. doi: 10.1128/mcb.12.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lamphier M. S., Ptashne M. Multiple mechanisms mediate glucose repression of the yeast GAL1 gene. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5922–5926. doi: 10.1073/pnas.89.13.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockington R., Scazzocchio C., Sequeval D., Mathieu M., Felenbok B. Regulation of alcR, the positive regulatory gene of the ethanol utilization regulon of Aspergillus nidulans. Mol Microbiol. 1987 Nov;1(3):275–281. doi: 10.1111/j.1365-2958.1987.tb01933.x. [DOI] [PubMed] [Google Scholar]
- Nehlin J. O., Carlberg M., Ronne H. Control of yeast GAL genes by MIG1 repressor: a transcriptional cascade in the glucose response. EMBO J. 1991 Nov;10(11):3373–3377. doi: 10.1002/j.1460-2075.1991.tb04901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlin J. O., Ronne H. Yeast MIG1 repressor is related to the mammalian early growth response and Wilms' tumour finger proteins. EMBO J. 1990 Sep;9(9):2891–2898. doi: 10.1002/j.1460-2075.1990.tb07479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateman J. A., Doy C. H., Olsen J. E., Norris U., Creaser E. H., Hynes M. Regulation of alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (AldDH) in Aspergillus nidulans. Proc R Soc Lond B Biol Sci. 1983 Feb 22;217(1208):243–264. doi: 10.1098/rspb.1983.0009. [DOI] [PubMed] [Google Scholar]
- Pickett M., Gwynne D. I., Buxton F. P., Elliott R., Davies R. W., Lockington R. A., Scazzocchio C., Sealy-Lewis H. M. Cloning and characterization of the aldA gene of Aspergillus nidulans. Gene. 1987;51(2-3):217–226. doi: 10.1016/0378-1119(87)90310-6. [DOI] [PubMed] [Google Scholar]
- Punt P. J., Dingemanse M. A., Jacobs-Meijsing B. J., Pouwels P. H., van den Hondel C. A. Isolation and characterization of the glyceraldehyde-3-phosphate dehydrogenase gene of Aspergillus nidulans. Gene. 1988 Sep 15;69(1):49–57. doi: 10.1016/0378-1119(88)90377-0. [DOI] [PubMed] [Google Scholar]
- Punt P. J., Oliver R. P., Dingemanse M. A., Pouwels P. H., van den Hondel C. A. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene. 1987;56(1):117–124. doi: 10.1016/0378-1119(87)90164-8. [DOI] [PubMed] [Google Scholar]
- Sequeval D., Felenbok B. Relationship between zinc content and DNA-binding activity of the DNA-binding motif of the transcription factor ALCR in Aspergillus nidulans. Mol Gen Genet. 1994 Jan;242(1):33–39. doi: 10.1007/BF00277345. [DOI] [PubMed] [Google Scholar]
- Stone G., Sadowski I. GAL4 is regulated by a glucose-responsive functional domain. EMBO J. 1993 Apr;12(4):1375–1385. doi: 10.1002/j.1460-2075.1993.tb05782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upshall A., Gilbert T., Saari G., O'Hara P. J., Weglenski P., Berse B., Miller K., Timberlake W. E. Molecular analysis of the argB gene of Aspergillus nidulans. Mol Gen Genet. 1986 Aug;204(2):349–354. doi: 10.1007/BF00425521. [DOI] [PubMed] [Google Scholar]