Abstract
The involvement of autoreactive T cells in the pathogenesis of rheumatoid arthritis (RA) as well as in autoimmune animal models of arthritis has been well established; however, unanswered questions, such as the role of joint-homing T cells, remain. Animal models of arthritis are superb experimental tools in demonstrating how T cells trigger joint inflammation, and thus can help to further our knowledge of disease mechanisms and potential therapies. In this Review, we discuss the similarities and differences in T-cell subsets and functions between RA and mouse arthritis models. For example, various T-cell subsets are involved in both human and mouse arthritis, but differences might exist in the cytokine regulation and plasticity of these cells. With regard to joint-homing T cells, an abundance of synovial T cells is present in humans compared with mice. On the other hand, local expansion of type 17 T helper (TH17) cells is observed in some animal models, but not in RA. Finally, whereas T-cell depletion essentially failed in RA, antibody targeting of T cells can work, at least preventatively, in most arthritis models. Clearly, additional human and animal studies are needed to fill the gap in our understanding of the specific contribution of T-cell subsets to arthritis in mice and men.
Introduction
“Arthritis: where are the T cells?” This question was raised by Kamradt and Frey in an editorial in a 2010 issue of Arthritis Research & Therapy.1 In their commentary, Kamradt and Frey referred to our study in which only a minuscule population of T cells was found in the joints of mice with proteoglycan-induced arthritis (PGIA).2 In the same study, depletion of T cells from the peripheral blood of mice, which led to a reduction of T-cell numbers in the synovial fluid, failed to diminish joint inflammation.2 This observation raises a more general question: how closely do animal models resemble human rheumatoid arthritis (RA)? Certainly a major, and clinically relevant, question is why T-cell depletion strategies failed in RA when, mostly preventatively but in some models also therapeutically, they worked in mouse arthritis.
RA is an autoimmune–inflammatory rheumatic disease characterized by synovitis of multiple joints eventually leading to cartilage and bone destruction.3 Although RA is a T-cell-dependent disease, no definitive ‘arthritogenic’ T-cell populations have yet been characterized. It is very likely that impaired immune regulation, resulting from deficiencies in the number and/or function of regulatory T (TREG) cells and the resistance of effector T cells to TREG-cell-mediated suppression, is involved in the development of synovial inflammation.4 Furthermore, in addition to the ‘loss of control’ within the inflammatory environment, autoreactive T cells outside the joints and autoantibodies generated via T-cell-dependent processes are key players in the pathogenesis of RA.
Animal models are needed for in-depth investigation of pathogenic pathways that are involved in RA but are not accessible in humans.5 For example, animals can be subjected to experimental procedures such as arthritis-inducing immunizations, cell depletion or transfer, selective cross-breeding, genetic manipulations, in vivo cell-homing studies and, most importantly, numerous preventative and therapeutic targeting strategies, most of which cannot be carried out in humans. Numerous arthritis models have been well characterized.5–7 In past decades, we have obtained considerable amounts of information about the function of T-cell subsets that drive the pathogenic processes in the mouse PGIA model.7–9
Here, we provide a review of our current understanding of autoreactive T cells, various T-cell subsets, joint-homing T cells and T-cell-dependent autoantibodies in arthritis. We briefly present data obtained in human RA and compare these findings with those obtained from studies on animal models of arthritis. As our greatest expertise is in PGIA, we focus primarily on this model. However, some studies performed in other inducible models, such as type II collagen (CII)-induced arthritis (CIA) and glucose-6-phosphate isomerase (G6PI)-induced arthritis, as well as in spontaneous arthritis in K/BxN or SKG mice, are also discussed. Finally, we touch on the question as to why most T-cell-targeting strategies failed in patients with RA and how suitable animal models are in predicting the clinical efficacy of T-cell-directed biologic agents in RA.
Rise and persistence of autoreactive T cells
The importance of T cells in arthritis
T cells have various roles in RA and in mouse models of the disease; the major similarities and differences between human and mouse disease with respect to T cells are summarized in Table 1. Several lines of evidence suggest that, similarly to in human RA, T cells have a critical role in inducible animal models of arthritis, including PGIA and CIA, as well as in spontaneous arthritis in K/BxN and SKG mice. T cells are also involved in the generation of ‘arthritogenic’ antibodies that can passively transfer arthritis following injection into naive mice. In PGIA, T-cell depletion using anti-CD4 antibodies led to complete inhibition of arthritis development, whereas treatment with anti-CD8 antibodies resulted in increased disease severity.10 As CD8+ TREG cells exist in human RA, depletion of these cells by anti-CD8 antibodies could indeed result in aggravation of the disease. CD4-depleting antibodies also suppressed CIA when administered before, but not after, arthritis development, suggesting a greater role of T helper (TH) cells in the initiation phase of the disease than in the effector phase.11 In the same study, in vivo activated CD4+ T cells specific for CII were found to be quite resistant to antibody-mediated depletion.11 This study could, at least in part, explain why most anti-CD4 antibody studies in human RA have failed. In adoptively transferred PGIA and CIA, which are induced in naive mice by the transfer of immune cells from mice with PGIA or CIA, removal of CD3+ T cells (that is, all T cells) or CD4+ T cells from the donor population inhibited the transfer of arthritis to severe combined immunodeficient (SCID) mice (which lack functional B and T cells).2, 12 Therapeutic depletion of CD4+ cells—after onset of arthritis—abrogated G6PI-induced arthritis.13 In conclusion, CD4+ T cells possibly have an important role in the development of arthritis in various mouse models. Their involvement might be crucial in the early phase of the disease, suggesting that anti-CD4 antibody therapy could be effective early in the disease course.
Table 1.
Similarities and differences between mouse arthritis models and human RA with respect to T cells
| Category | Feature | Mouse models(reference) | Human RA(reference) |
|---|---|---|---|
| Autoreactive T-cell development and function | |||
| MHC restriction | Yes8,21 | Yes17,18 | |
| T-cell responses to CII | Yes11 | Yes17,24 | |
| T-cell responses to citrullinated proteins | To citPG9 and citFib27 | To multiple citrullinated epitopes18,25 | |
| T-cell subsets | |||
| TH17–TREG cell plasticity | Yes4,32 | Yes4 | |
| TH17 → TH1 cell conversion | Yes38,43 | Yes40,46 | |
| TH2 → TH17 cell conversion | No32 | No32 | |
| TGF-β-dependent TH17 cell induction | No32 | Yes32 | |
| IL-6–STAT3- dependent TH17 cell development | Yes32 | Yes32 | |
| IL-1-dependent TH17 cell induction | In some studies110 | Yes32 | |
| IL-2-dependent stimulation | TREG cells4,32 | TH17 cells, IL-1732 | |
| IL-21-dependent positive feedback loop | Yes32 | In some studies111 | |
| IL-22 production by TH17 cells | In some studies110 | Yes32 | |
| IL-23-dependent maintenance of TH17 cell responses | Yes32 | Yes32 | |
| TGF-β-dependent TREG cell growth | Yes4,32 | Yes4, 32 | |
| TNF effects on TREG cells | Promote expansion4,71 | Inhibit expansion4,69 | |
| Ectopic lymphoid structures in synovium | NR | Yes49 | |
| Role of PD1 in TFH cells | Yes56 | Yes54 | |
| TH1–TFH cell plasticity | Yes57 | NR | |
| Joint-homing T cells | |||
| Level of T-cell recruitment to synovium | Low, majority TREG cells65,76,77 | High, majority TH1 cells30,32 | |
| Synovial TH17 cells | Locally expanded4 | Limited local expansion4 | |
| Diminished TREG cell suppressor capacity in arthritic joints | NR | Yes4 | |
| T-cell-dependent autoantibodies | |||
| ACPA, RF | Yes98 | Yes87,91 | |
| Anti-CII antibodies | Yes21 | Yes100 | |
| Therapeutic interventions | |||
| Efficacy of T-cell- depleting therapies | Yes (mostly preventatively)11,22 | No112 | |
| Rituximab effects on effector T cells | Yes37 | Yes105,107 | |
| Rituximab effects on TREG cells | Increased TREG cell numbers37 | Controversial105,106 |
Abbreviations: ACPA, anticitrullinated protein antibodies; citFib, citrullinated fibrinogen; citPG, citrullinated proteoglycan; NR, not reported; PD1, programmed cell death protein 1; RA, rheumatoid arthritis; RF, rheumatoid factor; STAT3, signal transducer and activator of transcription 3; TGF-β, transforming growth factor β; TFH cell, follicular helper T cell; TH cell, helper T cell; TREG, regulatory T cell.
Abnormal T-cell selection
Abnormal T-cell selection and low T-cell signalling capacity might have a role in the development of autoimmune arthritis. Normally, most self-reactive T cells are deleted in the thymus during their development or are deleted or suppressed in the periphery. In SKG mice, however, a point mutation in the gene encoding ZAP70, a tyrosine kinase involved in T-cell receptor signal transduction, results in aberrant TCR signalling, which might enable self-reactive T cells to escape thymic deletion.14 Such self-reactive T cells (of as-yet-unknown antigen specificity) are thus thought to drive the development of spontaneous autoimmune arthritis in SKG mice, which have an arthritis-prone BALB/c background.15 Similarly, in humans, RA and other autoimmune diseases such as SLE and scleroderma have also been associated with low signalling capacity of self-reactive T cells, which might protect these cells against negative selection.14, 16
Autoreactive T-cell activation
MHC restriction of antigen recognition
Antigen presentation to CD4+ T cells is MHC class II restricted. Thus, in both RA and its rodent models (Table 1), the recognition of particular autoantigens can depend on the MHC class II genotype of the individual. For example, the major T-cell epitope (amino acids 259–273) in CII—an autoantigen that might contribute to autoimmunity in a proportion of patients with RA—is preferentially presented by antigen-presenting cells (APCs) that express HLA-DRB1*04 (HLA-DR4) molecules in RA.17 Similarly, T-cell responses to citrullinated (deiminated) self proteins are detected more frequently in patients with RA who express the HLA-DRB1 ‘shared epitope’ (a five-amino-acid sequence present in some HLA-DRB1 alleles) than in those negative for shared-epitope-containing HLA class II molecules.18 In line with these findings, genetic studies have established that certain HLA-DRB1*01 and HLA-DRB1*04 alleles containing sequences encoding the shared epitope are associated with RA.19,20
As in patients with RA, the MHC haplotype (referred to as H2 in mice) is a major determinant of arthritis susceptibility in mouse models. For example, only a few mouse strains are susceptible to PGIA or CIA.8, 21 Moreover, PGIA-susceptible BALB/c mice (which have the H2d haplotype) are resistant to CIA, and conversely CIA-susceptible DBA/1 mice (which have the H2q haplotype) are resistant to PGIA.8 An interesting dichotomy of MHC class II-dependent T-cell antigen recognition was described in mice carrying a KRN TCR transgene. In the wild-type strains, T cells expressing the transgenic KRN TCR recognize bovine ribonuclease in the context of Ak MHC class II molecules. However, when crossed with nonobese diabetic (NOD) mice to generate K/BxN hybrids, KRN TCR-transgenic T cells recognize the ubiquitous self-antigen G6PI in the context of the MHC class II molecule Ag7, which is derived from the NOD background.22, 23 These G6PI-specific T cells then provide help to B cells, leading to overproduction of anti-G6PI antibodies and spontaneous arthritis development in K/BxN mice.22 Not surprisingly, treatment with anti-CD4 antibodies prevented the development of arthritis in this model.23 Furthermore, transfer of T-cell-depleted K/BxN splenocytes to immunodeficient mice was unable to induce arthritis, also indicating the critical role of autoreactive T cells in the disease process.22
A number of non-HLA genes related to T cells have also been associated with RA and with mouse models of the disease. These genetic associations are discussed briefly in Box 1.
Box 1. Associations of T-cell-related genes with RA.
SNPs within numerous chromosome regions (loci) have been reported in GWAS of patients with RA. Among the >30 confirmed non-HLA loci contributing to RA risk, probably the strongest associations have been found with loci containing the PTPN22 (protein tyrosine phosphatase, non-receptor type 22) and IL23R (IL-23 receptor) genes. In brief, these genes, as well as the CTLA4 (cytotoxic T-lymphocyte antigen 4), CCR6 (CC-chemokine receptor 6) and CD40 alleles that have also been associated with RA, are highly likely to be involved in T-cell function underlying the pathogenesis of RA.20,113 SNPs in PADI4 have also been identified in patients with RA. The PADI4 enzyme can catalyse the citrullination of proteins, which then can trigger the production of autoantibodies to citrullinated epitopes in patients with RA. 20,113 Autoantibody production is highly linked to pathogenic T cell involvement.
Arthritis-associated MHC and non-MHC loci have also been identified in GWAS carried out in CIA and proteoglycan-induced arthritis mouse models.20,114 Some of these mouse loci overlap between the two models as well as with RA susceptibility alleles.20,114
Abbreviations: CIA, collagen-induced arthritis; GWAS, genome-wide association studies; RA, rheumatoid arthritis; SNP, single nucleotide polymorphism.
Autoantigen specificities
Autoreactive T cells can recognize a number of autoantigens in patients with RA, such as various epitopes in CII17, 24 or the citrullinated form of the 5/4E8 epitope (amino acids 84–103) of the human cartilage proteoglycan aggrecan (citPG).18,25 Multifunctional T-cell responses directed towards both native and glycosylated variants of the CII259–273 epitope are sustained during the RA disease course.17 Similarly, citPG induces T-cell responses in patients with RA but not in healthy controls, whereas the non-citrullinated aggrecan epitope does not stimulate such responses.25 In addition, citrullinated proteins have been detected in fibrin deposits within RA synovial tissue, and citrullinated fibrin could be the major target of anti-filaggrin antibodies in the synovium in vivo.31 In a comparative study on T-cell responses towards various citrullinated peptides, citPG was the most immunogenic, especially in patients with RA carrying the HLA-DRB1 shared epitope.18 Interestingly, a T-cell response to the citPG epitope (or no response) was detected in early RA, whereas longstanding disease was associated with T-cell responses to citrullinated epitopes of multiple proteins.18 This varied responsiveness is a good example of epitope spreading, a process involved in the progression of autoimmunity. Such epitope spreading also occurred during sustained T-cell responses to CII.17
With regard to animal models, mouse CD4+ T-cell lines specific for citrullinated fibrinogen have been found to enhance arthritis severity when transferred to mice with CIA.27 Furthermore, T-cell responses to the mouse analogue of the citPG epitope after priming of BALB/c mice with the mouse citPG peptide were shown to be stronger than responses to the native (noncitrullinated) self epitope after priming with that peptide.9 Thus, T-cell responses against citrullinated protein antigens have been identified in both humans and mice.
T-cell clonal expansion
Clonal expansion of CD4+ T cells has been described in patients with RA.28,29 Indeed, in one early study of 15 patients with this disease, oligoclonally expanded T cells were identified in the peripheral blood of all the patients, whereas such T-cell subpopulations were found in few healthy controls or patients with other types of arthritis. These ‘RA-specific’ expanded T-cell subpopulations persisted for years and infiltrated the synovial tissue.28 Oligoclonal expansion of a unique CD4+ T-cell subset lacking the co-stimulatory molecule CD28 on their surface has also been described in RA. These CD4+CD28− T cells have been found to be resistant to apoptosis, which might explain the chronic persistence of the expanded populations in patients with RA.29
T-cell subsets in arthritis
Function and plasticity of TH1 and TH17 cells
A large body of literature suggests that the type 1 T helper (TH1)-cell and TH17-cell subsets have important pathogenic roles in RA. For many years, TH1 cells, which produce interferon γ (IFN-γ), and a disturbed TH1–TH2 balance have been thought to drive organ pathology in RA as well as in other autoimmune diseases.4, 30–32 However, the TH1-cell concept has significantly changed since the discovery of IL-17-producing TH17 cells. These cells, and IL-17, are major contributors to synovial inflammation, as well as to cartilage and bone damage.32,33 T-cell responses to CII and citPG in RA have been associated with increased production of IFN-γ and IL-17, respectively.17,18,24,25 Indeed, ex vivo stimulation of RA synovial fluid mononuclear cell populations (containing CII-reactive T cells) with CII resulted in increased production of the TH1-type cytokines IL-12 and IFN-γ but not of the TH2-type cytokine IL-4.17,24 Moreover, TH1-cell-associated epigenetic changes, such as demethylation of the IFNG locus, have been detected in CD4+ T-cells from RA synovial fluid.34 With regard to citPG, ex vivo stimulation of peripheral blood mononuclear cells with this epitope induced proliferative responses of TH17 cells and prominent IL-17 production in patients with RA.25 Similarly, another study showed that the proliferative T-cell response to citPG was associated with the release of the TH17-type cytokines IL-6, IL-17 and TNF.18
Among animal models of inflammatory arthritis, PGIA has been shown to be a TH1-cell-driven disease.35 IFN-γ is necessary for the onset and progression of the disease, and the absence of this cytokine leads to reduced incidence and severity of arthritis and weaker proteoglycan-specific IgG2a antibody responses.36 Increased IFN-γ levels decrease the number and percentage of activated TREG cells in PGIA.37 Moreover, compared with the standard protocol for induction of PGIA (in which BALB/c mice are injected with proteoglycan in complete Freund’s adjuvant [CFA]), immunization of BALB/c mice with proteoglycan in the synthetic adjuvant dimethyl-dioctadecyl ammonium bromide (DDA) induced a strong TH1 shift, and this effect led to a somewhat more severe manifestation of PGIA.38 Conversely, a shift towards a TH2-type response suppresses PGIA; for example, administration of TH2-type cytokines, such as IL-4 and IL-10, before the onset of arthritis prevented the development of histopathological changes in the joints and clinical arthritis. IL-4 also inhibited proteoglycan-specific IFN-γ responses and reduced pro-inflammatory cytokine expression at target sites in PGIA.35 Furthermore, arthritis was markedly more severe in IL-4-deficient BALB/c mice than in their wild-type counterparts.36
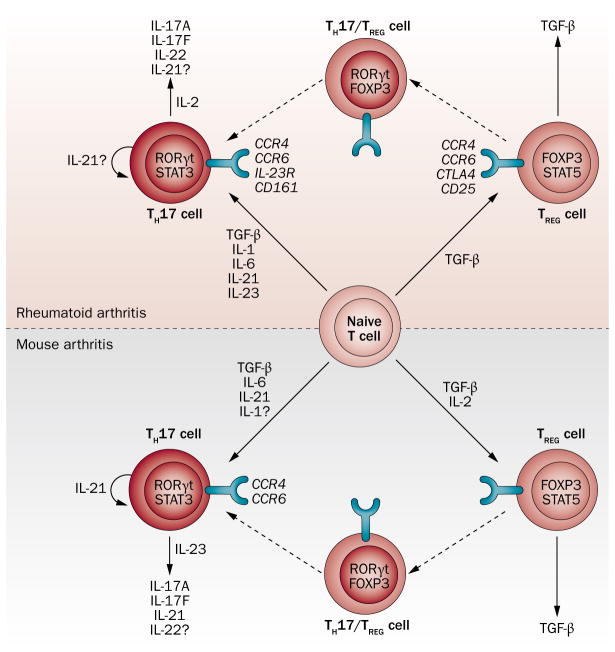
Substantial TH17-cell responses have been detected in both human and mouse arthritic joints.32,39,40 TH17 cells have a crucial role in CIA, as CIA was markedly suppressed in IL-17-deficient mice.41 Arthritis suppression in these mice was associated with reduced cellular and humoral responses to CII. By contrast, in PGIA, ablation of IL-17 had no effect on inflammatory cell recruitment or bone erosion in the arthritic joints, nor did it affect the generation of proteoglycan-specific T cells and IgG2a antibodies.42 In a comparative study, immunization of BALB/c mice with proteoglycan in CFA was associated with the dominance of TH17 over TH1 cells, whereas the same immunization protocol using DDA adjuvant had the opposite effect.43 Nonetheless, although TH1–TH17 polarization was clearly influenced by the adjuvant used, subset dominance had no significant impact on disease onset or severity.38,43 The cytokine regulation of TH17-cell regulation and function in human RA and its mouse models are illustrated in Figure 1 and discussed in Box 2.
Figure 1.
Cytokine regulation of the TH17–TREG cell axis in human RA and its mouse models. Naive T cells can differentiate into either TH17 or TREG cells and there is high plasticity between these T cell subsets. This figure summarizes inflammatory mediators enabling the differentiation of these T-cell subsets, and those mediators produced by these cells in humans and mice. Similarities and differences between human and mouse arthritis with respect to T-cell subsets are further explained in the main text and in Table 1. Abbreviations: CCR, CC-chemokine receptor; FOXP3, forkhead box P3; RA, rheumatoid arthritis; ROR, retinoic acid receptor-related orphan receptor; STAT, signal transducer and activator of transcription; TGF-β, transforming growth factor β; TREG, regulatory T; TH, T helper.
Box 2. Cytokine regulation of TH17-cell differentiation and function.
The STAT3-activating cytokine IL-6, in combination with other cytokines described below, is a major inducer of TH17-cell responses in both mice and humans.32 It was first shown in mice that TGF-β and IL-6 are the main cytokines involved in the induction of the key TH17-cell transcription factor RORγt and thus in TH17-cell development.32 Although early reports suggested that in RA, unlike in mouse models, TGF-β might not be needed for the development of TH17 cells, subsequent studies have confirmed that TGF-β is also required for human TH17-cell differentiation.66,67,115,116 In RA, TGF-β induces the expression of both RORγt and the key TREG-cell transcription factor FOXP3 in naive T cells and thus stimulates their differentiation into either TH17 or induced TREG cells, depending on the inflammatory environment.66,67,115,116 IL-1, which only transiently induces RORγt, also induces TH17-cell development in humans.66,67,115,116 Thus, in RA, IL-6 and IL-1, rather than TGF-β, might be the main inducers of TH17-cell development. However, IL-1 might not be as important in the induction of TH17 cells in mice, although this cytokine, especially in combination with IL-23, promoted TH17-cell proliferation in mice deficient of IL-1 receptor antagonist (Figure 1, Table 1).110 An IL-21 and STAT3-dependent positive feedback loop exists in mice, in which IL-21 produced by naive T cells promotes TH17-cell development, and these cells also produce IL-21.32 This positive IL-21-dependent feedback loop might also be present in RA;111 indeed, IL-21 is also required for human TH17-cell differentiation.115 IL-23, another STAT3-activating cytokine, also supports TH17-cell polarization in both species.32 Lastly, IL-2 stimulates the sustained production of the TH17-cell cytokines IL-17 and IL-22 in RA, and might induce TREG cells at the expense of TH17 cells in mice (Figure 1).4,32
Abbreviations: RA, rheumatoid arthritis; TGF-β, transforming growth factor β; TH17 cell, type 17 helper T cell; TREG, regulatory T cell.
In RA, the inflamed synovium contains an abundance of TH1 over TH17 cells, suggesting that the local expansion of TH17-cell populations within the arthritic joint is limited.4,44 The lower proportion of TH17 cells might be a result of the epigenetic instability of TH17-cell chromatin structure, which leads to phenotypic plasticity of this subset.45 Bifunctional TH1–TH17 cells producing both IFN-γ and IL-17 have been identified in RA synovial fluid, but not in peripheral blood.40,46 Moreover, in ex vivo cultures, synovial fluid, but not peripheral blood, TH17 cells produced both IL-17 and IFN-γ.4,40 On the other hand, addition of IL-12, a TH1-cell-promoting cytokine found in RA synovial fluid, enhanced the conversion of peripheral blood TH17 cells to the TH1 phenotype.40 Therefore, TH17 cells, under the influence of locally produced cytokines such as IL-12, might transform into TH1-like cells, leading to an increased ratio of TH1:TH17 cell numbers at inflammatory sites such as arthritic joints.4,40
The developmental plasticity of mouse TH17 cells, by means of their conversion to the TH1 phenotype, has been described in in vitro studies and in an animal model of colitis.47 Whether the inflammatory microenvironment in arthritic mouse joints facilitates the TH17 to TH1 cell conversion, as it does in RA,4,40 remains to be determined. In addition, mouse TREG cells can be converted into IL-17-secreting cells, indicating plasticity between TH17 and TREG cells in the mouse system.32 By contrast, mouse TH1 and TH2 cells seem to be phenotypically stable, terminally differentiated subsets.32
Follicular helper T cells
B-cell maturation in germinal centres (GCs) and autoantibody production are regulated by T cells.48–50 GC-like structures, which are signs of lymphoid neogenesis, have been described in the severely inflamed RA synovium,49 and these ectopic GCs have been shown to contain CD4+ T cells.49 However, unlike in severe RA, ectopic lymphoid structures have never been reported in the arthritic mouse synovium.
Within GCs, the fate of B cells is highly dependent on their ability to present antigens to a specialized subset of T cells known as follicular helper T (TFH) cells. TFH cells uniquely co-express CXC-chemokine receptor 5 (CXCR5; required for their homing to B-cell follicles) and the inhibitory receptor programmed cell death protein 1 (PD1).50,51 They also express the transcription factor BCL6, a master regulator of TFH-cell function.50,51 Through downregulation of IL-2 production, PD1 is thought to help maintain high levels of BCL6 in TFH cells.51 Within GCs, TFH cells promote the development of high-affinity memory B cells and long-lived plasma cells. Elevated numbers of TFH cells have been associated with RA,48 and given the critical role of these cells in B-cell activation and antibody production, the failure of TFH cells to maintain self-tolerance and their potential contribution to autoimmunity have drawn much attention.48,52,53 PD1 is abundantly expressed by RA synovial TFH cells, yet these cells are fairly resistant to PD1–PD1 ligand 1 (PDL1)-mediated inhibition of proliferation (Table 1).,55 The decreased response of synovial T cells to PD1 ligation might be attributable in part to the presence of a soluble isoform of PD1 in RA synovial fluid, which can interfere with, although not completely block, PDL1 binding to these cells.55
In mice, expansion of TFH cells might occur in the spleen, as observed in Pdl1-deficient BALB/c mice following induction of PGIA.56 PDL1 regulates autoimmunity by suppressing autoreactive CD4+ T-cell responses. In addition, expression of PDL1 on a non-B and non-T cell type promotes B-cell survival by restraining the expansion and activation of TFH cells through interaction with PD1. In the PGIA model, Pdl1-deficient mice exhibited increased autoreactive TH1-cell responses, as well as an elevated frequency and activation status of TFH cells, which were associated with death of GC B cells in the spleen and worsening of arthritis symptoms.56
Developmental plasticity of mouse TFH cells has also been described. Early TH1-cell differentiation was associated with transient appearance of T cells with TFH or dual TH1–TFH phenotypes. At a later phase of T-cell development, there was a repression of TFH-cell function that permitted full TH1-cell differentiation.57 Such plasticity has not yet been described in RA (Table 1).
Regulatory T cells
The role of TREG cells and the loss of regulatory control of effector T cells in the pathogenesis of RA has been reviewed in this journal by Wehrens et al.,4 so this issue is only briefly discussed here. Crucially, the resistance of effector T cells to TREG-cell suppression might be a key explanation for the loss of control and the perpetuation of autoimmune inflammation in RA. The two main populations of TREG cells—thymus-derived ‘natural’ TREG cells and peripherally induced TREG cells—have both shared and distinct properties, but they both can suppress effector T cells. In addition to emerging markers distinguishing between them, the signature TREG-cell transcription factor FOXP3 has been shown to be constitutively expressed in thymus-derived TREG cells, whereas its expression requires the presence of TGF-β in induced TREG cells.58 In RA, diminished function of thymus-derived TREG cells has been associated with defects in CTLA4-mediated signalling, and disease control via anti-TNF therapy cannot overcome this defect.59 By contrast, anti-TNF therapy in RA increases the number and function of induced TREG cells, in a TGF-β-dependent manner, and these cells seem to be able to suppress pathogenic T cells.60–62
TREG cells also have an important role in regulating arthritis in animal models. Transfer of CD4+CD25+ TREG cells into mice with CIA slowed disease progression and reduced acute-phase protein production, although CII-specific T-cell and antibody responses were not affected.63 In mice with PGIA that underwent a therapeutic bone marrow transplantation (BMT), CD25+FOXP3− memory TREG cells contributed to rapid improvement of arthritis 1 week after BMT, and 3 weeks after BMT FOXP3+ TREG cells emerged that stabilized the disease at a lower severity level than before BMT.64 Conversely, depletion of the TREG-cell subset by administration of an anti-CD25 antibody led to severe arthritis in asymptomatic proteoglycan-immunized mice.64 Moreover, reduced disease severity in PGIA following administration of a B-cell-depleting anti-CD20 antibody, rituximab, was associated with increased numbers of FOXP3+ TREG cells as well as diminished B-cell numbers.37 Indeed, depletion of these treatment-induced TREG cells using an anti-CD25 antibody restored the severity of PGIA to a level equal to that observed in untreated mice.37
Although T cells constitute a minor population of cells in the synovial fluid of arthritic K/BxN mice, 45–70% of these T cells belong to the CD25+FOXP3+ TREG-cell subset.65 In the absence of TREG cells, for example in K/BxN mice harbouring a loss-of-function mutation in the Foxp3 gene, a higher degree of local destruction in the joints was observed and otherwise unaffected joints also became inflamed.65 TREG cells seemingly accumulated in the arthritic joints regardless of antigen specificity, as TREG cells were also found in elevated numbers in non-K/BxN animals following passive transfer of arthritis with serum from K/BxN mice.65 Thus, TREG cells accumulate in arthritic joints through either recruitment or conversion from another phenotype. Such cells might locally suppress effector T cells; however, as shown in RA, their potency might be reduced in the inflammatory environment.
In both mice and humans, a reciprocal relationship exists in the development of TH17 and TREG cells, with a high degree of plasticity between these T-cell subsets.4,66,67,68 Under pro-inflammatory conditions, such as in the inflamed joint, TH17-cell development is enhanced by various cytokines, including IL-6, TGF-β and IL-1β, and also potentially through IL-2 consumption by TREG cells. Furthermore, in the inflammatory milieu, a small proportion of unstable induced TREG cells might be converted into effector TH17 cells that produce IL-17 (Figure 1).4,67,68 In patients with RA treated with TNF antagonists, TREG cells can gain control over TH17 cells, at least in part, by inhibiting IL-6 production.62 Thus, mouse models of arthritis have helped us to understand the role of TREG cells in RA. Passive transfer experiments and assessment of TREG plasticity are more accessible in the animal models.
Of note, some differences exist between mouse and human TREG cells. For example, IL-2 promotes TREG-cell development at the expense of TH17 cells in mice, but not in humans (Figure 1).32 With regard to TNF, both human and mouse TREG cells express TNF receptor 2 (TNFR2).4,69 In humans, TNF inhibits TREG-cell expansion4,69 and, correspondingly, neutralization of this cytokine by anti-TNF biologic agents promotes TREG-cell expansion in patients with RA.60,61 Furthermore, in RA, inhibition of TNF by various anti-TNF biologic agents or defective TNFR2 signalling improves the suppressive function of TREG cells. It has been suggested that human TREG-cell function is dependent on the phosphorylation status of FOXP3. In accordance with this hypothesis, TNF-induced FOXP3 dephosphorylation is associated with impaired TREG-cell function,70 and anti-TNF treatment improves defective TREG-cell function in RA by restoring FOXP3 serine phosphorylation.70 By contrast, in mice, TNF promotes the expansion of TREG cells4,71 and, at least in some studies, TNF neutralization was shown to suppress the function of this subset.72 Thus, TNF or anti-TNF treatment might have opposite effects on TREG cells in mice and humans.4
Joint-homing T cells in arthritis
Phenotype and function
As described above, the local inflammatory milieu within the RA joint highly influences the proportion and function of TH1, TH17 and TREG-cell subsets.4 Local enrichment and differentiation of TH1 and TREG-cell subpopulations is accompanied by a relatively limited local expansion of TH17 cells within the inflamed synovium, possibly triggered by cells with APC function.4,73 Pro-inflammatory cytokines downregulate FOXP3 expression and stimulate IL-17 production by CD25+FOXP3+ synovial TREG cells, leading to their differentiation towards the TH17-cell phenotype.4,74 Yet, TH17 cells are present in relatively low quantities in the synovial fluid of patients with RA, which might be a consequence of TH17-cell conversion to the TH1 phenotype.39,40 The inflammatory environment also influences the suppressive function of TREG cells in the RA joint. A substantial population of TREG cells is present in RA synovial fluid, and these cells have the capacity to suppress effector T cells. However, the presence of pro-inflammatory mediators in this environment abrogates such suppressor activity.4,75
Unlike in RA, T cells constitute a very minor population of joint-homing cells in mouse models including PGIA,2,76 CIA77 and K/BxN arthritis.65 In adoptively transferred PGIA, serial in vivo visualization of fluorochrome-labelled donor T cells failed to identify T cells entering the joints of recipient mice, whereas accumulation of donor T cells was readily detectable in the lymph nodes of the host following transfer and during arthritis development.2 In CIA, introduction of a human RA-susceptibility HLA-DR4 transgene into MHC class II-deficient DBA/1 mice made it possible to identify T cells specific for HLA-DR4-restricted CII261–273 peptide in the joints ex vivo.77 These CII-specific CD44+ (activated) T cells were detectable in acutely arthritic joints, but they disappeared from the synovial fluid by 2 weeks after the onset of CIA. Although the phenotype of joint-homing T cells was not investigated in this study, given the preferential attraction of the CD25+FOXP3+ subset to sites of inflammation63,65 it is possible that the majority of these cells were TREG cells.
Recruitment to the joint
Chemokines and their receptors provide directional cues for T-cell migration to lymphoid organs and inflammatory sites. In RA and animal models of arthritis, mostly CC-type chemokines, together with their receptors, are involved in T-cell homing to the joints.78 Among the CC-type chemokines, CCL2 (also known as MCP1), CCL3 (also known as MIP1α), CCL5 (also known as RANTES), CCL7 (also known as MCP3), CCL8 (also known as MCP2), CCL13 (also known as MCP4), CCL17 (also known as TARC), CCL18 (also known as PARC), CCL19 (also known as ELC), CCL20 (also known as MIP3α) and CCL21 (also known as SLC) have all been detected in the sera and joints of patients with RA.78,79 CCL20 preferentially recruits TH17 cells via CCR6.80 CCR1, CCR2, CCR5 and CCR7 are abundantly expressed in the RA synovium and on various cells in the synovial tissue.79 CCR5 is the most prominent CC-chemokine receptor in TH1-cell-dominated inflammatory infiltrates.81,82 Moreover, increased homing of CCR5+ and CXCR3+ T cells was observed in patients with seronegative arthritis, underscoring the significance of these T cell subsets in the development of the disease.83 Expression of CCR6 on TH17 cells, which supports the ingress of these cells into the RA joint, is attenuated by anti-TNF therapy.80,84,85 CCR4 and CCR6 are expressed by both TH17 and TREG cells, as well as on T cells with a dual TH17/TREG cell phenotype (Figure 1).66 Thus, CCR1, CCR4, CCR5, CCR6 and their ligands, CCL3, CCL5, CCL7, CCL8 and CCL20, seem to be the most relevant chemokine receptor–ligand pairs involved in T-cell recruitment to the RA joint.78,79
Animal models lend themselves to real-time in vivo analyses of T-cell recruitment and behaviour in lymphoid organs that are not accessible for in vivo studies in humans. For example, T-cell motility changes associated with antigen presentation, T-cell–APC interactions, and competition between antigen-experienced and naive T cells for access to antigen-bearing APCs can be visualized in real time in the joint-draining lymph nodes of mice with PGIA.2,76,86 However, as described above, monitoring of T-cell influx into distal joints in vivo revealed poor T-cell recruitment in these sites.2 This limited recruitment might be a drawback of all popular mouse arthritis models when it comes to studying T cells within the joints. In addition, the small volume of synovial fluid in rodent joints and the paucity of joint-homing T cells make it difficult to isolate such T cells and study their function ex vivo.5,8
T-cell-dependent autoantibody production
RF and ACPA
Probably the best-characterized autoimmune feature of RA is the presence of antibodies against self IgG (rheumatoid factor [RF]) and against citrullinated proteins (anti-citrullinated protein antibodies [ACPA]) in the sera of patients, with ACPA being more disease-specific than RF.87,88 Most of these autoantibodies have undergone isotype switching, suggesting that their production by B cells requires T-cell help.87,89,90 Numerous citrullinated proteins have been identified in RA synovial tissue and fluid.87,91–93 However, the broad epitope repertoire and the apparent lack of joint (that is, cartilage matrix protein) specificity of ACPA make it difficult to identify an ‘arthritogenic’ population of ACPA. Several ACPA specificities target serum proteins, such as citrullinated fibrinogen, or intracellular proteins including vimentin and α-enolase.94–96 Although antibodies reacting with filaggrin, mutated citrullinated vimentin, and citrullinated cyclic peptides (CCP) have been used for diagnostic purposes by several laboratories, to date fibrinogen, fibrin and vimentin have been the main citrullinated proteins identified in the RA synovium. In fact, citrullinated fibrin chains are a major target of anti-filaggrin antibodies.26
It has been demonstrated that epitope spreading of ACPA starts in the preclinical phase of RA, and is, at least in part, responsible for the perpetuation of the disease.94 Moreover, ACPA fine-specificities have been associated with clinical responses to biologic agents in patients with RA.95 However, as mentioned above, arthritogenic ACPA specificities have not yet been elucidated, although the synovial T-cell repertoire is quite different in ACPA-positive and ACPA-negative subsets of patients with RA. For example, an altered distribution of TCR complementarity-determining region 3 (CDR3) lengths, which is associated with monoclonal or oligoclonal T-cell expansion, was detected more frequently in ACPA-positive than ACPA-negative RA, suggesting the preferential involvement of clonally expanded T cells in seropositive disease.97
In animal models, ACPA responses to intracellular proteins develop during the early stages of CIA, and mice with chronic CIA exhibit expansion of antibody reactivity against citrullinated peptides.98 Both ACPA and RF are produced in PGIA, which makes this model, as well as human PG G1 domain-induced arthritis,99 more relevant to seropositive RA than other mouse models, and also a suitable model for investigating the potential pathogenic role of ACPA in RA development.
Other autoantibodies
T-cell–B-cell crosstalk is clearly involved in autoantibody production in various mouse models of RA. In CIA, autoantibodies of the IgG2 isotype that crossreact with mouse CII have been shown to cause chondrocyte death in the presence of complement;21 thus, T-cell help in switching to the IgG2 isotype is crucial for producing pathogenic antibodies that can mediate cell death. As mentioned above, citrullinated-fibrinogen-specific T cells transferred to mice with CIA enhanced the production of anti-CII autoantibodies, providing further evidence of T-cell–B-cell crosstalk.27 These findings might also be of relevance to human disease, as anti-CII antibodies have been detected in patients with early RA.100 In PGIA, antibodies against mouse proteoglycan are generated after the second immunization with human proteoglycan, and the progression from anti-human to self-reactive anti-mouse proteoglycan antibodies correlates with the development of arthritis.8 IgG-containing deposits are detected in the joints before arthritis onset, indicating that the formation of complement-fixing immune complexes containing proteoglycan and anti-proteoglycan antibodies might be the primary event that initiates the local activation of synovial macrophages and fibroblasts and the recruitment of leukocytes, which collectively drive inflammation and tissue destruction.7,8 T cells are involved in the initiation of B cell activation and autoantibody production, as well as the maintenance of autoimmunity throughout the disease course.
In K/BxN arthritis, linked T-cell and B-cell recognition of G6PI leads to overproduction of self-reactive anti-G6PI antibodies.22 It has been suggested that immune complexes formed inside the joints of K/BxN mice can fix complement and activate Fc receptors, thereby initiating leukocyte recruitment as well as local inflammatory mediator production.22 However, although treatment of K/BxN mice expressing human CD20 with the anti-human-CD20 antibody rituximab markedly reduced serum levels of anti-G6PI antibodies, it failed to suppress arthritis, suggesting that the level of circulating autoantibodies does not necessarily correlate with disease severity. On the other hand, transfer of arthritic K/BxN serum to naive mice can induce disease in a wide range of mouse strains, including B-cell-deficient or lymphocyte-deficient hosts, via G6PI-specific autoantibodies.22,101 However, passive induction of arthritis is not a universal phenomenon, as serum from mice rendered arthritic by immunization with G6PI,13 or from other models including PGIA,102 cannot transfer disease. This finding indicates that a certain threshold level of autoantibodies, perhaps of a particular antigen specificity and isotype, in the circulation might be required for arthritis induction. Although inflammation induced by arthritic K/BxN serum transfer recapitulates the major histopathological features of disease seen in the donor mice, arthritis is transient. Inflammation declines 2 weeks after serum transfer, and previously swollen joints show little or no evidence of inflammation or cartilage destruction. Arthritis transience can be overcome by repeated injections of serum from arthritic K/BxN mice, suggesting that a continuous supply of even the most potent autoantibodies is necessary for sustained inflammation.22 Although arthritis cannot be transferred via serum injection in the PGIA model, administration of immune sera accelerates the development of arthritis induced by lymphocyte transfer.102
In conclusion, the production of autoantibodies, including ACPA and RF, is characteristic of both RA and mouse arthritis, but among the mouse models ACPA and RF are produced concurrently only in PGIA. ACPA specificities may differ substantially between RA and animal models. Antibodies to citrullinated fibrinogen (and perhaps other, unknown antigens) might have a central role in humans and mice. Transferability of arthritis by autoantibody-containing sera to naive mice (which certainly cannot be tested in humans) might challenge the current paradigm regarding a direct contribution of joint-homing T cells to arthritis initiation.
Therapeutic targeting of T cells
Therapies targeting T cells in RA have held great promise since the 1990s. Several anti-CD4, anti-CD5, anti-CD7 and anti-CD52 antibodies have been developed and tested, but no clear-cut clinical efficacy could be demonstrated in clinical trials, and no correlation has been found between antibody-induced T-cell depletion and disease status in RA.103 To date, only one strategy of T-cell inhibition—blockade of signalling through the co-stimulatory molecule CD28 using a CTLA4-Ig fusion protein known as abatacept—has shown efficacy in clinical trials, leading to the registration of abatacept for the treatment of RA.104
With regard to indirect T-cell blockade, B-cell inhibition by the anti-CD20 antibody rituximab also affects T cells. Rituximab administration might decrease the activated phenotype of peripheral and tissue-resident T cells by abolishing antigen presentation by B cells.105 Much controversy has surrounded the effect of B-cell blockade on TREG cells. Rituximab treatment might enhance the numbers and function of these cells;105 however, in another study, anti-CD20 therapy did not influence the frequency of TREG cells in RA.106 Finally, a CD20+ T-cell subset has been characterized and shown to comprise terminally differentiated T cells with immunoregulatory and pro-inflammatory properties. Rituximab also depleted this T-cell population, an effect that might have a role in the beneficial effects of B-cell targeting in RA (Table 1).107
As CC chemokines and their receptors have been implicated in T-cell homing to the joint, we briefly touch on the efficacy of chemokine receptor blockade. To date, antibody-mediated blockade of CCR2 and CCR5 has yielded disappointing results in RA, and variable outcomes have been reported for CCR1 blockade. However, the fact that CC-chemokine receptor blockade failed in most clinical trials does not necessarily mean that chemokine receptors are not legitimate targets in RA. Rather, it seems to be critical to achieve very high levels of receptor occupancy in order to continuously inhibit leukocyte migration into the synovial compartment.79,108 Of note, patients with RA treated with TNF antagonists exhibit low expression levels of CCR6 in TH17 cells and reduced proportions of these cells in the joints.85
In contrast to RA, numerous T-cell-targeting therapies using anti-CD4 antibodies or genetic manipulations have shown great efficacy in preventing arthritis in animals, including in the PGIA,10 CIA11 and K/BxN22 models. There might be important reasons for this discrepancy. RA is characterized by a ‘waxing and waning’ of disease symptoms, and a persistence of polyclonally or oligoclonally expanded T cells including self-reactive populations. By contrast, arthritis is ‘monophasic’ in most rodent models, and joint inflammation is preceded by a robust and single-antigen-focused T-cell response, only a part of which is directed against self. It is relatively easy, therefore, to prevent arthritis in animal models by deleting T cells or knocking out molecules essential for the effector function of T cells. The early involvement of T cells in arthritis also means that anti-CD4 treatment works best when applied preventatively, before the appearance of clinical symptoms.11 In the CIA model, anti-CD4 treatment administered after arthritis onset failed to suppress disease or eliminate the CII-reactive pathogenic population of T cells,11 which might, in part, explain the inefficacy of a similar treatment in established RA. In addition, in the majority of animal models, counter-regulatory mechanisms mainly involving innate immune cells such as joint-infiltrating myeloid-derived suppressor cells109 kick in early during the course of inflammation. These innate immune cells can effectively reduce T-cell responses and inflammation in a reasonably short period of time, thereby masking the potentially beneficial effects of T-cell-targeting therapeutics administered after disease onset (Table 1).
As indicated by the clinical success of abatacept-mediated blockade of T-cell co-stimulation,104 treatment strategies that indirectly influence T cells may be more effective in RA than antibody-induced depletion or neutralization of these cells. For example, as mentioned above, adoptive transfer of CD25+ TREG cells and TREG-cell-inducing BMT suppressed ongoing CIA63 and PGIA,64 respectively, suggesting that in vivo induction or transfer of ex vivo-induced autologous TREG cells could be an effective therapy in RA. B-cell depletion via anti-CD20 antibody administration, which is also highly effective in RA, not only reduced T-cell-dependent autoantibody production in PGIA and the K/BxN model,37, 68 but also diminished proteoglycan-specific T-cell responses and increased TREG-cell activity in PGIA.37 These animal studies suggest that B-cell depletion via rituximab in RA probably has an indirect effect on T cells by shifting the balance between pathogenic and suppressive subsets in favour of suppressive TREG cells.
In summary, the inefficacy of T-cell depletion in RA may be explained by differences between RA and mouse arthritis in disease course as well as in the potency or persistence of autoreactive T cells. Even in mouse models, anti-CD4 treatment works only when initiated before the onset of arthritis, and not in established disease. Animal studies suggest that therapies that restore or stabilize the number and suppressive function of TREG cells are the key to further success in the treatment of RA.
Conclusions
It is widely accepted that autoreactive T cells have a major role in the pathogenesis of RA as well as in a number of animal models of the human disease. MHC-restricted autoreactive T-cell responses and T-cell-dependent autoantibody production have been extensively characterized in both species. However, the specific contribution of T cells to joint inflammation is not clear in either RA or mouse models of arthritis. Despite similarities in T-cell subset composition, there are differences between humans and mice in the cytokine regulation of TH-cell polarization, the developmental plasticity of T cells, and the TH17 cell–TREG cell axis. Finally, whereas most T-cell depletion strategies have failed in RA, elimination of T cells has prevented or suppressed arthritis in numerous animal models. In conclusion, although animal models such as PGIA or CIA bear significant resemblance to the human disease, additional studies are needed to refine our understanding of T-cell pathology in autoimmune arthritis and to further enhance the value of preclinical animal studies in predicting the outcome of T-cell-focused therapeutic interventions in RA.
Supplementary Material
Key points.
Autoreactive T cells have an important role in the pathogenesis of both rheumatoid arthritis (RA) and mouse models of the disease
In addition to similarities, some differences exist in the regulation of the development and plasticity of TH1, TH17 and TREG cell subsets in humans and mice
The contribution of joint-homing T cells to local inflammation is not clear in either species
Unlike in mouse arthritis, most T-cell-targeting strategies failed in RA; only a few of them (such as abatacept) succeeded
Anti-cytokine and anti-B-cell targeting strategies might indirectly suppress pathogenic T cells in human and mouse arthritis
Review criteria.
Relevant full-text English-language papers published between 1980 and 2013 were collected from PubMed. The search terms used were: “rheumatoid arthritis”, “arthritis models”, “proteoglycan-induced arthritis”, “collagen-induced arthritis”, “G6PI-induced arthritis”, “SKG arthritis”, “K/BxN arthritis”, “autoreactive T cell”, “TH1”, “TH17”, “TFH”, “TREG”, “joint homing T cells”, “RF”, “ACPA”, “autoantibodies”, “TNF-α”, “cytokines”, “chemokines”, “MHC” and “genetics of RA”.
Acknowledgments
Z. Szekanecz is supported by the Medical Research Council of Hungary research grant ETT 315/2009 and by the European Union and the European Social Fund co-financed projects TÁMOP 4.2.1/B-09/1/KONV-2010-0007 and TÁMOP-4.2.2.A-11/1/KONV-2012-0031. T. T. Glant is supported by NIH grant R01 AR059356. K. Mikecz is supported by NIH grants R01 AR064206 and R21 AR062332.
Footnotes
Competing interests: The authors declare no competing interests.
Author contributions
All authors made substantial contributions to researching data for the article and writing the manuscript. In addition, K. Mikecz, T. T. Glant and Z. Szekanecz contributed to discussions of the content of the article, and T. Kobezda, K. Mikecz, T. T. Glant and Z. Szekanecz reviewed/edited the manuscript prior to submission.
Contributor Information
Tamás Kobezda, Department of Rheumatology, Institute of Medicine, University of Debrecen Medical and Health Science Center, Negyerdei str 98, Debrecen, H-4032, Hungary.
Sheida Ghassemi-Nejad, Department of Rheumatology, Institute of Medicine, University of Debrecen Medical and Health Science Center, Negyerdei str 98, Debrecen, H-4032, Hungary.
Katalin Mikecz, Section of Molecular Medicine, Departments of Orthopedic Surgery, Biochemistry and Rheumatology, Rush University Medical Center, 1735 West Harrison Street, Chicago, IL 60612, USA.
Tibor T. Glant, Section of Molecular Medicine, Departments of Orthopedic Surgery, Biochemistry and Rheumatology, Rush University Medical Center, 1735 West Harrison Street, Chicago, IL 60612, USA
Zoltán Szekanecz, Department of Rheumatology, Institute of Medicine, University of Debrecen Medical and Health Science Center, Negyerdei str 98, Debrecen, H-4032, Hungary.
References
- 1.Kamradt T, Frey O. Arthritis: where are the T cells? Arthritis Res Ther. 2010;12:122. doi: 10.1186/ar3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angyal A, et al. Development of proteoglycan-induced arthritis depends on T cell-supported autoantibody production, but does not involve significant influx of T cells into the joints. Arthritis Res Ther. 2010;12:R44. doi: 10.1186/ar2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005;4:130–6. doi: 10.1016/j.autrev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Wehrens EJ, Prakken BJ, van Wijk F. T cells out of control--impaired immune regulation in the inflamed joint. Nat Rev Rheumatol. 2013;9:34–42. doi: 10.1038/nrrheum.2012.149. [DOI] [PubMed] [Google Scholar]
- 5.Wooley PH. Animal models of rheumatoid arthritis. Curr Opin Rheumatol. 1991;3:407–20. doi: 10.1097/00002281-199106000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Alzabin S, Williams RO. Effector T cells in rheumatoid arthritis: lessons from animal models. FEBS Lett. 2011;585:3649–59. doi: 10.1016/j.febslet.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Glant TT, Mikecz K, Arzoumanian A, Poole AR. Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum. 1987;30:201–12. doi: 10.1002/art.1780300211. [DOI] [PubMed] [Google Scholar]
- 8.Glant TT, Finnegan A, Mikecz K. Proteoglycan-induced arthritis: immune regulation, cellular mechanisms, and genetics. Crit Rev Immunol. 2003;23:199–250. doi: 10.1615/critrevimmunol.v23.i3.20. [DOI] [PubMed] [Google Scholar]
- 9.Misjak P, et al. The role of citrullination of an immunodominant proteoglycan (PG) aggrecan T cell epitope in BALB/c mice with PG-induced arthritis. Immunol Lett. 2013;152:25–31. doi: 10.1016/j.imlet.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee S, Webber C, Poole AR. The induction of arthritis in mice by the cartilage proteoglycan aggrecan: roles of CD4+ and CD8+ T cells. Cell Immunol. 1992;144:347–57. doi: 10.1016/0008-8749(92)90250-s. [DOI] [PubMed] [Google Scholar]
- 11.Goldschmidt TJ, Andersson M, Malmstrom V, Holmdahl R. Activated type II collagen reactive T cells are not eliminated by in vivo anti-CD4 treatment. Implications for therapeutic approaches on autoimmune arthritis. Immunobiology. 1992;184:359–71. doi: 10.1016/S0171-2985(11)80593-0. [DOI] [PubMed] [Google Scholar]
- 12.Kadowaki KM, Matsuno H, Tsuji H, Tunru I. CD4+ T cells from collagen-induced arthritic mice are essential to transfer arthritis into severe combined immunodeficient mice. Clin Exp Immunol. 1994;97:212–8. doi: 10.1111/j.1365-2249.1994.tb06070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schubert D, Maier B, Morawietz L, Krenn V, Kamradt T. Immunization with glucose-6-phosphate isomerase induces T cell-dependent peripheral polyarthritis in genetically unaltered mice. J Immunol. 2004;172:4503–9. doi: 10.4049/jimmunol.172.7.4503. [DOI] [PubMed] [Google Scholar]
- 14.Thomas R, Turner M, Cope AP. High avidity autoreactive T cells with a low signalling capacity through the T-cell receptor: central to rheumatoid arthritis pathogenesis? Arthritis Res Ther. 2008;10:210. doi: 10.1186/ar2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakaguchi STT, Hata T, Nomura T, Sakaguchi N. SKG mice a new genetic model of rheumatoid arthritis. Arthritis Res Ther. 2003;5 (Suppl 3):10. [Google Scholar]
- 16.Cope AP. Altered signalling thresholds in T lymphocytes cause autoimmune arthritis. Arthritis Res Ther. 2004;6:112–6. doi: 10.1186/ar1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snir O, et al. Multifunctional T cell reactivity with native and glycosylated type II collagen in rheumatoid arthritis. Arthritis Rheum. 2012;64:2482–8. doi: 10.1002/art.34459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Law SC, et al. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis Res Ther. 2012;14:R118. doi: 10.1186/ar3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–13. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 20.Kurko J, et al. Genetics of Rheumatoid Arthritis - A Comprehensive Review. Clin Rev Allergy Immunol. 2013 doi: 10.1007/s12016-012-8346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brand DD, et al. Autoantibodies to murine type II collagen in collagen-induced arthritis: a comparison of susceptible and nonsusceptible strains. J Immunol. 1996;157:5178–84. [PubMed] [Google Scholar]
- 22.Korganow AS, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–61. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 23.Kouskoff V, et al. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–22. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 24.Park SH, et al. Shift toward T helper 1 cytokines by type II collagen-reactive T cells in patients with rheumatoid arthritis. Arthritis Rheum. 2001;44:561–9. doi: 10.1002/1529-0131(200103)44:3<561::AID-ANR104>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 25.von Delwig A, Locke J, Robinson JH, Ng WF. Response of Th17 cells to a citrullinated arthritogenic aggrecan peptide in patients with rheumatoid arthritis. Arthritis Rheum. 2010;62:143–9. doi: 10.1002/art.25064. [DOI] [PubMed] [Google Scholar]
- 26.Masson-Bessiere C, et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001;166:4177–84. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- 27.Cordova KN, Willis VC, Haskins K, Holers VM. A citrullinated fibrinogen-specific T cell line enhances autoimmune arthritis in a mouse model of rheumatoid arthritis. J Immunol. 2013;190:1457–65. doi: 10.4049/jimmunol.1201517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waase I, Kayser C, Carlson PJ, Goronzy JJ, Weyand CM. Oligoclonal T cell proliferation in patients with rheumatoid arthritis and their unaffected siblings. Arthritis Rheum. 1996;39:904–13. doi: 10.1002/art.1780390606. [DOI] [PubMed] [Google Scholar]
- 29.Schirmer M, Vallejo AN, Weyand CM, Goronzy JJ. Resistance to apoptosis and elevated expression of Bcl-2 in clonally expanded CD4+CD28− T cells from rheumatoid arthritis patients. J Immunol. 1998;161:1018–25. [PubMed] [Google Scholar]
- 30.Miossec P, van den Berg W. Th1/Th2 cytokine balance in arthritis. Arthritis Rheum. 1997;40:2105–15. doi: 10.1002/art.1780401203. [DOI] [PubMed] [Google Scholar]
- 31.De Carli M, D’Elios MM, Zancuoghi G, Romagnani S, Del Prete G. Human Th1 and Th2 cells: functional properties, regulation of development and role in autoimmunity. Autoimmunity. 1994;18:301–8. doi: 10.3109/08916939409009532. [DOI] [PubMed] [Google Scholar]
- 32.Laurence A, O’Shea JJ. T(H)-17 differentiation: of mice and men. Nat Immunol. 2007;8:903–5. doi: 10.1038/ni0907-903. [DOI] [PubMed] [Google Scholar]
- 33.van den Berg WB, Miossec P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:549–53. doi: 10.1038/nrrheum.2009.179. [DOI] [PubMed] [Google Scholar]
- 34.Janson PC, et al. Profiling of CD4+ T cells with epigenetic immune lineage analysis. J Immunol. 2011;186:92–102. doi: 10.4049/jimmunol.1000960. [DOI] [PubMed] [Google Scholar]
- 35.Finnegan A, Mikecz K, Tao P, Glant TT. Proteoglycan (aggrecan)-induced arthritis in BALB/c mice is a Th1-type disease regulated by Th2 cytokines. J Immunol. 1999;163:5383–90. [PubMed] [Google Scholar]
- 36.Kaplan C, et al. Th1 and Th2 cytokines regulate proteoglycan-specific autoantibody isotypes and arthritis. Arthritis Res. 2002;4:54–8. doi: 10.1186/ar383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamel KM, et al. B cell depletion enhances T regulatory cell activity essential in the suppression of arthritis. J Immunol. 2011;187:4900–6. doi: 10.4049/jimmunol.1101844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanyecz A, et al. Achievement of a synergistic adjuvant effect on arthritis induction by activation of innate immunity and forcing the immune response toward the Th1 phenotype. Arthritis Rheum. 2004;50:1665–76. doi: 10.1002/art.20180. [DOI] [PubMed] [Google Scholar]
- 39.Nistala K, et al. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 2008;58:875–87. doi: 10.1002/art.23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nistala K, et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci U S A. 2010;107:14751–6. doi: 10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–7. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 42.Doodes PD, et al. Development of proteoglycan-induced arthritis is independent of IL-17. J Immunol. 2008;181:329–37. doi: 10.4049/jimmunol.181.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoop JN, Tibbitt CA, van Eden W, Robinson JH, Hilkens CM. The choice of adjuvant determines the cytokine profile of T cells in proteoglycan-induced arthritis but does not influence disease severity. Immunology. 2012;138:68–75. doi: 10.1111/imm.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santarlasci V, et al. Rarity of human T helper 17 cells is due to retinoic acid orphan receptor-dependent mechanisms that limit their expansion. Immunity. 2012;36:201–14. doi: 10.1016/j.immuni.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Mukasa R, et al. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–27. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen CJ, et al. Human Th1 and Th17 cells exhibit epigenetic stability at signature cytokine and transcription factor loci. J Immunol. 2011;187:5615–26. doi: 10.4049/jimmunol.1101058. [DOI] [PubMed] [Google Scholar]
- 47.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chakera A, et al. The phenotype of circulating follicular-helper T cells in patients with rheumatoid arthritis defines CD200 as a potential therapeutic target. Clin Dev Immunol. 2012;2012:948218. doi: 10.1155/2012/948218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weyand CM, Goronzy JJ. Ectopic germinal center formation in rheumatoid synovitis. Ann N Y Acad Sci. 2003;987:140–9. doi: 10.1111/j.1749-6632.2003.tb06042.x. [DOI] [PubMed] [Google Scholar]
- 50.Yu D, Vinuesa CG. The elusive identity of T follicular helper cells. Trends Immunol. 2010;31:377–83. doi: 10.1016/j.it.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Kamphorst AO, Ahmed R. Manipulating the PD-1 pathway to improve immunity. Curr Opin Immunol. 2013;25:381–8. doi: 10.1016/j.coi.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linterman MA, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–76. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morita R, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–21. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raptopoulou AP, et al. The programmed death 1/programmed death ligand 1 inhibitory pathway is up-regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis. Arthritis Rheum. 2010;62:1870–80. doi: 10.1002/art.27500. [DOI] [PubMed] [Google Scholar]
- 55.Wan B, et al. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol. 2006;177:8844–50. doi: 10.4049/jimmunol.177.12.8844. [DOI] [PubMed] [Google Scholar]
- 56.Hamel KM, et al. B7-H1 expression on non-B and non-T cells promotes distinct effects on T- and B-cell responses in autoimmune arthritis. Eur J Immunol. 2010;40:3117–27. doi: 10.1002/eji.201040690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakayamada S, et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity. 2012;35:919–31. doi: 10.1016/j.immuni.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin X, et al. Advances in distinguishing natural from induced Foxp3(+) regulatory T cells. Int J Clin Exp Pathol. 2013;6:116–23. [PMC free article] [PubMed] [Google Scholar]
- 59.Flores-Borja F, Jury EC, Mauri C, Ehrenstein MR. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2008;105:19396–401. doi: 10.1073/pnas.0806855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J Exp Med. 2007;204:33–9. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bayry J, Siberil S, Triebel F, Tough DF, Kaveri SV. Rescuing CD4+CD25+ regulatory T-cell functions in rheumatoid arthritis by cytokine-targeted monoclonal antibody therapy. Drug Discov Today. 2007;12:548–52. doi: 10.1016/j.drudis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 62.McGovern JL, et al. Th17 cells are restrained by Treg cells via the inhibition of interleukin-6 in patients with rheumatoid arthritis responding to anti-tumor necrosis factor antibody therapy. Arthritis Rheum. 2012;64:3129–38. doi: 10.1002/art.34565. [DOI] [PubMed] [Google Scholar]
- 63.Morgan ME, et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–21. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 64.Roord ST, et al. Autologous bone marrow transplantation in autoimmune arthritis restores immune homeostasis through CD4+CD25+Foxp3+ regulatory T cells. Blood. 2008;111:5233–41. doi: 10.1182/blood-2007-12-128488. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen LT, Jacobs J, Mathis D, Benoist C. Where FoxP3-dependent regulatory T cells impinge on the development of inflammatory arthritis. Arthritis Rheum. 2007;56:509–20. doi: 10.1002/art.22272. [DOI] [PubMed] [Google Scholar]
- 66.Crome SQ, Wang AY, Levings MK. Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol. 2010;159:109–19. doi: 10.1111/j.1365-2249.2009.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Afzali B, Mitchell P, Lechler RI, John S, Lombardi G. Translational mini-review series on Th17 cells: induction of interleukin-17 production by regulatory T cells. Clin Exp Immunol. 2010;159:120–30. doi: 10.1111/j.1365-2249.2009.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Connor RA, Taams LS, Anderton SM. Translational mini-review series on Th17 cells: CD4 T helper cells: functional plasticity and differential sensitivity to regulatory T cell-mediated regulation. Clin Exp Immunol. 2010;159:137–47. doi: 10.1111/j.1365-2249.2009.04040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valencia X, et al. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–61. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ehrenstein MR, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nie H, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat Med. 2013;19:322–8. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- 72.Ma HL, et al. Tumor necrosis factor alpha blockade exacerbates murine psoriasis-like disease by enhancing Th17 function and decreasing expansion of Treg cells. Arthritis Rheum. 2010;62:430–40. doi: 10.1002/art.27203. [DOI] [PubMed] [Google Scholar]
- 73.Herrath J, et al. The inflammatory milieu in the rheumatic joint reduces regulatory T-cell function. Eur J Immunol. 2011;41:2279–90. doi: 10.1002/eji.201041004. [DOI] [PubMed] [Google Scholar]
- 74.Koenen HJ, et al. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–52. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 75.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 76.Gal I, et al. Visualization and in situ analysis of leukocyte trafficking into the ankle joint in a systemic murine model of rheumatoid arthritis. Arthritis Rheum. 2005;52:3269–78. doi: 10.1002/art.21532. [DOI] [PubMed] [Google Scholar]
- 77.Svendsen P, et al. Tracking of proinflammatory collagen-specific T cells in early and late collagen-induced arthritis in humanized mice. J Immunol. 2004;173:7037–45. doi: 10.4049/jimmunol.173.11.7037. [DOI] [PubMed] [Google Scholar]
- 78.Szekanecz Z, Vegvari A, Szabo Z, Koch AE. Chemokines and chemokine receptors in arthritis. Front Biosci (Schol Ed) 2010;2:153–67. doi: 10.2741/s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szekanecz Z, Koch AE, Tak PP. Chemokine and chemokine receptor blockade in arthritis, a prototype of immune-mediated inflammatory diseases. Neth J Med. 2011;69:356–66. [PubMed] [Google Scholar]
- 80.Hirota K, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–12. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loetscher P, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–5. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 82.Qin S, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aggarwal A, Agarwal S, Misra R. Chemokine and chemokine receptor analysis reveals elevated interferon-inducible protein-10 (IP)-10/CXCL10 levels and increased number of CCR5+ and CXCR3+ CD4 T cells in synovial fluid of patients with enthesitis-related arthritis (ERA) Clin Exp Immunol. 2007;148:515–9. doi: 10.1111/j.1365-2249.2007.03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsui T, et al. Selective recruitment of CCR6-expressing cells by increased production of MIP-3 alpha in rheumatoid arthritis. Clin Exp Immunol. 2001;125:155–61. doi: 10.1046/j.1365-2249.2001.01542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aerts NE, et al. Increased IL-17 production by peripheral T helper cells after tumour necrosis factor blockade in rheumatoid arthritis is accompanied by inhibition of migration-associated chemokine receptor expression. Rheumatology (Oxford) 2010;49:2264–72. doi: 10.1093/rheumatology/keq224. [DOI] [PubMed] [Google Scholar]
- 86.Kobezda T, Ghassemi-Nejad S, Glant TT, Mikecz K. In vivo two-photon imaging of T cell motility in joint-draining lymph nodes in a mouse model of rheumatoid arthritis. Cell Immunol. 2012;278:158–65. doi: 10.1016/j.cellimm.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Szekanecz Z, et al. Anti-citrullinated protein antibodies in rheumatoid arthritis: as good as it gets? Clin Rev Allergy Immunol. 2008;34:26–31. doi: 10.1007/s12016-007-8022-5. [DOI] [PubMed] [Google Scholar]
- 88.Nell-Duxneuner V, et al. Autoantibody profiling in patients with very early rheumatoid arthritis - a follow-up study. Ann Rheum Dis. 2009 doi: 10.1136/ard.2008.100677. [DOI] [PubMed] [Google Scholar]
- 89.Klareskog L, Widhe M, Hermansson M, Ronnelid J. Antibodies to citrullinated proteins in arthritis: pathology and promise. Curr Opin Rheumatol. 2008;20:300–5. doi: 10.1097/BOR.0b013e3282fbd22a. [DOI] [PubMed] [Google Scholar]
- 90.Lakos G, et al. Anti-cyclic citrullinated peptide antibody isotypes in rheumatoid arthritis: association with disease duration, rheumatoid factor production and the presence of shared epitope. Clin Exp Rheumatol. 2008;26:253–60. [PubMed] [Google Scholar]
- 91.Baeten D, et al. Specific presence of intracellular citrullinated proteins in rheumatoid arthritis synovium: relevance to antifilaggrin autoantibodies. Arthritis Rheum. 2001;44:2255–62. doi: 10.1002/1529-0131(200110)44:10<2255::aid-art388>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 92.Chang X, et al. Localization of peptidylarginine deiminase 4 (PADI4) and citrullinated protein in synovial tissue of rheumatoid arthritis. Rheumatology (Oxford) 2005;44:40–50. doi: 10.1093/rheumatology/keh414. [DOI] [PubMed] [Google Scholar]
- 93.Caspi D, et al. Synovial fluid levels of anti-cyclic citrullinated peptide antibodies and IgA rheumatoid factor in rheumatoid arthritis, psoriatic arthritis, and osteoarthritis. Arthritis Rheum. 2006;55:53–6. doi: 10.1002/art.21691. [DOI] [PubMed] [Google Scholar]
- 94.Sokolove J, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7:e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fisher BA, et al. Heterogeneity of anticitrullinated peptide antibodies and response to anti-tumor necrosis factor agents in rheumatoid arthritis. J Rheumatol. 2012;39:929–32. doi: 10.3899/jrheum.111315. [DOI] [PubMed] [Google Scholar]
- 96.Lundberg K, et al. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008;58:3009–19. doi: 10.1002/art.23936. [DOI] [PubMed] [Google Scholar]
- 97.Cantaert T, et al. Alterations of the synovial T cell repertoire in anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheum. 2009;60:1944–56. doi: 10.1002/art.24635. [DOI] [PubMed] [Google Scholar]
- 98.Kidd BA, et al. Epitope spreading to citrullinated antigens in mouse models of autoimmune arthritis and demyelination. Arthritis Res Ther. 2008;10:R119. doi: 10.1186/ar2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Glant TT, et al. Proteoglycan-induced arthritis and recombinant human proteoglycan aggrecan G1 domain-induced arthritis in BALB/c mice resembling two subtypes of rheumatoid arthritis. Arthritis Rheum. 2011;63:1312–21. doi: 10.1002/art.30261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raza K, Mullazehi M, Salmon M, Buckley CD, Ronnelid J. Anti-collagen type II antibodies in patients with very early synovitis. Ann Rheum Dis. 2008;67:1354–5. doi: 10.1136/ard.2007.084277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang H, Benoist C, Mathis D. Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc Natl Acad Sci U S A. 2010;107:4658–63. doi: 10.1073/pnas.1001074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mikecz K, Glant TT, Buzas E, Poole AR. Proteoglycan-induced polyarthritis and spondylitis adoptively transferred to naive (nonimmunized) BALB/c mice. Arthritis Rheum. 1990;33:866–76. doi: 10.1002/art.1780330614. [DOI] [PubMed] [Google Scholar]
- 103.Keystone E. Treatments no longer in development for rheumatoid arthritis. Ann Rheum Dis. 2002;61(Suppl 2):ii43–5. doi: 10.1136/ard.61.suppl_2.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Genovese MC, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–23. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 105.Liossis SN, Sfikakis PP. Rituximab-induced B cell depletion in autoimmune diseases: potential effects on T cells. Clin Immunol. 2008;127:280–5. doi: 10.1016/j.clim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 106.Feuchtenberger M, et al. Frequency of Regulatory T Cells is Not Affected by Transient B Cell Depletion Using Anti-CD20 Antibodies in Rheumatoid Arthritis. Open Rheumatol J. 2008;2:81–8. doi: 10.2174/1874312900802010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wilk E, et al. Depletion of functionally active CD20+ T cells by rituximab treatment. Arthritis Rheum. 2009;60:3563–71. doi: 10.1002/art.24998. [DOI] [PubMed] [Google Scholar]
- 108.Vergunst CE, et al. MLN3897 plus methotrexate in patients with rheumatoid arthritis: safety, efficacy, pharmacokinetics, and pharmacodynamics of an oral CCR1 antagonist in a phase IIa, double-blind, placebo-controlled, randomized, proof-of-concept study. Arthritis Rheum. 2009;60:3572–81. doi: 10.1002/art.24978. [DOI] [PubMed] [Google Scholar]
- 109.Egelston C, et al. Suppression of dendritic cell maturation and t cell proliferation by synovial fluid myeloid cells from mice with autoimmune arthritis. Arthritis Rheum. 2012;64:3179–88. doi: 10.1002/art.34494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marijnissen RJ, et al. Increased expression of interleukin-22 by synovial Th17 cells during late stages of murine experimental arthritis is controlled by interleukin-1 and enhances bone degradation. Arthritis Rheum. 2011;63:2939–48. doi: 10.1002/art.30469. [DOI] [PubMed] [Google Scholar]
- 111.Niu X, et al. IL-21 regulates Th17 cells in rheumatoid arthritis. Hum Immunol. 2010;71:334–41. doi: 10.1016/j.humimm.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 112.Keystone EC. Abandoned therapies and unpublished trials in rheumatoid arthritis. Curr Opin Rheumatol. 2003;15:253–8. doi: 10.1097/00002281-200305000-00012. [DOI] [PubMed] [Google Scholar]
- 113.de Vries R. Genetics of rheumatoid arthritis: time for a change! Curr Opin Rheumatol. 2011;23:227–32. doi: 10.1097/BOR.0b013e3283457524. [DOI] [PubMed] [Google Scholar]
- 114.Besenyei T, et al. Non-MHC risk alleles in rheumatoid arthritis and in the syntenic chromosome regions of corresponding animal models. Clin Dev Immunol. 2012:284751. doi: 10.1155/2012/284751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang L, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–2. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012;181:8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.